Force-Based Characterization of the Wetting Properties of LDPE Surfaces Treated with CF4 and H2 Plasmas
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Treatment
2.2. Surface Texturing of LDPE
2.3. Measurements of Wetting Properties Using Contact Angles
2.4. Measurements of Wetting Properties using Force-Based Friction Measurements
3. Results
3.1. Contact Angle and Surface Free Energy Measurements
3.2. Sliding Angle Measurements
3.3. Force-Based Friction Measurements on T-LDPE Surfaces
3.4. SEM and AFM Images of Altered LDPE Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Švorčík, V.; Kotál, V.; Slepička, P.; Bláhová, O.; Špírková, M.; Sajdl, P.; Hnatowicz, V. Modification of Surface Properties of Polyethylene by Ar Plasma Discharge. Nucl. Instrum. Methods Phys. Res. B 2006, 244, 365–372. [Google Scholar] [CrossRef]
- Ataeefard, M.; Moradian, S.; Mirabedini, M.; Ebrahimi, M.; Asiaban, S. Investigating the Effect of Power/Time in the Wettability of Ar and O2 Gas Plasma-Treated Low-Density Polyethylene. Prog. Org. Coat. 2009, 64, 482–488. [Google Scholar] [CrossRef]
- Sanchis, M.R.; Blanes, V.; Blanes, M.; Garcia, D.; Balart, R. Surface Modification of Low Density Polyethylene (LDPE) Film by Low Pressure O2 Plasma Treatment. Eur. Polym. J. 2006, 42, 1558–1568. [Google Scholar] [CrossRef]
- Olifirenko, A.S.; Novak, I.; Rozova, E.Y.; Saprykina, N.N.; Mitilineos, A.G.; Elyashevich, G.K. Hydrophilization of Porous Polyethylene Films by Cold Plasma of Different Types. Polym. Sci. Ser. B 2009, 51, 247–255. [Google Scholar] [CrossRef]
- Drnovská, H.; Lapčík, L.; Buršíková, V.; Zemek, J.; Barros-Timmons, A.M. Surface Properties of Polyethylene after Low-Temperature Plasma Treatment. Colloid. Polym. Sci. 2003, 281, 1025–1033. [Google Scholar] [CrossRef]
- Xie, T.; McAuley, K.B.; Hsu, J.C.C.; Bacon, D.W. Gas Phase Ethylene Polymerization: Production Processes, Polymer Properties, and Reactor Modeling. Ind. Eng. Chem. Res. 1994, 33, 449–479. [Google Scholar] [CrossRef]
- Nowlin, T.E. Low Pressure Manufacture of Polyethylene. Prog. Polym. Sci. 1985, 11, 29–55. [Google Scholar] [CrossRef]
- Dilks, A. Polymer Surfaces. Anal. Chem. 1981, 53, 802A–816A. [Google Scholar] [CrossRef]
- Shenton, M.J.; Lovell-Hoare, M.C.; Stevens, G.C. Adhesion Enhancement of Polymer Surfaces by Atmospheric Plasma Treatment. J. Phys. D Appl. Phys. 2001, 34, 2754–2760. [Google Scholar] [CrossRef]
- Patra, N.; Hladik, J.; Pavlatová, M.; Militký, J.; Martinová, L. Investigation of Plasma-Induced Thermal, Structural and Wettability Changes on Low Density Polyethylene Powder. Polym. Degrad. Stab. 2013, 98, 1489–1494. [Google Scholar] [CrossRef]
- Popelka, A.; Kronek, J.; Novák, I.; Kleinová, A.; Mičušík, M.; Špírková, M.; Omastová, M. Surface Modification of Low-Density Polyethylene with Poly(2-Ethyl-2-Oxazoline) Using a Low-Pressure Plasma Treatment. Vacuum 2014, 100, 53–56. [Google Scholar] [CrossRef]
- Farzam, M.; Beitollahpoor, M.; Solomon, S.E.; Ashbaugh, H.S.; Pesika, N.S. Advances in the Fabrication and Characterization of Superhydrophobic Surfaces Inspired by the Lotus Leaf. Biomimetics 2022, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Gupta, M. Roll-to-Roll Surface Modification of Cellulose Paper via Initiated Chemical Vapor Deposition. Ind. Eng. Chem. Res. 2018, 57, 11675–11680. [Google Scholar] [CrossRef]
- Nejati, S.; Mirbagheri, S.A.; Waimin, J.; Grubb, M.E.; Peana, S.; Warsinger, D.M.; Rahimi, R. Laser Functionalization of Carbon Membranes for Effective Immobilization of Antimicrobial Silver Nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104109. [Google Scholar] [CrossRef]
- Strobel, M.; Branch, M.C.; Ulsh, M.; Kapaun, R.S.; Kirk, S.; Lyons, C.S. Flame Surface Modification of Polypropylene Film. J. Adhes. Sci. Technol. 1996, 10, 515–539. [Google Scholar] [CrossRef]
- Netravali, A.N.; Caceres, J.M.; Thompson, M.O.; Renk, T.J. Surface Modification of Ultra-High Strength Polyethylene Fibers for Enhanced Adhesion to Epoxy Resins Using Intense Pulsed High-Power Ion Beam. J. Adhes. Sci. Technol. 1999, 13, 1331–1342. [Google Scholar] [CrossRef]
- Mukherjee, J.; Peczonczyk, S.; Maldonado, S. Wet Chemical Functionalization of III−V Semiconductor Surfaces: Alkylation of Gallium Phosphide Using a Grignard Reaction Sequence. Langmuir 2010, 26, 10890–10896. [Google Scholar] [CrossRef]
- Liston, E.M.; Martinu, L.; Wertheimer, M.R. Plasma Surface Modification of Polymers for Improved Adhesion: A Critical Review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Primc, G. Surface Modification of Polyamides by Gaseous Plasma—Review and Scientific Challenges. Polymers 2020, 12, 3020. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Zhang, Q.-Z.; Zhao, K.; Zhang, Y.-R.; Gao, F.; Song, Y.-H.; Wang, Y.-N. Fundamental Study towards a Better Understanding of Low Pressure Radio-Frequency Plasmas for Industrial Applications. Chin. Phys. B 2022, 31, 085202. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-M.; Ko, T.-M.; Hiraoka, H. Polymer Surface Modification by Plasmas and Photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Friedrich, J.F.; Geng, S.; Unger, W.; Lippitz, A.; Erdmann, J.; Gorsler, H.-V.; Wöll, C.; Schertel, A.; Bierbaum, K. Plasma Functionalization and Reorientation of Macromolecules at Polymer Surfaces. Surf. Coat. Technol. 1995, 74–75, 664–669. [Google Scholar] [CrossRef]
- Nuzzo, R.G.; Smolinsky, G. Preparation and Characterization of Functionalized Polyethylene Surfaces. Macromolecules 1984, 17, 1013–1019. [Google Scholar] [CrossRef]
- Harth, K.; Hibst, H. Surface Modification of Polypropylene in Oxygen and Nitrogen Plasmas. Surf. Coat. Technol. 1993, 59, 350–355. [Google Scholar] [CrossRef]
- Mühlhan, C.; Weidner, S.; Friedrich, J.; Nowack, H. Improvement of Bonding Properties of Polypropylene by Low-Pressure Plasma Treatment. Surf. Coat. Technol. 1999, 116–119, 783–787. [Google Scholar] [CrossRef]
- Bhowmik, S.; Bonin, H.W.; Bui, V.T.; Chaki, T.K. Physicochemical and Adhesion Characteristics of High-Density Polyethylene When Treated in a Low-Pressure Plasma under Different Electrodes. J. Adhes. 2006, 82, 1–18. [Google Scholar] [CrossRef]
- Sanchis, R.; Fenollar, O.; García, D.; Sánchez, L.; Balart, R. Improved Adhesion of LDPE Films to Polyolefin Foams for Automotive Industry Using Low-Pressure Plasma. Int. J. Adhes. Adhes. 2008, 28, 445–451. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Yu, X.; Hörst, S.M.; He, C.; McGuiggan, P.; Kristiansen, K.; Zhang, X. Surface Energy of the Titan Aerosol Analog “Tholin. ” Astrophys. J. 2020, 905, 88. [Google Scholar] [CrossRef]
- de Gennes, P.G. Wetting: Statics and Dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]
- Hejda, F.; Solar, P.; Kousal, J. Surface Free Energy Determination by Contact Angle Measurements—A Comparison of Various Approaches. In Proceedings of the 19th Annual Conference of Doctoral Students, WDS’10 “Week of Doctoral Students 2010”, Charles University, Faculty of Mathematics and Physics, Prague, Czech Republic, 1–4 June 2010; Volume 19, pp. 25–30. [Google Scholar]
- Beitollahpoor, M.; Farzam, M.; Pesika, N.S. Determination of the Sliding Angle of Water Drops on Surfaces from Friction Force Measurements. Langmuir 2022, 38, 2132–2136. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Lamichhane, P.; Acharya, T.R.; Kaushik, N.; Nguyen, L.N.; Lim, J.S.; Hessel, V.; Kaushik, N.K.; Choi, E.H. Non-Thermal Argon Plasma Jets of Various Lengths for Selective Reactive Oxygen and Nitrogen Species Production. J. Environ. Chem. Eng. 2022, 10, 107782. [Google Scholar] [CrossRef]
- Sode, M.; Jacob, W.; Schwarz-Selinger, T.; Kersten, H. Measurement and Modeling of Neutral, Radical, and Ion Densities in H 2 -N 2 -Ar Plasmas. J. Appl. Phys. 2015, 117, 083303. [Google Scholar] [CrossRef]
- Chu, P. Plasma-Surface Modification of Biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Švorčík, V.; Kolářová, K.; Dvořánková, B.; Michálek, J.; Krumbholcová, E.; Hnatowicz, V. Plasma Modification of HEMA and EOEMA Surface Properties. Radiat. Eff. Defects Solids 2006, 161, 15–19. [Google Scholar] [CrossRef]
- Švorčík, V.; Kolářová, K.; Slepička, P.; Macková, A.; Novotná, M.; Hnatowicz, V. Modification of Surface Properties of High and Low Density Polyethylene by Ar Plasma Discharge. Polym. Degrad. Stab. 2006, 91, 1219–1225. [Google Scholar] [CrossRef]
- Polat, O.; Bhethanabotla, V.R.; Ayyala, R.S.; Sahiner, N. Carbon Tetrafluoride, Oxygen, and Air RF Plasma Modified Low-Density Polyethylene and Polydimethylsiloxane. Plasma Chem. Plasma Process. 2023, 43, 737–756. [Google Scholar] [CrossRef]
- Gizer, S.G.; Bhethanabotla, V.R.; Ayyala, R.S.; Sahiner, N. Low-Pressure Plasma Treated Polycarbonate and Polymethyl Methacrylate (PMMA) Sheets with Different Surface Patterns to Change Their Surface Properties. Surf. Interfaces 2023, 37, 102646. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Nitschke, M.; Janke, A.; Helbig, R.; D’Souza, F.; Donnelly, G.T.; Willemsen, P.R.; Werner, C. Fluorination of Poly(Dimethylsiloxane) Surfaces by Low Pressure CF4 Plasma—Physicochemical and Antifouling Properties. Express Polym. Lett. 2009, 3, 70–83. [Google Scholar] [CrossRef]
- Nitschke, M.; König, U.; Lappan, U.; Minko, S.; Simon, F.; Zschoche, S.; Werner, C. Low Pressure Plasma-Based Approaches to Fluorocarbon Polymer Surface Modification. J. Appl. Polym. Sci. 2007, 103, 100–109. [Google Scholar] [CrossRef]
- Sawada, Y.; Kogama, M. Plasma-Polymerized Tetrafluoroethylene Coatings on Silica Particles by Atmospheric-Pressure Glow Discharge. Powder Technol. 1997, 90, 245–250. [Google Scholar] [CrossRef]
- Zisman, W.A. Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution. In Contact Angle, Wettability, and Adhesion; American Chemical Society: Washington, DC, USA, 1964; pp. 1–51. [Google Scholar]
- Dalvi, V.H.; Rossky, P.J. Molecular Origins of Fluorocarbon Hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef] [PubMed]
- Dussan, V.E.B.; Chow, R.T.-P. On the Ability of Drops or Bubbles to Stick to Non-Horizontal Surfaces of Solids. J. Fluid. Mech. 1983, 137, 1–29. [Google Scholar] [CrossRef]
- Joanny, J.F.; de Gennes, P.G. A Model for Contact Angle Hysteresis. J. Chem. Phys. 1984, 81, 552–562. [Google Scholar] [CrossRef]
- Olde Riekerink, M.B.; Terlingen, J.G.A.; Engbers, G.H.M.; Feijen, J. Selective Etching of Semicrystalline Polymers: CF 4 Gas Plasma Treatment of Poly(Ethylene). Langmuir 1999, 15, 4847–4856. [Google Scholar] [CrossRef]
- Acharya, T.R.; Chaudhary, D.K.; Gautam, S.; Singh, A.K.; Shrestha, R.; Adhikari, B.C.; Lamichhane, P.; Paudyal, B.; Kaushik, N.K.; Choi, E.H. Influence of Nanoparticle Size on the Characterization of ZnO Thin Films for Formaldehyde Sensing at Room Temperature. Sens. Actuators A Phys. 2023, 351, 114175. [Google Scholar] [CrossRef]
- Nitschke, M.; Meichsner, J. Low-Pressure Plasma Polymer Modification from the FTIR Point of View. J. Appl. Polym. Sci. 1997, 65, 381–390. [Google Scholar] [CrossRef]
- Grace, J.M.; Gerenser, L.J. Plasma Treatment of Polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Liu, N.; Li, Z.; Chen, G.; Chen, Q.; Li, S. Space Charge Dynamics of CF 4 Fluorinated LDPE Samples from Different Fluorination Conditions and Their DC Conductivities. Mater. Res. Express 2017, 4, 075308. [Google Scholar] [CrossRef]
- Egitto, F.D. Plasma Etching and Modification of Organic Polymers. Pure Appl. Chem. 1990, 62, 1699–1708. [Google Scholar] [CrossRef]
- Riekerink, M.B.O. Structural and Chemical Modification of Polymer Surfaces by Gas Plasma Etching; Printpartners Ipskamp: Enschede, The Netherlands, 2001; ISBN 9789036516433. [Google Scholar]

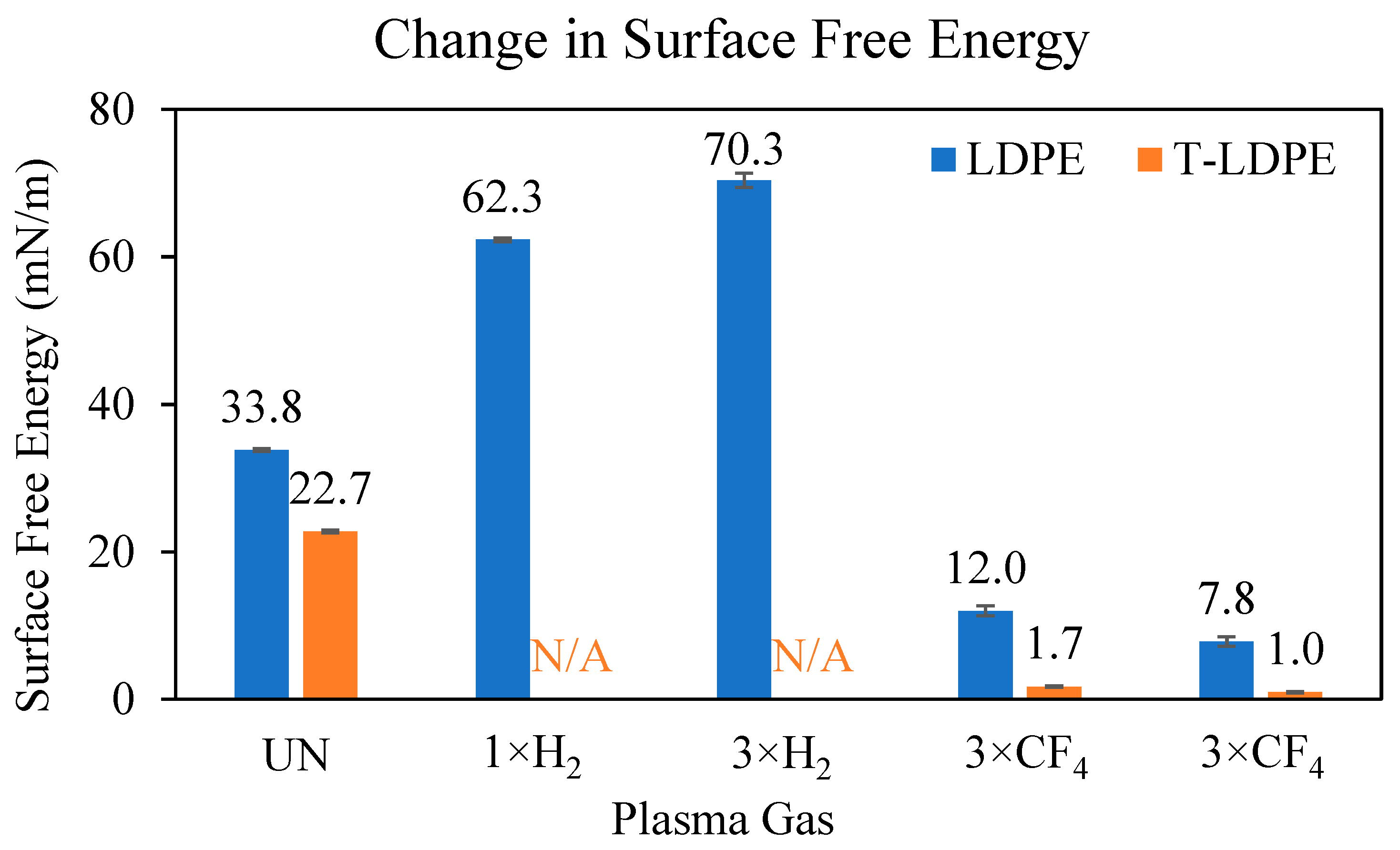
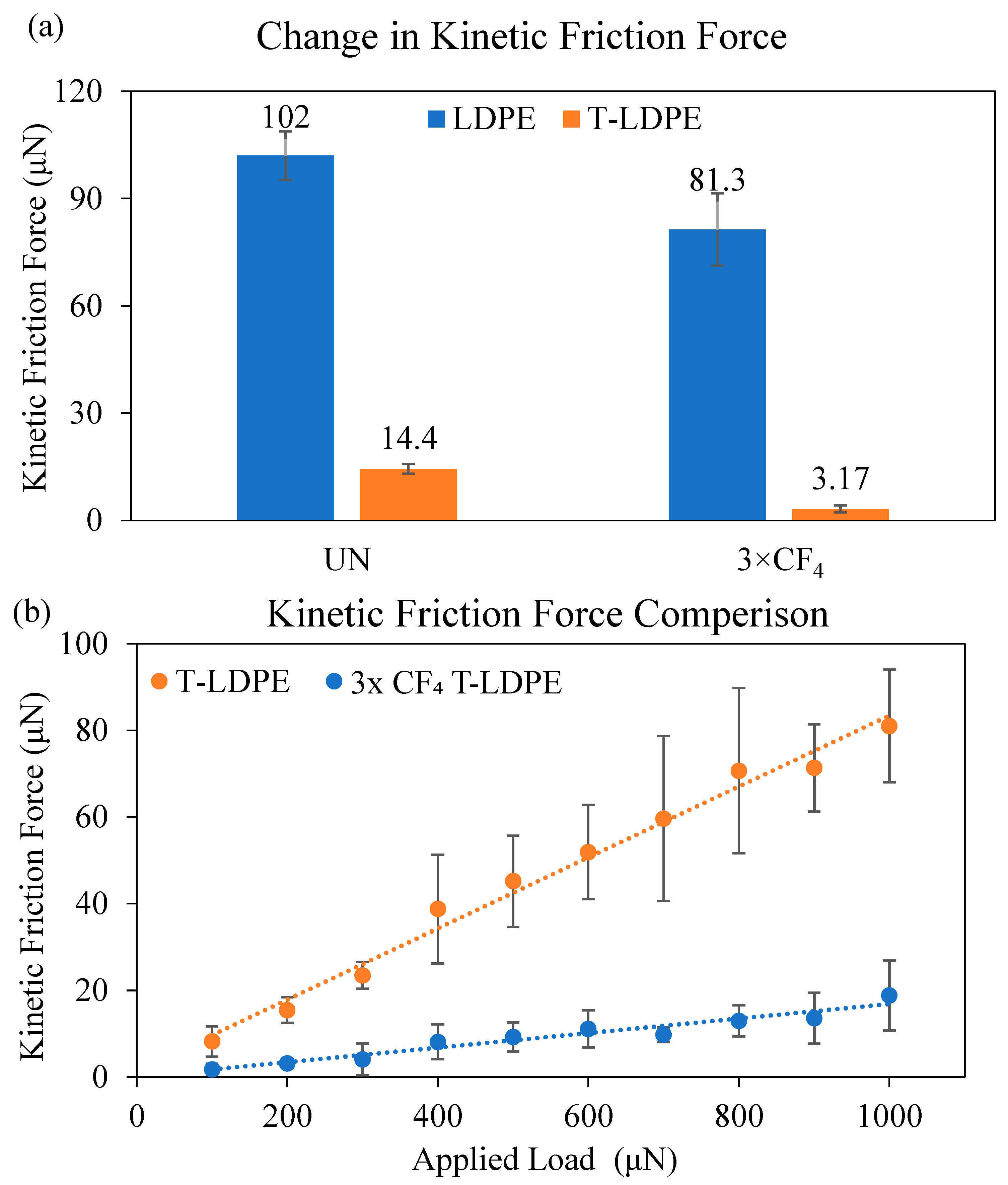

| Sample | Sliding Angle, α (°) | Advancing Contact Angle, θa (°) | Receding Contact Angle, θr (°) | Contact Angle Hysteresis, CAH = θa − θr (°) |
|---|---|---|---|---|
| LDPE | 12.5 ± 0.4 | 102.0 ± 0.8 | 80.1 ± 2.7 | 21.9 |
| T-LDPE | 6.9 ± 0.3 | 130.2 ± 3.5 | 110.5 ± 4.8 | 19.7 |
| LDPE, 3× H₂ | 14.7 ± 1.9 | 47.6 ± 0.6 | 7.6 ± 0.5 | 40 |
| T-LDPE, 3× H₂ | 38.2 ± 2.5 | 107.1 ± 3.5 | 23.2 ± 3.8 | 83.9 |
| LDPE, 3× CF4 | 27.2 ± 2.7 | 141.1 ± 1.0 | 81.1 ± 2.1 | 59.9 |
| T-LDPE, 3× CF4 | 1.7 ± 0.1 | 121.2 ± 0.7 | 118.3 ± 0.9 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktas, C.; Polat, O.; Beitollahpoor, M.; Farzam, M.; Pesika, N.S.; Sahiner, N. Force-Based Characterization of the Wetting Properties of LDPE Surfaces Treated with CF4 and H2 Plasmas. Polymers 2023, 15, 2132. https://doi.org/10.3390/polym15092132
Aktas C, Polat O, Beitollahpoor M, Farzam M, Pesika NS, Sahiner N. Force-Based Characterization of the Wetting Properties of LDPE Surfaces Treated with CF4 and H2 Plasmas. Polymers. 2023; 15(9):2132. https://doi.org/10.3390/polym15092132
Chicago/Turabian StyleAktas, Cihan, Osman Polat, Mohamadreza Beitollahpoor, Melika Farzam, Noshir S. Pesika, and Nurettin Sahiner. 2023. "Force-Based Characterization of the Wetting Properties of LDPE Surfaces Treated with CF4 and H2 Plasmas" Polymers 15, no. 9: 2132. https://doi.org/10.3390/polym15092132
APA StyleAktas, C., Polat, O., Beitollahpoor, M., Farzam, M., Pesika, N. S., & Sahiner, N. (2023). Force-Based Characterization of the Wetting Properties of LDPE Surfaces Treated with CF4 and H2 Plasmas. Polymers, 15(9), 2132. https://doi.org/10.3390/polym15092132













