Preparation of Polytetrafluoroethylene Superhydrophobic Materials by Femtosecond Laser Processing Technology
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
2.2. Characterization
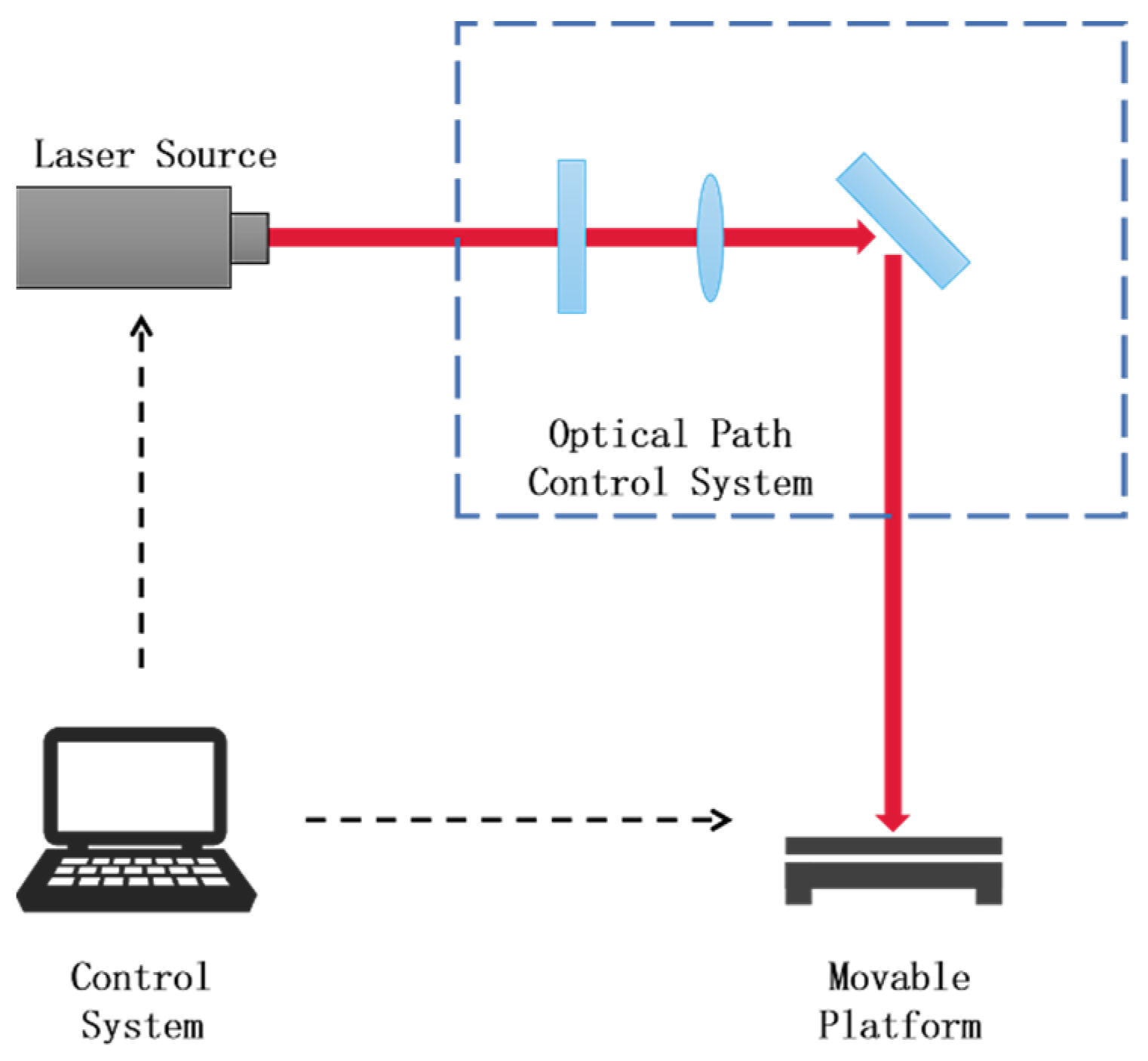
2.3. Processing
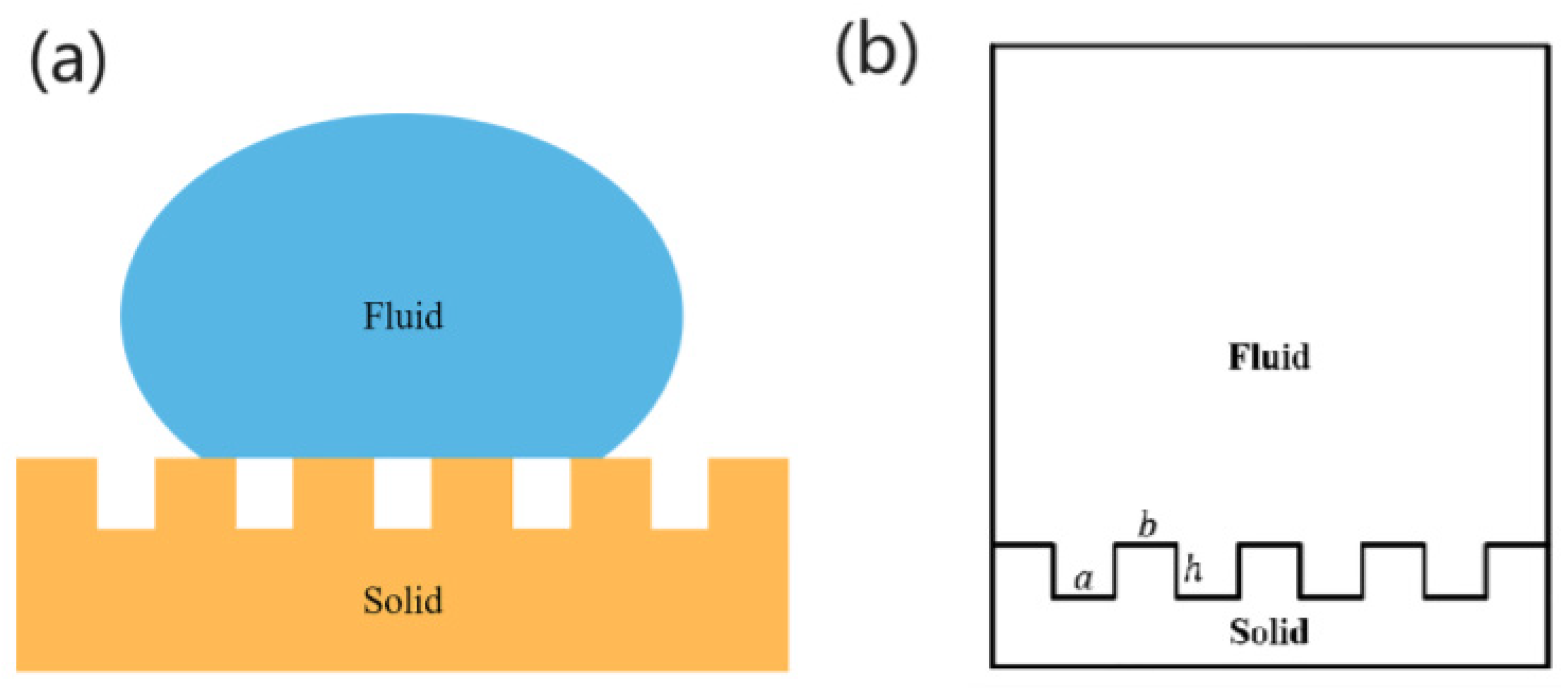
2.4. Theoretical Analysis
2.5. Simulation
3. Results and Discussion
3.1. Simulation Result
- The contact area of the water droplets on the solid–liquid contact surface, relative to the projected area of the solid–liquid contact surface, decreases;
- The contact angle of the water droplets on the solid surface increases;
- The hydrophobicity of the solid surface is significantly enhanced.
3.2. Hydrophobic and Oleophobic Properties
4. Conclusions
- Combining theoretical derivation based on the Cassie model with experimental data, the study concludes that for PTFE surfaces with different microstructure patterns, the larger the percentage of droplet–solid contact area relative to the projection area of the solid–liquid contact interface, the higher the contact angle and the better the superhydrophilicity of the surface;
- The study uses ANSYS software to simulate droplet states on various microstructure surfaces, guiding the design of the dimensions for femtosecond laser processing. The simulation results are in good agreement with the experimental results. The study successfully applies femtosecond laser processing technology to micro–nanostructuring the PTFE surface, producing a superhydrophobic surface with a contact angle of 166° and an oil contact angle of 135°.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.D.; Zhu, Z.Y.; Xu, T.Z.; Abadikhah, H.; Wang, J.W.; Xu, X.; Agathopoulos, S. Fabrication and characterization of robust hydrophobic lotus leaf-like surface on Si3N4 porous membrane via polymer-derived SiNCO inorganic nanoparticle modification. Ceram. Int. 2018, 44, 16443–16449. [Google Scholar] [CrossRef]
- Yang, L.; Shen, X.; Yang, Q.; Liu, J.; Wu, W.; Li, D.; Du, J.; Zhang, B.; Fan, S. Fabrication of biomimetic anisotropic super-hydrophobic surface with rice leaf-like structures by femtosecond laser. Opt. Mater. 2021, 112, 110740. [Google Scholar] [CrossRef]
- Tingkun, C.; Qian, C.; Yingchun, Q.; Jingfu, J.; Kwang-Leong, C.; Jie, Z. Hydrophobic durability characteristics of butterfly wing surface after freezing cycles towards the design of nature inspired anti-icing surfaces. PLoS ONE 2018, 13, e0188775. [Google Scholar]
- Zang, D.; Xun, X.; Gu, Z.; Dong, J.; Pan, T.; Liu, M. Fabrication of superhydrophobic self-cleaning manganese dioxide coatings on mg alloys inspired by lotus flower. Ceram. Int. 2020, 46, 20328–20334. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Sun, S.; Zhang, H.; Zhou, R.; Li, Y.; Liang, Y.; Wang, J. Preparation of a temperature-sensitive superhydrophobic self-cleaning sio2-tio2@pdms coating with photocatalytic activity. Surf. Coat. Technol. 2021, 408, 126853. [Google Scholar] [CrossRef]
- Jin, Z.; Mei, H.; Pan, L.; Liu, H.; Cheng, L. Superhydrophobic self-cleaning hierarchical micro-/nanocomposite coating with high corrosion resistance and durability. ACS Sustain. Chem. Eng. 2021, 9, 4111–4121. [Google Scholar] [CrossRef]
- Wang, P.; Yao, T.; Li, Z.; Wei, W.; Xie, Q.; Duan, W.; Han, H. A superhydrophobic/electrothermal synergistically anti-icing strategy based on graphene composite—Sciencedirect. Compos. Sci. Technol. 2020, 198, 108307. [Google Scholar] [CrossRef]
- Zhu, T.; Cheng, Y.; Huang, J.; Xiong, J.; Ge, M.; Mao, J.; Liu, Z.; Dong, X.; Chen, Z.; Lai, Y. A transparent superhydrophobic coating with mechanochemical robustness for anti-icing, photocatalysis and self-cleaning. Chem. Eng. J. 2020, 399, 125746. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Liu, H.; Guan, Y. One-step fabrication of robust superhydrophobic steel surfaces with mechanical durability, thermal stability, and anti-icing function. ACS Appl. Mater. Interfaces 2019, 11, 25586–25594. [Google Scholar] [CrossRef]
- Guo, Z.; Long, B.; Gao, S.; Luo, J.; Wang, L.; Huang, X.; Wang, D.; Xue, H.; Gao, J. Carbon nanofiber based superhydrophobic foam composite for high performance oil/water separation. J. Hazard. Mater. 2021, 402, 123838. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Sadasivuni, K.K.; Liu, S. Superhydrophobic surfaces for oil-water separation—Sciencedirect. In Superhydrophobic Polymer Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 339–356. [Google Scholar]
- Ren, G.; Song, Y.; Li, X.; Zhou, Y.; Zhang, Z.; Zhu, X. A superhydrophobic copper mesh as an advanced platform for oil-water separation. Appl. Surf. Sci. 2018, 428, 520–525. [Google Scholar] [CrossRef]
- SSeo, J.; Lee, S.K.; Lee, J.; Seung Lee, J.; Kwon, H.; Cho, S.W.; Ahn, J.H.; Lee, T. Path-programmable water droplet manipulations on an adhesion controlled superhydrophobic surface. Sci. Rep. 2015, 5, 12326. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, J.; Guo, Z. Tomato-lotus inspired edible superhydrophobic artificial lotus. Chem. Eng. J. 2020, 400, 125883. [Google Scholar]
- Ragesh, P.; Ganesh, V.A.; Nair, S.V.; Nair, A.S. A review on ‘self-cleaning and multifunctional materials’. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Gardiner, J. Fluoropolymers: Origin, production, and industrial and commercial applications. Aust. J. Chem. 2014, 68, 13–22. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, C.-H.; Cao, M.; Zhang, M.-M.; Li, M.; Ma, J.-Z. Highly transparent fluorine-free superhydrophobic silica nanotube coating. Chem. Eng. J. 2017, 320, 244–252. [Google Scholar] [CrossRef]
- Roslizar, A.; Dottermusch, S.; Vüllers, F.; Kavalenka, M.N.; Guttmann, M.; Schneider, M.; Paetzold, U.W.; Hölscher, H.; Richards, B.S.; Klampaftis, E. Self-cleaning performance of superhydrophobic hot-embossed fluoropolymer films for photovoltaic modules. Sol. Energy Mater. Sol. Cells 2019, 189, 188–196. [Google Scholar] [CrossRef]
- Li, K.; Hernandez-Castro, J.A.; Turcotte, K.; Morton, K.; Veres, T. Superhydrophobic thermoplastic surfaces with hierarchical micro-nanostructures fabricated by hot-embossing. In 2020 IEEE 20th International Conference on Nanotechnology (IEEE-NANO); IEEE: New York, NY, USA, 2020. [Google Scholar]
- Zhu, Y.; Sun, F.; Qian, H.; Wang, H.; Mu, L.; Zhu, J. A biomimetic spherical cactus superhydrophobic coating with durable and multiple anti-corrosion effects. Chem. Eng. J. 2018, 338, 670–679. [Google Scholar] [CrossRef]
- Wang, F.; Ci, J.; Di, Y.; Zhang, W. Preparation and properties of multiwalled carbon nanotube/waterborne polyurethane superhydrophobic conductive coatings using electrostatic spraying. J. Mater. Eng. Perform. 2022, 31, 7228–7239. [Google Scholar] [CrossRef]
- Ren, X.; Guo, H.; Ma, X.; Hou, G.; Chen, L.; Xu, X.; Chen, Q.; Feng, J.; Si, P.; Zhang, L.; et al. Improved interfacial floatability of superhydrophobic and compressive s, n co-doped graphene aerogel by electrostatic spraying for highly efficient organic pollutants recovery from water. Appl. Surf. Sci. 2018, 457, 780–788. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Das, R.; Hamid, S.B.A.; Hashim, N.A.; AlOmar, M.K.; Alayan, H.M.; Novikov, M.; Alsalhy, Q.F.; Hashim, M.A. Optimization of the synthesis of superhydrophobic carbon nanomaterials by chemical vapor deposition. Sci. Rep. 2018, 8, 2778. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, E.; Azizian, S.; Noei, N. Preparation of robust superhydrophobic sand by chemical vapor deposition of polydimethylsiloxane for oil/water separation. Macromol. Mater. Eng. 2020, 305, 2000425. [Google Scholar] [CrossRef]
- Pour, F.Z.; Karimi, H.; Avargani, V.M. Preparation of a superhydrophobic and superoleophilic polyester textile by chemical vapor deposition of dichlorodimethylsilane for water–oil separation. Polyhedron 2019, 159, 54–63. [Google Scholar] [CrossRef]
- Li, B.; Ouyang, Y.; Haider, Z.; Zhu, Y.; Qiu, R.; Hu, S.; Niu, H.; Zhang, Y.; Chen, M. One-step electrochemical deposition leading to superhydrophobic matrix for inhibiting abiotic and microbiologically influenced corrosion of Cu in seawater environment. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 616, 126337. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Zhao, G.; Taleb, A.; Jin, Y. Fabrication of ni co coating by electrochemical deposition with high super-hydrophobic properties for corrosion protection. Surf. Coat. Technol. 2019, 363, 352–361. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Y.; Ban, C.; Xue, Y.; Taleb, A.; Jin, Y. Fabrication of robust tungsten carbide particles reinforced co ni super-hydrophobic composite coating by electrochemical deposition. Surf. Coat. Technol. 2020, 385, 125390. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.; Tang, J.; Gong, H.; Liu, Y.; Wang, Z.; Shi, P.; Zhang, J.; Chen, X. A novel preparation of polystyrene film with a superhydrophobic surface using a template method. J. Phys. D Appl. Phys. 2007, 40, 3485–3489. [Google Scholar] [CrossRef]
- Yan, Z.; Liang, X.; Shen, H.; Liu, Y. Preparation and basic properties of superhydrophobic silicone rubber with micro-nano hierarchical structures formed by picosecond laser-ablated template. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1743–1750. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, A.-D.; Li, B.; Cui, T.-H.; Lu, Y.-F. Electrons dynamics control by shaping femtosecond laser pulses in micro/nanofabrication: Modeling, method, measurement and application. Light. Sci. Appl. 2018, 7, 17134. [Google Scholar] [CrossRef]
- Yao, Y.L.; Chen, H.; Zhang, W. Time scale effects in laser material removal: A review. Int. J. Adv. Manuf. Technol. 2005, 26, 598–608. [Google Scholar] [CrossRef]
- Alvarez, P.; Azucena, A. Micro/Nano Patterning of Silicon and Nip/Al Disks by Nanosecond and Femtosecond Laser Sources. Ph.D. Thesis, University of Manchester, Manchester, UK, 2012. [Google Scholar]
- Yin, K.; Wu, J.; Deng, Q.; Wu, Z.; Wu, T.; Luo, Z.; Jiang, J.; Duan, J.A. Tailoring micro/nanostructured porous polytetrafluoroethylene surfaces for dual-reversible transition of wettability and transmittance. Chem. Eng. J. 2022, 434, 134756. [Google Scholar] [CrossRef]
- De Palo, R.; Mazzarone, A.E.; Volpe, A.; Gaudiuso, C.; Mezzapesa, F.P.; Spagnolo, V.; Ancona, A. Investigation of Laser-Induced Surface Structures (LIPSS) on quartz and evaluation of their influence on material wettability. Opt. Laser Technol. 2024, 169, 110097. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.-T.; Xu, S.; Yang, H.; Sun, H.-B. Bioinspired superhydrophobic surfaces via laser-structuring. Front. Chem. 2020, 8, 835. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Gaudiuso, C.; Ancona, A. Laser fabrication of anti-icing surfaces: A review. Materials 2020, 13, 5692. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, J.; Yin, K.; Zhu, Z.; Xiao, S.; Wu, Z.; Duan, J.-A. Robust hierarchical porous ptfe film fabricated via femtosecond laser for self-cleaning passive cooling. Nano Lett. 2021, 21, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, T.; Abhinanadhan, K.; Anilkumar, C.; Sathyanarayan, S. Experimental study on mechanical characteristics of ptfe material. Appl. Mech. Mater. 2019, 895, 224–229. [Google Scholar] [CrossRef]
- Womack, M.; Vendan, M.; Molian, P. Femtosecond pulsed laser ablation and deposition of thin films of polytetrafluoroethylene. Appl. Surf. Sci. 2004, 221, 99–109. [Google Scholar] [CrossRef]
- Dong, Y.; Chi, J.; Ren, Z.; Xiong, B.; Liu, Z.; Zhang, W.; Wang, L.; Fujii, S.; Armes, S.P.; Ning, Y. Controlled deformation of soft nanogel particles generates artificial biominerals with ordered internal structure. Angew. Chem. 2023, 62, e202300031. [Google Scholar] [CrossRef]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Della Volpe, C.; Hołysz, L.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2019, 8, 3–27. [Google Scholar] [CrossRef]
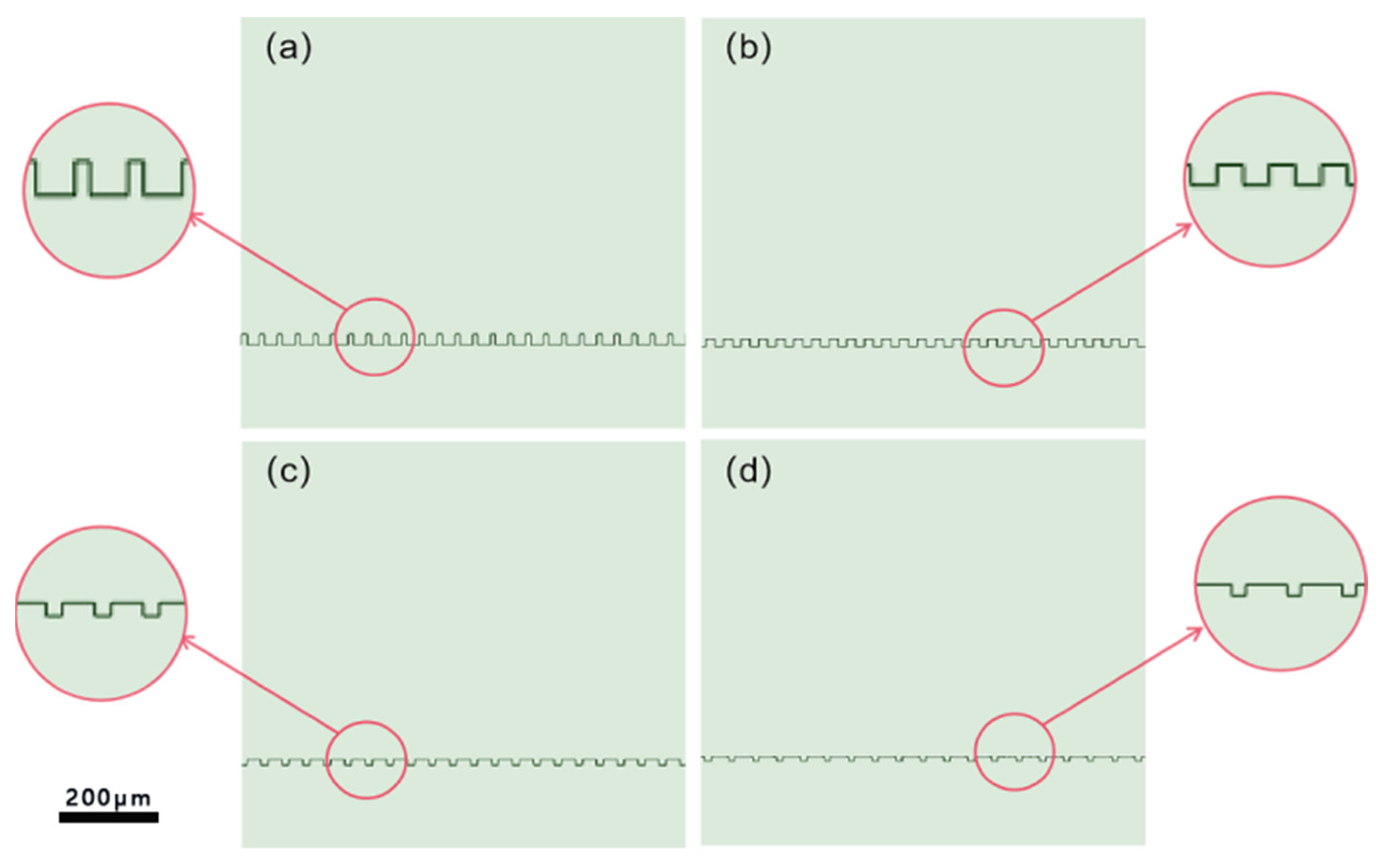



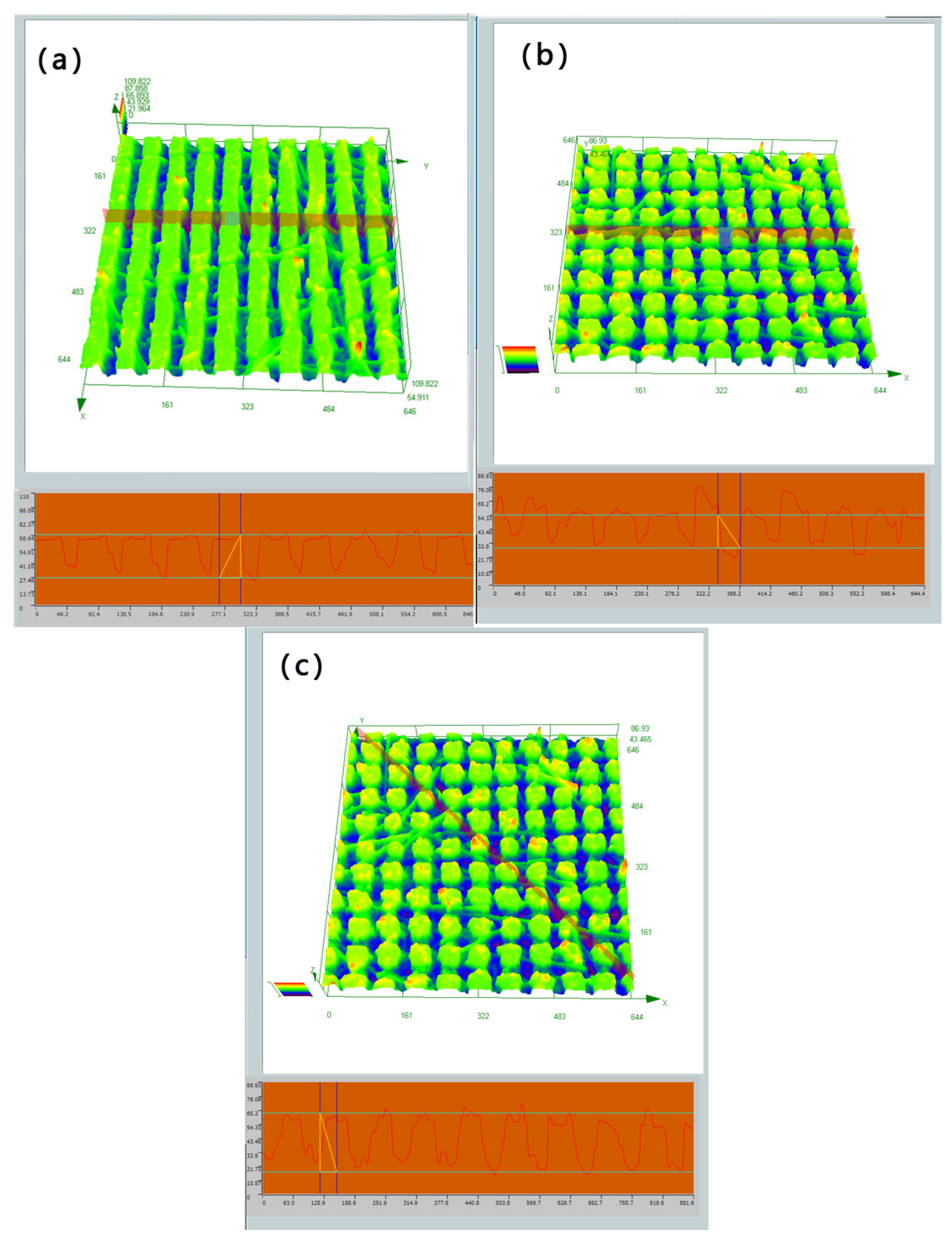
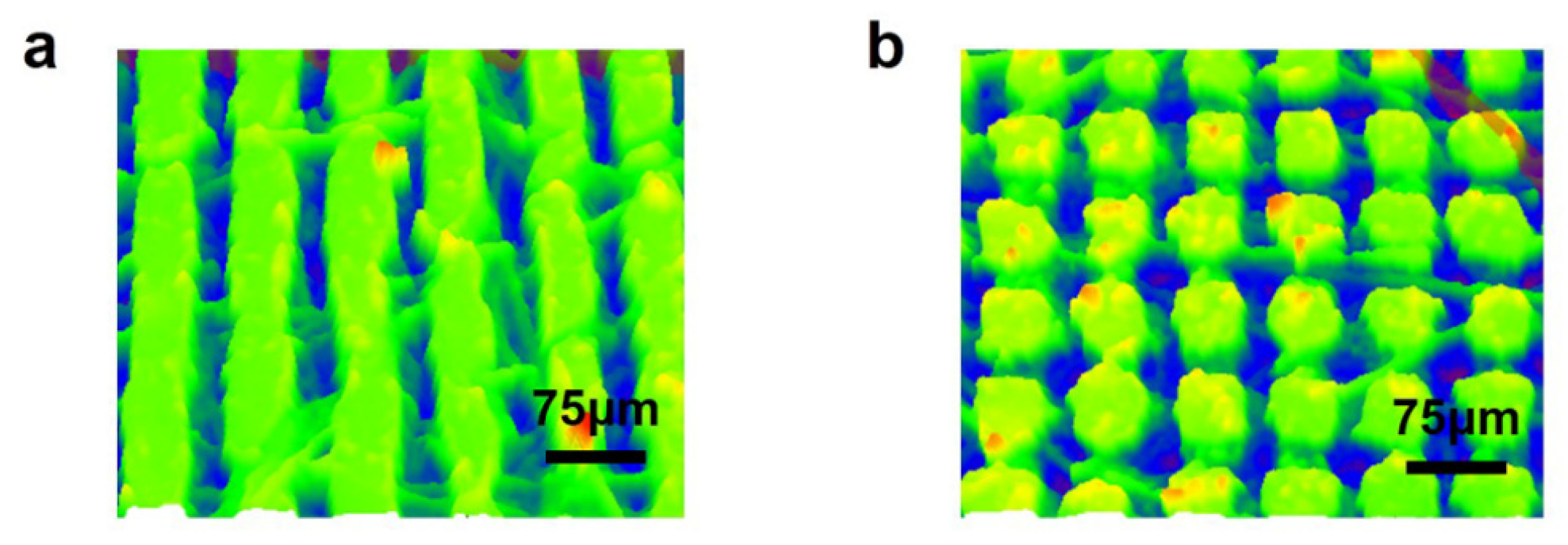


| Processing Parameters | Laser Fluence | Scanning Speed | Repetition Rate | Pulse Duration | Scanning Pitch | Scanning Route |
|---|---|---|---|---|---|---|
| Grid | 1.43 J/cm2 | 500 mm/s | 500 kHz | 500 fs | 50/100 μm | Lines |
| Columnar | 1.43 J/cm2 | 500 mm/s | 500 kHz | 500 fs | 50/100 μm | Crossed |
| T | 1:3 | 1:2 | 1:1 | 5:2 |
|---|---|---|---|---|
| Simulation Results | 146° | 146.4° | 160.4° | 165.9° |
| Calculation Results | 132° | 137.4° | 148.4° | 162.1° |
| Error Value | 9.6% | 6.1% | 7.5% | 2.3% |
| 0.75 | 0.67 | 0.5 | 0.29 |
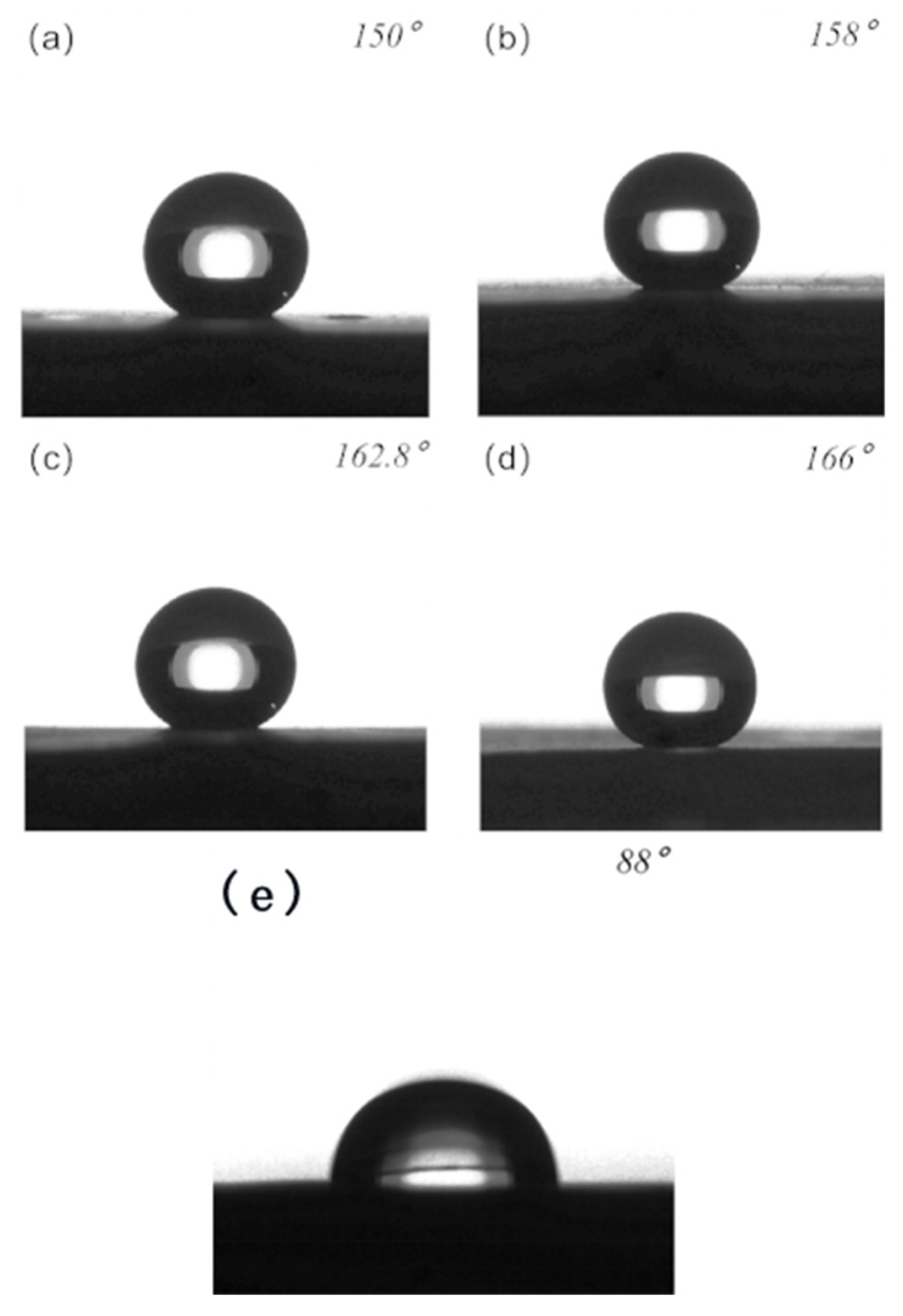
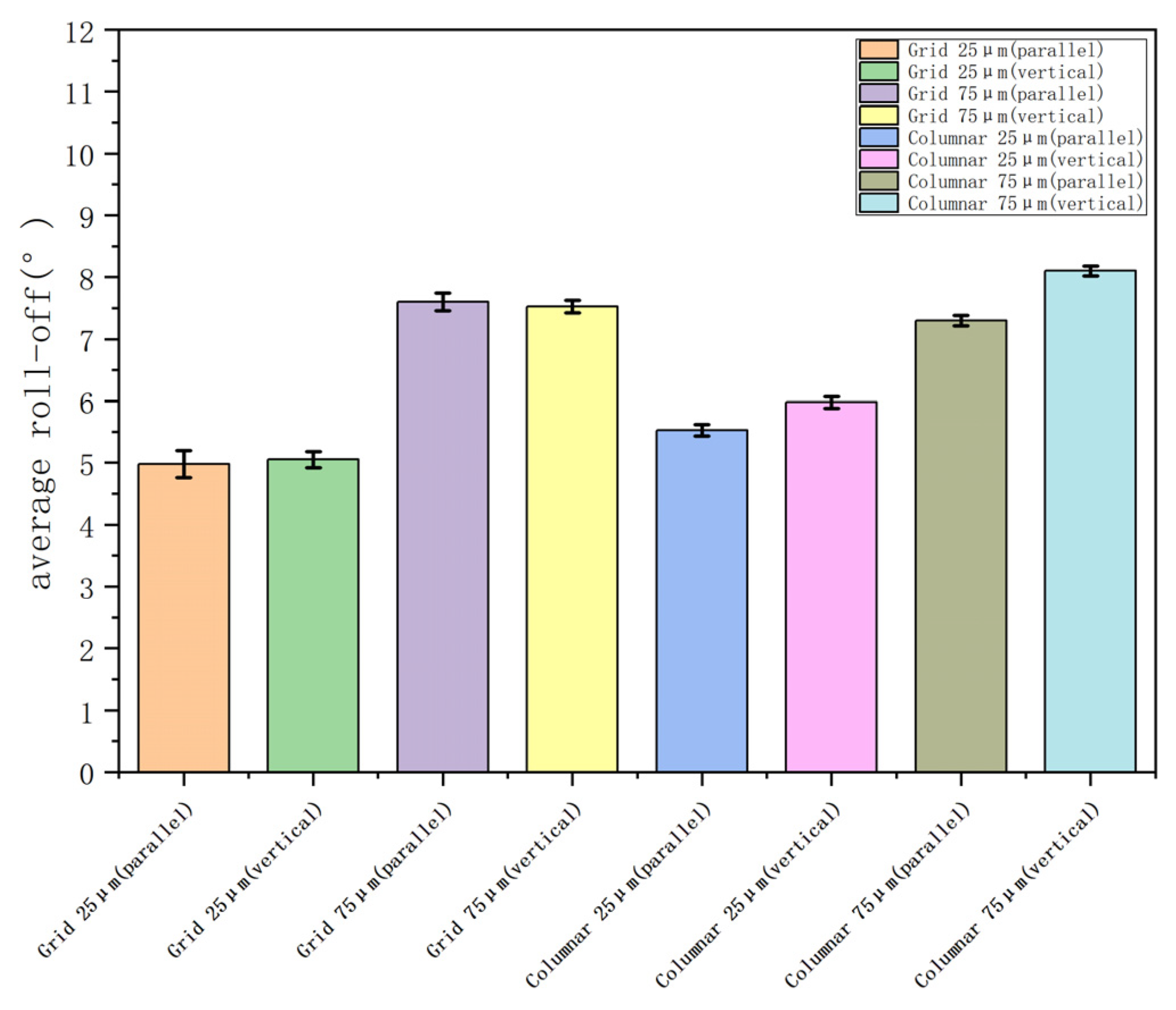
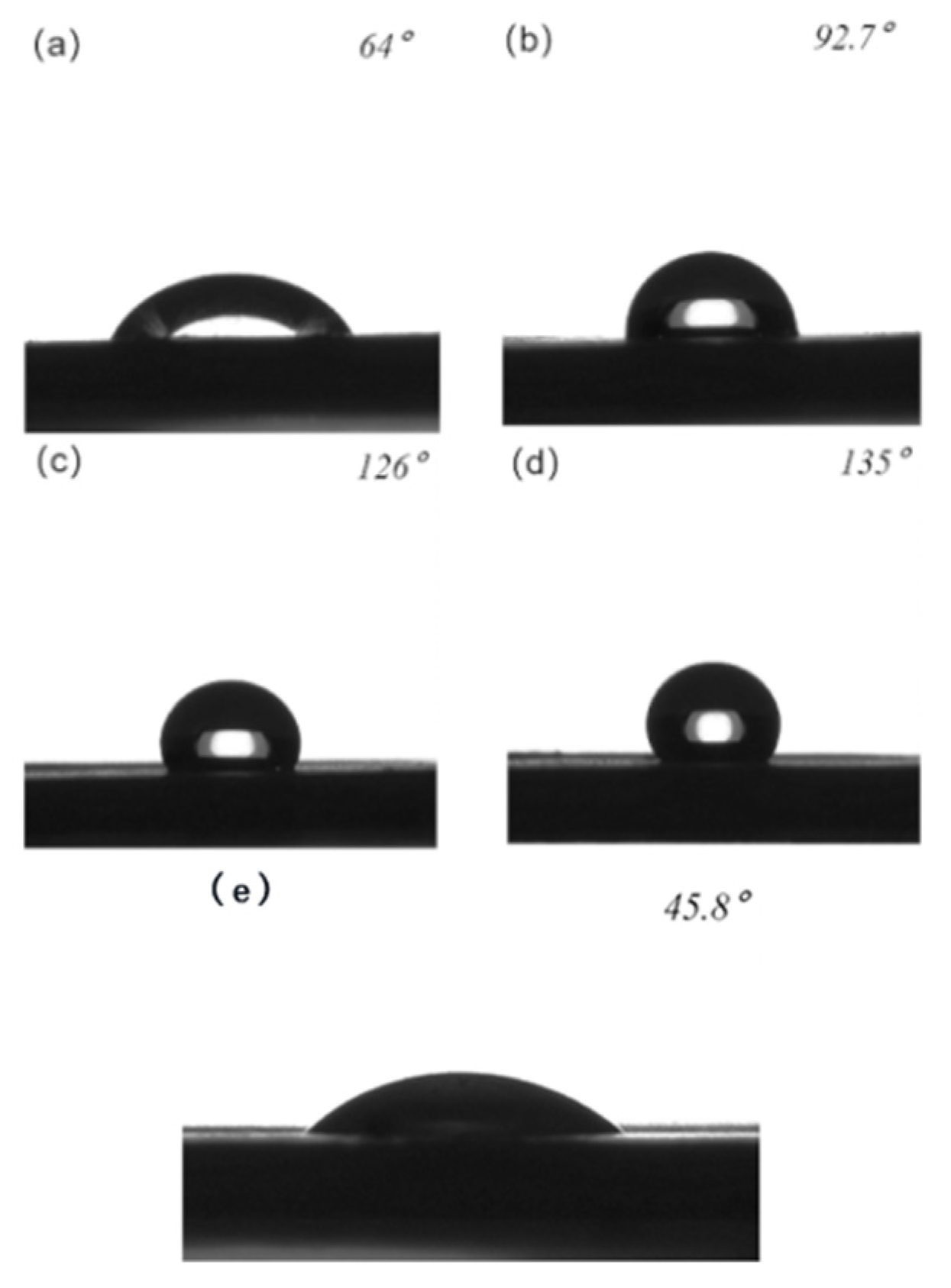
| Structure | Columnar 75 μm | Columnar 25 μm | Grid 75 μm | Grid 25 μm |
|---|---|---|---|---|
| 0.75 | 0.5 | 0.56 | 0.25 | |
| Contact angle | 150° | 158° | 162.8° | 166° |
| Oil contact angle | 64° | 92.7° | 126° | 135° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Hu, Y.; Huang, Y.; Xu, H.; Wu, D.; Wu, D.; Gao, X. Preparation of Polytetrafluoroethylene Superhydrophobic Materials by Femtosecond Laser Processing Technology. Polymers 2024, 16, 43. https://doi.org/10.3390/polym16010043
Zhou S, Hu Y, Huang Y, Xu H, Wu D, Wu D, Gao X. Preparation of Polytetrafluoroethylene Superhydrophobic Materials by Femtosecond Laser Processing Technology. Polymers. 2024; 16(1):43. https://doi.org/10.3390/polym16010043
Chicago/Turabian StyleZhou, Shuangquan, Yayue Hu, Yao Huang, Hong Xu, Daming Wu, Dong Wu, and Xiaolong Gao. 2024. "Preparation of Polytetrafluoroethylene Superhydrophobic Materials by Femtosecond Laser Processing Technology" Polymers 16, no. 1: 43. https://doi.org/10.3390/polym16010043
APA StyleZhou, S., Hu, Y., Huang, Y., Xu, H., Wu, D., Wu, D., & Gao, X. (2024). Preparation of Polytetrafluoroethylene Superhydrophobic Materials by Femtosecond Laser Processing Technology. Polymers, 16(1), 43. https://doi.org/10.3390/polym16010043






