Anthocyanin-Loaded Polymers as Promising Nature-Based, Responsive, and Bioactive Materials
Abstract
1. Introduction
2. Extraction Procedures for Anthocyanins
3. Biological Properties of Anthocyanins
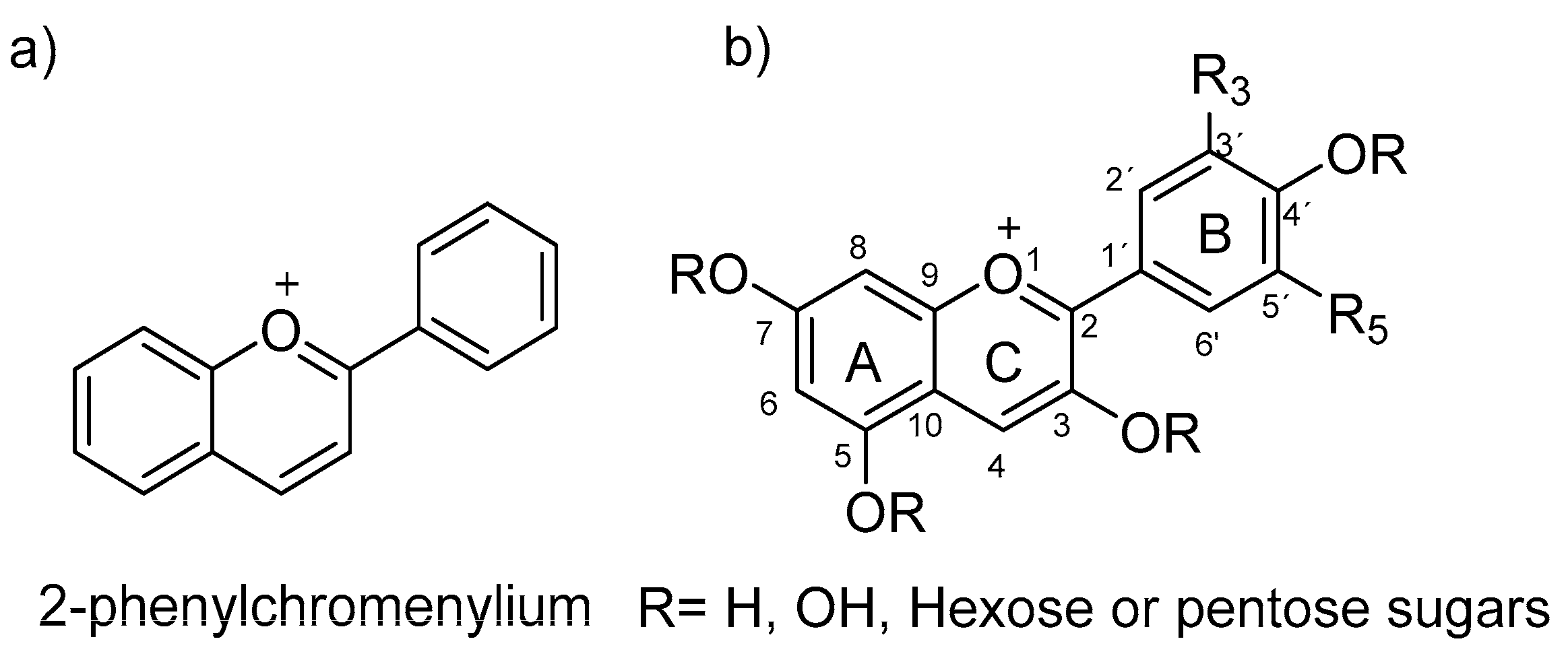
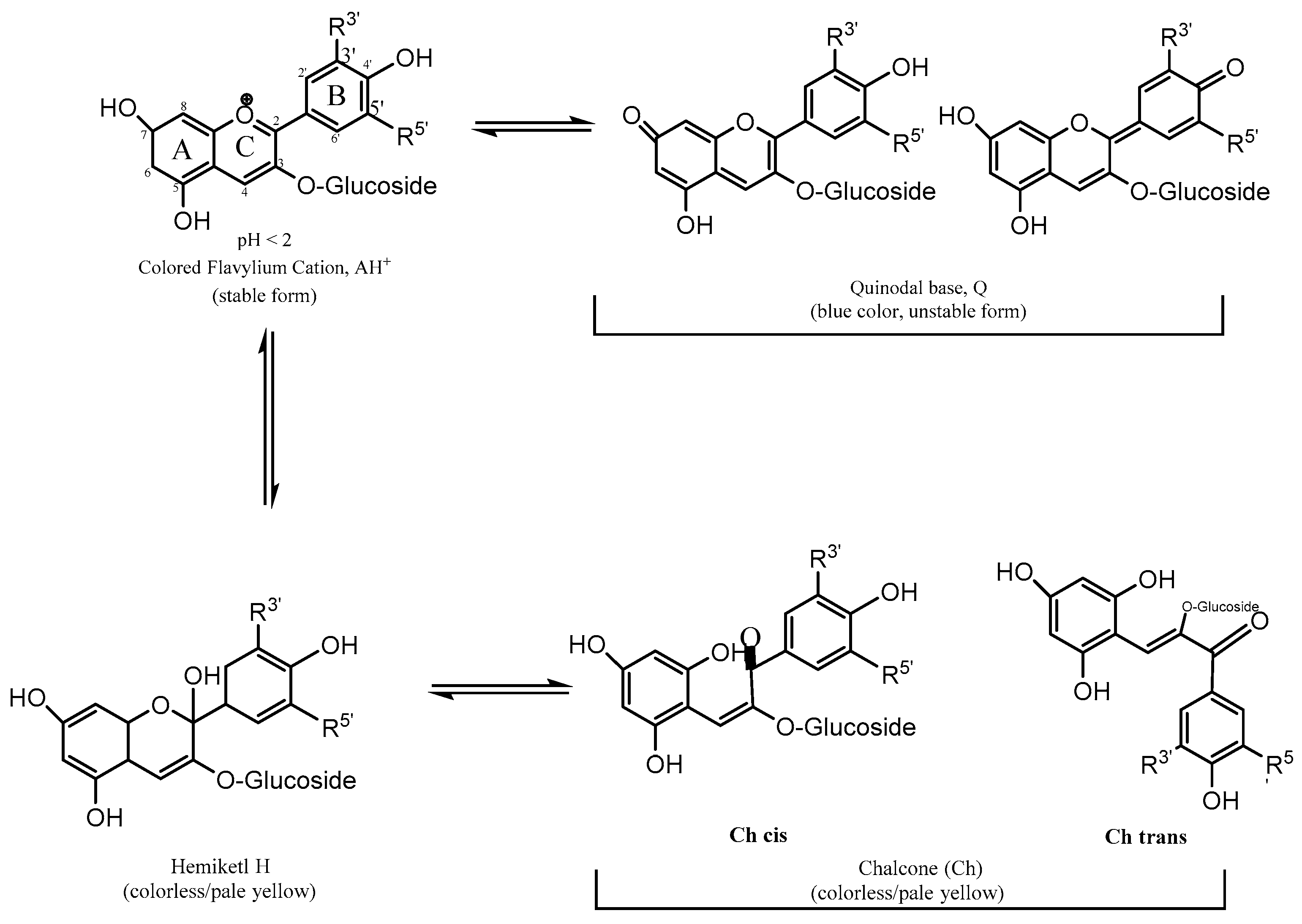
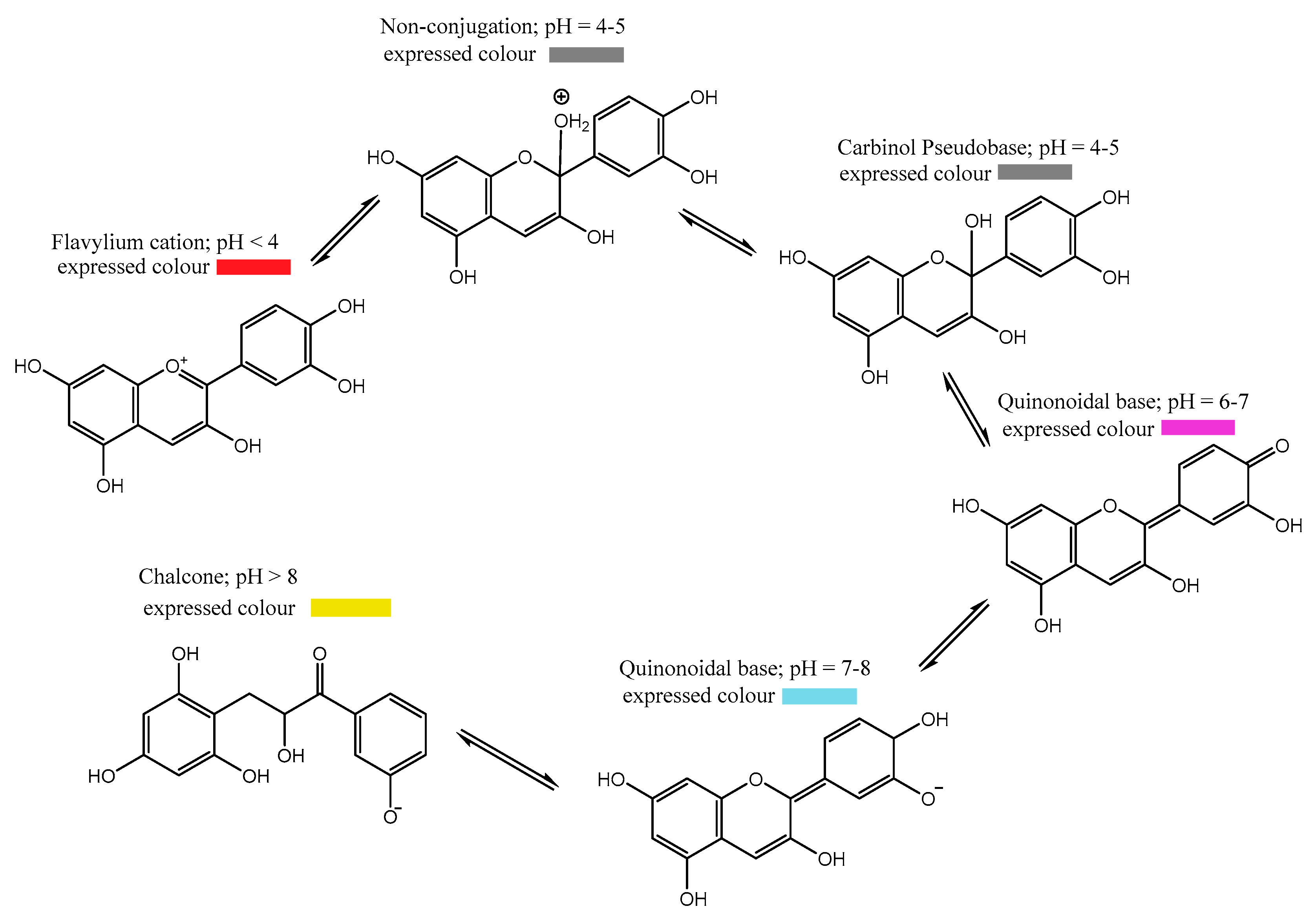
4. Halochromic Properties of Anthocyanins
5. Anthocyanin-Loaded Polymers: Preparation and Characterization Methods
5.1. Films
5.2. Mats and Fibers
5.3. Hydrogels
5.4. Polyelectrolyte Complexes
5.5. Nanoparticles
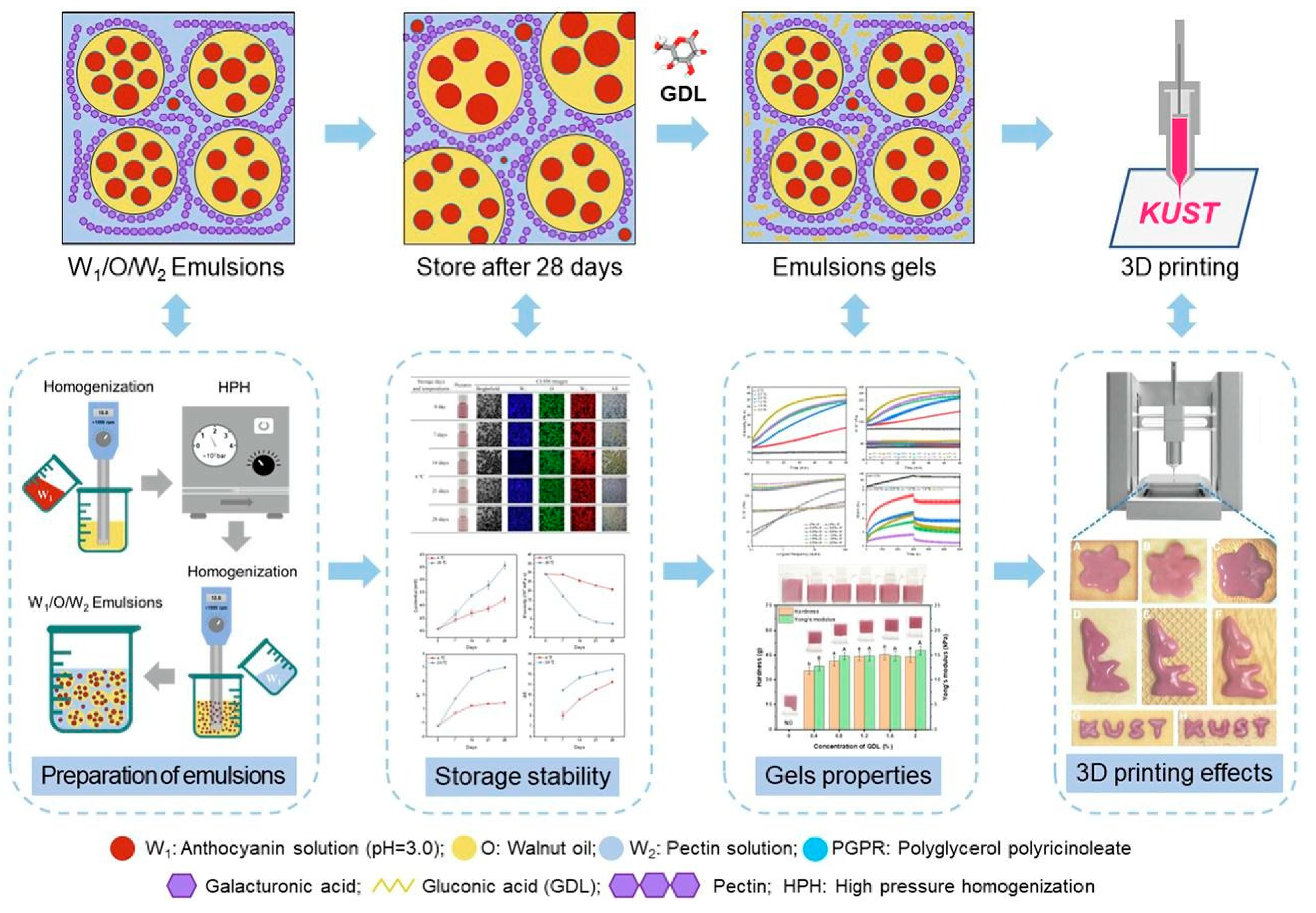
5.6. Emulsions
5.7. Self-Assembled Liposomes, Proteins, Peptides, and Phospholipids
5.8. Microencapsulates
5.9. Specific Characterization of Anthocyanin-Loaded Polymer
6. Anthocyanin-Based Polymers for Food Applications
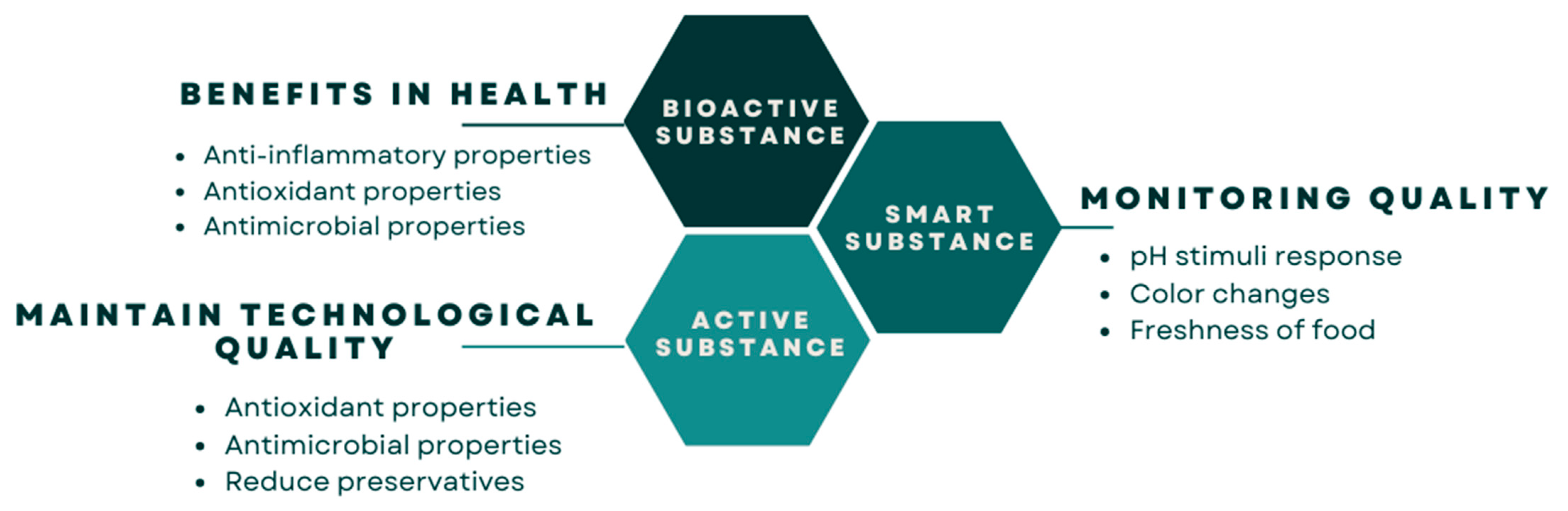
6.1. Anthocyanins as Bioactive Substances
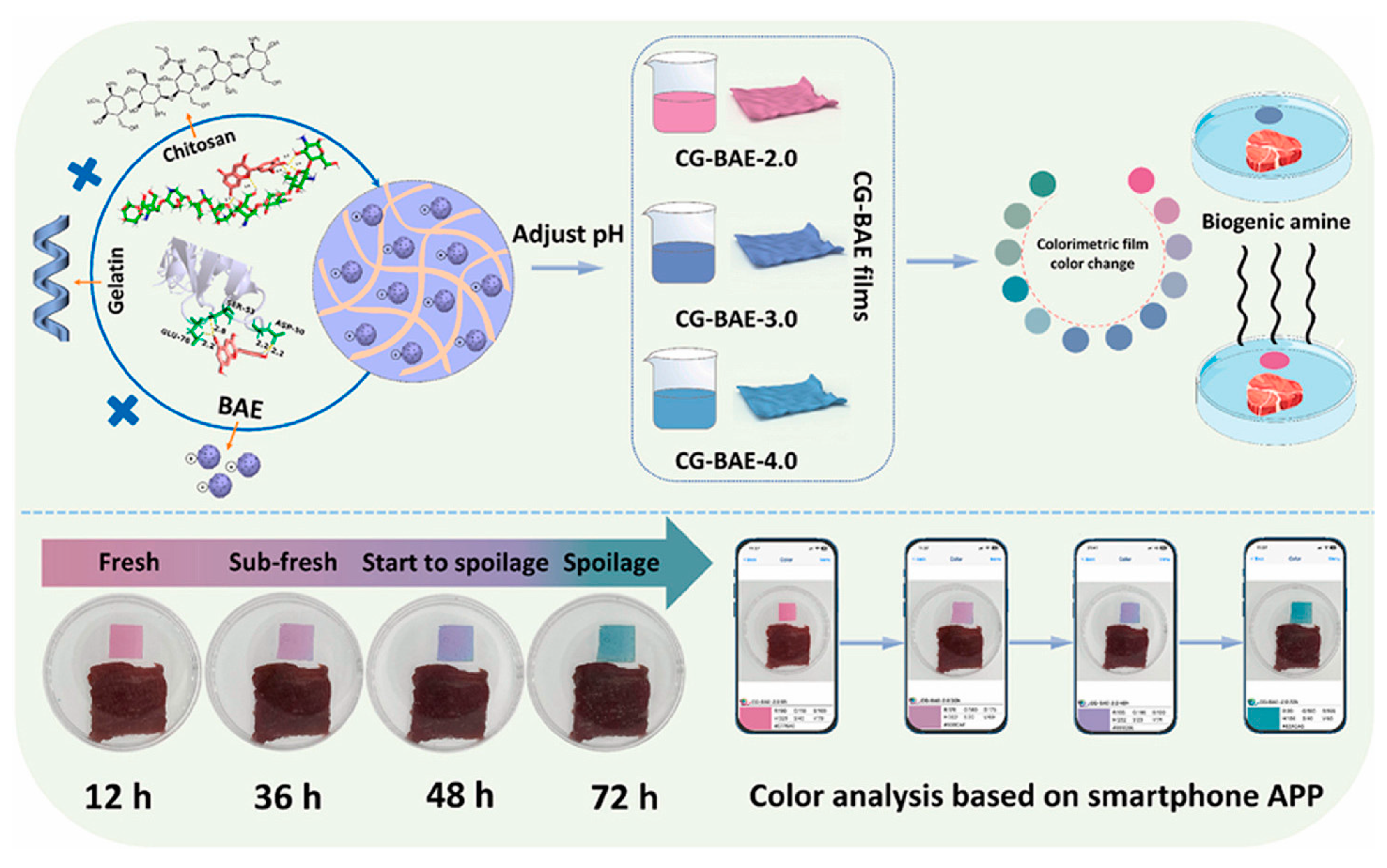
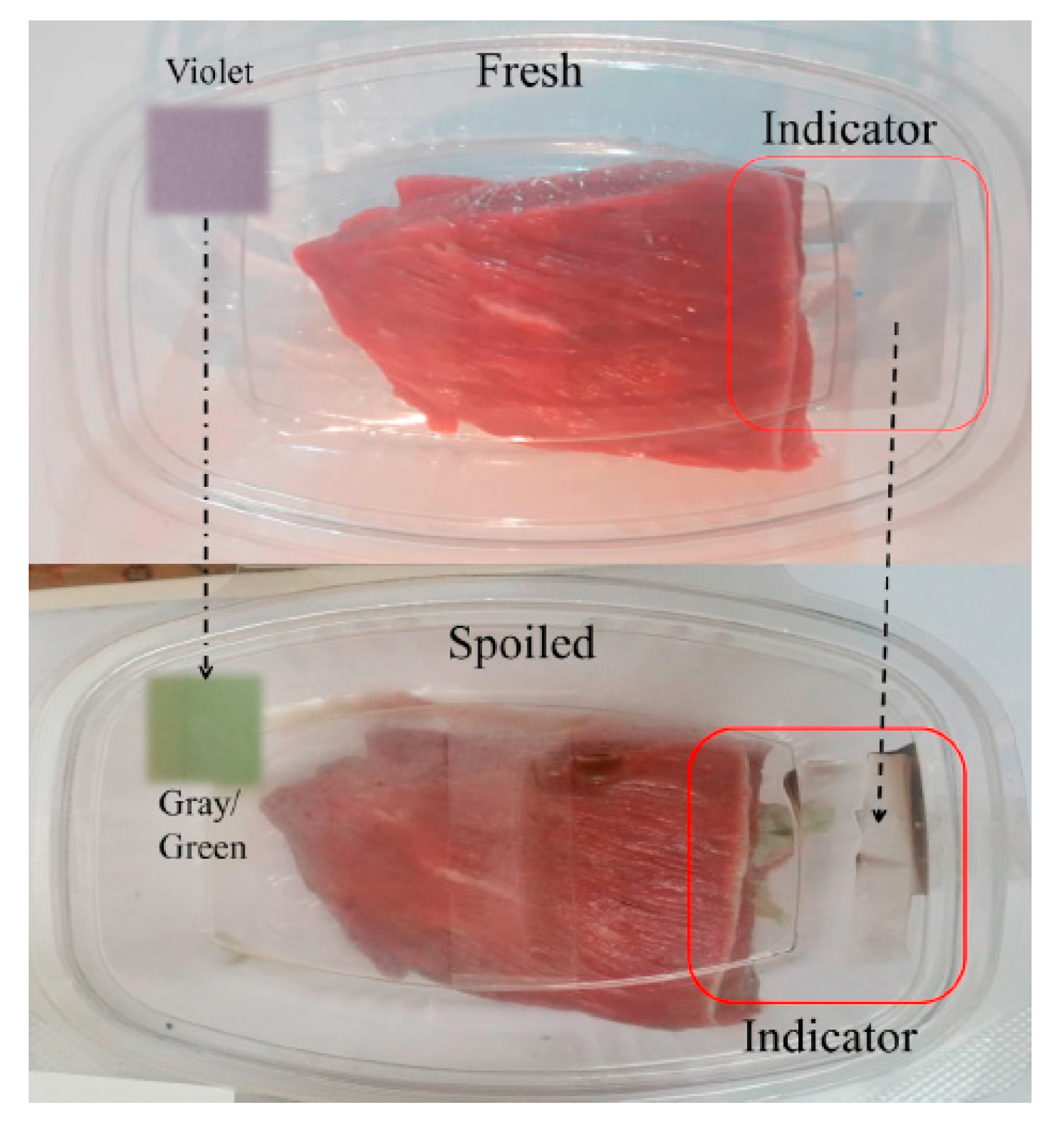
6.2. Smart Anthocyanin-Based Polymers
| Source of Anthocyanins | Type of Studied Anthocyanin | Anthocyanin Role | Type of Tested Food | Polymer Matrix | Ref. |
|---|---|---|---|---|---|
| Purple corn powder | Cyanidin-3-glucoside | pH indicator for detection of NH3, DMA, and TMA. | Muscle food products | Alginate hydrogel beads | [43] |
| Roselle | Not indicated | pH indicator for real-time freshness monitoring | Penaeus vannamei (white shrimp) | PVA/HEMC/RAE/OA films | [118] |
| Sumac powder | Not indicated | pH indicator for detection of ammonia vapors | Shrimp | Pectin (PC)/chitosan nanofiber (ChNF) films | [113] |
| Powdered barberry fruit and saffron petals | Cyanidin-3-glucoside | pH indicator for detection of ammonia vapors | Fish | Gelatin/chitosan nanofibers films | [114] |
| Common poppy | Not indicated | pH indicator for detection of ammonia vapors. | Fish | Gelatin (G)/rosemary essential oil (REO) | [115] |
| Saffron petals | Cyanidin-3-glucoside | pH indicator for detection of ammonia vapors | Meat product (lamb) | Methyl cellulose/chitosan nanofibers film | [116] |
| Viola odorata petals | Delphinidin-3-(4-p-coumaroyl)- rutinoside-5-glucoside and cyanidin-3-O-glucoside | pH indicator for detection of ammonia vapors | Pacific white shrimps, minced lamb meat, chicken fillets, and rainbow trout fillets | Double-layer polymers based on carboxymethyl cellulose/cellulose nanocrystals and poly(lactic acid) | [117] |
| Lycium ruthenicum anthocyanins | Not indicated | pH indicator for detection of volatile acids | Milk | Alginate-konjac/glucomannan films | [119] |
| Red cabbage (Brassica oleracea var. capitata f. rubra) | Not indicated | pH indicator for detection of ammonia vapors | Not studied | Chitosan/chitin nanocrystals with curcuma oil | [120] |
| Jacaranda cuspidifolia petals | Not indicated | pH indicator for detection of ammonia vapors | Fish | Chitosan/polyvinyl alcohol | [121] |
6.3. Active Anthocyanin-Based Polymers
7. Anthocyanin-Based Polymers for Healthcare Applications
7.1. Biosensors
7.2. Nanoencapsulated Delivery Systems
8. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and Purification of Anthocyanins: A Review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Horbowicz, M.; Grzesiuk, A.; DĘBski, H.; Kosson, R. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human. Veg. Crops Res. Bull. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as Developmental Regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Kossyvaki, D.; Contardi, M.; Athanassiou, A.; Fragouli, D. Colorimetric Indicators Based on Anthocyanin Polymer Composites: A Review. Polymers 2022, 14, 4129. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3244–3294. [Google Scholar] [CrossRef]
- Mirmoeini, S.S.; Moradi, M.; Tajik, H.; Almasi, H.; Gama, F.M. Cellulose/Salep-Based Intelligent Aerogel with Red Grape Anthocyanins: Preparation, Characterization and Application in Beef Packaging. Food Chem. 2023, 425, 136493. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and PH Degradation Kinetics of Anthocyanins in Natural Food Colorant Prepared from Black Rice Bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef]
- Fossen, T.; Cabrita, L.; Andersen, M. Colour and Stability of Pure Anthocyanins In¯uenced by PH Including the Alkaline Region. Food Chem. 1998, 4, 435–440. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Asuero, A.G. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef]
- Gomez Mattson, M.; Sozzi, A.; Corfield, R.; Gagneten, M.; Franceschinis, L.; Schebor, C.; Salvatori, D. Colorant and Antioxidant Properties of Freeze-Dried Extracts from Wild Berries: Use of Ultrasound-Assisted Extraction Method and Drivers of Liking of Colored Yogurts. J. Food Sci. Technol. 2022, 59, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanins in Cereals. J. Chromatogr. A 2004, 1054, 129–141. [Google Scholar] [CrossRef]
- Van Nguyen, S.; Lee, B.K. PVA/CNC/TiO2 Nanocomposite for Food-Packaging: Improved Mechanical, UV/Water Vapor Barrier, and Antimicrobial Properties. Carbohydr. Polym. 2022, 298, 120064. [Google Scholar] [CrossRef]
- Gravier-Rodríguez, G.; Jurado-Basante, S.; Arias-Contreras, A.N.; Gamarra Castillo, O.J.; Porras, A.; Sánchez-Camargo, A.d.P. Ultrasound-Assisted Extraction of Anthocyanins from Andean Blackberry and Their Use as an Indicator in Sustainable Smart Biofilms Developed with Cocoa Bean Shells as Natural Fiber-Filled PLA Composite Materials. Food Packag. Shelf Life 2023, 40, 101165. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 1286, 1265–1286. [Google Scholar] [CrossRef]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef]
- Różańska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef]
- Osorio, M.; Posada, L.; Martínez, E.; Estrada, V.; Quintana, G.; Maldonado, M.E.; Peresin, S.; Orozco, J.; Castro, C. Bacterial Nanocellulose Spheres Coated with Meta Acrylic Copolymer: Vaccinium Meridionale Swartz Extract Delivery for Colorectal Cancer Chemoprevention. Food Hydrocoll. 2024, 147, 109310. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Bhandari, B.; Yang, C. Novel PH-Sensitive Films Containing Curcumin and Anthocyanins to Monitor Fish Freshness. Food Hydrocoll. 2020, 100, 109310. [Google Scholar] [CrossRef]
- Rakić, Å.V.; Rinnan, T.; Polak, M.; Skrt, M.; Miljković, N.P. Ulrih, pH-induced structural forms of cyanidin and cyanidin 3-O-β-glucopyranoside. Dye. Pigment. 2019, 165, 71–80. [Google Scholar] [CrossRef]
- Câmara, J.S.; Locatelli, M.; Pereira, J.A.M.; Oliveira, H.; Arlorio, M.; Fernandes, I.; Perestrelo, R.; Freitas, V.; Bordiga, M. Behind the Scenes of Anthocyanins—From the Health Benefits to Potential Applications in Food, Pharmaceutical and Cosmetic Fields. Nutrients 2022, 14, 5133. [Google Scholar] [CrossRef]
- Cruz, L.; Basílio, N.; Mateus, N.; De Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Y. Biopolymer-Based Encapsulation of Anthocyanins as Reinforced Natural Colorants for Food Applications. J. Agric. Food Res. 2023, 11, 100488. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Feng, H.; Zhuang, D.; Zhu, J. An Intelligent Chitosan/Gelatin Film via Improving the Anthocyanin-Induced Color Recognition Accuracy for Beef Sub-Freshness Differentiation Monitoring. Food Hydrocoll. 2024, 146, 109219. [Google Scholar] [CrossRef]
- Neves, D.; Andrade, P.B.; Videira, R.A.; de Freitas, V.; Cruz, L. Berry Anthocyanin-Based Films in Smart Food Packaging: A Mini-Review. Food Hydrocoll. 2022, 133, 107885. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent Advances in the Preparation, Physical and Functional Properties, and Applications of Anthocyanins-Based Active and Intelligent Packaging Films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Nadi, M.; Razavi, S.M.A.; Shahrampour, D. Fabrication of Green Colorimetric Smart Packaging Based on Basil Seed Gum/Chitosan/Red Cabbage Anthocyanin for Real-Time Monitoring of Fish Freshness. Food Sci. Nutr. 2023, 11, 6360–6375. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C. A Double-Layer Smart Film Based on Gellan Gum/Modified Anthocyanin and Sodium Carboxymethyl Cellulose/Starch/Nisin for Application in Chicken Breast. Int. J. Biol. Macromol. 2023, 232, 123464. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The Potential of Anthocyanins in Smart, Active, and Bioactive Eco-Friendly Polymer-Based Films: A Review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Mirza Alizadeh, A.; Mohammadi, M.; Mirzakhani, E.; Sabouri, S.; Pourjafar, H.; Hosseini, S.M. Application and Development of Electrospun Nanofibers as an Efficient Platform for the Delivery of Anthocyanin Compounds in the Food Industry. Food Bioproc Tech. 2023. [Google Scholar] [CrossRef]
- Khaledian, Y.; Moshtaghi, H.; Shahbazi, Y. Development and Characterization of Smart Double-Layer Nanofiber Mats Based on Potato Starch-Turnip Peel Anthocyanins and Guar Gum-Cinnamaldehyde. Food Chem. 2024, 434, 137462. [Google Scholar] [CrossRef]
- Nath, V.A.; Vijayakumar, R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Co-Electrospun-Electrosprayed Ethyl Cellulose-Gelatin Nanocomposite PH-Sensitive Membrane for Food Quality Applications. Food Chem. 2022, 394, 133420. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Osman, B.; Göktalay, G.; Özer, E.T.; Şahan, Y.; Becerir, B.; Karaca, E. Design and in Vivo Evaluation of Alginate-Based PH-Sensing Electrospun Wound Dressing Containing Anthocyanins. J. Polym. Res. 2021, 28, 50. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Song, X.; Guo, M.; Wang, Z.; Geng, F.; Zhou, X.; Nie, S. Compound Hydrogels Derived from Gelatin and Gellan Gum Regulates the Release of Anthocyanins in Simulated Digestion. Food Hydrocoll. 2022, 127, 107487. [Google Scholar] [CrossRef]
- Jin, W.; Xiang, L.; Peng, D.; Liu, G.; He, J.; Cheng, S.; Li, B.; Huang, Q. Study on the Coupling Progress of Thermo-Induced Anthocyanins Degradation and Polysaccharides Gelation. Food Hydrocoll. 2020, 105, 105822. [Google Scholar] [CrossRef]
- Lotfinia, F.; Norouzi, M.R.; Ghasemi-Mobarakeh, L.; Naeimirad, M. Anthocyanin/Honey-Incorporated Alginate Hydrogel as a Bio-Based PH-Responsive/Antibacterial/Antioxidant Wound Dressing. J. Funct. Biomater. 2023, 14, 72. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Kurek, M.; Jensen, I.J.; Lerfall, J. The Potential of Anthocyanin-Loaded Alginate Hydrogel Beads for Intelligent Packaging Applications: Stability and Sensitivity to Volatile Amines. Curr. Res. Food Sci. 2023, 7, 100560. [Google Scholar] [CrossRef]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and Intelligent Starch-Based Films: A Review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Förster, S.; Schmidt, M. Polyelectrolytes in Solution. In Physical Properties of Polymers, Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Tan, C.; Huang, M.; Wang, J.; Sun, B. Biopolyelectrolyte Complex (BioPEC)-Based Carriers for Anthocyanin Delivery. Food Hydrocoll. Health 2021, 1, 100037. [Google Scholar] [CrossRef]
- Tan, C.; Celli, G.B.; Abbaspourrad, A. Copigment-Polyelectrolyte Complexes (PECs) Composite Systems for Anthocyanin Stabilization. Food Hydrocoll. 2018, 81, 371–379. [Google Scholar] [CrossRef]
- Tan, C.; Sun, Y.; Yao, X.; Zhu, Y.; Jafari, S.M.; Sun, B.; Wang, J. Stabilization of anthocyanins by simultaneous encapsulation-copigmentation via protein-polysaccharide polyelectrolyte complexes. Food Chem. 2023, 416, 135732. [Google Scholar] [CrossRef]
- Yuan, Y.; Fan, Q.; Xu, X.; Wang, O.; Zhao, L.; Zhao, L. Nanocarriers Based on Polysaccharides for Improving the Stability and Bioavailability of Anthocyanins: A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100346. [Google Scholar] [CrossRef]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/Microencapsulation of Anthocyanins; a Systematic Review and Meta-Analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Cai, S.; Yi, J.; Zhou, L. Fabrication of Anthocyanin–Rich W1/O/W2 Emulsion Gels Based on Pectin–GDL Complexes: 3D Printing Performance. Food Res. Int. 2023, 168, 112782. [Google Scholar] [CrossRef]
- Baba Shekh, A.O.; Abdul Wahab, R.; Yahya, N.A. Formulation of Roselle Extract Water-in-Oil Nanoemulsion for Controlled Pulmonary Delivery. J. Dispers. Sci. Technol. 2023, 44, 1830–1841. [Google Scholar] [CrossRef]
- Kenari, R.E.; Razavi, R. Encapsulation of Bougainvillea (Bougainvillea spectabilis) Flower Extract in Urtica dioica L. Seed Gum: Characterization, Antioxidant/Antimicrobial Properties, and in Vitro Digestion. Food Sci. Nutr. 2022, 10, 3436–3443. [Google Scholar] [CrossRef] [PubMed]
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Laokuldilok, T. Microencapsulation of Copigmented Anthocyanins Using Double Emulsion Followed by Complex Coacervation: Preparation, Characterization and Stability. LWT 2020, 133, 110154. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Liu, Y.; Wang, L.; Wang, H.; Hu, Z.; Dong, H.; Yu, Z.; Yuan, Y. A Review of Curcumin in Food Preservation: Delivery System and Photosensitization. Food Chem. 2023, 424, 136464. [Google Scholar] [CrossRef] [PubMed]
- Wulansari, A.; Jufri, M.; Budianti, A. Studies on the Formulation, Physical Stability, and in Vitro Antibacterial Activity of Tea Tree Oil (Melaleuca Alternifolia) Nanoemulsion Gel. Int. J. Appl. Pharm. 2017, 9, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, J.; Li, L.; Ren, J.; Lu, J.; Luo, F. Advances in Embedding Techniques of Anthocyanins: Improving Stability, Bioactivity and Bioavailability. Food Chem. X 2023, 20, 100983. [Google Scholar] [CrossRef] [PubMed]
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of Phenolic Compounds within Nano/Microemulsion Systems: A Review. Food Chem. 2021, 364, 130376. [Google Scholar] [CrossRef]
- Nazareth, M.S.; Shreelakshmi, S.V.; Rao, P.J.; Shetty, N.P. Micro and Nanoemulsions of Carissa Spinarum Fruit Polyphenols, Enhances Anthocyanin Stability and Anti-Quorum Sensing Activity: Comparison of Degradation Kinetics. Food Chem. 2021, 359, 129876. [Google Scholar] [CrossRef]
- Gorantla, S.; Wadhwa, G.; Jain, S.; Sankar, S.; Nuwal, K.; Mahmood, A.; Dubey, S.K.; Taliyan, R.; Kesharwani, P.; Singhvi, G. Recent Advances in Nanocarriers for Nutrient Delivery. Drug Deliv. Transl. Res. 2022, 12, 2359–2384. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Asghar, W.; Akhtar, A.; Ayub, H.; Aslam, I.; Khalid, N.; Al-Mssallem, M.Q.; Alessa, F.M.; Ghazzawy, H.S.; Attimarad, M. Anthocyanin Delivery Systems: A Critical Review of Recent Research Findings. Appl. Sci. 2022, 12, 12347. [Google Scholar] [CrossRef]
- Chen, B.H.; Inbaraj, B.S. Nanoemulsion and Nanoliposome Based Strategies for Improving Anthocyanin Stability and Bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Fan, C.; Wang, X.; Wang, X.; Xia, S.; Huang, Q. Food-Grade Pickering Emulsions and High Internal Phase Pickering Emulsions Encapsulating Cinnamaldehyde Based on Pea Protein-Pectin-EGCG Complexes for Extrusion 3D Printing. Food Hydrocoll. 2022, 124, 107265. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stănciuc, N.; Yan, Y.; Ghinea, I.O.; Ungureanu, C.; Cîrciumaru, A.; Wang, D.; Poklar Ulrih, N.; Râpeanu, G. Polymers and Protein-Associated Vesicles for the Microencapsulation of Anthocyanins from Grape Skins Used for Food Applications. J. Sci. Food Agric. 2021, 101, 2676–2686. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Yang, H.; Gao, S.; Li, L.; Fu, X.; Wei, Q. Research Progress on Self-Assembled Nanodrug Delivery Systems. J. Mater. Chem. B 2022, 10, 1908–1922. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Oliveira, H.; Mateus, N.; Ye, S.; Jiang, S.; He, J.; Wu, M. Preparation of Nanoliposomes Loaded with Anthocyanins from Grape Skin Extracts: Stability, Gastric Absorption and Antiproliferative Properties. Food Funct. 2022, 13, 10912–10922. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of Nanoliposomal Carriers to Improve the Stability of Anthocyanins. LWT 2019, 109, 101–107. [Google Scholar] [CrossRef]
- Surekha, Y.N.; Saravanan, R.; Jyothi, Y.; Swaroop Seetharam, S. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Indo Am. J. Pharm. Sci. 2023, 10, 236–255. [Google Scholar]
- Yin, Z.; Zheng, T.; Ho, C.T.; Huang, Q.; Wu, Q.; Zhang, M. Improving the Stability and Bioavailability of Tea Polyphenols by Encapsulations: A Review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A Comprehensive Review on Innovative and Advanced Stabilization Approaches of Anthocyanin by Modifying Structure and Controlling Environmental Factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Yavuz-Düzgün, M.; Kareth, S.; Özçelik, B.; Weidner, E. Black Carrot Extract Loaded-Potato Protein Particles by PGSS-Drying: Physico-Chemical Properties and in Vitro Bioaccessability. J. Supercrit. Fluids 2023, 203, 106065. [Google Scholar] [CrossRef]
- Rosales-Chimal, S.; Navarro-Cortez, R.O.; Bello-Perez, L.A.; Vargas-Torres, A.; Palma-Rodríguez, H.M. Optimal Conditions for Anthocyanin Extract Microencapsulation in Taro Starch: Physicochemical Characterization and Bioaccessibility in Gastrointestinal Conditions. Int. J. Biol. Macromol. 2023, 227, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.A.; Majek, M.; Onopiuk, A.; Szpicer, A.; Napiórkowska, A.; Samborska, K. Encapsulation of Anthocyanins from Chokeberry (Aronia Melanocarpa) with Plazmolyzed Yeast Cells of Different Species. Food Bioprod. Process. 2023, 137, 84–92. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Palomino-Rincón, H.; Taipe-Pardo, F.; Landa, J.P.A.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.; Quispe-Quezada, U.R.; Huamán-Carrión, M.L.; et al. Nanoencapsulation of Phenolic Extracts from Native Potato Clones (Solanum tuberosum spp. Andigena) by Spray Drying. Molecules 2023, 28, 4961. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; de Oliveira, T.J.P.; Mendes, R.F.; Mattoso, L.H.C. Pectin-Based Color Indicator Films Incorporated with Spray-Dried Hibiscus Extract Microparticles. Food Res. Int. 2022, 162, 111914. [Google Scholar] [CrossRef] [PubMed]
- Nadali, N.; Pahlevanlo, A.; Sarabi-Jamab, M.; Balandari, A. Effect of Maltodextrin with Different Dextrose Equivalents on the Physicochemical Properties of Spray-Dried Barberry Juice (Berberis vulgaris L.). J. Food Sci. Technol. 2022, 59, 2855–2866. [Google Scholar] [CrossRef]
- Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and Other Flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
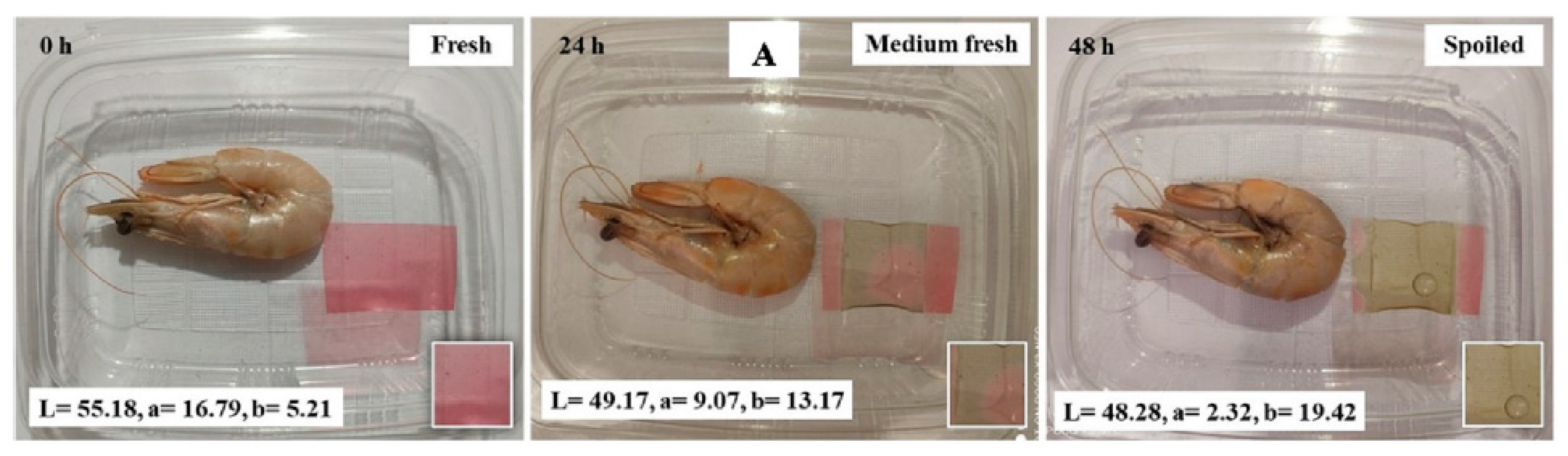
- Zhang, K.; Huang, T.S.; Yan, H.; Hu, X.; Ren, T. Novel PH-Sensitive Films Based on Starch/Polyvinyl Alcohol and Food Anthocyanins as a Visual Indicator of Shrimp Deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active Chitosan/PVA Films with Anthocyanins from Brassica Oleraceae (Red Cabbage) as Time-Temperature Indicators for Application in Intelligent Food Packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Wei, S.; Xu, K.; Xia, J.; Wu, Q.; Lü, X.; Wang, L. Development and Application of Multifunctional Films Based on Modified Chitosan/Gelatin Polyelectrolyte Complex for Preservation and Monitoring. Food Hydrocoll. 2024, 147, 109336. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, J.; Yu, S.; Niu, R.; Yang, Z.; Wang, H.; Cheng, H.; Ye, X.; Liu, D.; Wang, W. Active Chitosan/Gum Arabic-Based Emulsion Films Reinforced with Thyme Oil Encapsulating Blood Orange Anthocyanins: Improving Multi-Functionality. Food Hydrocoll. 2023, 134, 108094. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Chen, S.; Liu, D.; Ye, X.; Tian, J. Development and Characterization of PH Responsive Sodium Alginate Hydrogel Containing Metal-Phenolic Network for Anthocyanin Delivery. Carbohydr. Polym. 2023, 320, 121234. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Koh, E. Effect of Encapsulation on Stability of Anthocyanins and Chlorogenic Acid Isomers in Aronia during in Vitro Digestion and Their Transformation in a Model System. Food Chem. 2024, 434, 137443. [Google Scholar] [CrossRef]
- Tassanawat, S.; Phandee, A.; Magaraphan, R.; Nithitanakul, M.; Manuspiya, H. PH-Sensitive PP/Clay Nanocomposites for Beverage Smart Packaging. In Proceedings of the 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Bangkok, Thailand, 16–19 January 2007. [Google Scholar]
- Xue Mei, L.; Mohammadi Nafchi, A.; Ghasemipour, F.; Mat Easa, A.; Jafarzadeh, S.; Al-Hassan, A.A. Characterization of PH Sensitive Sago Starch Films Enriched with Anthocyanin-Rich Torch Ginger Extract. Int. J. Biol. Macromol. 2020, 164, 4603–4612. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Long, T.; Yuan, S.; Qi, P.; Han, L.; Hao, J. A PH-Indicating Smart Tag Based on Porous Hydrogel as Food Freshness Sensors. J. Colloid Interface Sci. 2023, 647, 32–42. [Google Scholar] [CrossRef]
- Goudarzi, J.; Moshtaghi, H.; Shahbazi, Y. Kappa-Carrageenan-Poly(Vinyl Alcohol) Electrospun Fiber Mats Encapsulated with Prunus Domestica Anthocyanins and Epigallocatechin Gallate to Monitor the Freshness and Enhance the Shelf-Life Quality of Minced Beef Meat. Food Packag. Shelf Life 2023, 35, 101017. [Google Scholar] [CrossRef]
- He, H.; Song, Y.; Li, M.; Zhang, H.; Li, J.; Huang, H.; Li, Y. A Novel Anthocyanin Electrospun Film by Caffeic Acid Co-Pigmentation for Real-Time Fish Freshness Monitoring. Anal. Methods 2022, 15, 228–239. [Google Scholar] [CrossRef]
- Jang, Y.; Koh, E. Characterisation and Storage Stability of Aronia Anthocyanins Encapsulated with Combinations of Maltodextrin with Carboxymethyl Cellulose, Gum Arabic, and Xanthan Gum. Food Chem. 2023, 405, 135002. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A Novel PH-Sensing Indicator Based on Bacterial Cellulose Nanofibers and Black Carrot Anthocyanins for Monitoring Fish Freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef]
- Ebrahimi Tirtashi, F.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/Chitosan PH-Responsive Indicator Incorporated with Carrot Anthocyanins for Intelligent Food Packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Phan, M.A.T.; Arcot, J.; Chandrawati, R. Anthocyanin-Based Sensors Derived from Food Waste as an Active Use-by Date Indicator for Milk. Food Chem. 2020, 326, 127017. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of Active Fish Gelatin Films with Anthocyanins by Compression Molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Hanafy, N.A.N. Starch Based Hydrogel NPs Loaded by Anthocyanins Might Treat Glycogen Storage at Cardiomyopathy in Animal Fibrotic Model. Int. J. Biol. Macromol. 2021, 183, 171–181. [Google Scholar] [CrossRef]
- Choi, I.; Choi, H.; Lee, J.S.; Han, J. Novel Color Stability and Colorimetry-Enhanced Intelligent CO2 Indicators by Metal Complexation of Anthocyanins for Monitoring Chicken Freshness. Food Chem. 2023, 404, 134534. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, H.; Park, B.D.; Khanjanzadeh, H.; Park, H.J.; Cho, Y.J. Nanocellulose-Based Ammonia Sensitive Smart Colorimetric Hydrogels Integrated with Anthocyanins to Monitor Pork Freshness. Food Control 2023, 147, 109595. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Hao, M.; Zhao, F.; Wang, Y.; Zhou, Y.; Liu, Z.; An, X.; Gao, Z.; Wang, J.; Zheng, T.; et al. Fabrication of Silk Sericin-Anthocyanin Nanocoating for Chelating and Saturation-Visualization Detection of Metal Ions. Nanoscale 2022, 14, 17277–17289. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent Gelatin/Oxidized Chitin Nanocrystals Nanocomposite Films Containing Black Rice Bran Anthocyanins for Fish Freshness Monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef]
- Lu, X.; Huang, Q.; Xiao, J.; Wang, Y. Milled Miscellaneous Black Rice Particles Stabilized Pickering Emulsions with Enhanced Antioxidation Activity. Food Chem. 2022, 385, 132639. [Google Scholar] [CrossRef]
- de Azevedo, E.S.; Noreña, C.P.Z. Anthocyanin-Based Indicators Design by Polyelectrolyte Complexation: A Study on Structural and Thermodynamic Properties, and Application for Milk Freshness Assessment. Food Hydrocoll. 2024, 147, 109389. [Google Scholar] [CrossRef]
- Ferron, L.; Milanese, C.; Colombo, R.; Pugliese, R.; Papetti, A. A New Polysaccharide Carrier Isolated from Camelina Cake: Structural Characterization, Rheological Behavior, and Its Influence on Purple Corn Cob Extract’s Bioaccessibility. Foods 2022, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zaa, C.A.; Marcelo, Á.J.; An, Z.; Medina-Franco, J.L.; Velasco-Velázquez, M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules 2023, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The Anti-Inflammatory Effects of Dietary Anthocyanins against Ulcerative Colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-Inflammatory Effect of Anthocyanins via Modulation of Nuclear Factor-ΚB and Mitogen-Activated Protein Kinase Signaling Cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Deng, Q.; Zhao, Y. Antimicrobial Properties of Wine Grape (Cv. Merlot) Pomace Extract-Based Films. J. Food Sci. 2011, 76, 1–10. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Liaudanskas, M.; Ramanauskienė, K.; Janulis, V. Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder. Molecules 2021, 26, 1095. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Kulozik, U.; Schwarz, K.; Richling, E. Encapsulation of Anthocyanins from Bilberries—Effects on Bioavailability and Intestinal Accessibility in Humans. Food Chem. 2018, 248, 217–224. [Google Scholar] [CrossRef]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza sativa L. In Primary Dermal Fibroblasts. Oxid. Med. Cell Longev. 2019, 2089817, 1–18. [Google Scholar] [CrossRef]
- Tavassoli, M.; Khezerlou, A.; Moghaddam, T.N.; Firoozy, S.; Bakhshizadeh, M.; Sani, M.A.; Hashemi, M.; Ehsani, A.; Lorenzo, J.M. Sumac (Rhus Coriaria L.) Anthocyanin Loaded-Pectin and Chitosan Nanofiber Matrices for Real-Time Monitoring of Shrimp Freshness. Int. J. Biol. Macromol. 2023, 242, 125044. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; Jahed-khaniki, G.; Mcclements, D.J. Smart Biopolymer-Based Nanocomposite Materials Freshness Monitoring. Molecules 2022, 27, 3168. [Google Scholar] [CrossRef] [PubMed]
- Bakhshizadeh, M.; Ayaseh, A.; Hamishehkar, H.; Samadi, H.; Niknazar, T.; Baghban, P.; Tavassoli, M.; Amjadi, S.; Manuel, J. Multifunctional Performance of Packaging System Based on Gelatin / Alove Vera Gel Film Containing of Rosemary Essential Oil and Common Poppy Anthocyanins. Food Control 2023, 154, 110017–110028. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional Halochromic Packaging Materials: Saffron Petal Anthocyanin Loaded-Chitosan Nanofiber/Methyl Cellulose Matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Rezaei, F.; Tajik, H.; Shahbazi, Y. Intelligent Double-Layer Polymers Based on Carboxymethyl Cellulose-Cellulose Nanocrystals Film and Poly(Lactic Acid)-Viola Odorata Petal Anthocyanins Nanofibers to Monitor Food Freshness. Int. J. Biol. Macromol. 2023, 252, 126512. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sun, Y.; Tan, C. Recent Advances in Emerging Pectin-Derived Nanocarriers for Controlled Delivery of Bioactive Compounds. Food Hydrocoll. 2023, 140, 108682. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Han, M.; Zhuang, D.; Zhu, J. Intelligent Active Films of Sodium Alginate and Konjac Glucomannan Mixed by Lycium ruthenicum Anthocyanins and Tea Polyphenols for Milk Preservation and Freshness Monitoring. Int. J. Biol. Macromol. 2023, 253, 126674. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an Intelligent Film Based on Chitosan/Oxidized Chitin Nanocrystals Incorporating Black Rice Bran Anthocyanins for Seafood Spoilage Monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Amaregouda, Y.; Kamanna, K.; Gasti, T. Fabrication of Intelligent/Active Films Based on Chitosan/Polyvinyl Alcohol Matrices Containing Jacaranda Cuspidifolia Anthocyanin for Real-Time Monitoring of Fish Freshness. Int. J. Biol. Macromol. 2022, 218, 799–815. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.W. Carrageenan-Based Multifunctional Packaging Films Containing Zn-Carbon Dots/Anthocyanin Derived from Kohlrabi Peel for Monitoring Quality and Extending the Shelf Life of Shrimps. Food Chem. 2024, 432, 137215. [Google Scholar] [CrossRef]
- Stoll, L.; da Silva, A.M.; e.Silva Iahnke, A.O.; Costa, T.M.H.; Flôres, S.H.; de Oliveira Rios, A. Active Biodegradable Film with Encapsulated Anthocyanins: Effect on the Quality Attributes of Extra-Virgin Olive Oil during Storage. J. Food Process Preserv. 2017, 41, e13218. [Google Scholar] [CrossRef]
- Estévez, M.; Xiong, Y.L. Protein Oxidation in Foods: Mechanisms, Consequences, and Antioxidant Solutions. Foods 2021, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Xie, J.; Du, H.; Bian, X.; Wang, C.; Zhou, L.; Wen, Y. Antibacterial Smart Absorbent Pad with Janus Structure for Meat Preservation. Food Packag. Shelf Life 2023, 37, 101066. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial Activity of Anthocyanins and Catechins against Foodborne Pathogens Escherichia Coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Rashid, S.A.; Tong, W.Y.; Leong, C.R.; Abdul Ghazali, N.M.; Taher, M.A.; Ahmad, N.; Tan, W.-N.; Teo, S.H. Anthocyanin Microcapsule from Clitoria Ternatea: Potential Bio Preservative and Blue Colorant for Baked Food Products. Arab. J. Sci. Eng. 2021, 46, 65–72. [Google Scholar] [CrossRef]
- Wagh, R.V.; Khan, A.; Priyadarshi, R.; Ezati, P.; Rhim, J. Cellulose Nanofiber-Based Multifunctional Films Integrated with Carbon Dots and Anthocyanins from Brassica oleracea for Active and Intelligent Food Packaging Applications. Int. J. Biol. Macromol. 2023, 233, 123567. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Sun, L.; Liu, B.; Li, H.; Peng, L. Crosslinked Fish Scale Gelatin/Alginate Dialdehyde Functional Films Incorporated with Carbon Dots Derived from Pomelo Peel Waste for Active Food Packaging. Int. J. Biol. Macromol. 2023, 253, 127290. [Google Scholar] [CrossRef]
- Riahi, Z.; Khan, A.; Rhim, J.W.; Shin, G.H.; Kim, J.T. Gelatin/Poly(Vinyl Alcohol)-Based Dual Functional Composite Films Integrated with Metal-Organic Frameworks and Anthocyanin for Active and Intelligent Food Packaging. Int. J. Biol. Macromol. 2023, 249, 126040. [Google Scholar] [CrossRef]
- Riaz, R.S.; Elsherif, M.; Moreddu, R.; Rashid, I.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Anthocyanin-Functionalized Contact Lens Sensors for Ocular PH Monitoring. ACS Omega 2019, 4, 21792–21798. [Google Scholar] [CrossRef]
- Alsahag, M.; Alisaac, A.; Al-Hazmi, G.A.A.; Pashameah, R.A.; Attar, R.M.S.; Saad, F.A.; El-Metwaly, N.M. Preparation of Carboxymethyl Cellulose/Polyvinyl Alcohol Wound Dressing Composite Immobilized with Anthocyanin Extract for Colorimetric Monitoring of Wound Healing and Prevention of Wound Infection. Int. J. Biol. Macromol. 2023, 224, 233–242. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Alzahrani, H.K.; Azher, O.A.; Owidah, Z.O.; Abualnaja, M.; Habeebullah, T.M.; El-Metwaly, N.M. Immobilization of Anthocyanin-Based Red-Cabbage Extract onto Cellulose Fibers toward Environmentally Friendly Biochromic Diagnostic Biosensor for Recognition of Urea. J. Environ. Chem. Eng. 2021, 9, 105493. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Azher, O.A.; Felaly, R.; Subaihi, A.; Alkabli, J.; Alaysuy, O.; El-Metwaly, N.M. Development of Sponge-like Cellulose Colorimetric Swab Immobilized with Anthocyanin from Red-Cabbage for Sweat Monitoring. Int. J. Biol. Macromol. 2021, 182, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Lopes, J.A.; Silva, L.R. Employ of Anthocyanins in Nanocarriers for Nano Delivery: In Vitro and In Vivo Experimental Approaches for Chronic Diseases. Pharmaceutics 2022, 14, 2272. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioproc Tech. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Guterres, S.S.; Paese, K.; Pohlmann, A.R. Polymeric Nanoparticles. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–94. ISBN 9783030165734. [Google Scholar]
- Zhao, X.; Zhang, X.; Tie, S.; Hou, S.; Wang, H.; Song, Y.; Rai, R.; Tan, M. Facile Synthesis of Nano-Nanocarriers from Chitosan and Pectin with Improved Stability and Biocompatibility for Anthocyanins Delivery: An in Vitro and in Vivo Study. Food Hydrocoll. 2020, 109, 106114. [Google Scholar] [CrossRef]
- Sreerekha, P.R.; Dara, P.K.; Vijayan, D.K.; Chatterjee, N.S.; Raghavankutty, M.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Dietary Supplementation of Encapsulated Anthocyanin Loaded-Chitosan Nanoparticles Attenuates Hyperlipidemic Aberrations in Male Wistar Rats. Carbohydr. Polym. Technol. Appl. 2021, 2, 100051. [Google Scholar] [CrossRef]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Anthocyanin Encapsulated Nanoparticles as a Pulmonary Delivery System. Oxid. Med. Cell Longev. 2022, 2022, 1422929. [Google Scholar] [CrossRef]
- Jeong, D.; Na, K. Chondroitin Sulfate Based Nanocomplex for Enhancing the Stability and Activity of Anthocyanin. Carbohydr. Polym. 2012, 90, 507–515. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Z.; Jing, P. Black Rice Anthocyanins Embedded in Self-Assembled Chitosan/Chondroitin Sulfate Nanoparticles Enhance Apoptosis in HCT-116 Cells. Food Chem. 2019, 301, 125280–125289. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, B. A Novel Hyaluronic Acid-Black Rice Anthocyanins Nanocomposite: Preparation, Characterization, and Its Xanthine Oxidase (XO) -Inhibiting Properties. Front. Nutr. 2022, 9, 879354. [Google Scholar] [CrossRef]
- Unosson, E. Antibacterial Strategies for Titanium Biomaterials; Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1250; Acta Universitatis Upsaliensis: Uppsala, Sweden, 2015; p. 72. ISBN 978-91-554-9241-0. [Google Scholar]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins Encapsulated by PLGA@PEG Nanoparticles Potentially Improved Its Free Radical Scavenging Capabilities via P38/JNK Pathway against Aβ1-42-Induced Oxidative Stress. J. Nanobiotechnol. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Giaconia, M.A.; Ramos, S.d.P.; Neves, B.V.; Almeida, L.; Costa-Lotufo, L.; de Rosso, V.V.; Braga, A.R.C. Nanofibers of Jussara Pulp: A Tool to Prevent the Loss of Thermal Stability and the Antioxidant Activity of Anthocyanins after Simulated Digestion. Processes 2022, 10, 2343. [Google Scholar] [CrossRef]
- Ghiman, R.; Nistor, M.; Focșan, M.; Pintea, A.; Aștilean, S.; Rugina, D. Fluorescent Polyelectrolyte System to Track Anthocyanins Delivery inside Melanoma Cells. Nanomaterials 2021, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Valentão, P.; Oliveira, M.M.; Andrade, P.; Videira, R.A. A Nanophytosomes Formulation Based on Elderberry Anthocyanins and Codium Lipids to Mitigate Mitochondrial Dysfunctions. Biomed. Pharmacother. 2021, 143, 112157–112169. [Google Scholar] [CrossRef] [PubMed]
- Fidan-Yardimci, M.; Akay, S.; Sharifi, F.; Sevimli-Gur, C.; Ongen, G.; Yesil-Celiktas, O. A Novel Niosome Formulation for Encapsulation of Anthocyanins and Modelling Intestinal Transport. Food Chem. 2019, 293, 57–65. [Google Scholar] [CrossRef]
- Priprem, A.; Damrongrungruang, T.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Nukulkit, C.; Thapphasaraphong, S.; Limphirat, W. Topical Niosome Gel Containing an Anthocyanin Complex: A Potential Oral Wound Healing in Rats. AAPS PharmSciTech 2018, 19, 1681–1692. [Google Scholar] [CrossRef]
- Samadder, A.; Tarafdar, D.; Abraham, S.K.; Ghosh, K.; Khuda-Bukhsh, A.R. Nano-Pelargonidin Protects Hyperglycemic-Induced L6 Cells against Mitochondrial Dysfunction. Planta Med. 2017, 83, 468–475. [Google Scholar] [CrossRef]
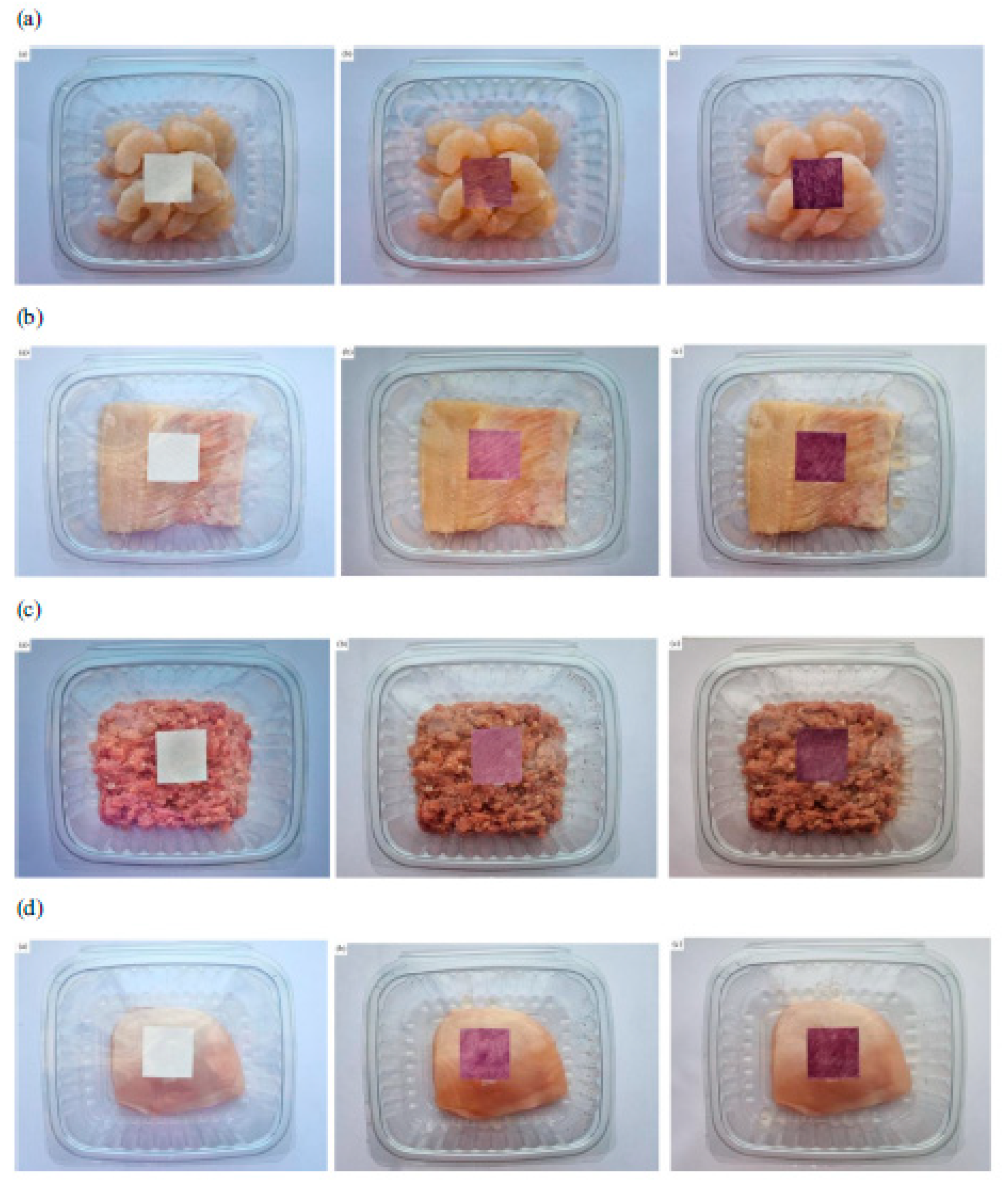
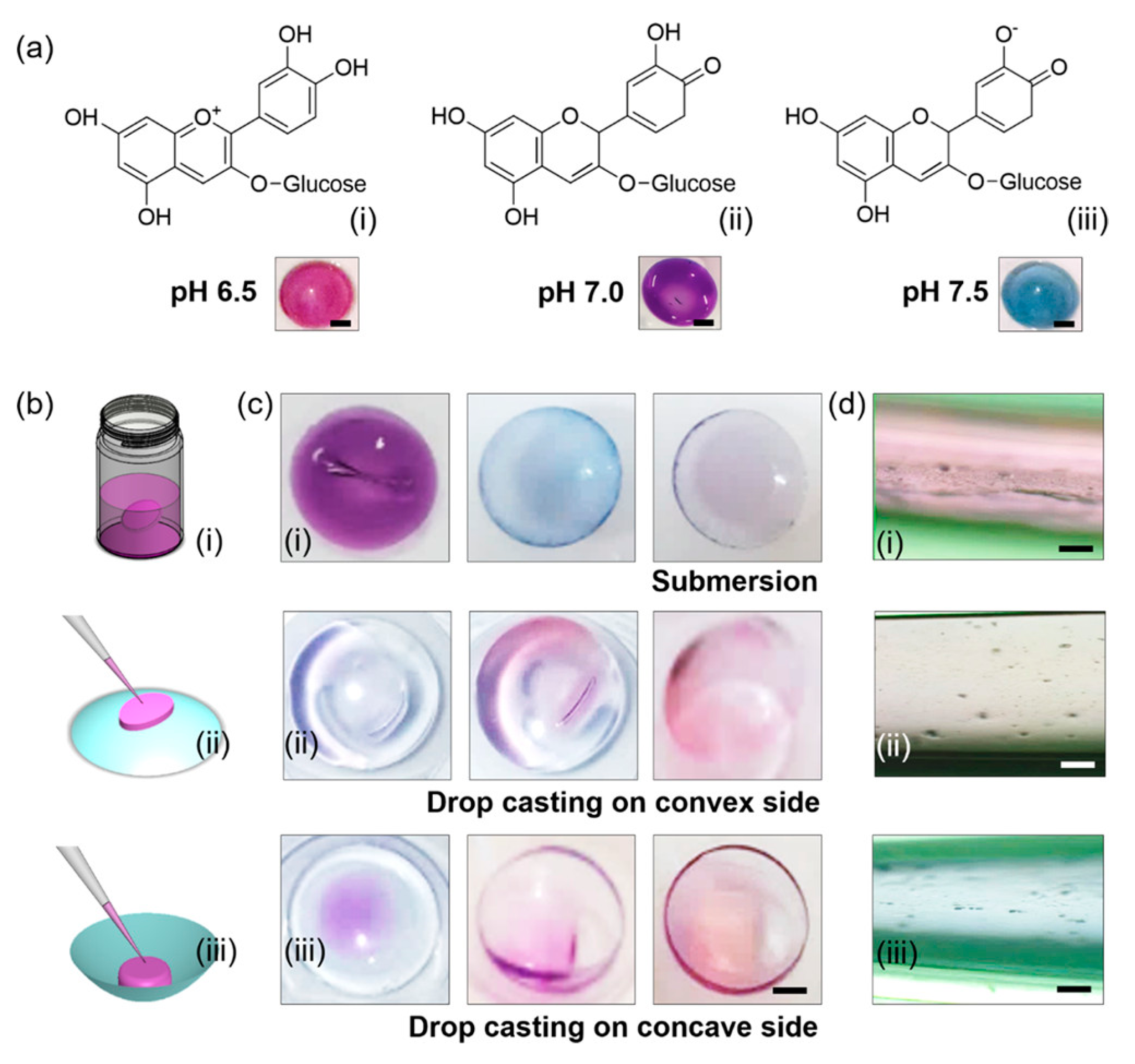
| Type of Material | Anthocyanin Function | Extract Used | Ref(s). |
|---|---|---|---|
| Aerogel | pH indicator | Red grape | [9] |
| Cryogel | pH indicator | Red radish | [88] |
| Double emulsions | Bioactive substance | Mulberry | [9] |
| Double-layer nanofiber mats | pH indicator | Turnip peel extract and potato starch–turnip peel extract | [37] |
| Electrospun fiber mats | pH indicator | Prunus domestica | [89] |
| Electrospun film | pH indicator | Blueberry | [90] |
| Nanoencapsulation/spray drying | Active ingredient | Aronia | [85,91] |
| Film | pH indicator | Black carrot | [92,93] |
| Film | pH indicator | Red cabbage | [94,95] |
| Hydrogel | pH indicator | Red beets, purple corn | [43,96] |
| Hydrogel | CO2-sensitive indicator | Black goji anthocyanin | [97] |
| Hydrogel | pH indicator | Aronia, liriope, and red cabbage | [98] |
| Hydrogel | Bioactive substance | Blueberry | [40,83] |
| Film | pH indicator | Red cabbage | [87] |
| Lipid nanoparticles | Bioactive substance | Purple sweet potato | [99] |
| Microencapsulation spray drying | Bioactive substance | Taro tubers | [100] |
| Microencapsulation spray drying | Bioactive substance | Chokeberry | [73] |
| Nanocoating | Colorimetric sensor | Cyanidin 3-O-glucoside | [100] |
| Films | pH indicator | Black rice | [101] |
| Nanoliposomes | Bioactive substance | Grape skin | [66] |
| Emulsion | Bioactive substance | Black rice | [102] |
| Polyelectrolyte complex | pH indicator | Non-pomace residue of grape juice powder | [103] |
| Polysaccharide assembly | Bioactive substance | Purple corn cob | [104] |
| Source of Anthocyanins | Polymer Matrix | Effects/Results | Reference |
|---|---|---|---|
| Clitoria ternatea flowers | Maltodextrin | Enhanced stability. Improved antibacterial effect. | [127] |
| Brassica oleracea (BO) extract | Cellulose nanofiber loaded with carbon dots | Reduced spoilage. Enhanced antioxidant effect. Improved thermal stability. UV barrier properties. | [128] |
| Pomelo peel | Fish scale gelatin (FSG)/alginate dialdehyde (ADA) loaded with carbon dots | Physiological qualities post-harvest and extended shelf-life. | [129] |
| Red cabbage (Brassica oleracea) | Gelatin/poly(vinyl alcohol)-based matrix integrated with metal–organic frameworks | Improved antibacterial and antioxidant effects. Spoilage detection. Enhanced shrimp preservation. | [130] |
| Purple kohlrabi peel | Carrageenan-based matrix containing Zn-carbon dots | Improved antioxidant and antimicrobial properties. Reduced spoilage. Excellent sensor for food quality. | [122] |
| Source of Anthocyanins | Type of Anthocyanin | Anthocyanin Role | Polymer Matrix | Applications | Ref. * |
|---|---|---|---|---|---|
| Brassica oleracea | Not indicated | pH indicator | ACUVUE® contact lenses and poly(hydroxyethyacrylamide) | Ocular biosensor | Riaz et al. [131] |
| Brassica oleracea L. Var. capitata | Not indicated | pH indicator | Carboxymethyl cellulose (CMC) and polyvinyl alcohol (PVA) | Wound dressing | Alsahag et al. [132] |
| Red cabbage | Not indicated | pH indicator | Calcium alginate and cellulose | Urea detection | Al-Qahtani et al. [133] |
| Red cabbage | Not indicated | pH indicator | Cellulose | Sweat fluid detection | Al-Qahtani et al. [134] |
| Anthocyanin Source | Anthocyanin Type | Polymer Matrix | Advantages | Application(s) | Ref. |
|---|---|---|---|---|---|
| Bilberry | Not indicated | Chitosan and pectin | Improved bioavailability Gastrointestinal protection | Nutraceutical | Zhao et al. [138] |
| Black carrot | Not indicated | Chitosan | Induced hypolipidemic effect | Nutraceutical as dietary supplement | Sreerekha et al. [139] |
| Haskap berry (Lonicera caerulea L.) | Cyanidin 3-O-glucoside (C3G) | PLGA, maltodextrin, and CMC | Reduced carcinogen-induced oxidative stress | Anticancer therapy | Amararathna et al. [140] |
| Black soybean | Not indicated | Chondroitin sulfate | Inhibited proliferation of cancer cells (HeLa) | Anticancer therapy | Jeong et al. [141] |
| Black rice | Not indicated | Chitosan/chondroitin sulfate | Improved gastrointestinal bioavailability Reduced cancer cell viability Induced colon cancer cell apoptosis | Nutraceutical, functional foods, and anticancer therapy | Liang et al. [142] |
| Black rice | Cyanidin-3-glucoside) | HA | Enhanced stability Reduced xanthine oxidase activity | Nutraceutical | Liu et al. [143] |
| Not indicated | Not indicated | Starch from corn | Reduced glycogen levels Improved cardiomyopathy | Cardiovascular diseases | Hanafy et al. [96] |
| Not indicated | Pelargonidin | PLGA | Increased protection and control of mitochondrial dysfunction | Nutraceutical for diabetes prevention | Samadder et al. [151] |
| Not indicated | Not indicated | PLGA/PEG-2000 | Protection against free radicals Excellent antioxidant, antiapoptotic, and anti-inflammatory effects Protection against Alzheimer’s disease | Prevention and treatment of neurological disorders | Amin et al. [145] |
| Jussara pulp | Not indicated | PEO | Improved thermal stability and enhanced antioxidant effect | Nutraceutical and food preservation | Giaconia et al. [146] |
| Chokeberries | Not indicated | PAA and PAH | Improved anti-tumoral performance Monitor and trafficking | Anticancer therapy and biosensor | Ghiman et al. [147] |
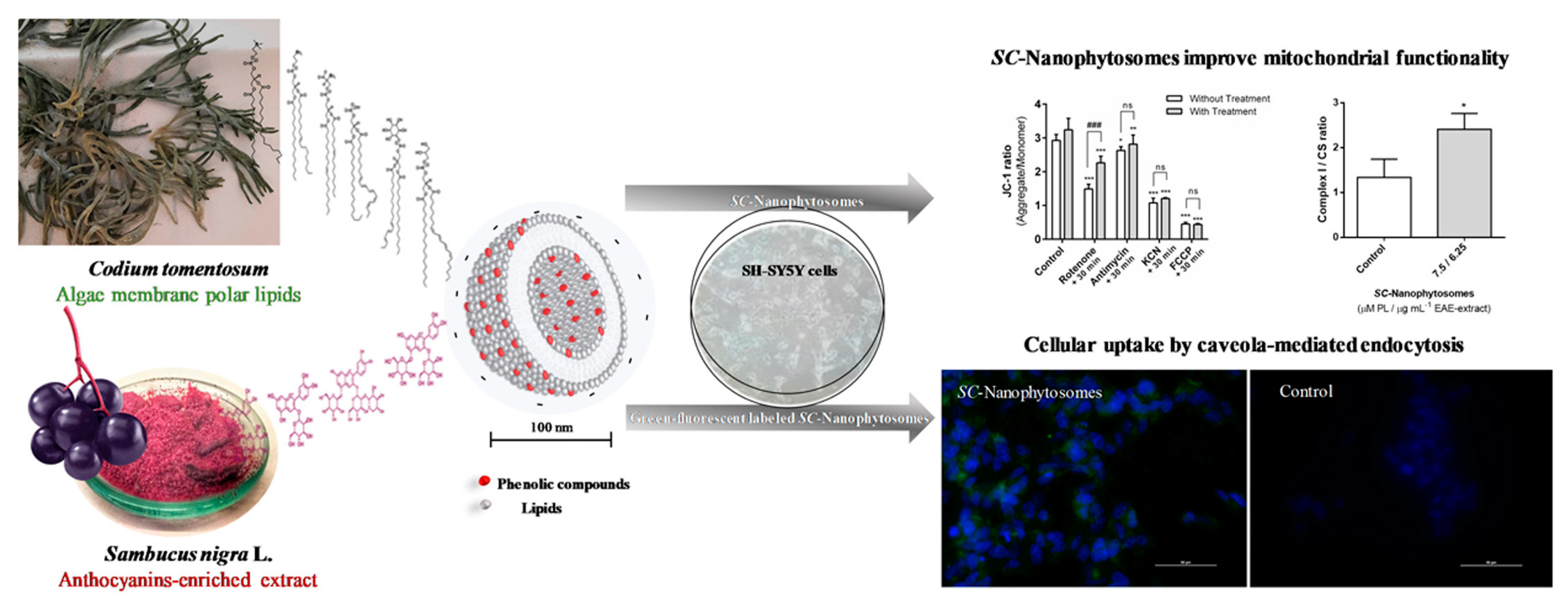
| Elderberries (Sambucus nigra) | Not indicated | Lipids (from Codium tomentosun) | Improved protection of mitochondrial membrane | Prevention and treatment of neurodegenerative diseases | Mendes et al. [148] |
| Black carrots | Not indicated | Niosome (cholesterol and non-ionic surfactants) | Improved bioavailability Reduced neuroblastoma cell viability | Pharmaceutical and biotechnological applications Anticancer therapy | Fidan et al. [149] |
| Black rice (Zea mays and Clitoria ternatea) | Not indicated | Niosome (cholesterol) | Enhanced bioavailability Promoted collagen production Improved anti-inflammatory effect | Wound healing systems | Priprem et al. [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales-Murillo, S.S.; Sánchez-Bodón, J.; Hernández Olmos, S.L.; Ibarra-Vázquez, M.F.; Guerrero-Ramírez, L.G.; Pérez-Álvarez, L.; Vilas-Vilela, J.L. Anthocyanin-Loaded Polymers as Promising Nature-Based, Responsive, and Bioactive Materials. Polymers 2024, 16, 163. https://doi.org/10.3390/polym16010163
Rosales-Murillo SS, Sánchez-Bodón J, Hernández Olmos SL, Ibarra-Vázquez MF, Guerrero-Ramírez LG, Pérez-Álvarez L, Vilas-Vilela JL. Anthocyanin-Loaded Polymers as Promising Nature-Based, Responsive, and Bioactive Materials. Polymers. 2024; 16(1):163. https://doi.org/10.3390/polym16010163
Chicago/Turabian StyleRosales-Murillo, S.S., Julia Sánchez-Bodón, S.L. Hernández Olmos, M.F. Ibarra-Vázquez, L.G. Guerrero-Ramírez, L. Pérez-Álvarez, and J.L. Vilas-Vilela. 2024. "Anthocyanin-Loaded Polymers as Promising Nature-Based, Responsive, and Bioactive Materials" Polymers 16, no. 1: 163. https://doi.org/10.3390/polym16010163
APA StyleRosales-Murillo, S. S., Sánchez-Bodón, J., Hernández Olmos, S. L., Ibarra-Vázquez, M. F., Guerrero-Ramírez, L. G., Pérez-Álvarez, L., & Vilas-Vilela, J. L. (2024). Anthocyanin-Loaded Polymers as Promising Nature-Based, Responsive, and Bioactive Materials. Polymers, 16(1), 163. https://doi.org/10.3390/polym16010163








