On the Suitability of Phosphonate-Containing Polyamidoamines as Cotton Flame Retardants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
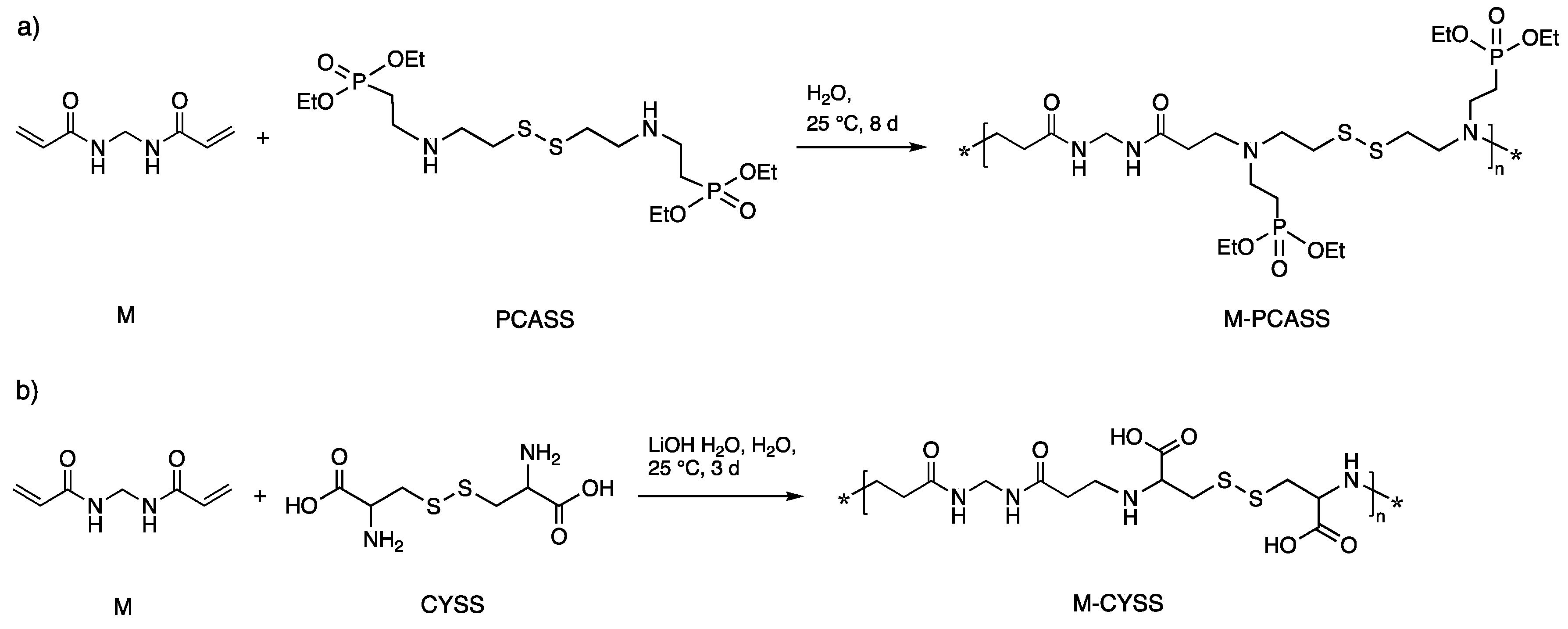
2.3. Synthesis of Tetraethyl(((disul-fanediylbis(ethane-2,1-diyl))bis(azanediyl))bis(ethane-2,1-diyl))bis(phosphonate) (PCASS)
2.4. Synthesis of Polyamidoamines
2.5. Treatment of Cotton Fabrics with PAAs
2.6. Combustion Tests of PAA-Treated Cotton Fabrics
3. Results and Discussion
3.1. Synthesis of PAAs
3.2. Thermal Stability of PAAs
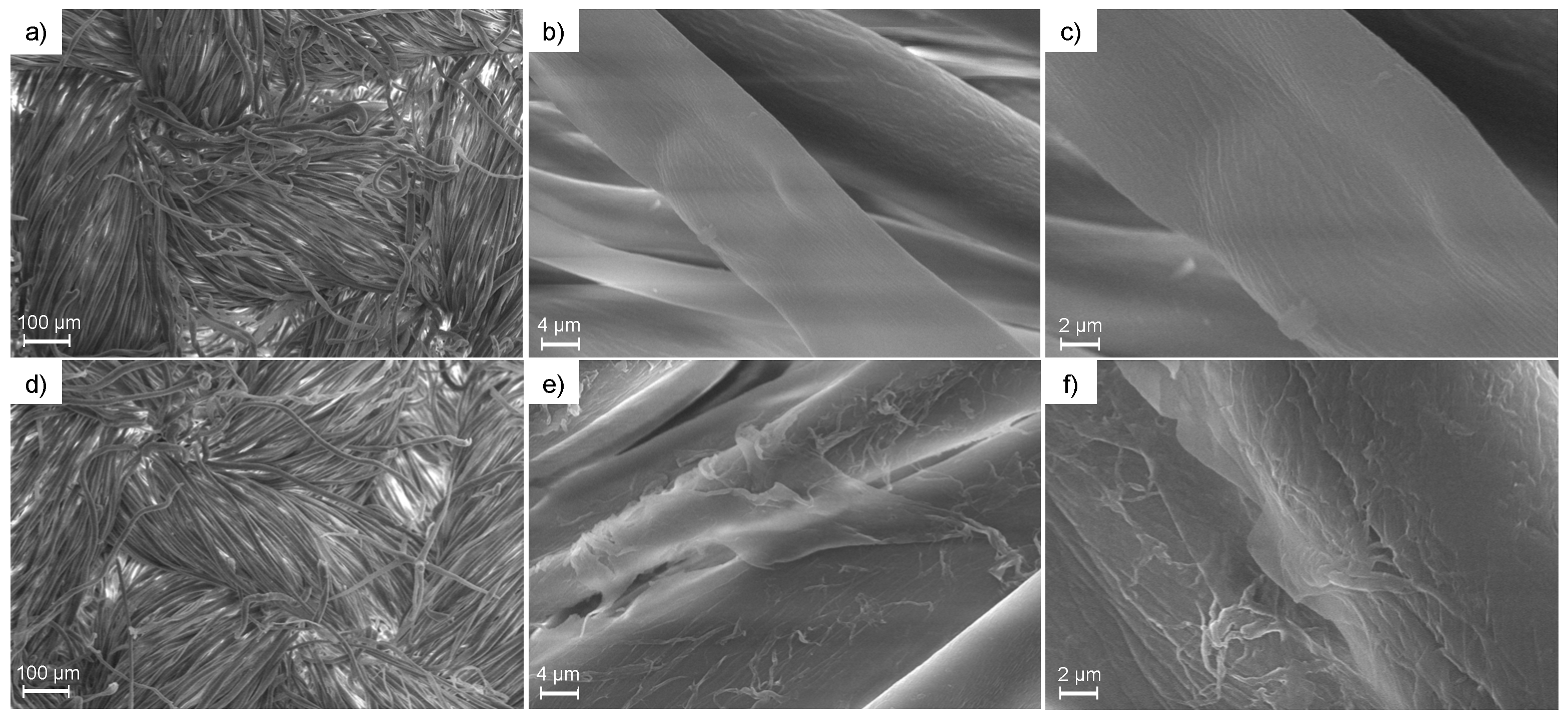
3.3. FT-IR and Morphological Characterization of PAA-Treated Cotton Fabrics
3.4. Thermal Characterization of PAA-Treated Cotton Fabrics
3.5. Combustion Tests of PAA-Treated Cotton Fabrics
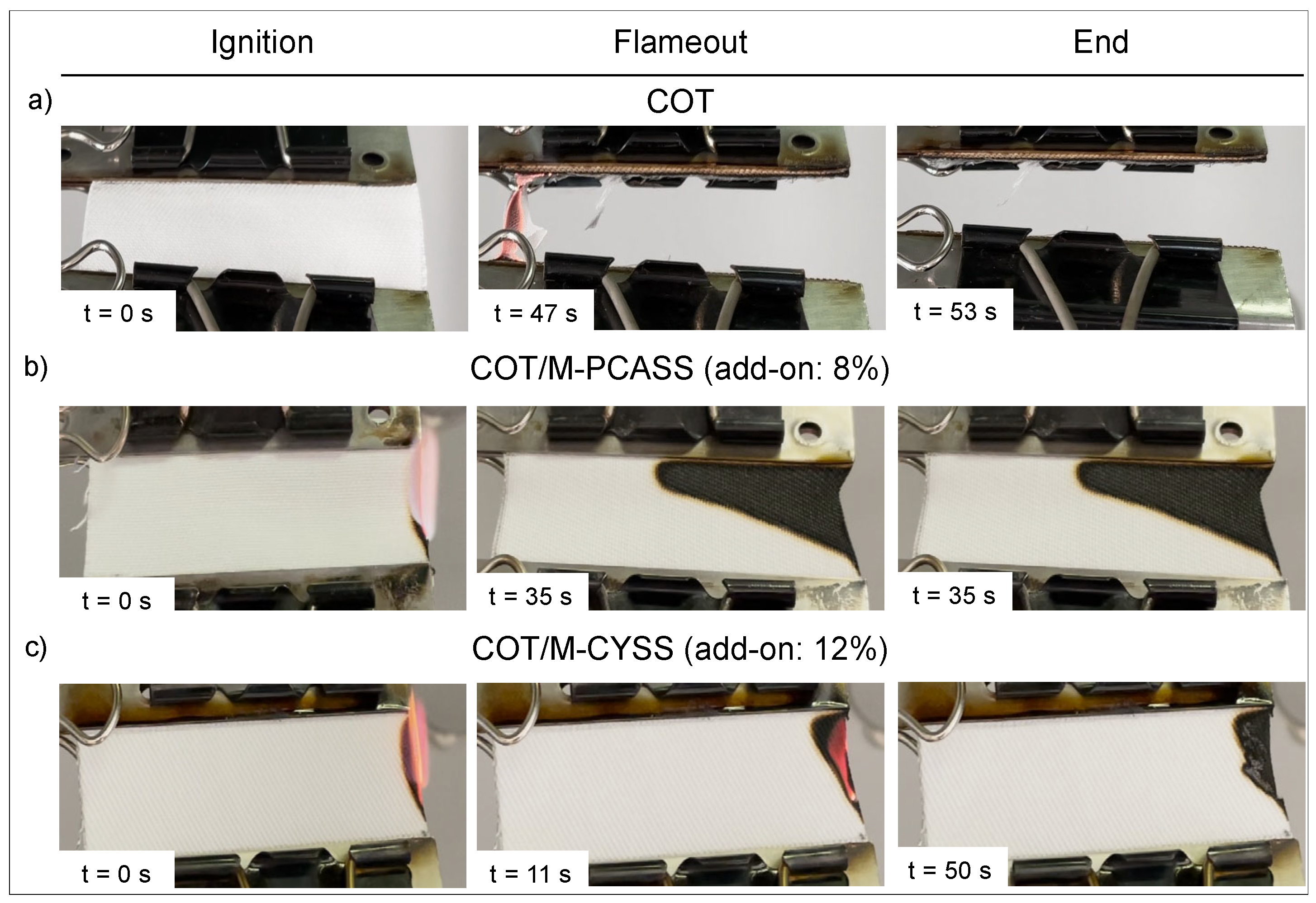
3.5.1. Horizontal Flame Spread Tests
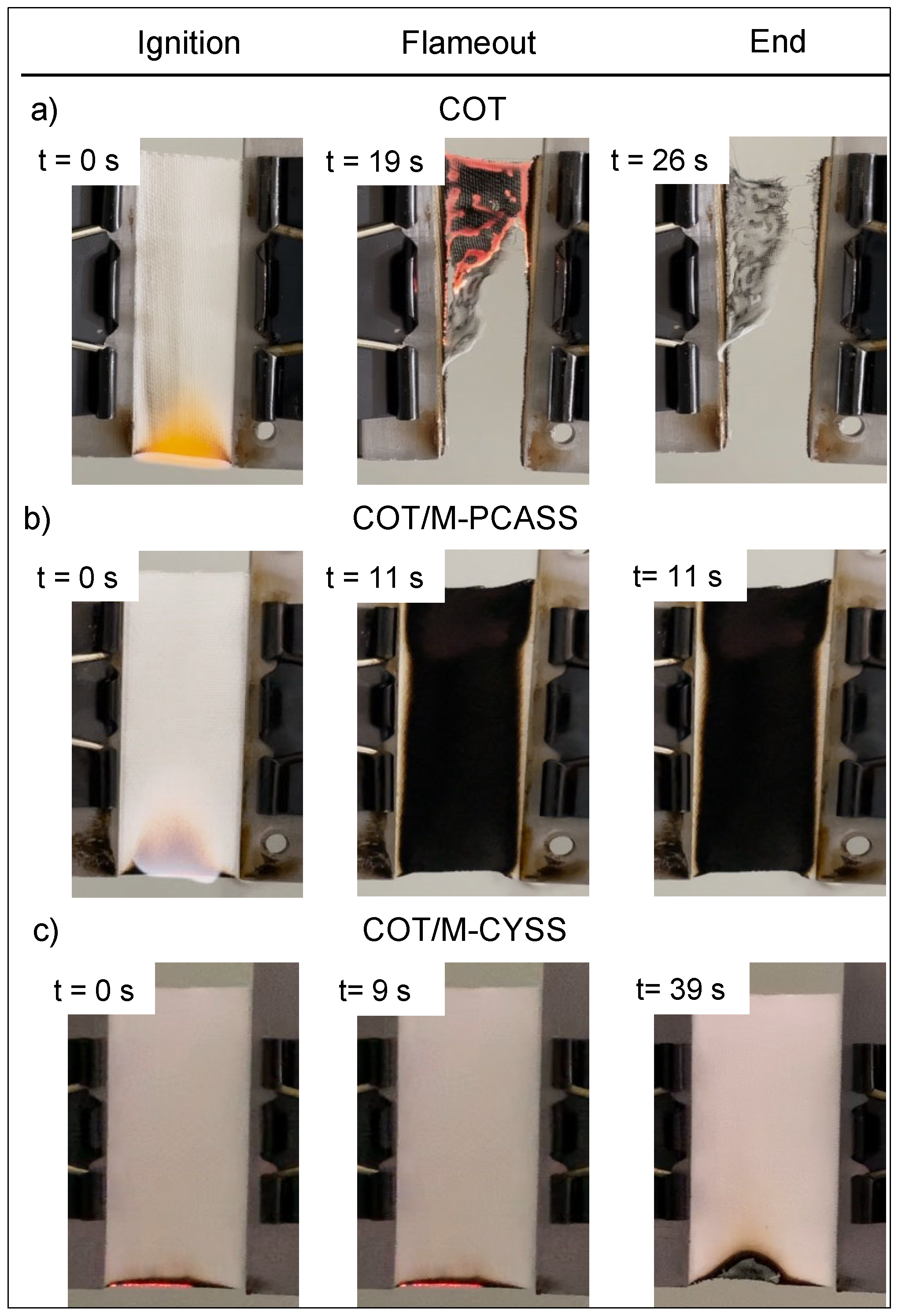
3.5.2. Vertical Flame Spread Tests
3.5.3. Oxygen-Consumption Cone Calorimetry Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Jovic, M.; Ragaisiene, A.; Rukuiziene, Z.; Milasius, R.; Mikucioniene, D.; Gaan, S. Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy. Polymers 2016, 8, 293. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horváth, P.G.; Alpár, T. Potential fabric-reinforced composites: A comprehensive review. J. Mater. Sci. 2021, 56, 14381–14415. [Google Scholar] [CrossRef]
- World Fire statistics n° 27, International Association of Fire and Rescue Service, CTIF 2022. Available online: https://www.ctif.org/news/ctif-world-fire-statistics-report-no-27-now-available-download (accessed on 1 August 2022).
- Islam, S.; van de Ven, T.G.M. Cotton-based flame retardant textile: A review. BioResources 2021, 16, 4354–4381. [Google Scholar] [CrossRef]
- Horrocks, R.A. Flame retardant challenges for textiles and fibres: New chemistry versus innovatory solutions. Polym. Degrad. Stabil. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Horrocks, R.A. Textile flammability research since 1980—Personal challenges and partial solutions. Polym. Degrad. Stabil. 2013, 98, 2813–2824. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent advances for flame retardancy of textiles based on phosphorus chemistry. Polymers 2016, 9, 319. [Google Scholar] [CrossRef]
- Kang, M.; Chen, S.; Yang, R.; Li, D.; Wenchao, Z. Fabrication of an Eco-Friendly Clay-Based Coating for Enhancing Flame Retardant and Mechanical Properties of Cotton Fabrics via LbL Assembly. Polymers 2022, 14, 4994. [Google Scholar] [CrossRef]
- Zilke, O.; Plohl, D.; Opwis, K.; Mayer-Gall, T.; Gutmann, J.S. A flame-retardant phytic-acid-based LbL-coating for cotton using polyvinylamine. Polymers 2020, 12, 1202. [Google Scholar] [CrossRef]
- Barbalini, M.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Phytic acid and biochar: An effective all bio-sourced flame retardant formulation for cotton fabrics. Polymers 2020, 12, 811. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Leng, Q.; Wang, Y. Eco-friendly flame retardant coating deposited on cotton fabrics from bio-based chitosan, phytic acid and divalent metal ions. Int. J. Biol. Macromol. 2019, 140, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Behary, N.; Giraud, S.; Perwuelz, A. In situ degradation of organophosphorus flame retardant on cellulosic fabric using advanced oxidation process: A study on degradation and characterization. Polym. Degrad. Stab. 2016, 126, 1–8. [Google Scholar] [CrossRef]
- Horrocks, A.R. Regulatory and Testing Requirements for Flame Retardant Textile Applications. In Update on Flame Retardant textiles: State of the Art, Environmental Issues and Innovative Solutions; Alongi, J., Carosio, F., Horrocks, A.R., Malucelli, G., Eds.; Smithers RAPRA, Chapter 3; Smithers MSE Limited: Shropshire, UK, 2013; pp. 53–122. ISBN 978-1-90903-017-6. [Google Scholar]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Ferruti, P. Polyamidoamines: Past, Present and Perspectives. J. Polym. Sci. Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Linear polyamidoamines as novel biocompatible phosphorus-free surface confined intumescent flame retardants for cotton fabrics. Polym. Degrad. Stabil. 2018, 151, 52–64. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Alongi, J.; Ranucci, E. Disulfide-containing polyamidoamines with remarkable flame retardant activity for cotton fabrics. Polym. Degrad. Stabil. 2018, 156, 28. [Google Scholar] [CrossRef]
- Alongi, J.; Ferruti, P.; Manfredi, A.; Carosio, F.; Feng, Z.; Hakkarainen, M.; Ranucci, E. Superior flame retardancy of cotton by synergistic effect of cellulose derived nano-graphene oxide carbon dots and disulphide-containing polyamidoamines. Polym. Degrad. Stabil. 2019, 169, 108993. [Google Scholar] [CrossRef]
- Beduini, A.; Ferruti, P.; Carosio, F.; Ranucci, E.; Alongi, J. Polyamidoamines derived from natural α-amino acids as effective flame retardants for cotton. Polymers 2021, 13, 3714. [Google Scholar] [CrossRef]
- Bingol, H.B.; Duman, F.D.; Acar, H.Y.; Yagci, M.B.; Avci, D. Redox-responsive phosphonate-functionalized poly(β-amino ester) gels and cryogels. Eur. Pol. J. 2018, 108, 57–68. [Google Scholar] [CrossRef]
- ISO 3795; Road Vehicles, and Tractors and Machinery for Agriculture and Forestry—Determination of Burning Behaviour of Interior Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 15025; Protective Clothing—Protection Against Flame—Method of Test for Limited Flame Spread. International Organization for Standardization: Geneva, Switzerland, 2016.
- Tata, J.; Alongi, J.; Carosio, F.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part I. Combustion behavior of polyester. Fire Mater. 2011, 35, 397–409. [Google Scholar] [CrossRef]
- ISO 5660 Fire Test; Reaction to Fire, Rate of Heat Release (Cone Calorimeter Method). International Organization for Standardization: Geneva, Switzerland, 2002.
- Schartel, B.; Bartholomai, M.; Knoll, U. Some comments on the main fire retardancy mechanisms in polymer nanocomposites. Polym. Adv. Technol. 2006, 17, 772–777. [Google Scholar] [CrossRef]
- Forte, C.; Alongi, J.; Beduini, A.; Borsacchi, S.; Calucci, L.; Carosio, F.; Ferruti, P.; Ranucci, E. The Thermo-Oxidative Behavior of Cotton Coated with an Intumescent Flame Retardant Glycine-Derived Polyamidoamine: A Multi-Technique Study. Polymers 2021, 13, 4382. [Google Scholar] [CrossRef]
- Price, D.; Horrocks, A.R.; Akalin, M.; Faroq, A.A. Influence of flame retardants on the mechanism of pyrolysis of cotton (celluIose) fabrics in air. J. Anal. Appl. Pyrolysis 1997, 40–41, 511–524. [Google Scholar] [CrossRef]
- Beduini, B.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Sulfur-Based Copolymeric Polyamidoamines as Efficient Flame-Retardants for Cotton. Polymers 2019, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Schartel, B.; Hull, R. Development of fire-retardant materials. Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]







| Sample | Tonset10% 1 (°C) | Tmax1 2 (°C) | Tmax2 3 (°C) | RMF800 4 (%) |
|---|---|---|---|---|
| Nitrogen | ||||
| M-PCASS | 149 | 243 | - | 25 |
| M-CYSS | 157 | 246 | - | 0 |
| Air | ||||
| M-PCASS | 154 | 238 | 600 | 17 |
| M-CYSS | 154 | 242 | 449 | 23 |
| Sample | Tonset10% 1 (°C) | Tmax1 2 (°C) | Tmax2 3 (°C) | RMF800 4 (%) |
|---|---|---|---|---|
| Nitrogen | ||||
| COT | 330 | 365 | - | 5 |
| COT/M-PCASS | 292 | 340 | - | 29 |
| COT/M-CYSS | 250 | 302 | - | 23 |
| Air | ||||
| COT | 328 | 345 | 480 | 0 |
| COT/M-PCASS | 256 | 301 | 509 | 2 |
| COT/M-CYSS | 256 | 299 | 507 | 6 |
| Sample | Add-on 1 (%) | Combustion Time 2 (s) | Afterglow | Extinguishment | RMF 3 (%) |
|---|---|---|---|---|---|
| COT | - | 53 | YES | NO | <1 |
| COT/M-PCASS | 8 | 34 | NO | YES | 81 |
| COT/M-CYSS | 12 | 19 | YES | YES | 93 |
| Sample | Add-on 1 (%) | Combustion Time 2 (s) | Afterglow | Extinguishment | RMF 3 (%) |
|---|---|---|---|---|---|
| COT | - | 53 | YES | NO | <1 |
| COT/M-PCASS | 16 | 11 | NO | NO | 45 |
| COT/M-CYSS | 16 | 20 | YES | YES | 96 |
| Sample | TTI 1 (s) | pkHRR 2 (kW∙m−2) (Reduction, %) | RMF 3 (%) |
|---|---|---|---|
| COT | 45 ± 5 | 109 ± 7 | <1 |
| COT/M-PCASS | 21 ± 1 | 92 ± 2 (−16) | 10.0 ± 0.1 |
| Sample | TSR 1 (m2∙m−2) | TSR(NFP) 2 (m2∙m−2) (Reduction, %) | TSR(FP) 3 (m2∙m−2) (Reduction, %) | (CO) (kg∙kg−1) | (CO2) (kg∙kg−1) |
|---|---|---|---|---|---|
| COT | 20.0 ± 0.5 | 15.8 ± 1.2 | 4.0 ± 0.7 | 0.0001 ± 0.0001 | 0.0055 ± 0.0025 |
| COT/M-PCASS | 4.5 ± 0.4 | 2.7 ± 0.7 (−83) | 1.8 ± 1.0 (−55) | 0.0001 ± 0 | 0.0025 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beduini, A.; Albanese, D.; Carosio, F.; Manfredi, A.; Ranucci, E.; Ferruti, P.; Alongi, J. On the Suitability of Phosphonate-Containing Polyamidoamines as Cotton Flame Retardants. Polymers 2023, 15, 1869. https://doi.org/10.3390/polym15081869
Beduini A, Albanese D, Carosio F, Manfredi A, Ranucci E, Ferruti P, Alongi J. On the Suitability of Phosphonate-Containing Polyamidoamines as Cotton Flame Retardants. Polymers. 2023; 15(8):1869. https://doi.org/10.3390/polym15081869
Chicago/Turabian StyleBeduini, Alessandro, Domenico Albanese, Federico Carosio, Amedea Manfredi, Elisabetta Ranucci, Paolo Ferruti, and Jenny Alongi. 2023. "On the Suitability of Phosphonate-Containing Polyamidoamines as Cotton Flame Retardants" Polymers 15, no. 8: 1869. https://doi.org/10.3390/polym15081869
APA StyleBeduini, A., Albanese, D., Carosio, F., Manfredi, A., Ranucci, E., Ferruti, P., & Alongi, J. (2023). On the Suitability of Phosphonate-Containing Polyamidoamines as Cotton Flame Retardants. Polymers, 15(8), 1869. https://doi.org/10.3390/polym15081869












