Effect of Silicone Modifier on the Physical Properties of Flexible Silica Aerogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Synthesis of Flexible Silica Aerogels
3. Results and Discussion
3.1. Material Characterization
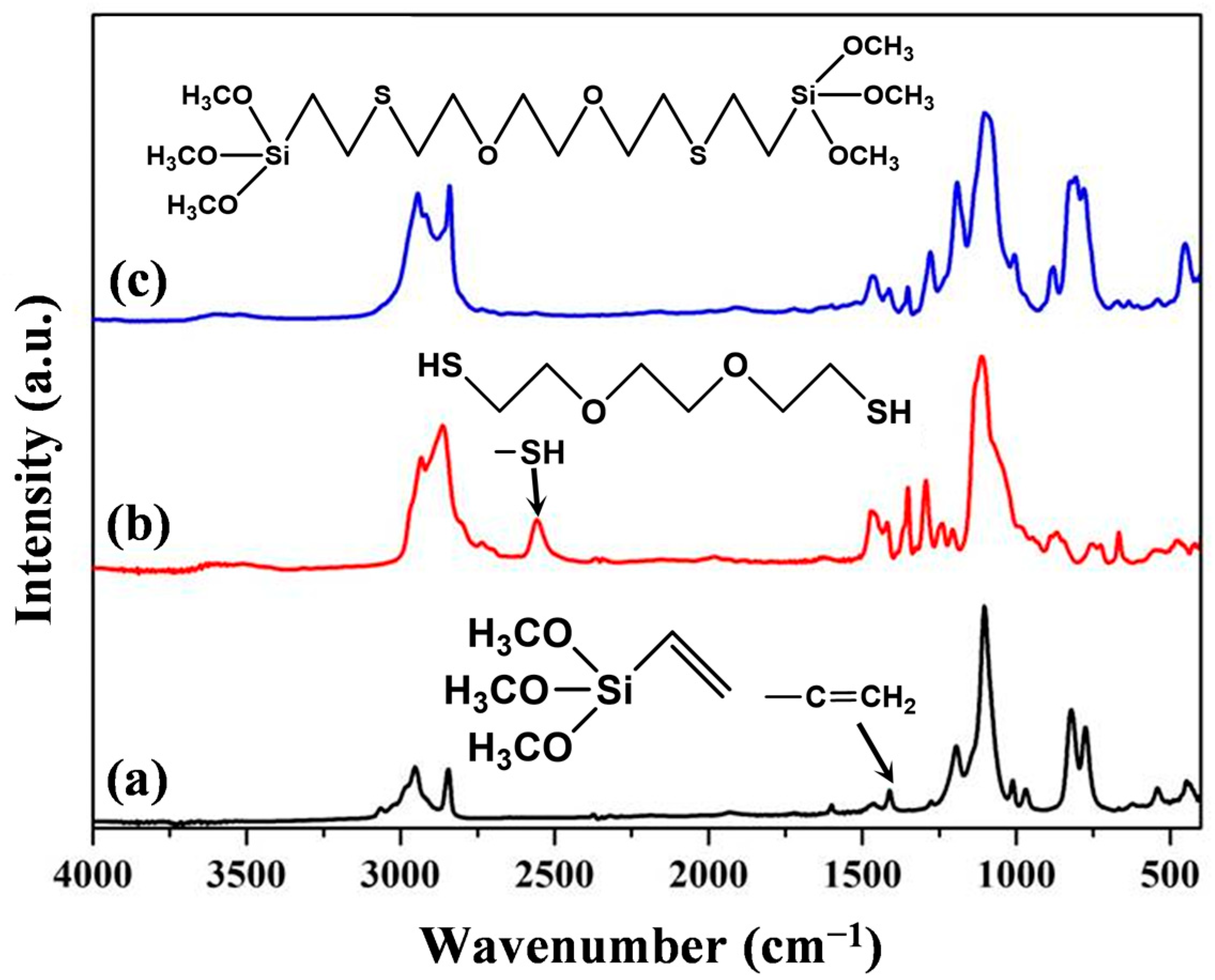
3.1.1. Spectroscopic Studies (FTIR and NMR) of EDDET, VTMS, Precursor
3.1.2. Characterization of Flexible Silica Aerogels (FSA, FSA-M, and FSA-T)
Spectroscopic Studies of Flexible Silica Aerogels (FSAs)
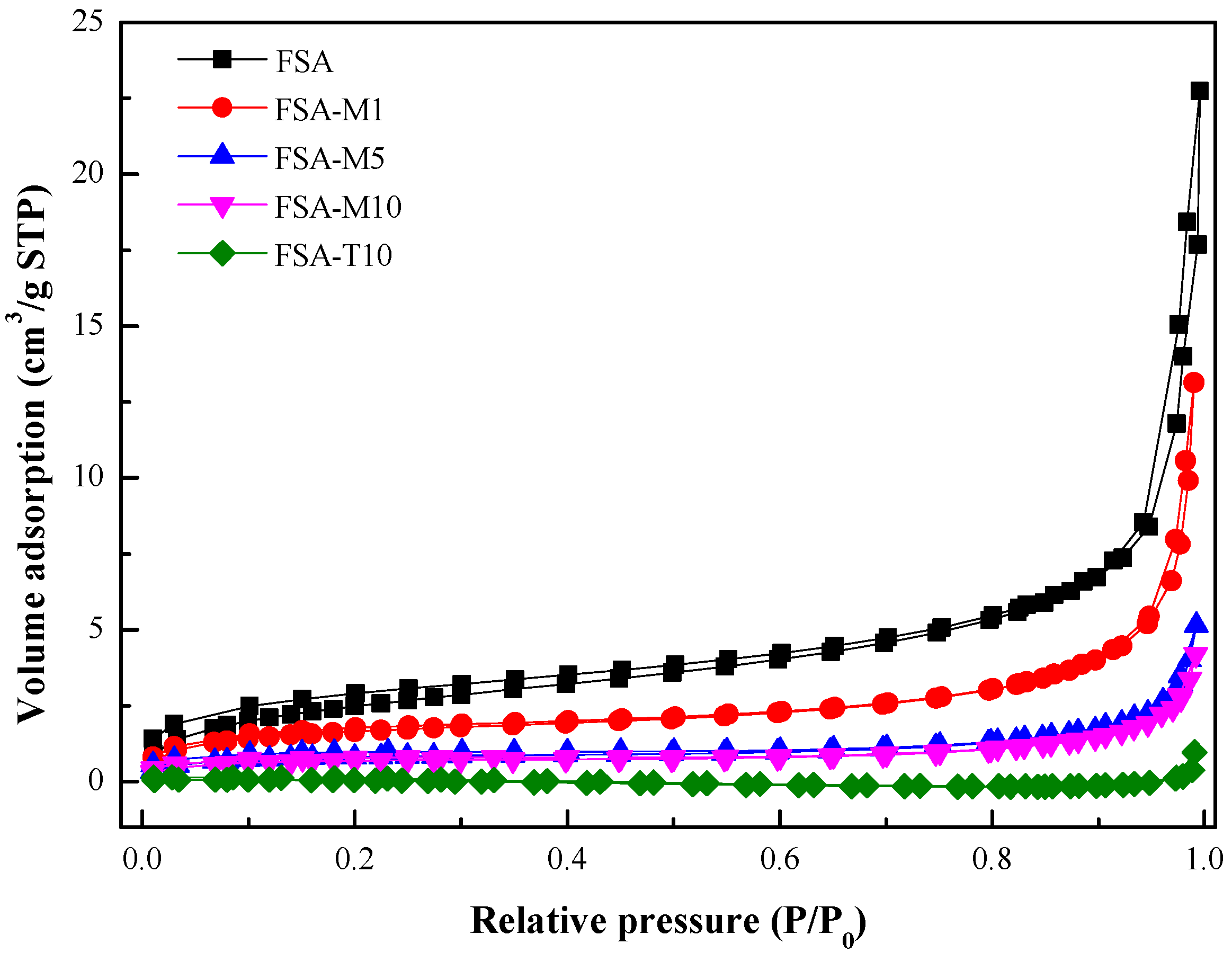
Porous Structure Characterization of Flexible Silica Aerogels
3.2. Surface Morphology
3.3. Thermal Properties of FSAs
3.3.1. Thermal Conductivity (k) of FSAs
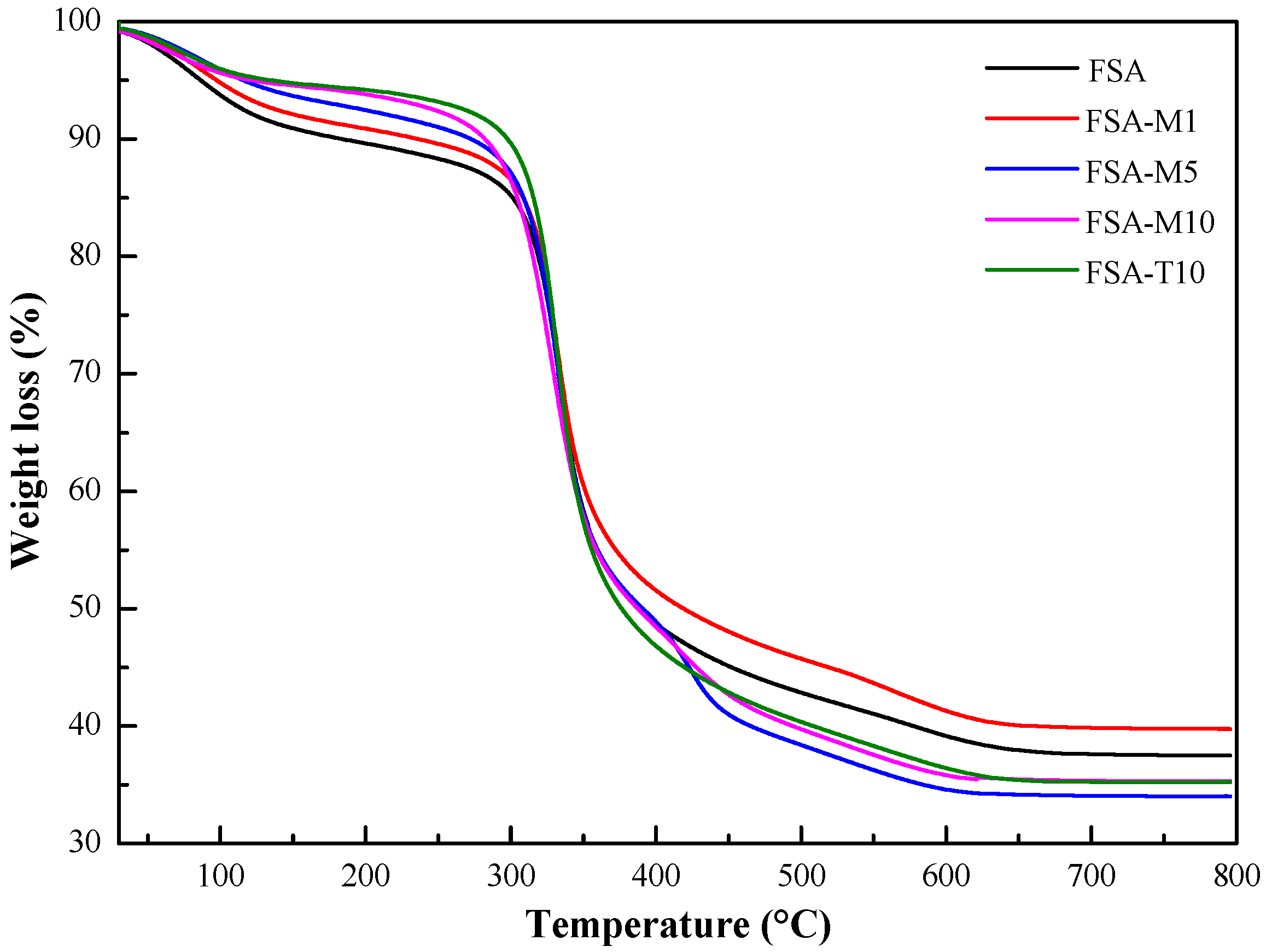
3.3.2. Thermal Stability of FSAs
3.4. Mechanical Properties of FSAs
3.4.1. Surface Hardness of FSAs Determined by Durometer
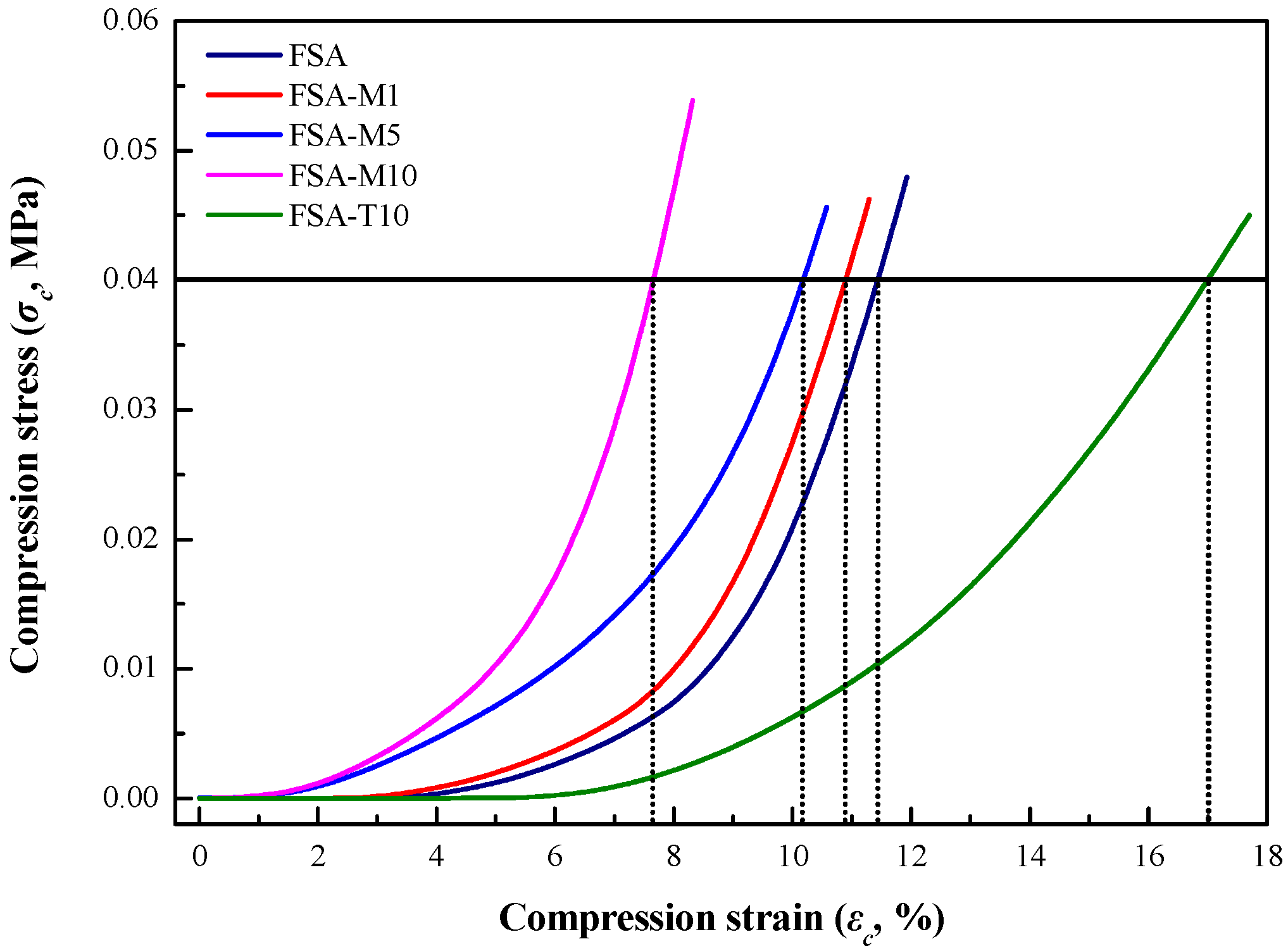
3.4.2. Bulk Mechanical Strength of FSAs Determined by DMA in Compression Mode
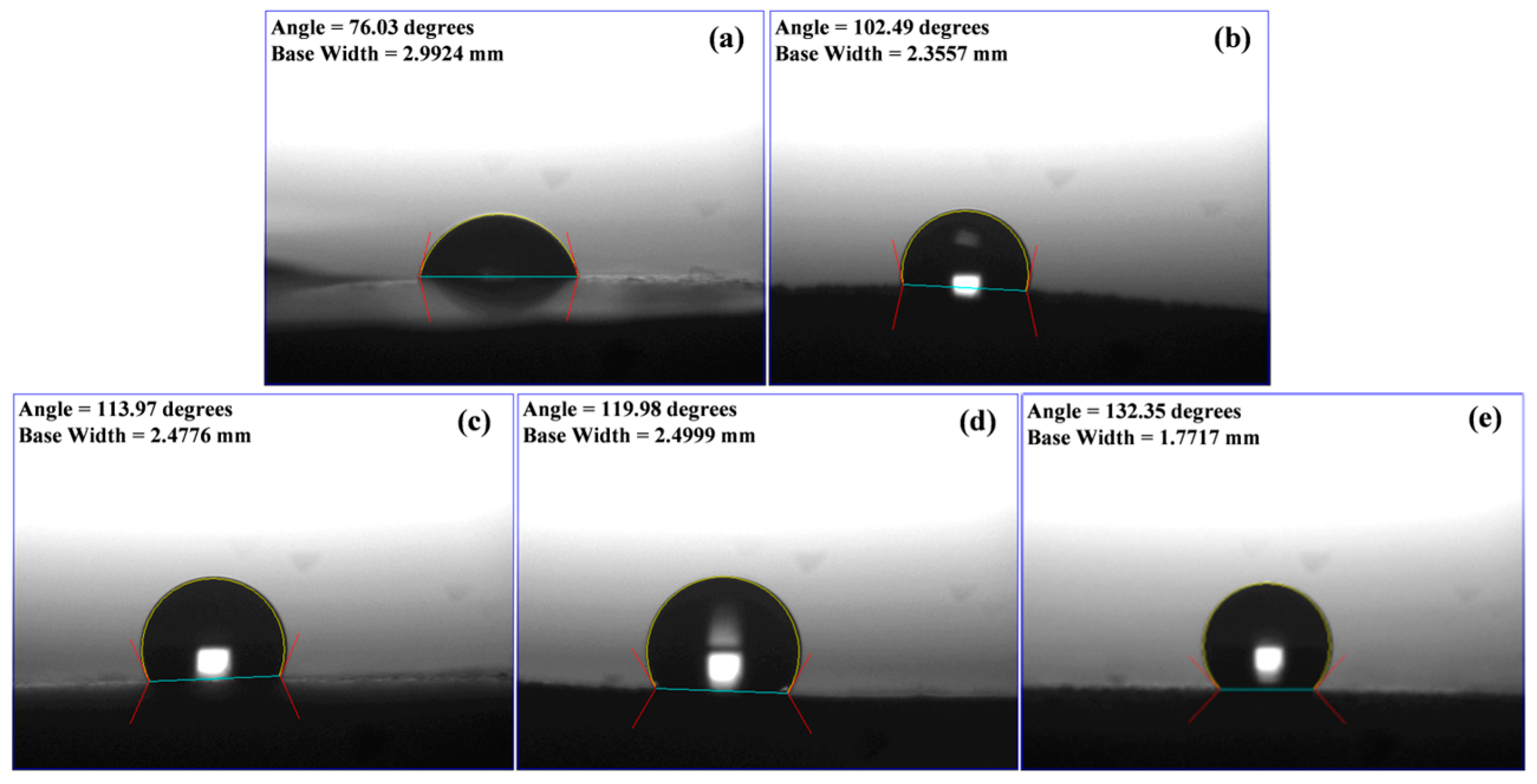
3.5. Surface Wettability of FSAs Determined by CA Measurement of Water Droplets
3.6. Comparison & Application Prospect of As-Prepared Modified FSAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushika, N.D.; Sumathy, K. Solar transparent insulation materials: A review. Renew. Sustain. Energy Rev. 2003, 7, 317–351. [Google Scholar] [CrossRef]
- Reim, M.; Körner, W.; Manara, J.; Korder, S.; Arduini-Schuster, M.; Ebert, H.P.; Fricke, J. Silica aerogel granulate material for thermal insulation and daylighting. Sol. Energy 2005, 79, 131–139. [Google Scholar] [CrossRef]
- Jensen, K.I.; Schultz, J.M.; Kristiansen, F.H. Development of windows based on highly insulating aerogel glazings. J. Non-Cryst. Solids 2004, 350, 351–357. [Google Scholar] [CrossRef]
- Stegmaier, T.; Linke, M.; Planck, H. Bionics in textiles: Flexible and translucent thermal insulations for solar thermal applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1749–1758. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.; Guo, J.; Cakmak, M. Mechanically Strong, Flexible Polyimide Aerogels Cross-Linked with Aromatic Triamine. ACS Appl. Mater. Interfaces 2012, 4, 536–544. [Google Scholar] [CrossRef]
- Pan, Y.; He, S.; Gong, L.; Cheng, X.; Li, C.; Li, Z.; Liu, Z.; Zhang, H. Low thermal-conductivity and high thermal stable silica aerogel based on MTMS/Water-glass co-precursor prepared by freeze drying. Mater. Des. 2017, 113, 246–253. [Google Scholar] [CrossRef]
- Bangi, U.K.H.; Kavale, M.S.; Baek, S.; Park, H.-H. Synthesis of MWCNTs doped sodium silicate based aerogels by ambient pressure drying. J. Sol-Gel Sci. Technol. 2012, 62, 201–207. [Google Scholar] [CrossRef]
- Parale, V.G.; Mahadik, D.B.; Mahadik, S.A.; Kavale, M.S.; Venkateswara Rao, A.; Wagh, P.B. Wettability study of surface modified silica aerogels with different silylating agents. J. Sol-Gel Sci. Technol. 2012, 63, 573–579. [Google Scholar] [CrossRef]
- Bangi, U.K.H.; Park, C.-S.; Baek, S.; Park, H.-H. Improvement in optical and physical properties of TEOS based aerogels using acetonitrile via ambient pressure drying. Ceram. Int. 2012, 38, 6883–6888. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.-K.; Kim, H.-K.; Kim, H.-T. High specific surface area TEOS-based aerogels with large pore volume prepared at an ambient pressure. Appl. Surf. Sci. 2007, 254, 574–579. [Google Scholar] [CrossRef]
- Kim, G.; Hyun, S.; Park, H. Synthesis of Low—Dielectric Silica Aerogel Films by Ambient Drying. J. Am. Ceram. Soc. 2004, 84, 453–455. [Google Scholar] [CrossRef]
- Bheekhun, N.; Abu Talib, A.R.; Hassan, M.R. Aerogels in Aerospace: An Overview. Adv. Mater. Sci. Eng. 2013, 2013, 406065. [Google Scholar] [CrossRef]
- Kueh, A.B.H.; Razali, A.W.; Lee, Y.Y.; Hamdan, S.; Yakub, I.; Suhaili, N. Acoustical and mechanical characteristics of mortars with pineapple leaf fiber and silica aerogel infills—Measurement and modeling. Mater. Today Commun. 2023, 35, 105540. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Fei, Z.; Li, K.; Chen, G.; Chen, J.; Zhang, P.; Yang, Z. Self-catalyzed Gelling Synthesis of Aerogels with Inorganic and Organic Nanocomponents for Thermal Insulation and CO2 Capture. ACS Appl. Nano Mater. 2023, 6, 1927–1936. [Google Scholar] [CrossRef]
- Islam, S.R.; Patoary, M.K.; Estifanos, H.D.; Lugoloobi, I.; Yousif, A.H.D.; Jiang, J.; Shao, H. Hydrophobic and oleophilic 3D weft-knitted spacer fabrics coated by silica aerogels with five different concentrations. J. Ind. Text. 2022, 52, 15280837221118063. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Yang, J.; Yang, K.-H.; Ju, S.; Lim, T.; Jeong, S.-M. Hydrophobic halochromic aerogel capable of reversibly measuring acidic and basic vapors. AIP Adv. 2021, 11, 115115. [Google Scholar] [CrossRef]
- Mao, B.; Xia, X.; Qin, R.; Li, P.; Yang, G.; Fu, H.; Lv, H.; Li, X.; Jia, X.; Xu, D. Microstructure evolution and microwave absorbing properties of novel double-layered SiC reinforced SiO2 aerogel. J. Alloys Compd. 2023, 936, 168314. [Google Scholar] [CrossRef]
- Xie, H.; He, Z.; Liu, Y.; Zhao, C.; Guo, B.; Zhu, C.; Xu, J. Efficient Antibacterial Agent Delivery by Mesoporous Silica Aerogel. ACS Omega 2022, 7, 7638–7647. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Shafi, S.; Rasheed, T.; Naz, R.; Majeed, S.; Bilal, M. Supercritical CO2 drying of pure silica aerogels: Effect of drying time on textural properties of nanoporous silica aerogels. J. Sol-Gel Sci. Technol. 2021, 98, 478–486. [Google Scholar] [CrossRef]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of Silica Aerogel by Supercritical Drying Method. Procedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z.; Wu, J.; Zhao, N.; Xu, J. Vacuum-Dried Robust Bridged Silsesquioxane Aerogels. Adv. Mater. 2013, 25, 4494–4497. [Google Scholar] [CrossRef] [PubMed]
- Aravind, P.R.; Niemeyer, P.; Ratke, L. Novel flexible aerogels derived from methyltrimethoxysilane/3-(2,3-epoxypropoxy)propyltrimethoxysilane co-precursor. Microporous Mesoporous Mater. 2013, 181, 111–115. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, J.; Feng, J. Synthesis and characterization of ambient-dried microglass fibers/silica aerogel nanocomposites with low thermal conductivity. J. Sol-Gel Sci. Technol. 2017, 83, 64–71. [Google Scholar] [CrossRef]
- Khedkar, M.V.; Somvanshi, S.B.; Humbe, A.V.; Jadhav, K.M. Surface modified sodium silicate based superhydrophobic silica aerogels prepared via ambient pressure drying process. J. Non-Cryst. Solids 2019, 511, 140–146. [Google Scholar] [CrossRef]
- Arkles, B.; Larson, G. Silicon Compounds: Silanes & Silicones; Barry Arkles, G.L.L., Ed.; Gelest Inc.: Morrisville, NC, USA, 2013; pp. 175–178. [Google Scholar]
- Yun, S.; Luo, H.; Gao, Y. Low-density, hydrophobic, highly flexible ambient-pressure-dried monolithic bridged silsesquioxane aerogels. J. Mater. Chem. A 2015, 3, 3390–3398. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Yan, M.; Luo, K.-H.; Wei, Y.; Yeh, J.-M. Comparative Studies of the Dielectric Properties of Polyester Imide Composite Membranes Containing Hydrophilic and Hydrophobic Mesoporous Silica Particles. Materials 2023, 16, 140. [Google Scholar] [CrossRef]
- Brooke, D. Introduction to powder surface area. By S. LOWELL. Wiley, 605 Third Ave., New York, NY 10016. 1979. 199 pp. 15 × 23 cm. Price $17.95. J. Pharm. Sci. 1980, 69, 486. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, P.; Dong, S. The Influence of Crosslink Density on the Failure Behavior in Amorphous Polymers by Molecular Dynamics Simulations. Materials 2016, 9, 234. [Google Scholar] [CrossRef]
- Liu, Y.J.; Peng, C.L.; Li, W.M.; Zhu, X.L.; Shen, M.G.; Liao, X.W.; Liu, K.; Wei, C.Y.; Yusuf, Y.A.; Yang, J.; et al. Effect of hollow insulation riser on shrinkage porosity and solidification structure of ingot. J. Iron Steel Res. Int. 2022, 29, 1951–1960. [Google Scholar] [CrossRef]
- Wagh, P.B.; Ingale, S.V. Comparison of some physico-chemical properties of hydrophilic and hydrophobic silica aerogels. Ceram. Int. 2002, 28, 43–50. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Bhagat, S.D.; Hirashima, H.; Pajonk, G.M. Synthesis of flexible silica aerogels using methyltrimethoxysilane (MTMS) precursor. J. Colloid Interface Sci. 2006, 300, 279–285. [Google Scholar] [CrossRef]
- Ferreiro-Rangel, C.A.; Gelb, L.D. Computational Study of Uniaxial Deformations in Silica Aerogel Using a Coarse-Grained Model. J. Phys. Chem. B 2015, 119, 8640–8650. [Google Scholar] [CrossRef]
- Xiong, P.; Gong, Y.; Yang, X.; Zhu, Y.; Chen, C.; Shen, J. Effect of hydrophobic silica aerogels in-situ on encapsulation the stability of CsPbBr3 quantum dots for white light-emitting diodes. J. Alloys Compd. 2023, 938, 168541. [Google Scholar] [CrossRef]
- Merillas, B.; Villafañe, F.; Rodríguez-Pérez, M.Á. Super-Insulating Transparent Polyisocyanurate-Polyurethane Aerogels: Analysis of Thermal Conductivity and Mechanical Properties. Nanomaterials 2022, 12, 2409. [Google Scholar] [CrossRef]
- Salimian, S.; Zadhoush, A.; Talebi, Z.; Fischer, B.; Winiger, P.; Winnefeld, F.; Zhao, S.; Barbezat, M.; Koebel, M.M.; Malfait, W.J. Silica Aerogel–Epoxy Nanocomposites: Understanding Epoxy Reinforcement in Terms of Aerogel Surface Chemistry and Epoxy–Silica Interface Compatibility. ACS Appl. Nano Mater. 2018, 1, 4179–4189. [Google Scholar] [CrossRef]
- Jahed, F.S.; Hamidi, S.; Zamani-Kalajahi, M.; Siahi-Shadbad, M. Biomedical applications of silica-based aerogels: A comprehensive review. Macromol. Res. 2023, 1–20. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, H.; Ding, Y.; Yin, S.; Wang, M.; Cao, A. Engineering thermal and mechanical properties of flexible fiber-reinforced aerogel composites. J. Sol-Gel Sci. Technol. 2012, 63, 445–456. [Google Scholar] [CrossRef]
- Hayase, G.; Kugimiya, K.; Ogawa, M.; Kodera, Y.; Kanamori, K.; Nakanishi, K. The thermal conductivity of polymethylsilsesquioxane aerogels and xerogels with varied pore sizes for practical application as thermal superinsulators. J. Mater. Chem. A 2014, 2, 6525–6531. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.; Jiang, Y.; Li, L.; Feng, J. Elastic methyltrimethoxysilane based silica aerogels reinforced with polyvinylmethyldimethoxysilane. RSC Adv. 2019, 9, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Shimizu, T.; Kanamori, K.; Zhu, Y.; Maeno, A.; Kaji, H.; Shen, J.; Nakanishi, K. Transparent, Superflexible Doubly Cross-Linked Polyvinylpolymethylsiloxane Aerogel Superinsulators via Ambient Pressure Drying. ACS Nano 2018, 12, 521–532. [Google Scholar] [CrossRef] [PubMed]







| Sample Code | Feed Composition (wt%) | Thickness (mm) | SBET (m2/g) b | Vt (cm3/g) c | Dp (nm) d | |
|---|---|---|---|---|---|---|
| Precursor | Modifier a | |||||
| FSA | 100 | 0 | 6.26 ± 0.1 | 9.41 | 0.0312 | 30.7 |
| FSA-M1 | 99 | 1 | 6.31 ± 0.1 | 3.21 | 0.0282 | 20.4 |
| FSA-M5 | 95 | 5 | 6.30 ± 0.1 | 1.47 | 0.0059 | 15.6 |
| FSA-M10 | 90 | 10 | 6.39 ± 0.1 | 1.18 | 0.0026 | 13.3 |
| FSA-T10 | 90 | 10 | 6.37 ± 0.2 | 0.23 | 0.0015 | 26.7 |
| Sample Code | Thermal Conductivity (W/mK) a | 5 wt% Loss Decomposition Temperature (T5d) b | Hardness (N/mm2) | Density (g/cm3) | Young’s Modulus (MPa) c | Contact Angle (°) |
|---|---|---|---|---|---|---|
| FSA | 0.114 | 86.46 | 34 | 0.419 | 0.35 | 76.03 |
| FSA-M1 | 0.120 | 97.45 | 35 | 0.423 | 0.37 | 102.49 |
| FSA-M5 | 0.131 | 115.98 | 55 | 0.448 | 0.39 | 113.97 |
| FSA-M10 | 0.135 | 121.77 | 67 | 0.456 | 0.52 | 119.98 |
| FSA-T10 | 0.091 | 135.33 | 20 | 0.333 | 0.23 | 132.35 |
| Sample Code | Density (g/cm3) | Thermal Conductivity (W/mK) | Young’s Modulus (MPa) | Contact Angle (°) | Ref. |
|---|---|---|---|---|---|
| MTMS-FSA | 0.423–0.456 | 0.120–0.135 | 0.37–0.52 | 102.49–119.98 | This work |
| TMES-FSA | 0.333 | 0.091 | 0.23 | 132.35 | This work |
| Silsesquioxane aerogel | 0.237 | 0.043 | 1.31 | 142.0 | [23] |
| TMCS-silica aerogel | 0.225 | 0.032 | 12.8 | not reported | [40] |
| polymethylsilsesquioxane aerogels | 0.450 | 0.015 | 27.0 | not reported | [41] |
| MTMS-silica aerogel with PVMDMS | 0.138 | 0.025 | ~1.5 | 136.9 | [42] |
| PVPMS silica aerogel | 0.22 | 0.015 | 4.7 | 131.0 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.-H.; Yan, M.-S.; Chang, C.-A.; Weng, C.-W.; Yeh, J.-M. Effect of Silicone Modifier on the Physical Properties of Flexible Silica Aerogels. Polymers 2023, 15, 2043. https://doi.org/10.3390/polym15092043
Luo K-H, Yan M-S, Chang C-A, Weng C-W, Yeh J-M. Effect of Silicone Modifier on the Physical Properties of Flexible Silica Aerogels. Polymers. 2023; 15(9):2043. https://doi.org/10.3390/polym15092043
Chicago/Turabian StyleLuo, Kun-Hao, Min-Si Yan, Chen-An Chang, Chih-Wei Weng, and Jui-Ming Yeh. 2023. "Effect of Silicone Modifier on the Physical Properties of Flexible Silica Aerogels" Polymers 15, no. 9: 2043. https://doi.org/10.3390/polym15092043
APA StyleLuo, K.-H., Yan, M.-S., Chang, C.-A., Weng, C.-W., & Yeh, J.-M. (2023). Effect of Silicone Modifier on the Physical Properties of Flexible Silica Aerogels. Polymers, 15(9), 2043. https://doi.org/10.3390/polym15092043







