Abstract
Alkyd resins are oil-based polymers that have been widely used for generations in the surface coating industry and beyond. Characterization of these resins is of high importance to understand the influence of its components on its behavior, compatibility with other resins, and final quality to ensure high durability. Here, NMR spectroscopy and GPC were used for characterizing differences in the chemical structure, molecular distribution, and dispersity between oil-based and fatty acid-based alkyd polymers made from sacha inchi and linseed oils. Sancha inchi (Plukentia volubilis L.) is a fruit-bearing plant native to South America and the Caribbean, and has a rich unsaturated fatty acid content. The effect of vegetable oil and polyol selection on the synthesis of alkyd resins for coating applications was analyzed. The influence of two different synthesis methods, monoglyceride and fatty acid processes, was also compared. Important structural differences were observed using NMR: one-dimensional spectra revealed the degree of unsaturated fatty acid chains along the polyester backbone, whereas, 2D NMR experiments facilitated chemical shift assignments of all signals. GPC analysis suggested that alkyd resins with homogeneous and high molecular weights can be obtained with the fatty acid process, and that resins containing pentaerythritol may have uniform chain lengths.
1. Introduction
Alkyd resins are polyesters modified using vegetable oils [1]. Conjugated carbon double-bonds from fatty acid chains, preferably catalyzed by metallic driers, afford the polymer the ability to generate radical autoxidation reactions [2], which facilitate its cross-linking and the formation of a dry film. This inherent characteristic gives alkyd resins the advantage of being single component coatings and, due to their oily content, they can better tolerate the presence of rust on the surface. In addition to being easy to apply, these vegetable oil-based coatings are cheap and have been successfully used in a variety of applications, including industrial, decorative, architectural or artistic coatings [3,4,5,6]. In recent years, they are being explored for self-healing applications to ensure the long-term durability of protective coatings [7,8]. Alkyd resins also have a great compatibility with other resins, and have been blended with other polymers to improve their performance [4].
The wide range of reagents that can be used for the synthesis of alkyd resins increase their versatility, and their structures and film properties can be tuned by their components [9]. Vegetable oils as fatty acid sources, polycarboxylic acids, such as phthalic anhydride (PA), and polyols, such as glycerol (GC) or pentaerythritol (PE), are commonly used for alkyd resin production [10]. Except for phthalic anhydride, alkyd polymers are based on natural resources and can be classified as biologically degradable materials [11]. Recent work has demonstrated the possibility to reduce non-renewable component and incorporate the use of waste products, such as polyethylene terephthalate (PET) from postconsumer bottles [12], to manufacture this type of resins.
We examined alkyd resins that contain different glycerol (GC): pentaerythritol (PE) weight ratios. Glycerol was the first polyol to be used in the production of alkyd resins. GC is a cheap and easily available renewable raw material, which contains two primary and one secondary hydroxyl groups [13]. On the other hand, pentaerythritol has the highest functionality, four hydroxyl groups per molecule, which causes the formation of more branched and higher molar mass alkyd resins [14]. It has been used as an alternative to glycerol in alkyd formulations to impart high viscosity, greater hardness, improved hydrolytic resistance, color stability, thermal stability, and external durability [15,16,17]. Due to the high reactivity of PE and its ability to increase branching [15], that could cause gelation or loss of resin, we examined different proportions of GC and PE in the alkyd resin synthesis.
Alkyd resins can be classified in accordance with their oil length or weight percentage of oil: long (>55%), medium (45–55%) and short oil (<45%) [3,18]. Long oil alkyd resins are used in clear lacquers for decorative or artistic paints, whereas medium oil alkyd resins have a wider industrial application, being used as primers, maintenance paints and metal finishes. Short oil alkyd resins are used in baking primers and enamels. [18]. On the other hand, the iodine value of oils or degree of unsaturation, determines its classification as drying, semi-drying or non-drying. Long and medium oil alkyd resins are frequently produced from semi-drying or drying oils [3,16]. Alkyd resins synthesized with non-drying oils are used as plasticizing agents [18]. The alkyd resins analyzed in this study have a medium chain length.
The properties of alkyd resins strongly depend on the type of vegetable oil precursor [17]. The high content of polyunsaturated fatty acids have turned linseed oil into one of the most used oil resource for alkyd manufacturing [19,20]. However, in recent years, non-traditional and new feedstocks of vegetable oils are being investigated for polymeric resin synthesis [21]. This work reports the nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC) characterization of the new alkyd polymers made of sacha inchi oil (Plukentia volubilis L.) and linseed oil (Linum usitatissimum L.). Linseed oil was used for comparison purposes. Sacha inchi oil, of Peruvian origin, was selected due to its rich unsaturated fatty acids content [22]. Its similarity to the composition of linseed oil [23] makes it a potential raw material for new alkyd coatings. Recent work has shown that sacha inchi oil is a new and effective feedstock for alkyd resins synthesis in the surface coating industry, either as pure oil [17,24,25,26], through its fatty acids [16] or as part of an oil mixture [27,28]. Its effectiveness has also been proven as a raw material for the creation of artistic products [6]. Currently, sacha inchi seeds are used for oils, cakes and protein meals, and are highly used in the cosmetic, food and medicine industry [28].
NMR analysis has been extensively used for elucidating the chemical composition of alkyd resins [29,30,31,32,33,34]. Moreover, the use of 2D NMR spectroscopy has been successfully applied to distinguish between multiple components of broad and/or featureless peaks in 1D NMR spectra [29]. On the other hand, GPC has been used to evaluate the molecular weight and dispersity of alkyd resins [21,30,34,35,36]. These properties are of crucial importance, as they can affect the quality of alkyd resins [35,37].
This work examines the structural differences between the new alkyd resins obtained from sacha inchi and linseed oils. Two different synthesis methods were used: (A) The monoglyceride process. (B) The fatty acid process. Both processes can be performed with the same starting materials, but fatty acids from the vegetable source must be first extracted in the fatty acid process, which makes it costly [16,38]. Due to the high reactivity of fatty acids, the synthesis time is shorter, however greater control is required to avoid gelation of the resin [16]. In this study, alkyd resins were manufactured with both methods, and with precursors that have similar fatty acid content, but that vary in content of polyols or that contain a mixture of polyols, and the same polyacid.
2. Experimental Section
2.1. Materials
All samples were provided by the Instituto de Corrosión y Protección, Pontificia Universidad Católica del Perú. All alkyd resins contain different glycerol (GC): pentaerythritol (PE) weight ratios. Linseed oil was used for comparison purposes. “A” alkyd samples were synthesized using the monoglyceride method, whereas “FA” alkyd samples were synthesized using the fatty acid process. Fatty acid fractions were obtained from oils using a base-catalyzed transesterification reaction [16]. (F)AS were prepared with sacha inchi oil/fatty acid, while (F)AL were prepared with linseed oil/fatty acid. Alkyd resins were identified with numbers from 1 to 3, depending on their content of polyol: (F)AS1 and (F)AL1 contain 1:0 GC:PE; (F)AS2 and (F)AL2 contain 0.5:0.5 GC:PE; (F)AS3 and (F)AL3 contain 0.2:0.8 GC:PE (Table 1). All samples were prepared with the same amount of phthalic anhydride and oil/fatty acid fraction [16,17].

Table 1.
Composition of alkyd resins. Letter “x” indicates the fatty acid source used.
2.2. NMR Spectroscopy
NMR analysis was performed in the Department of Physical Chemistry and Microreaction Technology at the Technische Universität Ilmenau (Ilmenau, Germany) with a Bruker Avance 300 spectrometer. Samples were dissolved with deuterated chloroform (10 wt.%). Data analysis was performed using the MestReNova v12.0.4-22023 software.
2.3. Gel Permeation Chromatography
Gel Permeation Chromatography (GPC) was performed in the Department of Chemical Engineering at The Pennsylvania State University (USA). Molecular weights and molecular weight distribution (Mw/Mn) were determined with the 1260 Agilent Technologies gel permeation chromatograph (GPC) equipped with a refractive index detector (RID), a viscometer (VS), a light scattering detector (LS) and a multiwavelength detector (MWD), using an Agilent PLgel-MIXED-LC column. Chlorobenzene was used as the mobile phase at 0.5 mL/min and the sample concentration was ~30 mg/mL. Universal GPC calibration curve was prepared with low polydispersed linear polystyrene standards (Agilent EasiVial PS-M, Agilent, Santa Clara, CA, USA).
3. Results and Discussion
3.1. 1D 1H-NMR Spectra
Similar 1H NMR (300 MHz, chloroform-d) spectra were obtained for all alkyd resins. Figure 1 shows the 1H NMR spectra that contains thirteen signals typical of an alkyd resin classified with letters (A) to (M), starting from the high field region. Possible location of protons corresponding to each peak in alkyd resin structures (representing possible repeating units) is also identified in Figure 1. The unmarked peak at δ 7.28 ppm corresponds to chloroform-d. The assignment of 1H-NMR chemical shifts in FAS3, AS3, AL3, AS1 and AL1 alkyd resins are summarized in Table 2. For the NMR resonance description, the “NMR Guidelines for ACS Journals” standard was used. For example, AS3 can be summarized as follows: δ 7.71 (singlet (s), 2H), δ7.54 (s, 2H), δ 5.38 (s), δ 4.70–4.31 (multiplet (m)), δ 4.29–4.08 (m), δ 3.83–3.47 (m), δ 2.96–2.70 (m), δ 2.43–2.23 (m), δ 2.19–1.96 (m), δ 1.61 (s), δ 1.30 (doublet), δ 1.07–0.95 (m), δ 0.95–0.84 (m).
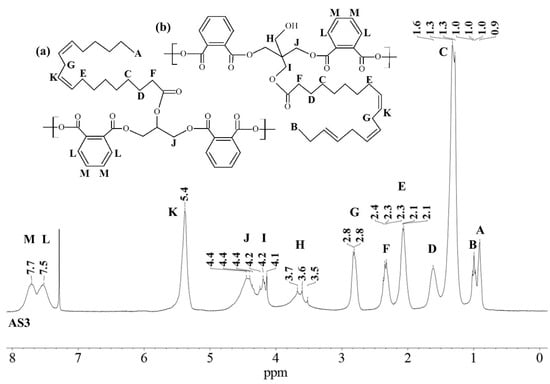
Figure 1.
1H-NMR spectra of a sacha inchi-based alkyd resin, AS3 at 300 MHz in deuterated chloroform. Polymer structure comparison: (a) glycerol-based alkyd resin with linoleic (C18:2) fatty acid chain; (b) pentaerythritol-based alkyd resin with linolenic (C18:3) fatty acid chain.

Table 2.
1H NMR peaks identified (Figure 1) on alkyd resins. The resonances were measured in deuterated chloroform solution and assigned to the polymer structure units [21,29,31,33,34].
The low field region of the spectrum at δ = 6.5–8.0 ppm aromatic ring protons, peaks (L) and (M), from the phthalic ester moieties. The resonance at about δ = 5.4 ppm, peak (K), was assigned to the vinylic protons. Intermediate signals δ = 3.4–4.8 ppm, composed of peaks (H), (I) and (J), correspond to the protons assigned to the polyol(s) CH2 groups. The resonance at about δ = 2.8 ppm, peak (G), was assigned to aliphatic CH2 groups shielded by two neighboring vinyl groups. Remaining peaks in the aliphatic region δ = 0–2.6 ppm correspond to sp3 C bound protons assigned to the fatty acid chains, as shown in Table 2. The proton resonance assignment of all peaks is summarized in Table 2 and they align with data in the literature [29,32,33,39]. Previous studies of alkyd resins were reported by Boruah et al. [21], Spyros [29], Chiplunkar and Pratap [31], Rämänen and Maunu [33], and Glenn et al. [34].
An expansion of the 1H spectra polyol region of the alkyd resins is shown in Figure 2. Through a visual comparison of all the spectra, a clear difference can be seen between alkyds prepared with glycerol (e.g., AS1, AL1) and those prepared with pentaerythritol (e.g., AS3, AL3, FAS3). As specified in Table 2, signal (H) correspond to methylene protons of the CH2OH groups. The degree of branching depends on the esterification degree of the polyol unit [33]. However, PE has a higher degree of OH-functionality than GC. Because of the steric differences, there is a higher probability that more unreacted CH2-OH groups remain in the PE structure in comparison to GC. This assumption is supported by the observation that the intensity of peak (H) is more intense in case of the PE alkyds (AL3, AS3, FAS3) compared to the GC alkyds (AL1, AS1). Peak (I), assigned to the esterified CH2-O- moieties of the PE-containing alkyd resins, has higher signal intensity because of the greater amounts of PE in the batch. On the other hand, peak (J)’s intensity is comparable for all samples, because the same amount of phthalic anhydride was used for the synthesis of all alkyd resins variants.
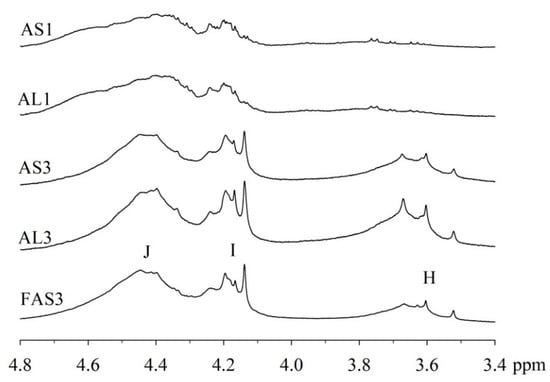
Figure 2.
Expanded 1H-NMR spectra of the polyol region (δ 3.4–4.8 ppm) of alkyd resins at 300 MHz in chloroform-d.
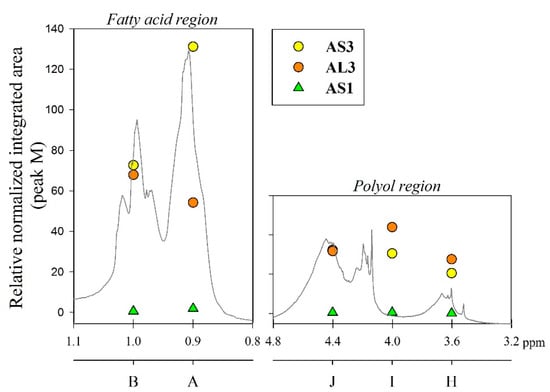
The integrated peak area (M) at δ 7.71 ppm, corresponding to two protons attached to the aromatic ring of the phthalic anhydride structure, was used for integral normalization of the full spectrum. The comparison of different alkyd resin samples revealed different relative proportions of polyol and fatty acid chain proton integrals. We assume that that all polymer samples had the same amount of phthalic anhydride, because the same relative amount of phthalic acid was used for the preparation of each batch. Peaks (H), (I) and (J) from the polyol region were chosen to make comparisons between the different samples. Peaks (A) and (B) were also selected, since they are representative signals of the linoleic and linolenic fatty acids, or the main components of vegetable oils used for alkyd synthesis. Other peaks were not taken into account because they might be present in a similar proportion in every possible structure of a repeating unit of an alkyd polymer.
Relative normalized integrated areas of the characteristic signals corresponding to the polyol and fatty acid chain protons are illustrated in Figure 3. Relative proportions of the polyol protons corroborated the visual comparison made with the 1H-NMR spectra. Protons in the polyol region are in greater proportion in PE-based alkyd resins. It was also noted that PE-based resins contain more fatty acid chains, due to the highest functionality of pentaerythritol. In the high field region, sacha inchi-based resins have more terminal methyl groups found in a linoleic acid (omega-6) structure (peak (A) from Figure 1) than linseed-based resins (Figure 3). It has been reported that sacha inchi has a similar omega-3 fatty acid composition to linseed oil; though, its omega-6 fatty acid content is higher [22]. A higher degree of unsaturation, i.e., large quantity of double bonds, could enhance resin drying properties and, therefore, the hardness of cured resins. The development of extensive cross-linking would lead to the obtainment of a better quality product [29,40].
3.2. 1D 13C-NMR Spectra
13C-NMR spectra of the alkyd resins were recorded and analyzed accordingly. Representative spectra of resins AS1 and AS3, and their resonance/structure assignment are shown in Figure 4. The 2D-heteronuclear correlation spectra (see Figure 5 and Figure 6) were considered during the assignment of the resonances. Carbonyl groups of phthalic and fatty acid esters (O=C-HC=CH-C=O) were observed at δ = 173.6 ppm (C1) and δ = 167.1 ppm (C2), respectively [12,34]. The region from δ = 127.1 ppm to δ = 131.9 ppm contained aromatic carbons, C3 to C5, and vinyl carbons of unsaturated fatty acid chains (C6) [12,29,34].
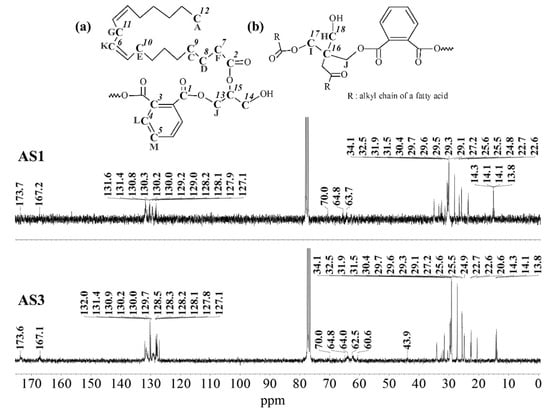
Figure 4.
13C-NMR spectra of alkyd resins AS1 and AS3 at 75 MHz in deuterated chloroform: (a) glycerol-based alkyd resin unit with linoleic (C18:2) fatty acid chain; (b) pentaerythritol-based alkyd resin unit.
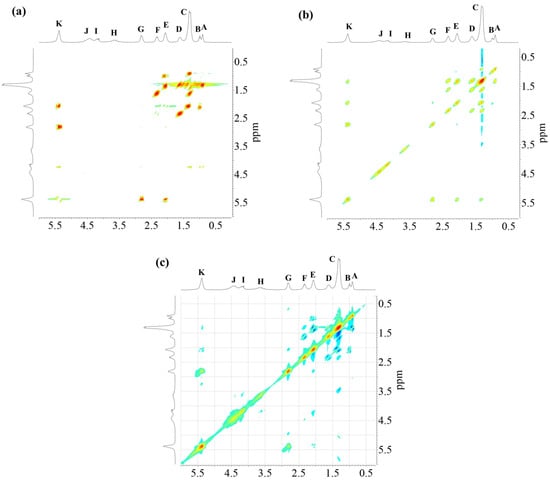
Figure 5.
1H -1H NMR-2D spectra of alkyd resin AS3: (a) COSY-90; (b) TOCSY; (c) ROESY.
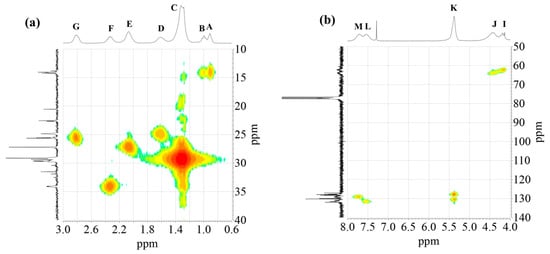
Figure 6.
1H-13C HMQC spectra of alkyd resin AS3: (a) high field region; (b) low field region.
Signals of terminal methyl carbons -CH3, and internal methylene carbons -(CH2)n- from the fatty acid chains, C7 to C12, appeared in the high field region, between δ = 10 and 40 ppm [12,29,33]. Carbon atoms, directly bonded to an oxygen atom in polyalcohol moieties, were perceived in the δ = 40–70 ppm region [34]. Glycerol-based resin AS1 presented three characteristic peaks: C13 at 64.8 ppm, C14 at 63.7–64.0 ppm and C15 at 70.0 ppm [29]. Whereas, resin AS3 prepared with pentaerythritol and glycerol showed the presence of a low intensity peak, corresponding to the quaternary carbon of pentaerythritol (C16) [29]. Moreover, pure glycerol peaks were observed, along with signals coming from the pentaerythritol-based resin units, such as peaks at δ = 62.5 ppm (C17), and δ = 60.6 ppm (C18).
3.3. 2D NMR Spectra
Sample AS3 was analyzed using different 2D NMR experiments in order to investigate the structure in greater detail. The 1H-1H COSY-90 (Figure 5a), 1H-1H TOCSY (Figure 5b), and 1H-1H ROESY (Figure 5c) correlation spectra were recorded. The analysis revealed clearly the coupling of peak (K) with peaks (E) and (G), located in the fatty acid chain (Figure 5a). Moreover, coupling of proton peaks (C), (D) and (F) were confirmed. It was corroborated that protons from terminal methyl groups of linoleic acid chain correspond to peak (A), as it has a strong coupling with protons from peak (C). Thus, peak (B), protons from terminal methyl groups of linolenic acid chain, showed a crosspeak with signal (E) in the COSY and ROESY measurements. It should be noted that methylene protons of peak (E) are also close to those of peak (C). The TOCSY-2D spectra (Figure 5b) additionally confirmed that protons of unsaturated carbons identified as peak (K), correlates with protons from internal methylene groups of the aliphatic chains (peak (C)).
The 2D 1H-13C HMQC spectra (Figure 6) were also acquired for AS3 sample. Figure 6a shows the expanded spectra containing identified 1H NMR peaks (A) to (G) (Figure 1), located in the δ 0.6 to 3.0 ppm region, and carbons C7 to C12 that appeared from δ 10 to 40 ppm in the 13C NMR spectra (Figure 4). Proton peaks (A) and (B) showed the expected connectivity with carbon peaks around δ 13.8–14.3 ppm, C12 identified in Figure 4, which represent terminal methyl groups on fatty acid chains.
Heteronuclear coupling was used for the identification of the different 13C NMR resonances to the methylene protons of the fatty acid chains. Here, peak (C) has a cross correlation with the carbon signals around δ = 29.1–29.7 ppm (identified in Figure 4 as carbon C9). The correlation of the proton peak at δ = 1.61ppm (peak (D)) with a carbon C8 peak at δ = 24.9 ppm, is attributed to the methylene groups located next to -CH2COO- group. Proton peak (E) has a cross correlation with carbon C10 peak at δ = 34.1 ppm. Other cross correlations observed in this spectrum originated from coupling between the couples proton peak (F)-carbon C7 peak, and proton peak (G)-carbon C11 peak, that appeared at δ 34.2 ppm and δ 25.5–25.6 ppm, respectively.
The 1H-13C HMQC spectra of the polyol and aromatic regions of an alkyd resin are presented in Figure 6b. Detected signals of the expanded spectra were found in the range of δ 4.0–8.0 ppm in the 1H NMR experiment, which included peaks (I) to (M) (Figure 1). It should be noted that peak (H) showed no clear correlation with a carbon peak. Carbon atoms located in the polyalcohol moiety near aromatic rings (proton peak (J) [32]) appear at higher chemical shifts (e.g., δ 64 ppm) than those closer to fatty acid chains (proton peak (I)). Unsaturated carbons of the fatty acid chains, identified as C6 in Figure 4 and assigned to the proton signal (K), exhibited chemical shifts around δ = 127 and δ = 130 ppm. Aromatic carbons attached to protons (L) and (M) (Figure 1) appeared at δ = 131 and δ = 129 ppm, respectively, which was specified using Spyros [29] as well.
3.4. GPC Analysis
The molecular weight averages, (weight-average molar mass) and (number-average molar mass), and dispersity (Đ) of alkyds are summarized in Table 3. With regard to Sacha inchi-based resins, and varied in the order (F)AS3 > (F)AS2 > (F)AS1, whereas, linseed-based resins did not show a specific trend. Linseed oil-based resins had the lowest values, which tend to decrease as the PE content increase. Linseed fatty acid-based alkyds prepared with pure glycerol presented the highest molecular weight averages values.

Table 3.
Molecular weight averages and molecular weight distribution of oil-based alkyd resins.
The molecular weight distribution was correlated with the viscosity of resins, as previously reported by our research group [16,17]. As seen in Figure 7a, oil-based resins that had Gardner viscosities in the range between Z5 and Z8 presented a lower than 1.4 × 105 g/mol. High molecular weights have been related to high degrees of cross-linking [41]. On the other hand, fatty acid-based resins, despite having a greater viscosity range from Z3 to Z10, presented higher and homogeneous degrees of cross-linking. It is important to remark that fatty acid-based resins had a near or even higher than 105 g/mol.
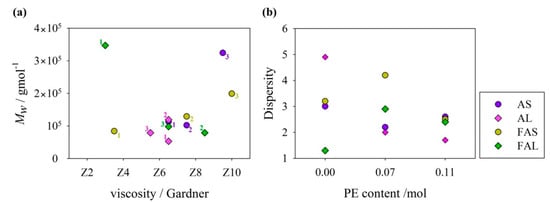
Figure 7.
GPC results of alkyd resins: (a) Molecular weight average molar mass versus Gardner viscosity (numbers from 1 to 3 represent the polyol rate as detailed in Table 1). (b) Dispersity versus molar content of pentaerythritol in alkyd composition.
Results from Table 3 clearly indicate that the size distribution of almost all resins is broad (Đ 2). There are some GPC studies of alkyd resins prepared only with glycerol as the main polyalcohol, which report the following dispersity values: (i) Jatropha Curcas oil-based alkyd, Đ = 1.15 [21]; (ii) Ricinodendron heudelotii oil-based alkyd, Đ = 1.5 [30]; (iii) Salvia Hispanica L. (Chia) oil-based alkyds, Đ = 1.2–4.3 [34]; (iv) rubber seed oil-based alkyd, Đ = 1.6 [35]. However, the oil chain length of these alkyd resins is different, as well as the saturation index of the oils used.
In general, dispersity values of oil-based resins decreased as the PE content increased (Figure 7b). GPC traces of PE-based alkyds tend to approximate a symmetric and narrow distribution, which would imply the formation of a more branched polymer (Figure 8) [42]. Đ of linseed oil-based alkyd resins is more dependent on the PE content than the alkyd resins from sacha inchi oil. Oil-based alkyd resins prepared with pure glycerol presented higher dispersity values, which could be attributed to side reactions, such as glycerol oligomerization [43]. In the side reactions, not only glycerol monoesters but also diglycerol and diglycerol monoesters (sub products) react in the polycondensation process, possibly creating polymers of different chain lengths.
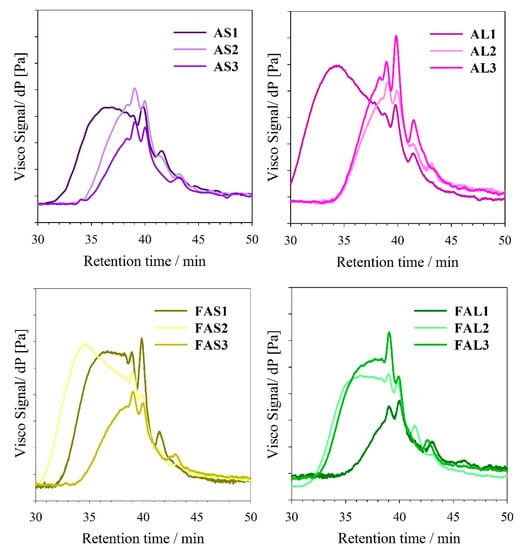
Figure 8.
GPC chromatograms of alkyd resins.
Linseed fatty acid-based alkyds had dispersity below 3, while SIFA-based resins have Đ values over 2.5. FAL1 sample had the lowest dispersity. On the other hand, samples with a higher content of PE (e.g., FAS3, FAL3) had similar Đ, despite being synthesized with different fatty acid sources. Đ trend is different from that observed in the case of oil-based alkyd resins, possibly because of the differences in reaction times or minimal differences in the preparation of the fatty acids during the synthesis process. However, the alkyds prepared with the highest PE content had the lowest variation in the dispersity values.
4. Conclusions
Alkyd resins were successfully characterized using NMR spectroscopy and GPC. Structural differences between polymers prepared with dissimilar polyols, glycerol and pentaerythritol, and diverse plant sources, sacha inchi and linseed oils, were found using the NMR spectroscopy. In the high field region of 1D NMR spectra, the peaks corresponding to terminal methyl groups, found in the δ = 0.8–1.1 ppm region, allowed us to identify the approximate amount and type of fatty acid with which the resins were prepared. On the other hand, 13C NMR resonances helped to identify the type of polyalcohol that were used in the synthesis of alkyd resins, by coupling carbon atoms with oxygen atoms in the δ = 40–70 ppm region.
The 2D NMR spectroscopy facilitated the interpretation of chemical resonance assignments of the resin components. The HMQC 2D NMR spectroscopy was used for the identification of 13C/1H resonance couples, especially for the identification of methylene carbons/proton cross correlations of the fatty acid chain moieties of sacha inchi oil-based alkyd resins. With this, we can compare the degree of unsaturation of the fatty acids chains present in the alkyd resins, enabling the prediction of which resin would have better film performance properties (e.g., drying, hardness, chemical resistance).
GPC results revealed that the molecular weight averages of sacha inchi-based resins increase considerably with more PE content. On the contrary, resins prepared with linseed oil generally decreased their weight-average molar mass by containing more PE. GPC analysis confirmed that dispersity of almost all alkyds was broad. The addition of PE reduced the dispersity values of resins possibly due to the high branching generated by the polyalcohol. Alkyds based on pure glycerol may have broader molecular weight distributions because of the possible formation of side reactions during the monoglyceride process. It was also corroborated that the fatty acid manufacturing process allows synthesizing alkyd resins with a more homogeneous molecular distribution, regardless of their viscosity. Alkyd resins with similar chain lengths can be obtained by working with PE.
Author Contributions
Conceptualization, A.H. and S.F.; Formal analysis, A.H. and G.A.G.; Funding acquisition, A.H., E.D.G. and G.A.G.; Investigation, A.H., A.E.M. and G.A.G.; Methodology, A.H., S.F. and G.A.G.; Resources, S.F., E.D.G. and G.A.G.; Supervision, S.F., E.D.G. and G.A.G.; Validation, A.H., S.F., E.D.G. and G.A.G.; Visualization, A.H. and G.A.G.; Writing—original draft, A.H.; Writing—review and editing, S.F., A.E.M., E.D.G. and G.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by Pontificia Universidad Católica del Perú (PUCP) under the postgraduate fellowship program CONCYTEC (Consejo Nacional de Ciencia Tecnología e Innovación Tecnológica) (A.H.), grant No. 236-2015-FONDECYT. Additional support has been provided by the German Academic Exchange Service (DAAD) in conjunction with FONDECYT (Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica) under grant No. 035-2016-FONDECYT. A.E.M. and E.D.G. acknowledge funding from the National Science Foundation under Award DMREF-1921854.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Acknowledgments
Special thanks to Amazon Health Products, Peru, for providing the sacha inchi oil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouwman, E.; Van Gorkum, R. A study of new manganese complexes as potential driers for alkyd paints. J. Coat. Technol. Res. 2007, 4, 491–503. [Google Scholar] [CrossRef]
- Simpson, N.; Maaijen, K.; Roelofsen, Y.; Hage, R. The Evolution of Catalysis for Alkyd Coatings: Responding to Impending Cobalt Reclassification with Very Active Iron and Manganese Catalysts, Using Polydentate Nitrogen Donor Ligands. Catalysts 2019, 9, 825. [Google Scholar] [CrossRef]
- Dizman, C.; Ozman, E. Preparation of rapid (chain-stopped) alkyds by incorporation of gum rosin and investigation of coating properties. Turk. J. Chem. 2020, 44, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, F.A.M.; Lin, L.; El-Wahab, H.A. Preparation and evaluation of high–performance modified alkyd resins based on and study of their anticorrosive properties for surface coating applications. E-Polymers 2022, 22, 781–792. [Google Scholar] [CrossRef]
- Ploeger, R.; Scalarone, D.; Chiantore, O. The characterization of commercial artists’ alkyd paints. J. Cult. Herit. 2008, 9, 412–419. [Google Scholar] [CrossRef]
- Bellatin Arciniega, L.; Meza Yapu, R.; Obregón Valencia, D.; Hadzich, A.; Costa, M.A.; Ispas, A.; Bund, A.; Flores, S. Alkyds with artistic applications based on drying oils, multifunctional polyalcohols and different polybasic acids. J. Appl. Polym. Sci. 2023, 140, e53746. [Google Scholar] [CrossRef]
- Khorasani, S.N.; Ataei, S.; Neisiany, R.E. Microencapsulation of a coconut oil-based alkyd resin into poly (melamine-urea-formaldehyde) as shell for self-healing purposes. Prog. Org. Coat. 2017, 111, 99–106. [Google Scholar] [CrossRef]
- Çömlekçi, G.K.; Ulutan, S. Encapsulation of linseed oil and linseed oil based alkyd resin by urea formaldehyde shell for self-healing systems. Prog. Org. Coat. 2018, 121, 190–200. [Google Scholar] [CrossRef]
- Taylor, S.R. Coatings for corrosion protection: An overview. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1263–1269. [Google Scholar] [CrossRef]
- Van Haveren, J.; Oostveen, E.A.; Miccichè, F.; Noordover, B.A.J.; Koning, C.E.; van Benthem, R.A.T.M.; Frissen, A.E.; Weijnen, J.G.J. Resins and additives for powder coatings and alkyd paints, based on renewable resources. J. Coat. Technol. Res. 2007, 4, 177–186. [Google Scholar] [CrossRef]
- Hofland, A. Alkyd resins: From down and out to alive and kicking. Prog. Org. Coat. 2012, 73, 274–282. [Google Scholar] [CrossRef]
- Spasojević, P.M.; Panić, V.V.; Džunuzović, J.V.; Marinković, A.D.; Woortman, A.J.J.; Loos, K.; Popović, I.G. High performance alkyd resins synthesized from postconsumer PET bottles. RSC Adv. 2015, 5, 62273–62283. [Google Scholar] [CrossRef]
- Tuck, N. Raw Materials Used for Alkyd Preparation. In Waterborne and Solvent Based Alkyds and Their End User Applications; Sita Technology Limited: Edinburgh, UK, 2000; Volume 6, pp. 19–47. [Google Scholar]
- Vallejo, P.P.; López, B.L.; Murillo, E.A. Hyperbranched phenolic-alkyd resins with high solid content. Prog. Org. Coat. 2015, 87, 213–221. [Google Scholar] [CrossRef]
- Prashantha, M.A.B.; Premachandra, B.A.J.K.; Amarasinghe, A.D.U.S. Synthesis of fast drying long oil alkyd resins using seed oil of Karawila (Momordica charantia). Indian J. Chem. Technol. 2017, 24, 47–54. [Google Scholar]
- Hadzich, A.; Gross, G.A.; Leimbach, M.; Ispas, A.; Bund, A.; Flores, S. Characterization of Plukenetia volubilis L. fatty acid-based alkyd resins. Pol. Test. 2020, 82, 106296. [Google Scholar] [CrossRef]
- Hadzich, A.; Gross, G.A.; Leimbach, M.; Ispas, A.; Bund, A.; Flores, S. Effect of polyalcohols on the anticorrosive behaviour of alkyd coatings prepared with drying oils. Prog. Org. Coat. 2020, 145, 105671. [Google Scholar] [CrossRef]
- Nanvaee, A.A.; Yahya, R.; Gan, S.N. Alkyd resins are still of major important binders in organic coatings. In Proceedings of the Malaysia Polymer International Conference (MPIC 2009), Kuala Lumpur, Malaysia, 21–22 October 2009; pp. 65–69. [Google Scholar]
- Onukwli, O.D.; Igbokwe, P.K. Production and Characterization of Castor Oil-Modified Alkyd Resins. J. Eng. Appl. Sci. 2008, 3, 161–165. [Google Scholar]
- Alam, M.; Alandis, N.M. Development of Ambient Cured Polyesteramide Coatings from Linseed Oil: A Sustainable Resource. J. Polym. Environ. 2011, 19, 391–397. [Google Scholar] [CrossRef]
- Boruah, M.; Gogoi, P.; Adhikari, B.; Dolui, S.K. Preparation and characterization of Jatropha Curcas oil based alkyd resin suitable for surface coating. Prog. Org. Coat. 2012, 74, 596–602. [Google Scholar] [CrossRef]
- Maurer, N.E.; Hatta-Sakoda, B.; Pascual-Chagman, G.; Rodriguez-Saona, L.E. Characterization and authentication of a novel vegetable source of omega-3 fatty acids, sacha inchi (Plukenetia volubilis L.) oil. Food Chem. 2012, 134, 1173–1180. [Google Scholar] [CrossRef]
- Popa, V.-M.; Gruia, A.; Raba, D.-N.; Dumbrava, D.; Moldovan, C.; Bordean, D.; Mateescu, C. Fatty acids composition and oil characteristics of linseed (Linum Usitatissimum L.) from Romania. J. Agroaliment. Process. Technol. 2012, 18, 136–140. [Google Scholar]
- Flores, S.; Flores, A.; Calderón, C.; Obregón, D. Synthesis and characterization of sacha inchi (Plukenetia volubilis L.) oil-based alkyd resin. Prog. Org. Coat. 2019, 136, 105289. [Google Scholar] [CrossRef]
- Obregón, D.; Toledo, C.; Hadzich, A.; Flores, S. Low viscosity alkyd resins based on trimethylolpropane and Peruvian oil. J. Polym. Res. 2021, 28, 203. [Google Scholar] [CrossRef]
- Obregón, D.; Hadzich, A.; Bellatin, L.; Flores, S. Microwave-assisted synthesis of alkyd resins using response surface methodology. Chem. Eng. Process. Process Intensif. 2023, 183, 109221. [Google Scholar] [CrossRef]
- Hadzich, A.; Flores, S. Physicochemical Characterization of Medium Alkyd Resins Prepared with a Mixture of Linum usitatissimum L. and Plukenetia volubilis L. Oils. IJCME 2019, 13, 319–322. [Google Scholar]
- Gómez, C.; Inciarte, H.; Orozco, L.M.; Cardona, S.; Villada, Y.; Rios, L. Interesterification and blending with Sacha Inchi oil as strategies to improve the drying properties of Castor Oil. Prog. Org. Coat. 2022, 162, 106572. [Google Scholar] [CrossRef]
- Spyros, A. Characterization of Unsaturated Polyester and Alkyd Resins Using One- and Two-Dimensional NMR Spectroscopy. J. Appl. Polym. Sci. 2003, 88, 1881–1888. [Google Scholar] [CrossRef]
- Assanvo, E.F.; Gogoi, P.; Dolui, S.K.; Baruah, S.D. Synthesis, characterization, and performance characteristics of alkyd resins based on Ricinodendron heudelotii oil and their blending with epoxy resins. Ind. Crops Prod. 2015, 65, 293–302. [Google Scholar] [CrossRef]
- Chiplunkar, P.P.; Pratap, A.P. Utilization of sunflower acid oil for synthesis of alkyd resin. Prog. Org. Coat. 2016, 93, 61–67. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Abu-sbeih, K.A.; Al-Trawneh, I.; Bourghli, L. Preparation and Characterization of Alkyd Resins of Jordan Valley Tomato Oil. J. Polym. Environ. 2014, 22, 553–558. [Google Scholar] [CrossRef]
- Rämänen, P.; Maunu, S.L. Structure of tall oil fatty acid-based alkyd resins and alkyd-acrylic copolymers studied by NMR spectroscopy. Prog. Org. Coat. 2014, 77, 361–368. [Google Scholar] [CrossRef]
- Glenn, A.; Jensen, A.T.; Machado, F. Salvia hispanica L. (Chia) Oil as a Potential Renewable Raw Material for the Production of Air-Dry Alkyd Resins. ACS Appl. Polym. Mater. 2021, 3, 6186–6197. [Google Scholar] [CrossRef]
- Aigbodion, A.I.; Pillai, C.K.S. Synthesis and molecular weight characterization of rubber seed oil-modified alkyd resins. J. Appl. Polym. Sci. 2001, 79, 2431–2438. [Google Scholar] [CrossRef]
- Mustafa, S.M.; Gan, S.N.; Yahya, R. Synthesis and Characterization of Novel Alkyds Derived From Palm Oil Based Polyester Resin. Asian J. Chem. 2013, 25, 8737–8740. [Google Scholar] [CrossRef]
- Yin, X.; Duan, H.; Wang, X.; Sun, L.; Sun, W.; Qi, H.; Ma, L. An investigation on synthesis of alkyd resin with sorbitol. Prog. Org. Coat. 2013, 77, 674–678. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Maliki, M.; Odiachi, I.J.; Aghedo, O.N.; Ohiocheoya, E.B. Review on Solvents Based Alkyd Resins and Water Borne Alkyd Resins: Impacts of Modification on Their Coating Properties. Chem. Afr. 2022, 5, 211–225. [Google Scholar] [CrossRef]
- Odetoye, T.E.; Ogunniyi, D.S.; Olatunji, G.A. Studies on the preparation of Parinari polyandra benth seed oil alkyd resins. J. Appl. Polym. Sci. 2012, 127, 4610–4616. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131, 9016–9028. [Google Scholar] [CrossRef]
- Kadam, A.; Pawar, M.; Yemul, O.; Thamke, V.; Kodam, K. Biodegradable biobased epoxy resin from karanja oil. Polymer 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Mańczyk, K.; Szewczyk, P. Highly branched high solids alkyd resins. Prog. Org. Coat. 2002, 44, 99–109. [Google Scholar] [CrossRef]
- Nosal, H.; Nowicki, J.; Warzała, M.; Semeniuk, I.; Sabura, E. Synthesis and characterization of alkyd resins based on Camelina sativa oil, glycerol and selected epoxidized vegetable oils as functional modifiers. Prog. Org. Coat. 2016, 101, 553–568. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).