Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
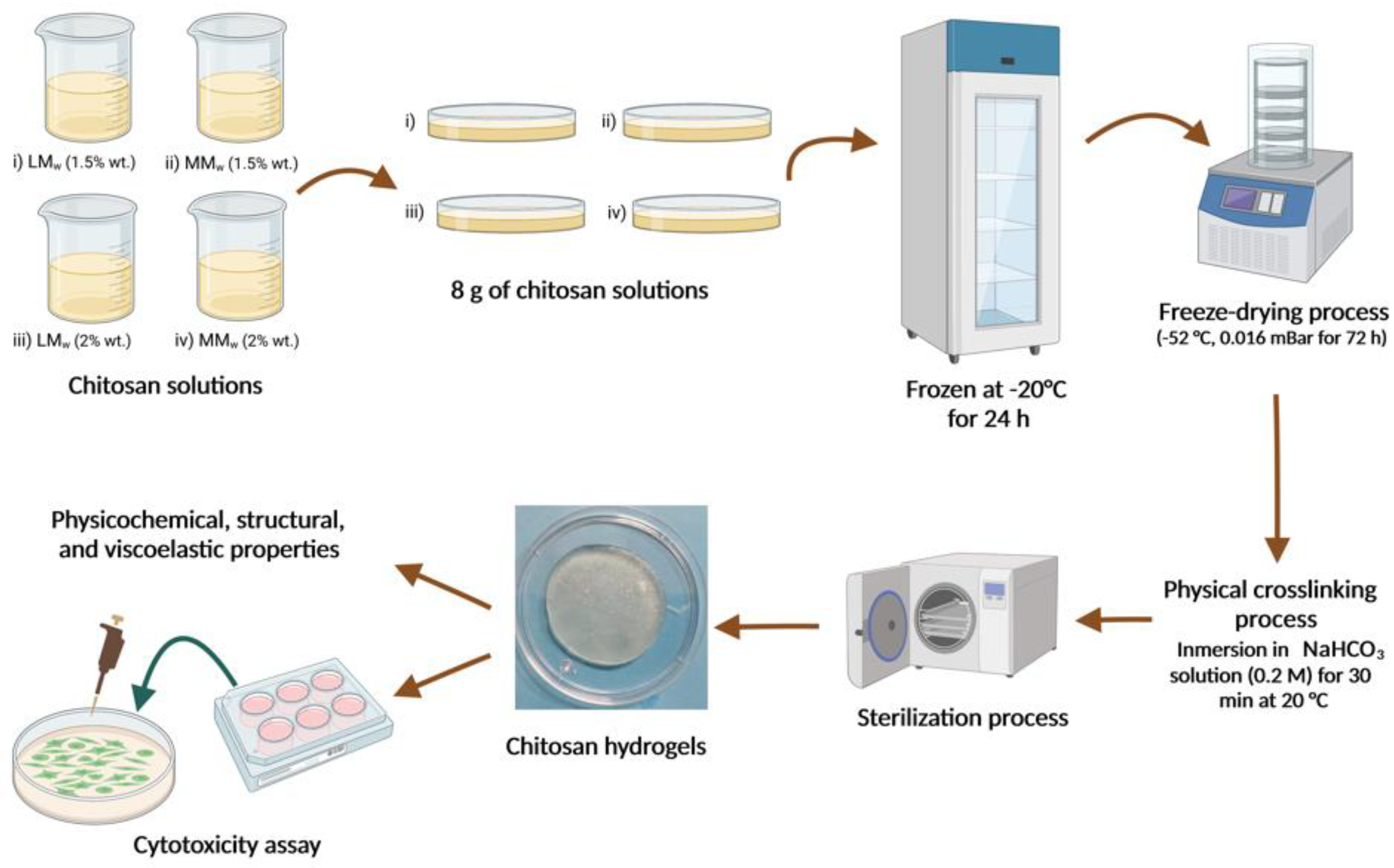
2.2. Fabrication of Hydrogels
2.3. Physicochemical Characterization of Chitosan Hydrogels
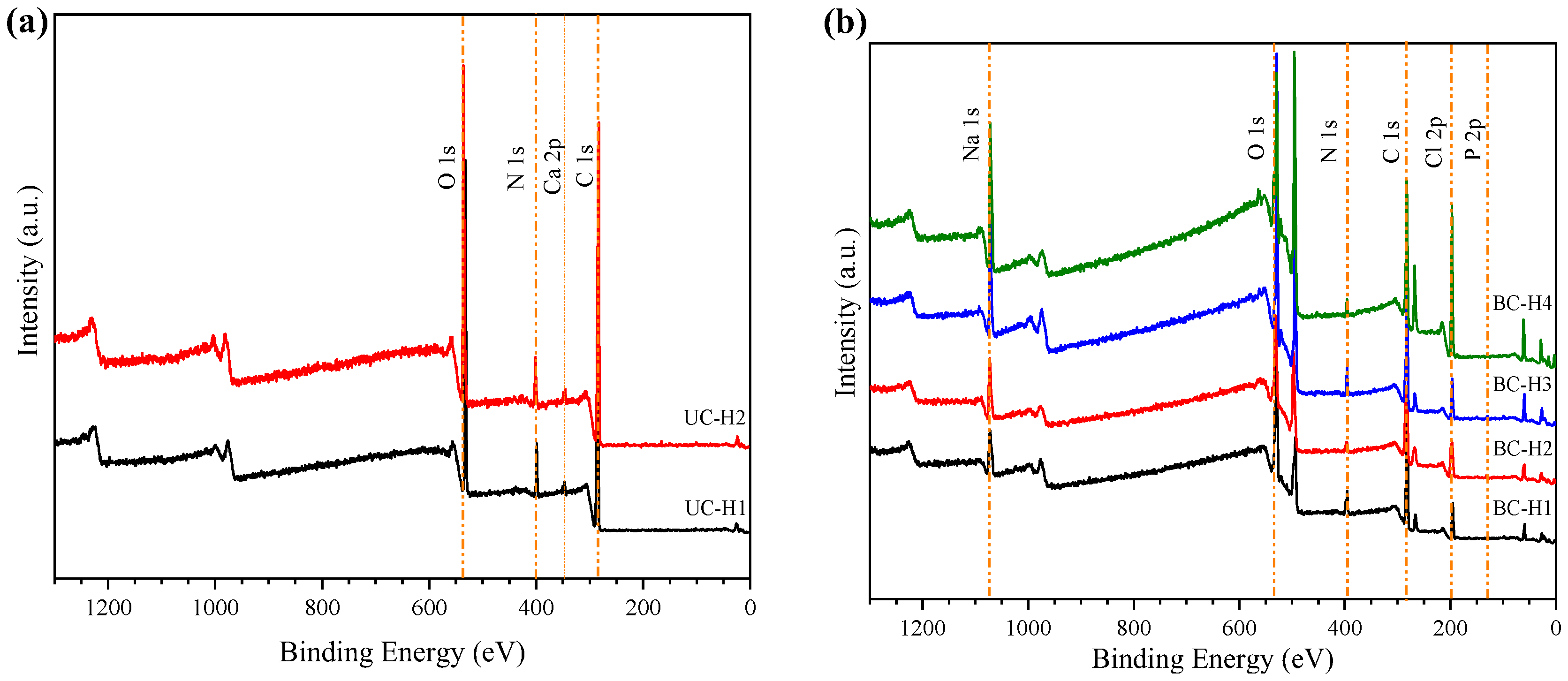
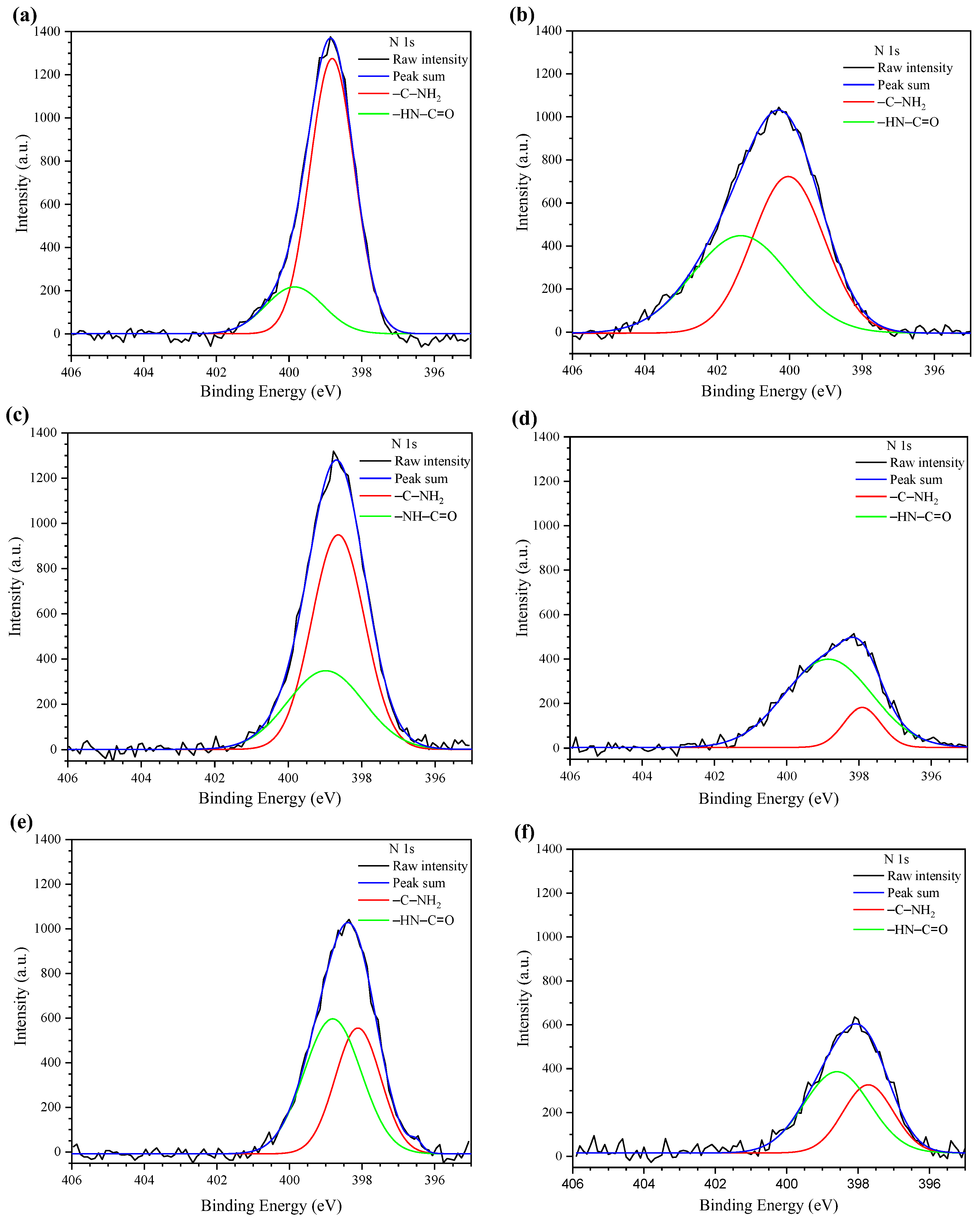
2.3.1. X-ray Photoelectron Spectroscopy
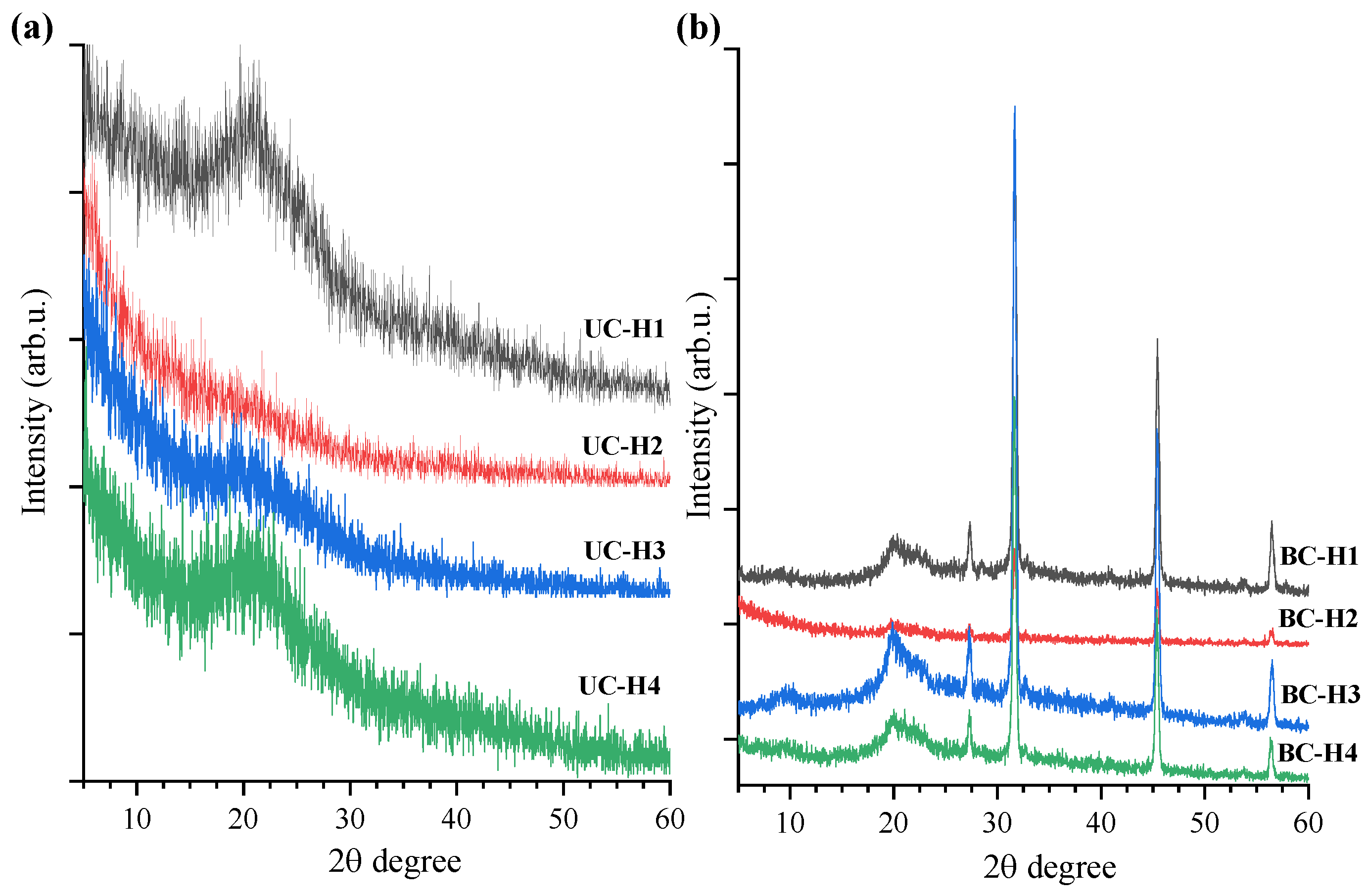
2.3.2. XRD Analysis
2.3.3. Morphological Properties
2.3.4. Equilibrium Water Content
2.3.5. Rheological Properties
2.4. In Vitro Cytotoxicity
Cytotoxicity of the Hydrogel Extract
2.5. Statistical Analysis
3. Results and Discussion
3.1. XRD Spectroscopy
3.2. X-ray Photoelectron Spectroscopy
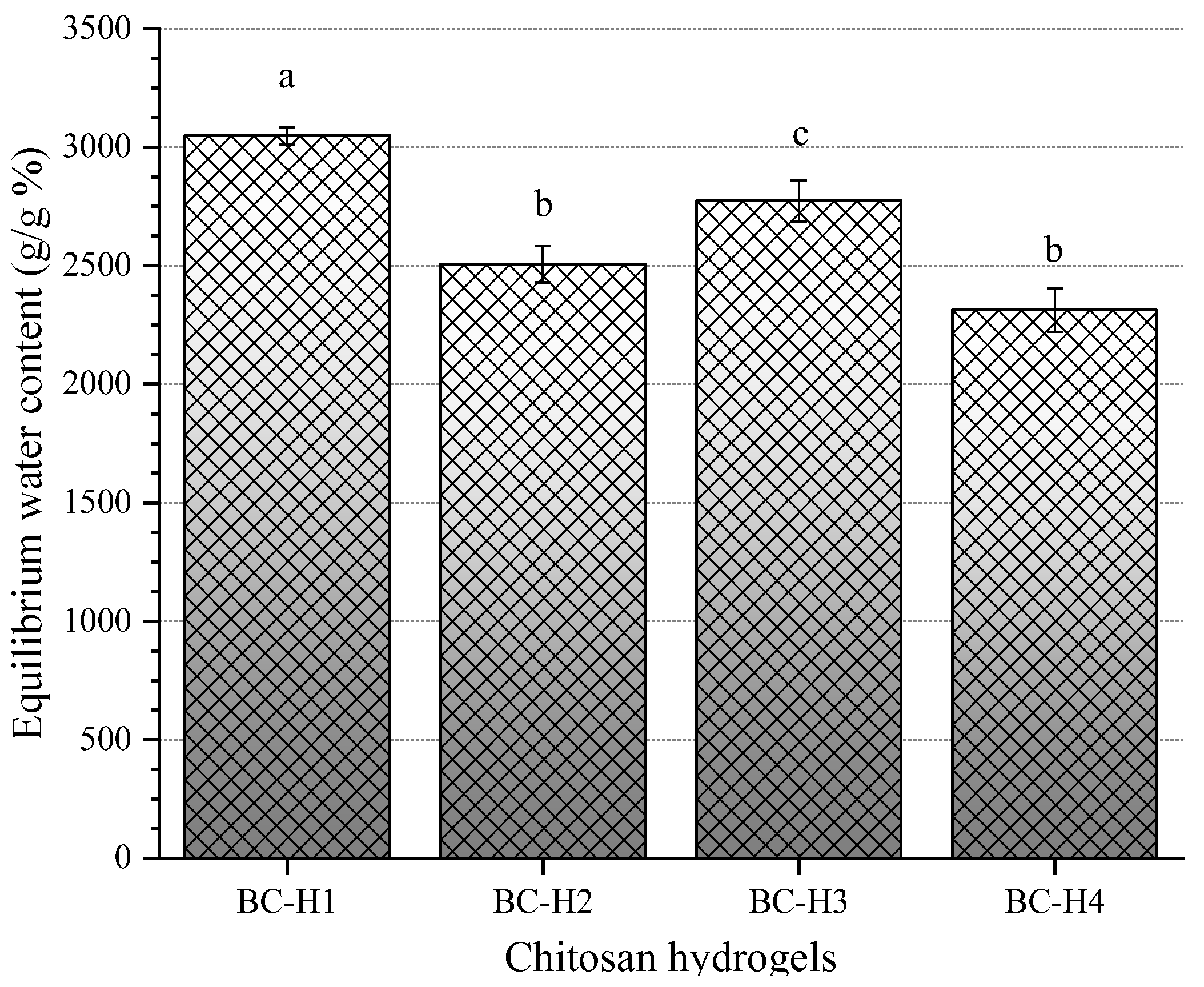
3.3. Equilibrium Water Content
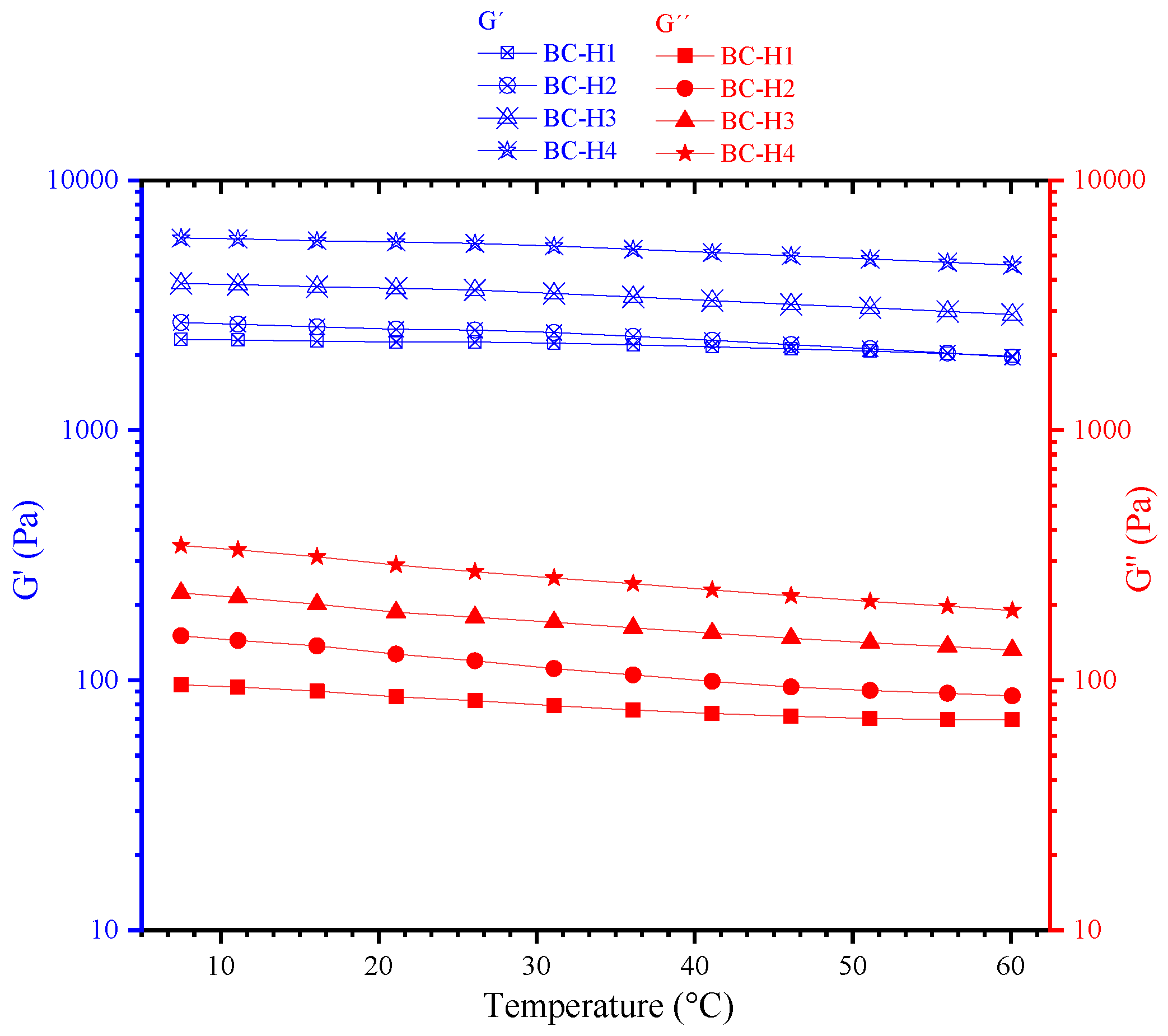
3.4. Viscoelastic Properties
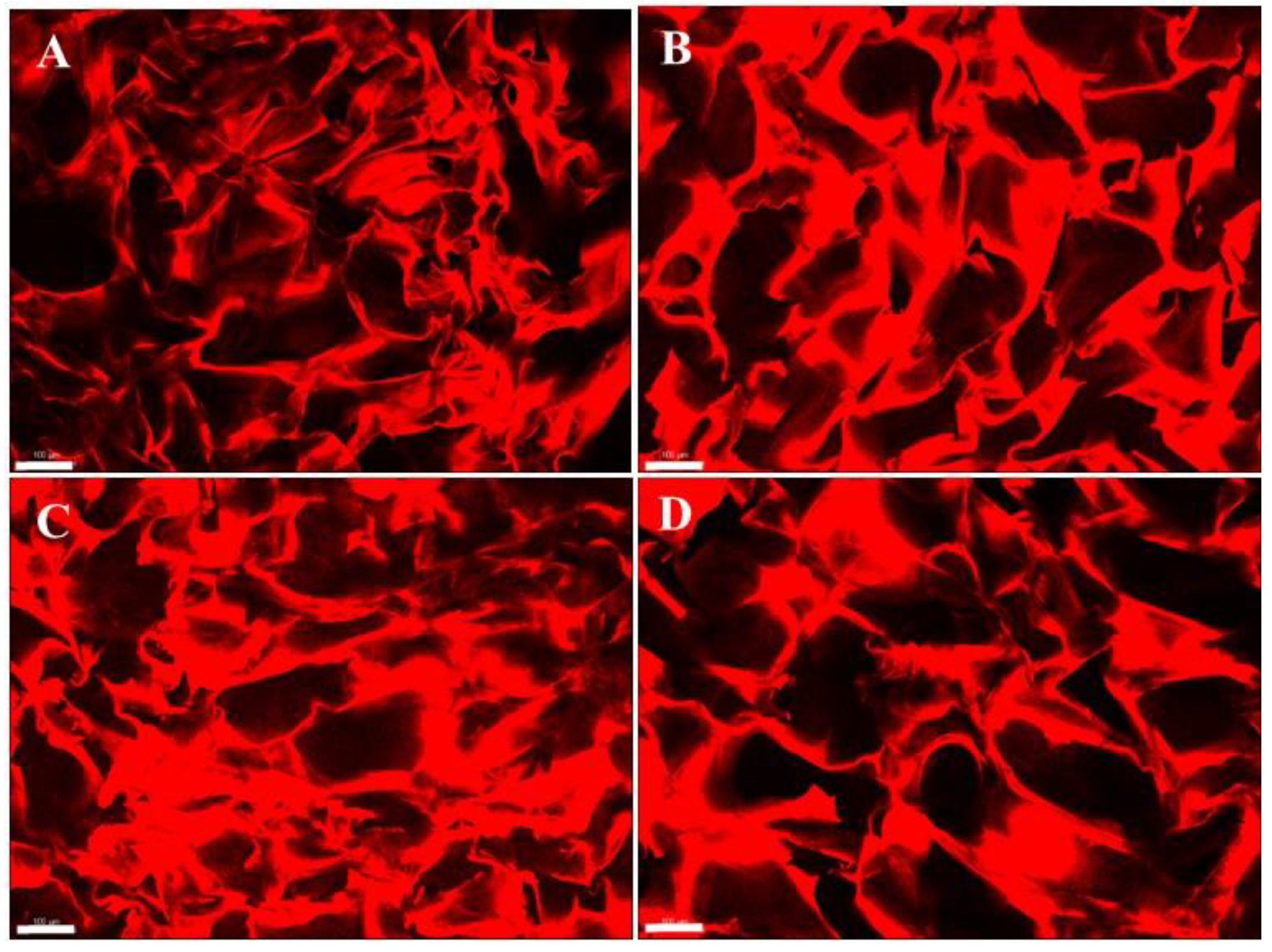
3.5. Microstructure by Confocal Microscopy
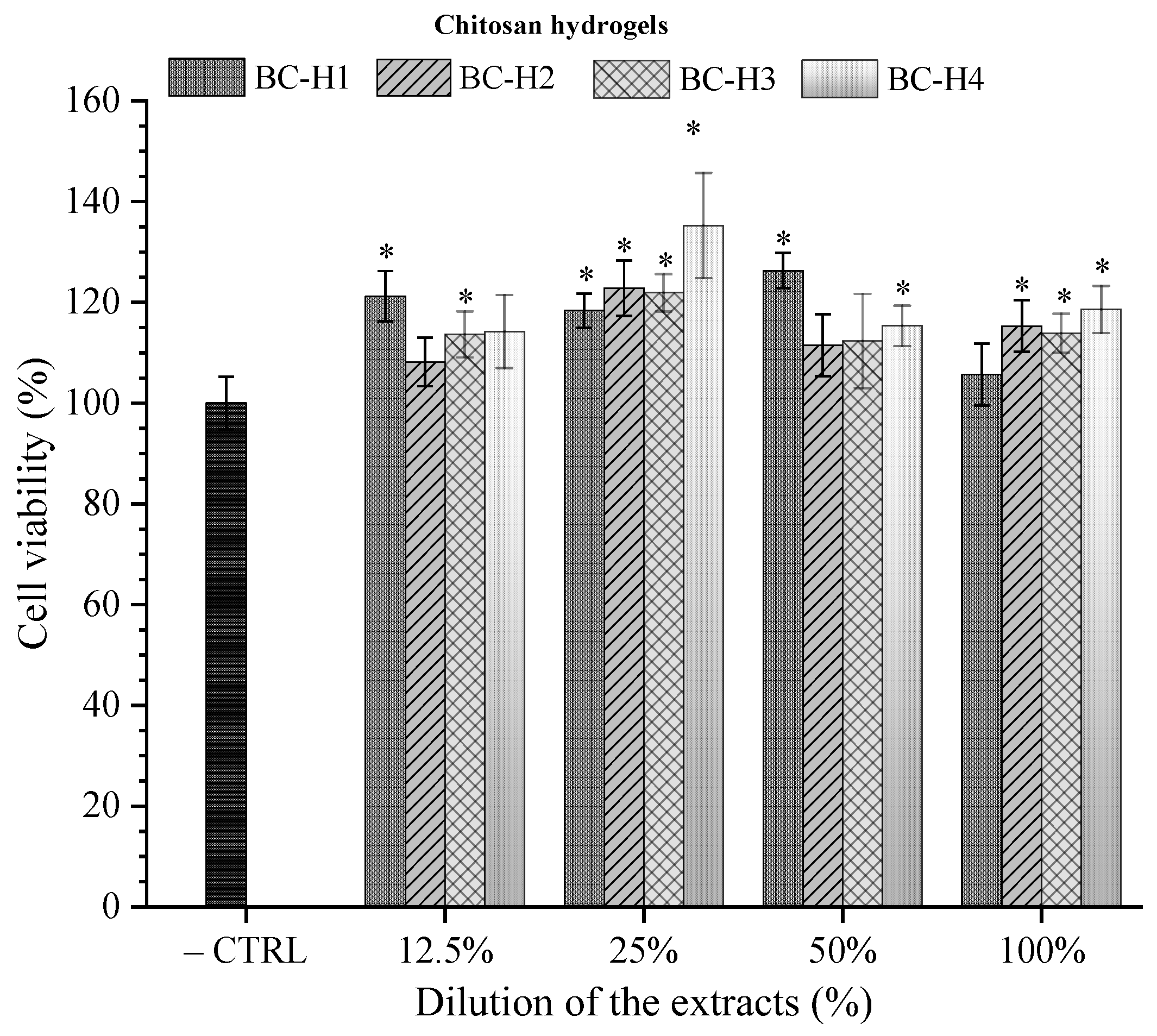
3.6. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176–110196. [Google Scholar] [CrossRef]
- Rumon, M.M.; Akib, A.A.; Sultana, F.; Moniruzzaman, M.; Niloy, M.S.; Shakil, M.S.; Roy, C.K. Self-Healing Hydrogels: Development, Biomedical Applications, and Challenges. Polymers 2022, 14, 4539–4561. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Zheng, C.; Chen, W.; Sun, N.; Gao, Q.; Liu, J.; Hu, F.; Pimpi, S.; Yan, X.; Zhang, Y.; et al. Current challenges and future applications of antibacterial nanomaterials and chitosan hydrogel in burn wound healing. Mater. Adv. 2022, 3, 6707–6727. [Google Scholar] [CrossRef]
- Petroni, S.; Tagliaro, I.; Antonini, C.; D’Arienzo, M.; Orsini, S.F.; Mano, J.F.; Brancato, V.; Borges, J.; Cipolla, L. Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications. Mar. Drugs 2023, 21, 147–201. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Park, K. Swelling agents and devices in oral drug delivery. J. Drug Deliv. Sci. Technol. 2008, 18, 83–93. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Velasquillo-Martínez, C.; Knauth, P.; López, Z.; Moreno-Valtierra, M.; Bravo-Madrigal, J.; Jiménez-Palomar, I.; Luna-Bárcenas, G.; Espinosa-Andrews, H.; García-Carvajal, Z.Y. Sterilized chitosan-based composite hydrogels: Physicochemical characterization and in vitro cytotoxicity. J. Biomed. Mater. Res. Part A 2020, 108, 81–93. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984–109996. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Morales-Hernández, N.; Lobato-Calleros, C.; Vernon-Carter, E.J. Mesquite gum/chitosan insoluble complexes: Relationship between the water state and viscoelastic properties. J. Dispers. Sci. Technol. 2019, 40, 1345–1352. [Google Scholar] [CrossRef]
- Rivas-Araiza, R.; Alcouffe, P.; Rochas, C.; Montembault, A.; David, L. Micron Range Morphology of Physical Chitosan Hydrogels. Langmuir 2010, 26, 17495–17504. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Enache, A.A.; David, L.; Puaux, J.-P.; Banu, I.; Bozga, G. Kinetics of chitosan coagulation from aqueous solutions. J. Appl. Polym. Sci. 2018, 135, 46062–46074. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Zahid Butt, M.T.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: A preclinical study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Romero, A.; Díaz, M.J.; de-Paz, M.V.; Perez-Puyana, V. Chitosan-based hydrogels obtained via photoinitiated click polymer IPN reaction. J. Mol. Liq. 2023, 379, 121735–121746. [Google Scholar] [CrossRef]
- Lu, Z.; Zou, L.; Zhou, X.; Huang, D.; Zhang, Y. High strength chitosan hydrogels prepared from NaOH/urea aqueous solutions: The role of thermal gelling. Carbohydr. Polym. 2022, 297, 120054–120064. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ohzuno, Y.; Saito, Y.; Fujiwara, Y.; Yoshida, M.; Takei, T. Autoclaving-Triggered Hydrogelation of Chitosan-Gluconic acid Conjugate Aqueous Solution for Wound Healing. Gels 2023, 9, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, J.; Chen, Y.; Cheng, B.; Yu, Z.; Zhao, Y.; Yan, X.; Tong, Z.; Jin, S. Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydr. Polym. 2017, 157, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Samal, S.K.; Douglas, T.E.L.; Schaubroeck, D.; Leeuwenburgh, S.C.; Van Der Voort, P.; Declercq, H.A.; Dubruel, P. Enzymatically biomineralized chitosan scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2017, 11, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, C.; Di Natale, A.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R.; Elviri, L. Study of 3D-printed chitosan scaffold features after different post-printing gelation processes. Sci. Rep. 2019, 9, 362–373. [Google Scholar] [CrossRef]
- Azueta-Aguayo, P.H.; Chuc-Gamboa, M.G.; Aguilar-Pérez, F.J.; Aguilar-Ayala, F.J.; Rodas-Junco, B.A.; Vargas-Coronado, R.F.; Cauich-Rodríguez, J.V. Effects of Neutralization on the Physicochemical, Mechanical, and Biological Properties of Ammonium-Hydroxide-Crosslinked Chitosan Scaffolds. Int. J. Mol. Sci. 2022, 23, 14822–14835. [Google Scholar] [CrossRef]
- Reddy, N.; Santosh, M.S.; Venkatesh, K.; Sakkara, S.; Nagananda, G.S. Alkali Treated 3D Chitosan Scaffolds with Enhanced Strength and Stability. J. Polym. Environ. 2021, 29, 3302–3310. [Google Scholar] [CrossRef]
- Xu, Y.; Han, J.; Lin, H. Fabrication and characterization of a self-crosslinking chitosan hydrogel under mild conditions without the use of strong bases. Carbohydr. Polym. 2017, 156, 372–379. [Google Scholar] [CrossRef]
- Ernesto, J.V.; Gasparini, Í.d.M.; Corazza, F.G.; Mathor, M.B.; Silva, C.F.d.; Leite-Silva, V.R.; Andréo-Filho, N.; Lopes, P.S. Physical, chemical, and biological characterization of biodegradable chitosan dressing for biomedical applications: Could sodium bicarbonate act as a crosslinking agent? Mater. Chem. Phys. 2023, 301, 127636–127648. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Velásquez-Ordoñez, C.; Cervantes-Uc, J.M.; Rodríguez-Rodríguez, R. Water behavior, thermal, structural, and viscoelastic properties of physically cross-linked chitosan hydrogels produced by NaHCO3 as a crosslinking agent. J. Mater. Sci. 2023, 58, 6025–6037. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; García-Carvajal, Z.Y.; Jiménez-Palomar, I.; Jiménez-Avalos, J.A.; Espinosa-Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2019, 136, 47149–47158. [Google Scholar] [CrossRef]
- Chijcheapaza-Flores, H.; Tabary, N.; Chai, F.; Maton, M.; Staelens, J.-N.; Cazaux, F.; Neut, C.; Martel, B.; Blanchemain, N.; Garcia-Fernandez, M.J. Injectable Chitosan-Based Hydrogels for Trans-Cinnamaldehyde Delivery in the Treatment of Diabetic Foot Ulcer Infections. Gels 2023, 9, 262–279. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, H.A.; Lopez-Romero, M.; Franco-Molina, M.A.; Manzano-Ramirez, A.; Velasquillo, C.; España-Sanchez, B.L.; Martinez-Hernandez, A.L.; Vergara-Castañeda, H.; Giraldo-Betancur, A.; Favela, S.; et al. Chitosan-G-Glycidyl Methacrylate/Au Nanocomposites Promote Accelerated Skin Wound Healing. Pharmaceutics 2022, 14, 1855–1873. [Google Scholar] [CrossRef]
- Pallela, R.; Venkatesan, J.; Janapala, V.R.; Kim, S.-K. Biophysicochemical evaluation of chitosan-hydroxyapatite-marine sponge collagen composite for bone tissue engineering. J. Biomed. Mater. Res. Part A 2012, 100, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Alghuwainem, Y.A.A.; Gouda, M.; Khalaf, M.M.; Elmushyakhi, A.; Abou Taleb, M.F.; El-Lateef, H.M.A. Synthesis and Characterization of Chitosan-Containing ZnS/ZrO2/Graphene Oxide Nanocomposites and Their Application in Wound Dressing. Polymers 2022, 14, 5195–5210. [Google Scholar] [CrossRef] [PubMed]
- Nady, N.; Kandil, S.H. Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications. Membranes 2018, 8, 2. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Linares-Fernandez, L.; Doehne, E.; Sebastian, E. Effects of ferrocyanide ions on NaCl crystallization in porous stone. J. Cryst. Growth 2002, 243, 503–516. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, Q.; Long, Y.; Xu, C.; Fang, H.; Chen, Y.; Dai, H. A chitosan based scaffold with enhanced mechanical and biocompatible performance for biomedical applications. Polym. Degrad. Stab. 2020, 181, 109322. [Google Scholar] [CrossRef]
- de Souza, M.F.; da Silva, H.N.; Rodrigues, J.F.B.; Macêdo, M.D.M.; de Sousa, W.J.B.; Barbosa, R.C.; Fook, M.V.L. Chitosan/Gelatin Scaffolds Loaded with Jatropha mollissima Extract as Potential Skin Tissue Engineering Materials. Polymers 2023, 15, 603–620. [Google Scholar] [CrossRef]
- Begum, E.R.A.; Rajaiah, S.; Bhavani, K.; Devi, M.; Karthika, K.; Gowri Priya, C. Evaluation of Extracted Chitosan from Portunus Pelagicus for the Preparation of Chitosan Alginate Blend Scaffolds. J. Polym. Environ. 2017, 25, 578–585. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Gołyńska, M.; Polkowska, I.; Szponder, T.; Nehrbass, D.; Osyczka, A.M. In vivo study on scaffolds based on chitosan, collagen, and hyaluronic acid with hydroxyapatite. Int. J. Biol. Macromol. 2018, 118, 938–944. [Google Scholar] [CrossRef]
- Seda-Tığlı, R.; Karakeçili, A.; Gümüşderelioğlu, M. In vitro characterization of chitosan scaffolds: Influence of composition and deacetylation degree. J. Mater. Sci. Mater. Med. 2007, 18, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Etxabide, A.; Seyfoddin, A.; Ramezani, M. Fabrication and characterisation of poly(vinyl alcohol)/chitosan scaffolds for tissue engineering applications. Mater. Today Proc. 2023, 1–8. [Google Scholar] [CrossRef]
- Zhong, X.; Ji, C.; Chan, A.K.L.; Kazarian, S.G.; Ruys, A.; Dehghani, F. Fabrication of chitosan/poly(ε-caprolactone) composite hydrogels for tissue engineering applications. J. Mater. Sci. Mater. Med. 2011, 22, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Tolstova, T.; Drozdova, M.; Popyrina, T.; Matveeva, D.; Demina, T.; Akopova, T.; Andreeva, E.; Markvicheva, E. Preparation and In Vitro Evaluation of Chitosan-g-Oligolactide Based Films and Macroporous Hydrogels for Tissue Engineering. Polymers 2023, 15, 907–924. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Development of chitosan-tripolyphosphate non-woven fibrous scaffolds for tissue engineering application. J. Mater. Sci. Mater. Med. 2012, 23, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Springer: Singapore, 2018; pp. 475–485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletes-Vargas, G.; Espinosa-Andrews, H.; Cervantes-Uc, J.M.; Limón-Rocha, I.; Luna-Bárcenas, G.; Vázquez-Lepe, M.; Morales-Hernández, N.; Jiménez-Ávalos, J.A.; Mejía-Torres, D.G.; Ramos-Martínez, P.; et al. Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers 2023, 15, 2203. https://doi.org/10.3390/polym15092203
Fletes-Vargas G, Espinosa-Andrews H, Cervantes-Uc JM, Limón-Rocha I, Luna-Bárcenas G, Vázquez-Lepe M, Morales-Hernández N, Jiménez-Ávalos JA, Mejía-Torres DG, Ramos-Martínez P, et al. Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers. 2023; 15(9):2203. https://doi.org/10.3390/polym15092203
Chicago/Turabian StyleFletes-Vargas, Gabriela, Hugo Espinosa-Andrews, José Manuel Cervantes-Uc, Isaías Limón-Rocha, Gabriel Luna-Bárcenas, Milton Vázquez-Lepe, Norma Morales-Hernández, Jorge Armando Jiménez-Ávalos, Dante Guillermo Mejía-Torres, Paris Ramos-Martínez, and et al. 2023. "Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties" Polymers 15, no. 9: 2203. https://doi.org/10.3390/polym15092203
APA StyleFletes-Vargas, G., Espinosa-Andrews, H., Cervantes-Uc, J. M., Limón-Rocha, I., Luna-Bárcenas, G., Vázquez-Lepe, M., Morales-Hernández, N., Jiménez-Ávalos, J. A., Mejía-Torres, D. G., Ramos-Martínez, P., & Rodríguez-Rodríguez, R. (2023). Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers, 15(9), 2203. https://doi.org/10.3390/polym15092203








