Synthesizing a Water-Soluble Polymeric Nitrification Inhibitor with Novel Soil-Loosening Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Materials
2.2. Methods
3. Results and Discussion
3.1. Content of DMPZ in WSPNI
3.2. Chemical Structures
3.3. Thermal Stability
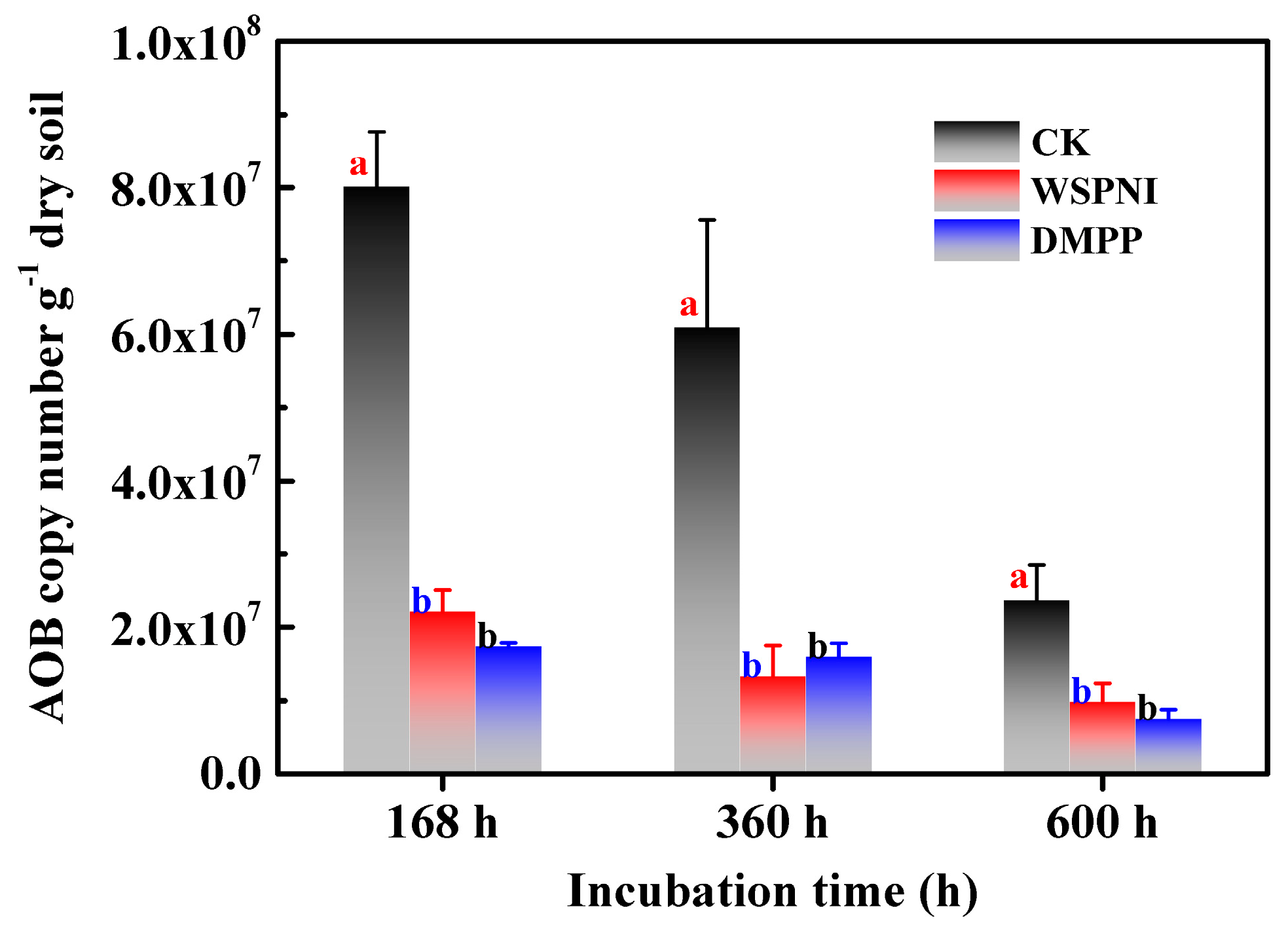
3.4. Nitrogen Fixation Ability
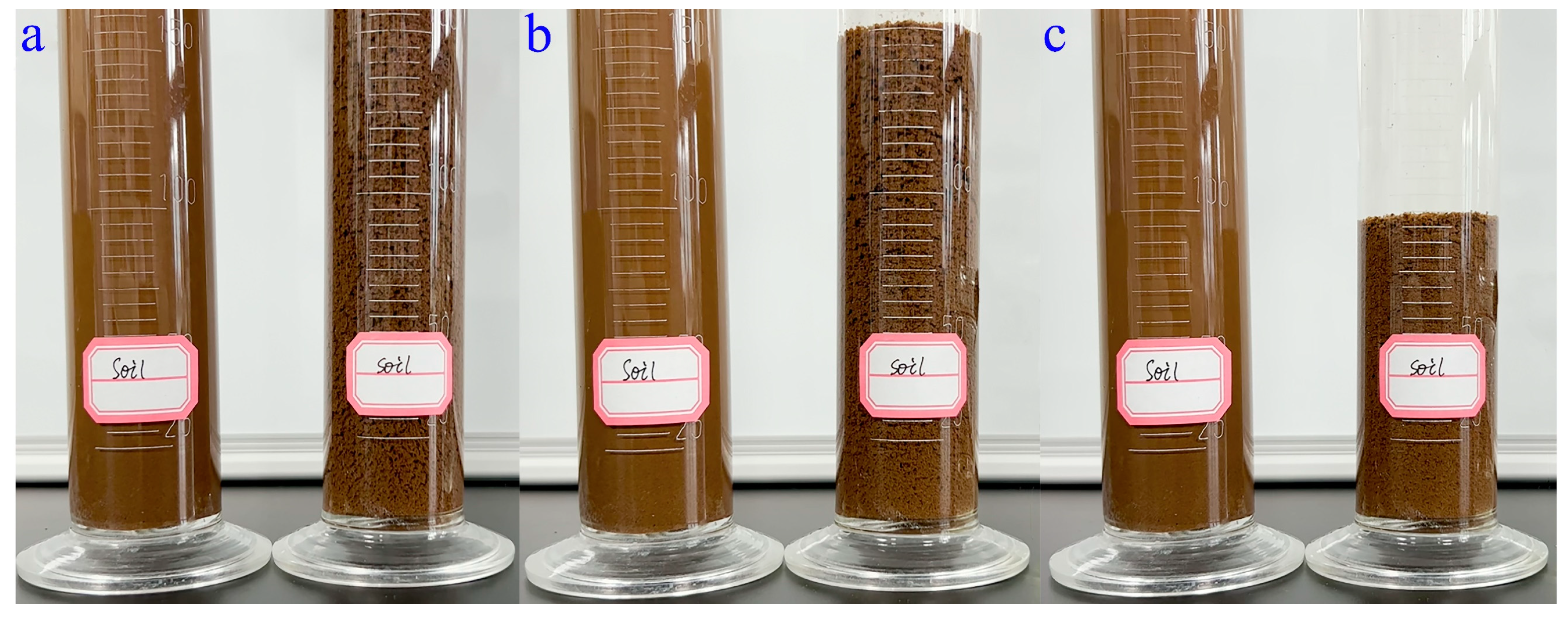
3.5. Soil-Loosening Ability
3.6. Phosphorous-Solubilizing Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Muller, J.; De Rosa, D.; Friedl, J.; De Antoni Migliorati, M.; Rowlings, D.; Grace, P.; Scheer, C. Combining nitrification inhibitors with a reduced N rate maintains yield and reduces N2O emissions in sweet corn. Nutr. Cycl. Agroecosyst. 2023, 125, 107–121. [Google Scholar] [CrossRef]
- Ren, B.; Huang, Z.; Liu, P.; Zhao, B.; Zhang, J. Urea ammonium nitrate solution combined with urease and nitrification inhibitors jointly mitigate NH3 and N2O emissions and improves nitrogen efficiency of summer maize under fertigation. Field Crop. Res. 2023, 296, 108909. [Google Scholar] [CrossRef]
- Min, J.; Sun, H.; Kronzucker, H.J.; Wang, Y.; Shi, W. Comprehensive assessment of the effects of nitrification inhibitor application on reactive nitrogen loss in intensive vegetable production systems. Agric. Ecosyst. Environ. 2021, 307, 107227. [Google Scholar] [CrossRef]
- Lan, T.; He, X.; Wang, Q.; Deng, O.; Zhou, W.; Luo, L.; Chen, G.; Zeng, J.; Yuan, S.; Zeng, M.; et al. Synergistic effects of biological nitrification inhibitor, urease inhibitor, and biochar on NH3 volatilization, N leaching, and nitrogen use efficiency in a calcareous soil-wheat system. Appl. Soil Ecol. 2022, 174, 104412. [Google Scholar]
- Gao, Y.; Cabrera Serrenho, A. Greenhouse gas emissions from nitrogen fertilizers could be reduced by up to one-fifth of current levels by 2050 with combined interventions. Nat. Food 2023, 4, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Friedl, J.; Warner, D.; Wang, W.; Rowlings, D.W.; Grace, P.R.; Scheer, C. Strategies for mitigating N2O and N2 emissions from an intensive sugarcane cropping system. Nutr. Cycl. Agroecosyst. 2023, 125, 295–308. [Google Scholar] [CrossRef]
- Li, H.; Song, X.; Bakken, L.R.; Ju, X. Reduction of N2O emissions by DMPP depends on the interactions of nitrogen sources (digestate vs. urea) with soil properties. J. Integr. Agric. 2023, 22, 251–264. [Google Scholar] [CrossRef]
- Liu, D.; Dong, H.; Ma, C.; Mo, Q.; Liu, B.; Irshad, A.; Li, H.; Yang, B.; Ding, R.; Shayakhmetoya, A.; et al. Inhibiting N2O emissions and improving environmental benefits by integrating garlic growing in grain production systems. Agric. Ecosyst. Environ. 2023, 347, 108371. [Google Scholar] [CrossRef]
- Lan, T.; Huang, Y.; Song, X.; Deng, O.; Zhou, W.; Luo, L.; Tang, X.; Zeng, J.; Chen, G.; Gao, X. Biological nitrification inhibitor co-application with urease inhibitor or biochar yield different synergistic interaction effects on NH3 volatilization, N leaching, and N use efficiency in a calcareous soil under rice cropping. Environ. Pollut. 2022, 293, 118499. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Li, Y.; Duan, W.; Zhai, X.; Luan, J.; Guo, F. Synthesis of dual-functional pyrazole-based transition metal complexes for improved urease and nitrification activities. Inorg. Chim. Acta 2022, 543, 121184. [Google Scholar] [CrossRef]
- Casali, L.; Broll, V.; Ciurli, S.; Emmerling, F.; Braga, D.; Grepioni, F. Facilitating nitrification inhibition through green, mechanochemical synthesis of a novel nitrapyrin complex. Cryst. Growth Des. 2021, 21, 5792–5799. [Google Scholar] [CrossRef]
- Wang, F.; Ge, S.; Lyu, M.; Liu, J.; Li, M.; Jiang, Y.; Xu, X.; Xing, Y.; Cao, H.; Zhu, Z.; et al. DMPP reduces nitrogen fertilizer application rate, improves fruit quality, and reduces environmental cost of intensive apple production in China. Sci. Total Environ. 2022, 802, 149813. [Google Scholar] [CrossRef] [PubMed]
- Hoshimov, A.A.; Seytnazarov, A.R.; Tadjiev, S.M.; Alimov, U.K.; Tojiev, R.R.; Madenov, B.D. NPSCA-containing fertilizers based on ammonium nitrate melt and powder Suprefos-NS. In Proceedings of the 2nd International Conference on Energetics, Civil and Agricultural Engineering 2021 (ICECAE 2021), Tashkent, Uzbekistan, 14–16 October 2021. [Google Scholar]
- Forster, S.P.; Dippold, E.; Chiang, T. Twin-screw melt granulation for oral solid pharmaceutical products. Pharmaceutics 2021, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Levett, I.; Liao, M.; Pratt, C.; Redding, M.; Laycock, B.; Pratt, S. Designing for effective controlled release in agricultural products: New insights into the complex nature of the polymer-active agent relationship and implications for use. J. Sci. Food Agric. 2020, 100, 4723–4733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, J. Preparation and characterization of multifunctional slow release fertilizer coated with cellulose derivatives. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 774–781. [Google Scholar] [CrossRef]
- Saurabh, K.; Kanchikeri Math, M.; Datta, S.C.; Thekkumpurath, A.S.; Kumar, R. Nanoclay polymer composites loaded with urea and nitrification inhibitors for controlling nitrification in soil. Arch. Agron. Soil Sci. 2019, 65, 478–491. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Xu, F. Synthesis of a novel polymer nitrification inhibitor with acrylic acid and 3,4-dimethylpyrazole. J. Agric. Food Chem. 2021, 69, 3307–3311. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Huang, W.; Xu, F. A novel high temperature resistant and multifunctional nitrification inhibitor: Synthesis, characterization, and application. J. Agric. Food Chem. 2022, 70, 13832–13838. [Google Scholar] [CrossRef]
- Davidson, R.A.; Davidson, C.F.; Roa-Espinosa, A. Linear anionic polyacrylamide as an effective post-fire soil treatment: Understanding the chemistry and physical science. J. Soil Water Conserv. 2009, 64, 243–252. [Google Scholar] [CrossRef]
- Levy, G.J.; Ben-Hur, M. Some uses of water-soluble polymers in soil. In Handbook of Soil Conditioners; Books in Soils, plants and the Environment; Marcel Dekker, Inc.: New York, NY, USA, 1988; pp. 399–428. [Google Scholar]
- Lentz, R.D. Polyacrylamide and biopolymer effects on flocculation, aggregate stability, and water seepage in a silt loam. Geoderma 2015, 241, 289–294. [Google Scholar] [CrossRef]
- Busscher, W.J.; Bjorneberg, D.L.; Sojka, R.E. Field application of PAM as an amendment in deep-tilled US southeastern coastal plain soils. Soil Tillage Res. 2009, 104, 215–220. [Google Scholar] [CrossRef]
- Watwood, M.E.; Kay-Shoemake, J.L. Impact of polyacrylamide treatment on sorptive dynamics and degradation of 2, 4-D and atrazine in agricultural soil. J. Soil Contam. 2000, 9, 133–147. [Google Scholar] [CrossRef]
- Li, J.; How, Z.T.; El-Din, M.G. Aerobic degradation of anionic polyacrylamide in oil sands tailings: Impact factor, degradation effect, and mechanism. Sci. Total Environ. 2023, 856, 159079. [Google Scholar] [CrossRef] [PubMed]
- Kebede, B.; Tsunekawa, A.; Haregeweyn, N.; Tsubo, M.; Mulualem, T.; Mamedov, A.I.; Meshesha, D.T.; Adgo, E.; Fenta, A.A.; Ebabu, K.; et al. Effect of polyacrylamide integrated with other soil amendments on runoff and soil loss: Case study from northwest Ethiopia. Int. Soil Water Conserv. Res. 2022, 10, 487–496. [Google Scholar] [CrossRef]
- Ng, C.W.W.; So, P.S.; Lau, S.Y.; Zhou, C.; Coo, J.L.; Ni, J.J. Influence of biopolymer on gas permeability in compacted clay at different densities and water contents. Eng. Geol. 2020, 272, 105631. [Google Scholar] [CrossRef]
- Pan, Y.; She, D.; Shi, Z.; Chen, X.; Xia, Y. Do biochar and polyacrylamide have synergistic effect on net denitrification and ammonia volatilization in saline soils? Environ. Sci. Pollut. Res. 2021, 28, 59974–59987. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A state-of-the-art review of polymers used in soil stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Hermens JG, H.; Jensma, A.; Feringa, B.L. Highly efficient biobased synthesis of acrylic acid. Angew. Chem. 2022, 134, e202112618. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; van Bronswijk, W. An in situ FTIR-ATR study of polyacrylate adsorbed onto hematite at high pH and high ionic strength. Langmuir 2004, 20, 4093–4100. [Google Scholar] [CrossRef]
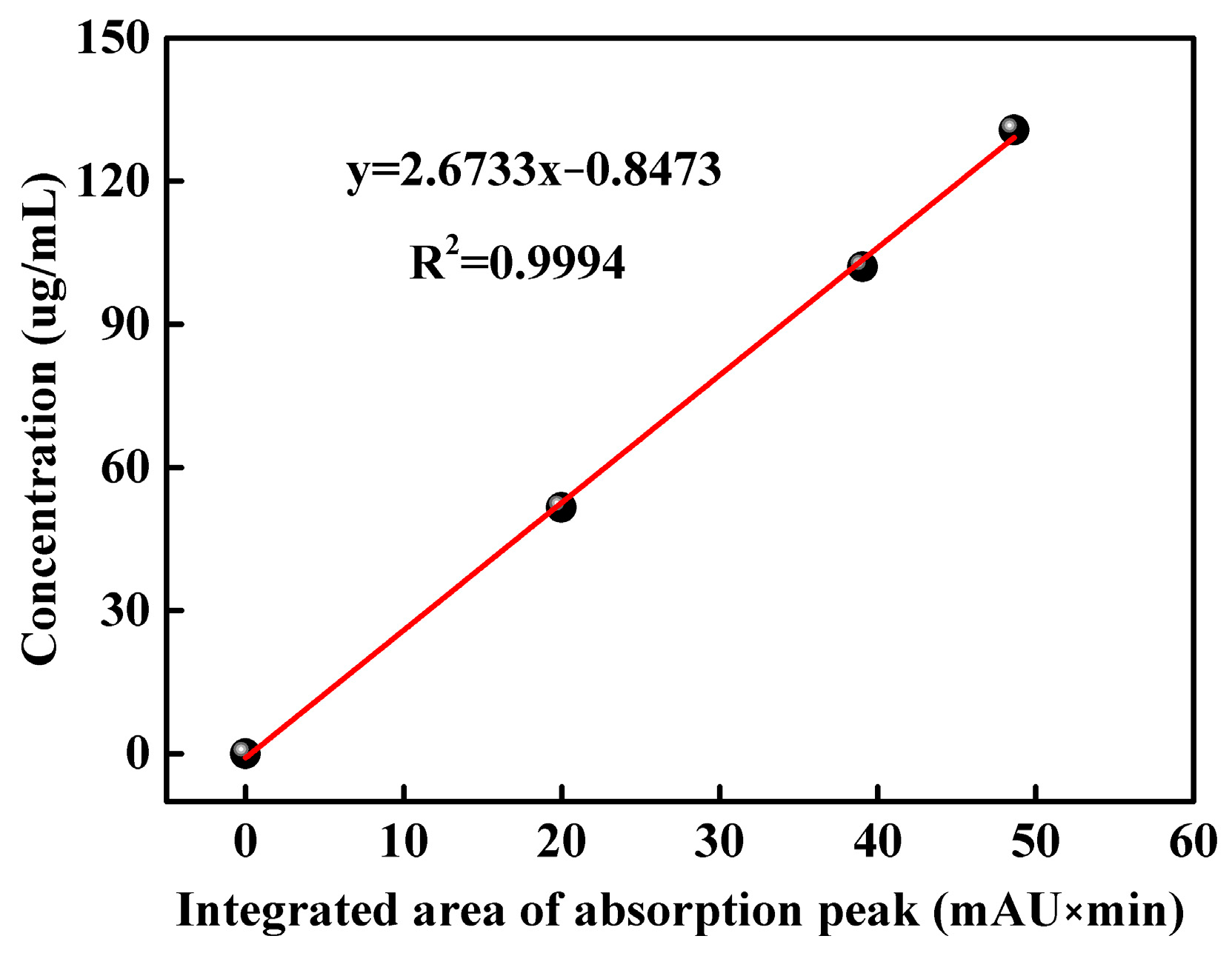
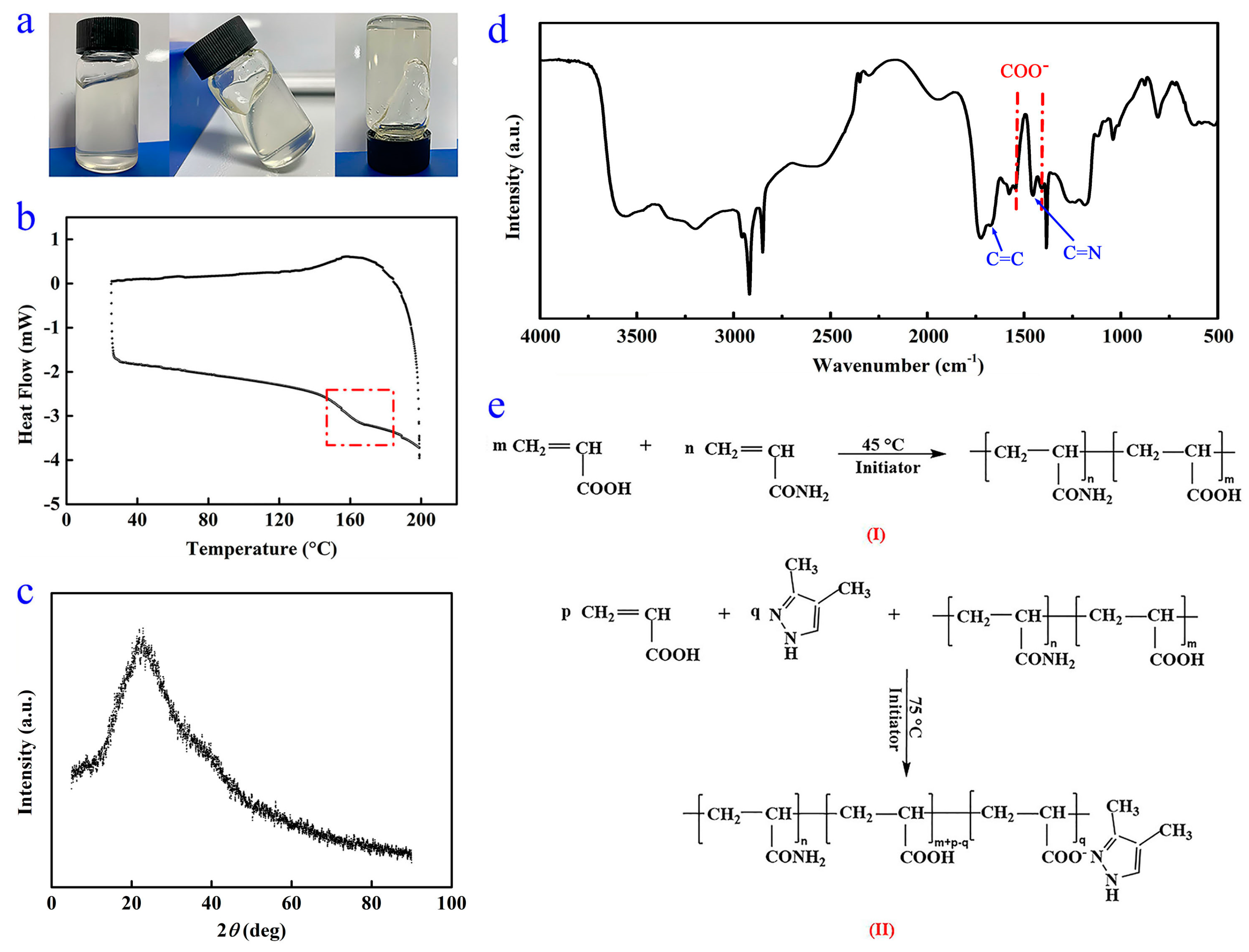
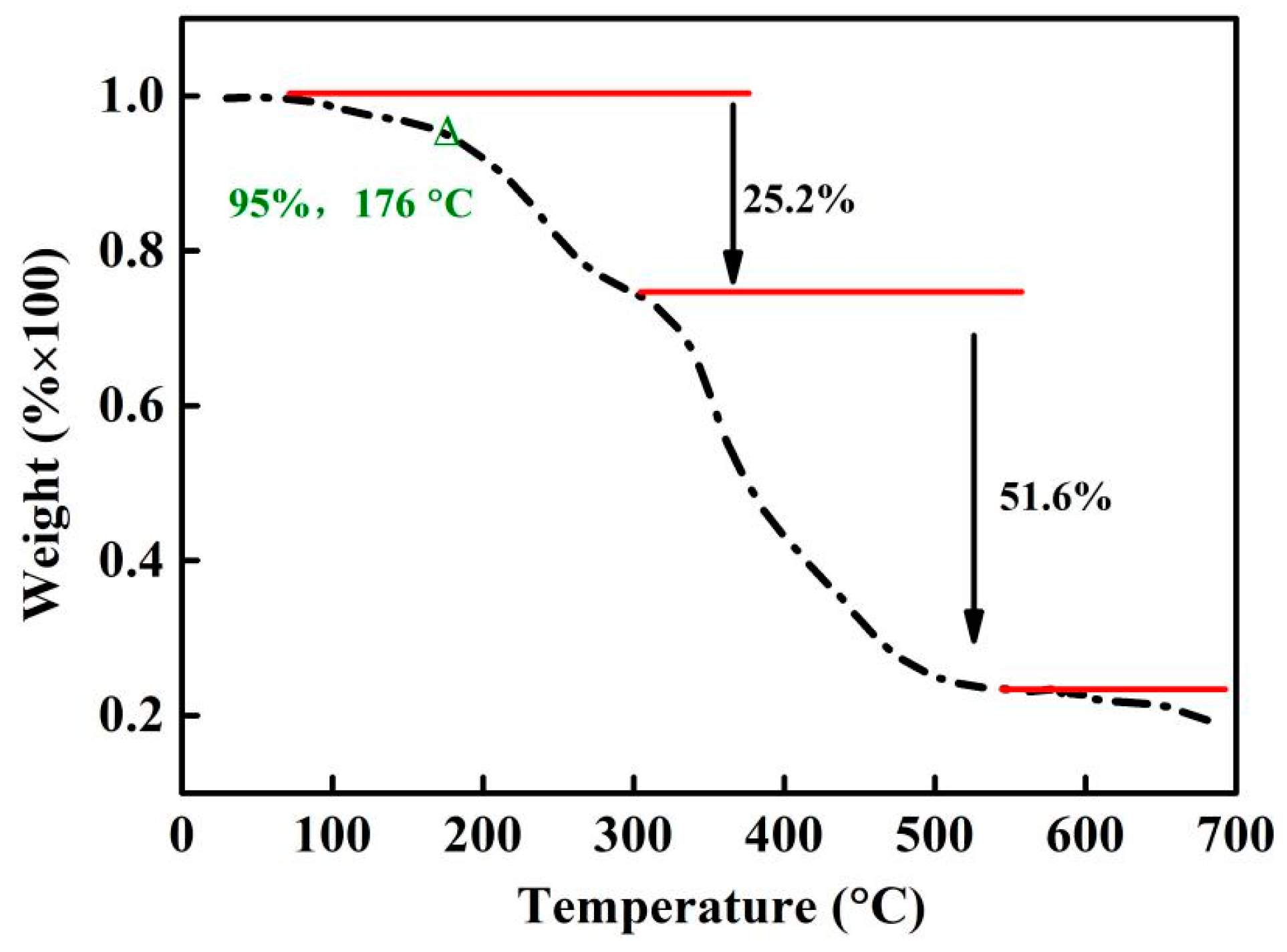
| Sample | Concentration (μg/mL) | Area of Peak at 8–10 min (mAU × min) | DMPZ Content (wt%) |
|---|---|---|---|
| DMPP-std | 51.60 | 19.97 | -- |
| DMPP-std | 102.04 | 39.06 | -- |
| DMPP-std | 130.76 | 48.63 | -- |
| WSPNI | 49.60 | 18.87 | 2.81 |
| Incubation Time (h) | Nitrification Inhibition Rate (%) | Soil Apparent Nitrification Rate (%) | |||
|---|---|---|---|---|---|
| WSPNI Treated | DMPP Treated | CK (Only Urea) | WSPNI Treated | DMPP Treated | |
| 48 | 75.1 ± 3.02 b | 85.0 ± 1.82 a | 26.9 ± 2.08 a | 24.9 ± 0.64 ab | 20.9 ± 0.99 b |
| 72 | 67.6 ± 0.31 a | 68.1 ± 0.30 a | 45.6 ± 0.92 a | 29.9 ± 1.10 b | 31.7 ± 1.18 b |
| 120 | 75.6 ± 1.68 a | 76.5 ± 1.62 a | 90.6 ± 1.41 a | 36.5 ± 1.99 b | 37.4 ± 2.49 b |
| 168 | 65.8 ± 0.51 b | 70.0 ± 0.44 a | 92.9 ± 2.09 a | 51.0 ± 3.59 b | 58.8 ± 3.85 b |
| 240 | 48.8 ± 0.72 a | 46.6 ± 0.75 a | 99.2 ± 1.33 a | 81.3 ± 2.12 b | 76.8 ± 1.19 b |
| 360 | 46.2 ± 2.49 a | 41.7 ± 2.7 a | 97.9 ± 0.53 a | 96.6 ± 0.65 a | 97.6 ± 0.61 a |
| 600 | 39.9 ± 0.75 b | 41.0 ± 0.74 b | 99.0 ± 0.15 a | 97.8 ± 0.61 b | 98.1 ± 0.10 ab |
| Sample | Density (g/cm3) | Compactness (N/cm2) | Porosity (%) |
|---|---|---|---|
| Without WSPNI | 1.38 ± 0.04 a | 417 ± 12 a | 48.0% |
| With WSPNI | 1.14 ± 0.07 a | 358 ± 13 a | 57.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gao, H.; Liu, S.; Li, J.; Kong, F. Synthesizing a Water-Soluble Polymeric Nitrification Inhibitor with Novel Soil-Loosening Ability. Polymers 2024, 16, 107. https://doi.org/10.3390/polym16010107
Liu Y, Gao H, Liu S, Li J, Kong F. Synthesizing a Water-Soluble Polymeric Nitrification Inhibitor with Novel Soil-Loosening Ability. Polymers. 2024; 16(1):107. https://doi.org/10.3390/polym16010107
Chicago/Turabian StyleLiu, Yu, Hui Gao, Shanshan Liu, Jinrong Li, and Fangong Kong. 2024. "Synthesizing a Water-Soluble Polymeric Nitrification Inhibitor with Novel Soil-Loosening Ability" Polymers 16, no. 1: 107. https://doi.org/10.3390/polym16010107
APA StyleLiu, Y., Gao, H., Liu, S., Li, J., & Kong, F. (2024). Synthesizing a Water-Soluble Polymeric Nitrification Inhibitor with Novel Soil-Loosening Ability. Polymers, 16(1), 107. https://doi.org/10.3390/polym16010107







