Hierarchical Emulsion-Templated Monoliths (polyHIPEs) as Scaffolds for Covalent Immobilization of P. acidilactici
Abstract
1. Introduction
2. Results and Discussion
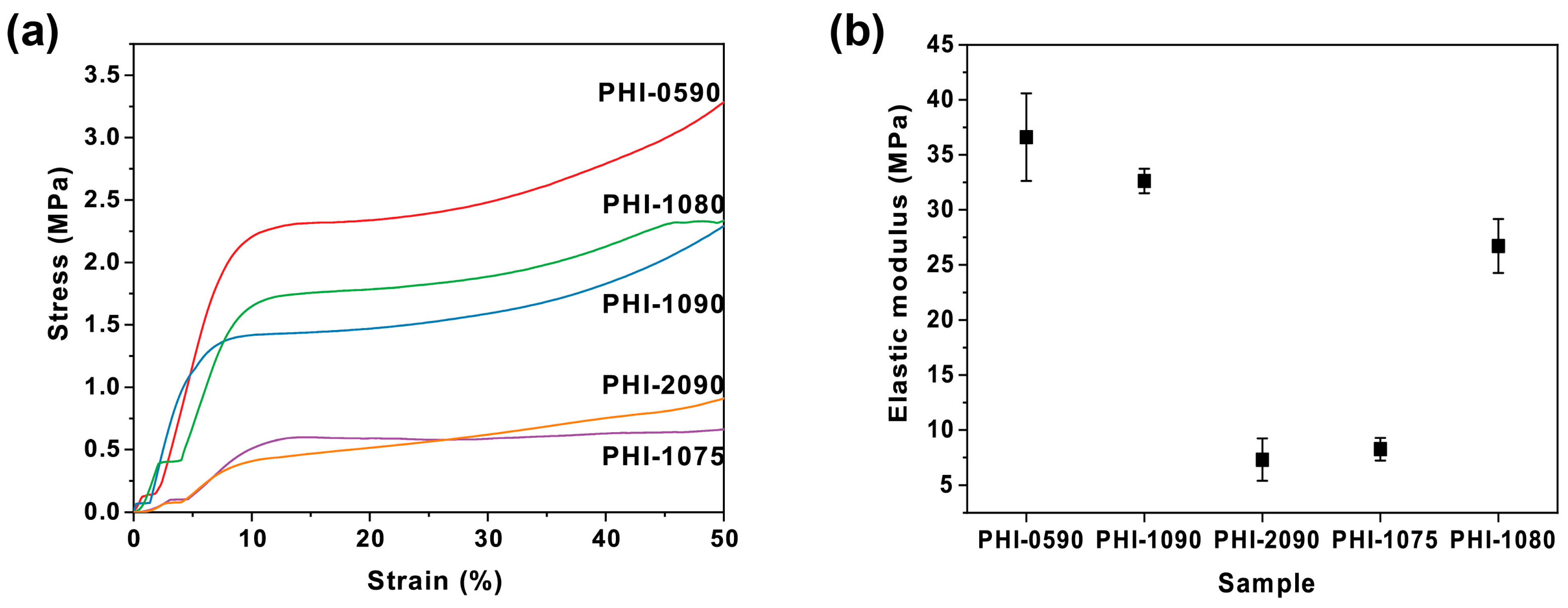
2.1. Preparation and Characterization of Immobilized polyHIPE Scaffold
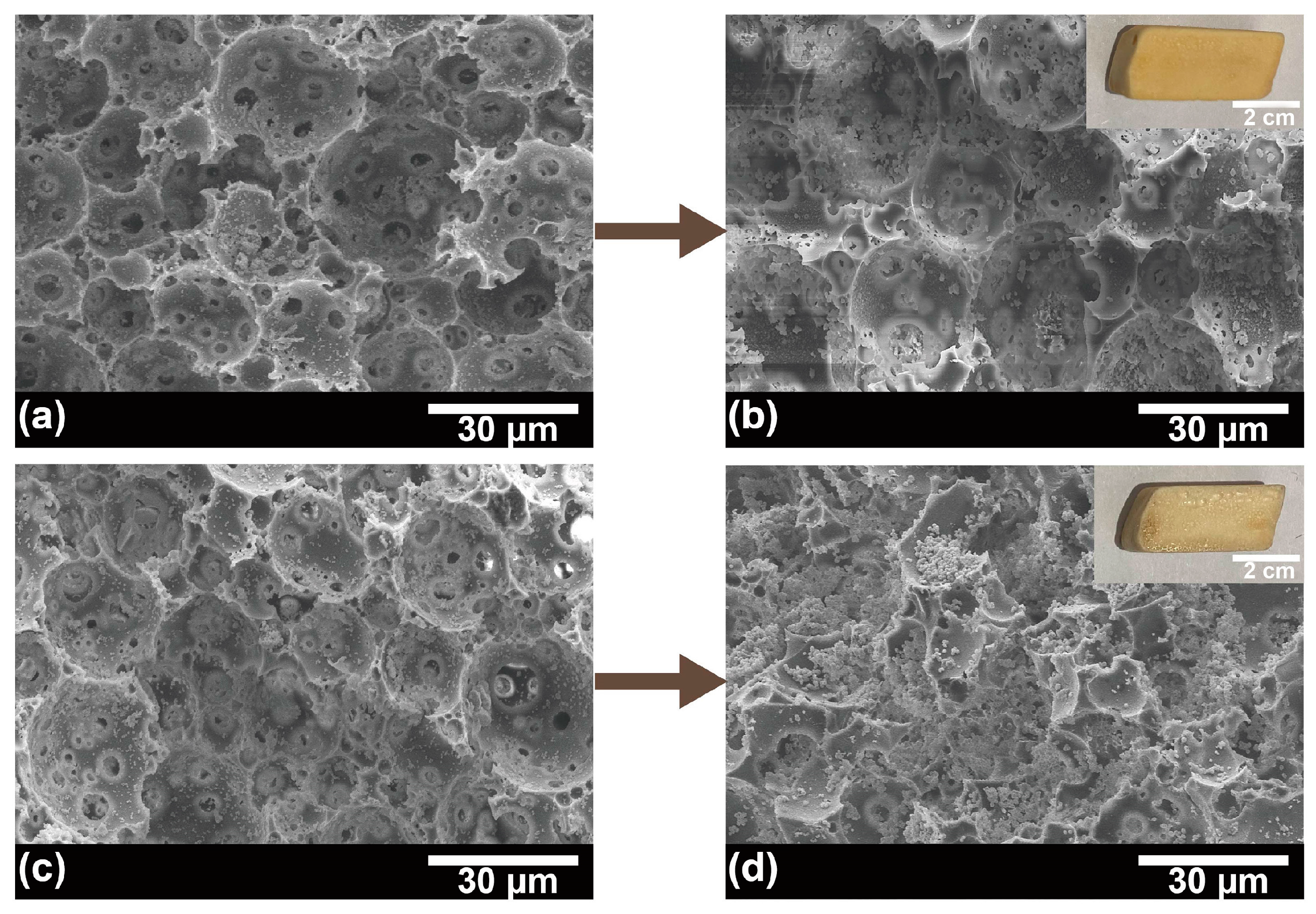
2.2. Immobilization Process
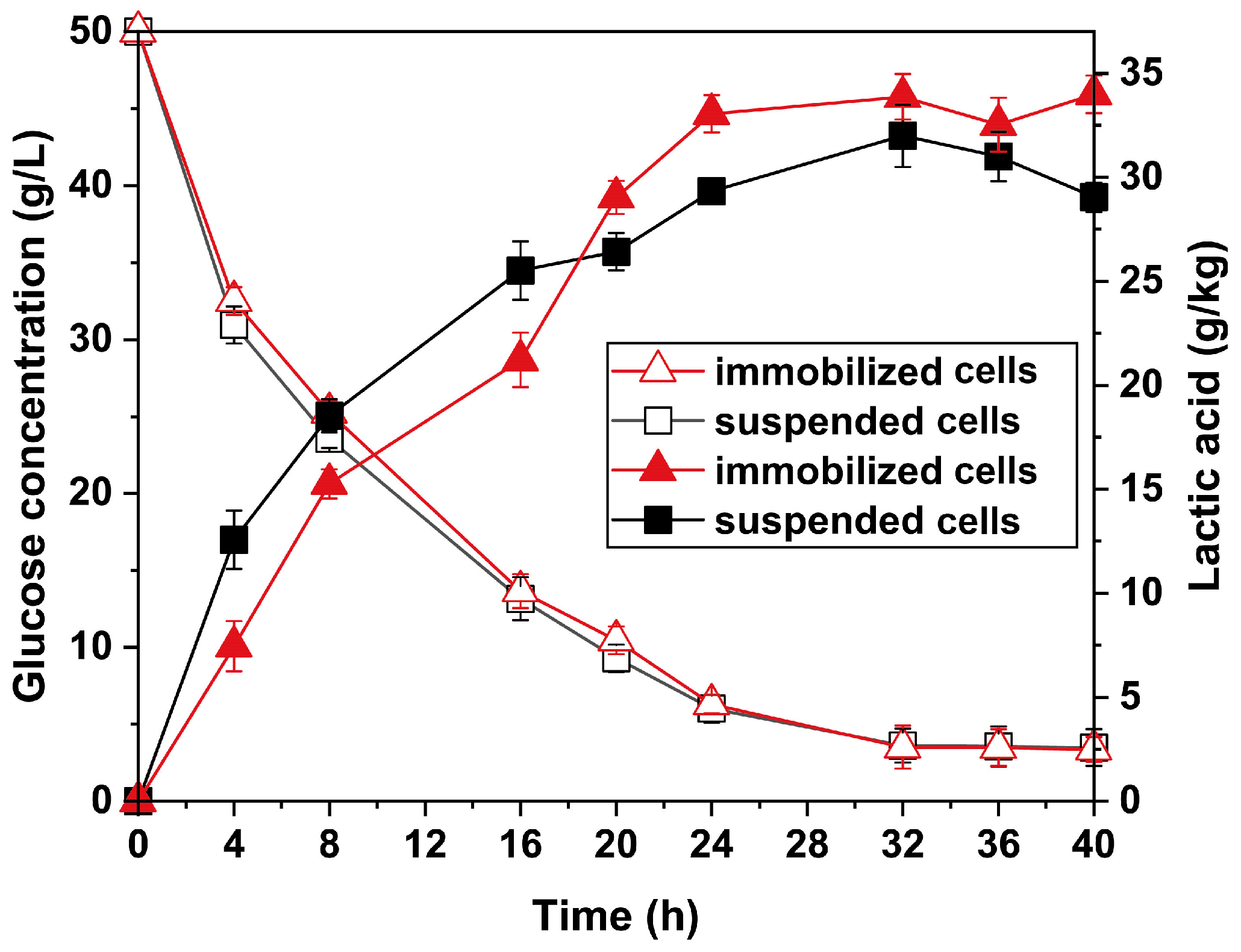
2.3. Fermentation Process of Immobilized P. acidilactici
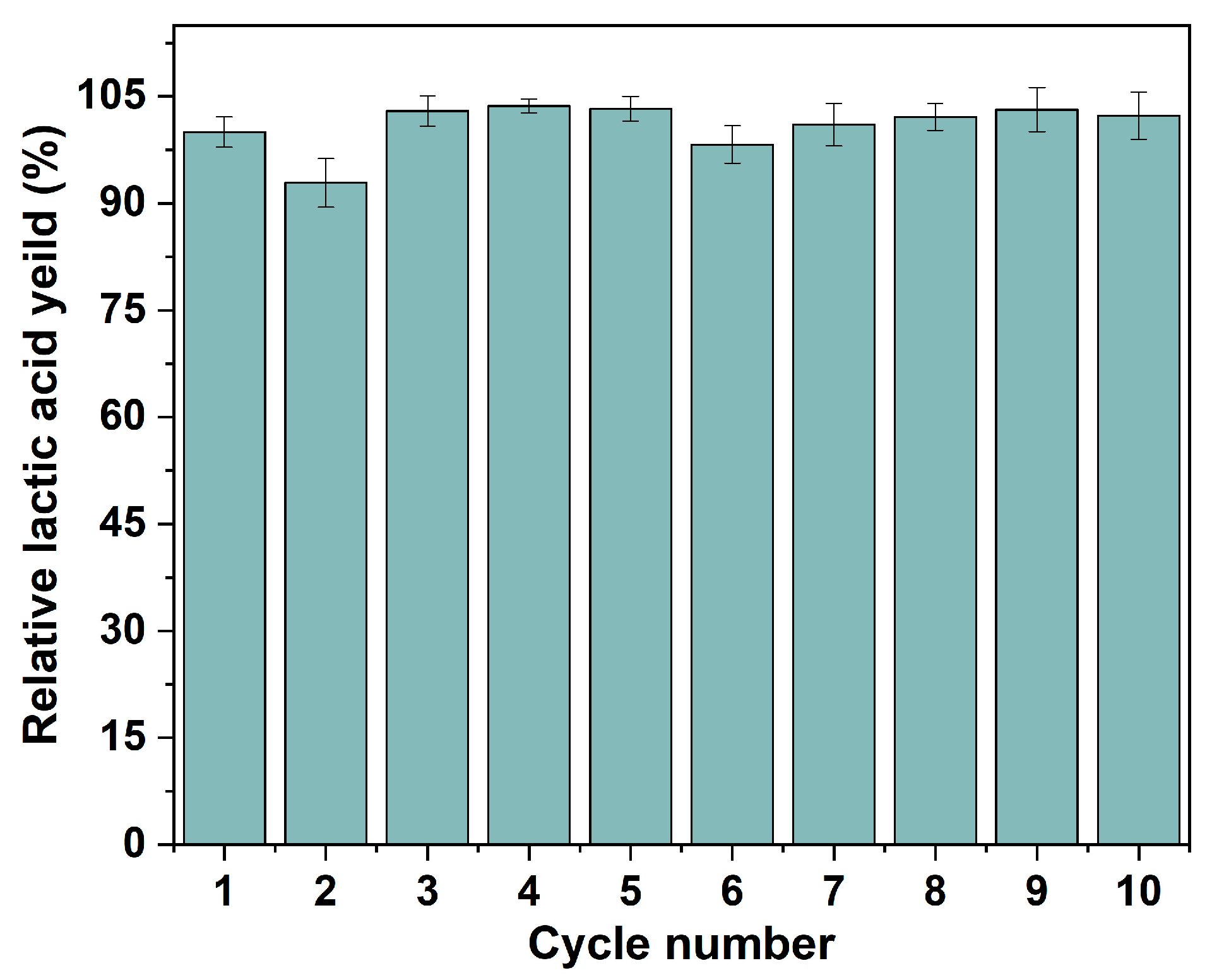
2.4. Recyclability and Reusability of Immobilized P. acidilactici
3. Experimental Section
3.1. Materials
3.2. Method
3.2.1. Preparation of polyHIPE Scaffold
3.2.2. Immobilization Process
3.2.3. Fermentation Process of Immobilized P. acidilactici
3.2.4. Recyclability and Reusability of Immobilized P. acidilactici
3.2.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.S. Developments in seaweed biorefinery research: A comprehensive review. Chem. Eng. J. 2023, 454, 140177. [Google Scholar] [CrossRef]
- Fu, X.; Zheng, Z.; Sha, Z.; Cao, H.; Yuan, Q.; Yu, H.; Li, Q. Biorefining waste into nanobiotechnologies can revolutionize sustainable agriculture. Trends Biotechnol. 2022, 40, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Anderson, E.; Addy, M.; Zhang, R.; Cheng, Y.; Peng, P.; Ma, Y.; Fan, L.; Zhang, Y.; Lu, Q.; et al. Breakthrough Technologies for the Biorefining of Organic Solid and Liquid Wastes. Engineering 2018, 4, 574–580. [Google Scholar] [CrossRef]
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef]
- Tuck, C.O. Valorization of biomass: Deriving more value from Waste. Science 2012, 337, 695–699, Erratum in: Science 2012, 338, 604–604. [Google Scholar] [CrossRef]
- Jouhten, P.; Huerta-Cepas, J.; Bork, P.; Patil, K.R. Metabolic anchor reactions for robust biorefining. Metab. Eng. 2017, 40, 1–4. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.H.; Koutinas, A.A.; Webb, C. Microbial biodegradable plastic production from a wheat-based biorefining strategy. Process Biochem. 2010, 45, 153–163. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Stevens, M.M. Exploring and Engineering the Cell-Surface Interface. Biophys. J. 2011, 100, 189. [Google Scholar] [CrossRef]
- Gao, H.; Lu, J.S.; Jiang, Y.J.; Fang, Y.; Tang, Y.H.; Yu, Z.Y.; Zhang, W.M.; Xin, F.X.; Jiang, M. Material-mediated cell immobilization technology in the biological fermentation proces. Biofuels Bioprod. Biorefin. 2021, 15, 1160–1173. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F.; Knill, C.J.; Kosseva, M.R. Applicability of pectate-entrapped Lactobacillus casei cells for L(+) lactic acid production from whey. Appl. Microbiol. Biotechnol. 2007, 74, 35–42. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Z.; Li, Y.; Zhao, Z.; Liu, J.; Jiang, L.; Xu, H.; Li, Z. “Fish-in-net”, a novel method for cell immobilization of Zymomonas mobilis. PLoS ONE 2013, 8, e79569. [Google Scholar] [CrossRef] [PubMed]
- Tope, A.M.; Srinivas, N.; Kulkarni, S.J.; Jamil, K. Mesoporous molecular sieve (MCM-41) as support material for microbial cell immobilization and transformation of 2,4,6-trinitrotoluene (TNT): A novel system for whole cell immobilization. J. Mol. Catal. B: Enzym. 2001, 16, 17–26. [Google Scholar] [CrossRef]
- Yiu, H.H.P.; Wright, P.A. Enzymes supported on ordered mesoporous solids: A special case of an inorganic–organic hybrid. J. Mater. Chem. 2005, 15, 3690–3700. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, X.; Wang, Z.; Tao, Y.; Niu, X.; Huang, X.; Liu, L.; Li, Z. Immobilization of Lactobacillus rhamnosus in mesoporous silica-based material: An efficiency continuous cell-recycle fermentation system for lactic acid production. J. Biosci. Bioeng. 2016, 121, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Belgrano, F.D.S.; Diegel, O.; Pereira, N., Jr.; Hatti-Kaul, R. Cell immobilization on 3D-printed matrices: A model study on propionic acid fermentation. Bioresour. Technol. 2018, 249, 777–782. [Google Scholar] [CrossRef]
- Barbetta, A.; Massimi, M.; Devirgiliis, L.C.; Dentini, M. Enzymatic Cross-Linking versus Radical Polymerization in the Preparation of Gelatin PolyHIPEs and Their Performance as Scaffolds in the Culture of Hepatocytes. Biomacromolecules 2006, 7, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Busby, W.; Cameron, N.R.; Jahoda, C.A.B. Emulsion-Derived Foams (PolyHIPEs) Containing Poly(E-caprolactone) as Matrixes for Tissue Engineering. Biomacromolecules 2001, 2, 154–164. [Google Scholar] [CrossRef]
- Busby, W.; Cameron, N.R.; Jahoda, C.A.B. Tissue engineering matrixes by emulsion templating. Polym. Int. 2002, 51, 871–881. [Google Scholar] [CrossRef]
- Lee, A.; Langford, C.R.; Rodriguez-Lorenzo, L.M.; Thissen, H.; Cameron, N.R. Bioceramic nanocomposite thiol-acrylate polyHIPE scaffolds for enhanced osteoblastic cell culture in 3D. Biomater. Sci. 2017, 5, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, S.; Wang, G.; Zhu, Y. Fabrication of Anisotropic Polyphosphazene/Bio-based Poly(urethane-acrylate) composite foams with High Thermal Insulation and Flame Retardancy. Polymer 2021, 231, 124108. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, S.; Yang, H.; Zhu, Y.; Chen, J. Preparation of emulsion-templated fluorinated polymers and their application in oil/water separation. J. Polym. Sci. A Polym. Chem. 2018, 56, 1508–1515. [Google Scholar] [CrossRef]
- Yang, X.; Yin, Z.; Zhang, X.; Zhu, Y.; Zhang, S. Fabrication of emulsion-templated macroporous poly(ε-caprolactone) towards highly effective and sustainable oil/water separation. Polymer 2020, 204, 122852. [Google Scholar] [CrossRef]
- Silverstein, M.S. Emulsion-templated porous polymers: A retrospective perspective. Polymer 2014, 55, 304–320. [Google Scholar] [CrossRef]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Foudazi, R. HIPEs to PolyHIPEs. React. Funct. Polym. 2021, 164, 104917. [Google Scholar] [CrossRef]
- Jing, D.; Song, S.; He, L. Reexamination of Murray’s law for tree-like rectangular microchannel network with constant channel height. Int. J. Heat Mass Transf. 2019, 128, 1344–1350. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X. Mixing performance of a fractal-like tree network micromixer based on Murray’s law. Int. J. Heat Mass Transf. 2019, 141, 346–352. [Google Scholar] [CrossRef]
- Severn, C.E.; Eissa, A.M.; Langford, C.R.; Parker, A.; Walker, M.; Dobbe, J.G.G.; Streekstra, G.J.; Cameron, N.R.; Toye, A.M. Ex vivo culture of adult CD34(+) stem cells using functional highly porous polymer scaffolds to establish biomimicry of the bone marrow niche. Biomaterials 2019, 225, 119533. [Google Scholar] [CrossRef]
- Wang, A.J.; Paterson, T.; Owen, R.; Sherborne, C.; Dugan, J.; Li, J.M.; Claeyssens, F. Photocurable high internal phase emulsions (HIPEs) containing hydroxyapatite for additive manufacture of tissue engineering scaffolds with multi-scale porosity. Mater. Sci. Eng. C Mater. Biol. App.l 2016, 67, 51–58. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Carlier, B.; Agniel, R.; Leroy-Dudal, J.; Vancaeyzeele, C.; Plesse, C. 4D smart porous scaffolds based on the polyHIPE architecture and electroactive PEDOT. J. Mater. Chem. C 2021, 9, 12388–12398. [Google Scholar] [CrossRef]
- Dikici, S.; Aldemir Dikici, B.; MacNeil, S.; Claeyssens, F. Decellularised extracellular matrix decorated PCL PolyHIPE scaffolds for enhanced cellular activity, integration and angiogenesis. Biomater. Sci. 2021, 9, 7297–7310. [Google Scholar] [CrossRef]
- Murphy, A.R.; Ghobrial, I.; Jamshidi, P.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Tailored emulsion-templated porous polymer scaffolds for iPSC-derived human neural precursor cell culture. Polym. Chem. 2017, 8, 6617–6627. [Google Scholar] [CrossRef]
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic l-lactic acid fermentation. Bioresour. Technol. 2018, 249, 9–15. [Google Scholar] [CrossRef]
- Qiao, H.; Guo, J.; Wang, L.; Sun, J.; Jiang, S.; Zhang, S.; Yang, W.; Gu, X.; Li, H. Effects of divinylbenzene-maleic anhydride copolymer hollow microspheres on crystallization behaviors, mechanical properties and heat resistance of poly(l-lactide acid). Polym. Adv. Technol. 2019, 31, 817–826. [Google Scholar] [CrossRef]
- Shabnam, R.; Miah, M.A.J.; Sharafat, M.K.; Alam, M.A.; Islam, H.M.T.; Ahmad, H. Cumulative effect of hydrophobic PLMA and surface epoxide groups in composite polymer particles on adsorption behavior of congo red and direct red-75. Arab. J. Chem. 2019, 12, 4989–4999. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Dangtangee, R. Effects of the actual diameters and diameter ratios of barrels and dies on the elastic swell and entrance pressure drop of natural rubber in capillary die flow. J. Appl. Polym. Sci. 2002, 86, 1762–1772. [Google Scholar] [CrossRef]
- Penwell, R.C.; Porter, R.S. Effect of pressure in capillary flow of polystyrene. J. Polym. Sci. Polym. Phys. 1971, 9, 463–482. [Google Scholar] [CrossRef]
- Lumelsky, Y.; Lalush-Michael, I.; Levenberg, S.; Silverstein, M.S. A degradable, porous, emulsion-templated polyacrylate. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 7043–7053. [Google Scholar] [CrossRef]
- Sanchez-Cardona, Y.; Echeverri-Cuartas, C.E.; Lopez, M.E.L.; Moreno-Castellanos, N. Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture. Polymers 2021, 13, 2372. [Google Scholar] [CrossRef]
- Johnston, T.G.; Yuan, S.F.; Wagner, J.M.; Yi, X.; Saha, A.; Smith, P.; Nelson, A.; Alper, H.S. Compartmentalized microbes and co-cultures in hydrogels for on-demand bioproduction and preservation. Nat. Commun. 2020, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, P.; Zhu, W.; Zhu, Y. Macroporous Lithium Adsorbent Monolith Prepared via High Internal Phase Emulsion Technique. ACS Appl. Polym. Mater. 2020, 2, 2563–2570. [Google Scholar] [CrossRef]
- Aydin, E.B.; Sezginturk, M.K. A comparison between LP(GMA) and CLP(GMA) polymer composites as an immobilization matrix for biosensing applications: A model immunosensor for IL 1alpha. Anal. Chim. Acta. 2019, 1077, 129–139. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Purohit, H.J.; Kalia, V.C. Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int. J. Hydrogen Energy 2010, 35, 10674–10681. [Google Scholar] [CrossRef]
- Devi, N.; Patel, S.K.S.; Kumar, P.; Singh, A.; Thakur, N.; Lata, J.; Pandey, D.; Thakur, V.; Chand, D. Bioprocess Scale-up for Acetohydroxamic Acid Production by Hyperactive Acyltransferase of Immobilized Rhodococcus Pyridinivorans. Catal. Lett. 2021, 152, 944–953. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Guo, H.; Jiang, S.; Zhang, J.; Ning, Y.; Fang, M.; Liu, S. Optimization of immobilization conditions for Lactobacillus pentosus cells. Bioprocess Biosyst. Eng. 2020, 43, 1071–1079. [Google Scholar] [CrossRef]
- Bartkiene, E.; Vizbickiene, D.; Bartkevics, V.; Pugajeva, I.; Krungleviciute, V.; Zadeike, D.; Zavistanaviciute, P.; Juodeikiene, G. Application of Pediococcus acidilactici LUHS29 immobilized in apple pomace matrix for high value wheat-barley sourdough bread. LWT Food Sci. Technol. 2017, 83, 157–164. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lan, C.-C.; Pan, C.-L.; Huang, M.-Y.; Chew, C.-H.; Hung, C.-C.; Chen, P.-H.; Lin, H.-T.V. Repeated-batch lactic acid fermentation using a novel bacterial immobilization technique based on a microtube array membrane. Process Biochem. 2019, 87, 25–32. [Google Scholar] [CrossRef]
- Cuny, L.; Pfaff, D.; Luther, J.; Ranzinger, F.; Odman, P.; Gescher, J.; Guthausen, G.; Horn, H.; Hille-Reichel, A. Evaluation of productive biofilms for continuous lactic acid production. Biotechnol. Bioeng. 2019, 116, 2687–2697. [Google Scholar] [CrossRef]
- Shinmyo, A.; Kimura, H.; Okada, H. Physiology of α-amylase production by immobilized Bacillus amyloliquefaciens. Eur. J. Appl. Microbiol. Biotechnol. 1982, 14, 7–12. [Google Scholar] [CrossRef]
- Senthuran, A.; Senthuran, V.; Hatti-Kaul, R.; Mattiasson, B. Lactic acid production by immobilized Lactobacillus casei in recycle batch reactor: A step towards optimization. J. Biotechnol. 1999, 73, 61–70. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Jia, J.; Qiu, Z.; Fang, C.; Liden, G.; Liu, X.; Bao, J. Cyclic l-lactide synthesis from lignocellulose biomass by biorefining with complete inhibitor removal and highly simultaneous sugars assimilation. Biotechnol. Bioeng. 2022, 119, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Su, R.; Liu, X.; Xu, W.; Zhang, Y.; Wang, R.; Ouyang, P.; Wu, J.; Ge, J.; Liu, Z. Lectin corona enhances enzymatic catalysis on the surface of magnetic nanoparticles. Biochem. Eng. J. 2018, 129, 26–32. [Google Scholar] [CrossRef]
- Zada, N.S.; Belduz, A.O.; Guler, H.I.; Sahinkaya, M.; Khan, S.I.; Saba, M.; Bektas, K.I.; Kara, Y.; Kolayli, S.; Badshah, M.; et al. Cloning, biochemical characterization and molecular docking of novel thermostable beta-glucosidase BglA9 from Anoxybacillus ayderensis A9 and its application in de-glycosylation of Polydatin. Int. J. Biol. Macromol. 2021, 193, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
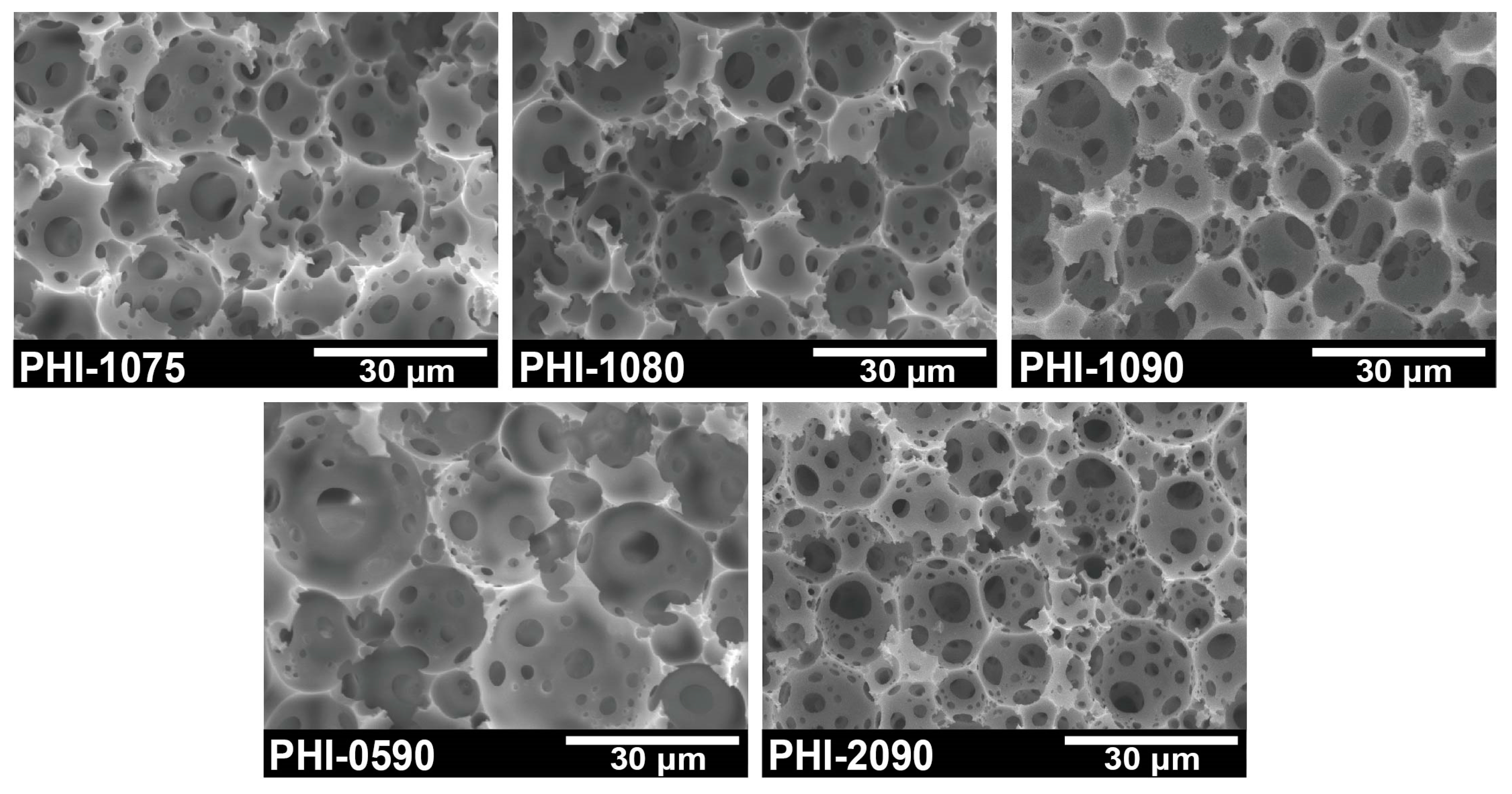




| Sample | Span80 (wt.%) [a] | ƒinternal phase (vol.%) [b] | D (μm) [c] | d (μm) [d] | Interconnectivity (%) [e] |
|---|---|---|---|---|---|
| PHI-0590 | 5 | 90 | 18.9 ± 5.9 | 3.4 ± 1.8 | 6.1 |
| PHI-1090 | 10 | 90 | 12.5 ± 3.1 | 3.2 ± 1.9 | 12.3 |
| PHI-2090 | 20 | 90 | 11.6 ± 4.9 | 3.6 ± 1.4 | 25.3 |
| PHI-1075 | 10 | 75 | 16.1 ± 4.6 | 3.3 ± 1.4 | 10.5 |
| PHI-1080 | 10 | 80 | 14.1 ± 3.8 | 3.1 ± 1.2 | 11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Z.; Zhang, S.; Liu, X. Hierarchical Emulsion-Templated Monoliths (polyHIPEs) as Scaffolds for Covalent Immobilization of P. acidilactici. Polymers 2023, 15, 1862. https://doi.org/10.3390/polym15081862
Yin Z, Zhang S, Liu X. Hierarchical Emulsion-Templated Monoliths (polyHIPEs) as Scaffolds for Covalent Immobilization of P. acidilactici. Polymers. 2023; 15(8):1862. https://doi.org/10.3390/polym15081862
Chicago/Turabian StyleYin, Zhengqiao, Shengmiao Zhang, and Xiucai Liu. 2023. "Hierarchical Emulsion-Templated Monoliths (polyHIPEs) as Scaffolds for Covalent Immobilization of P. acidilactici" Polymers 15, no. 8: 1862. https://doi.org/10.3390/polym15081862
APA StyleYin, Z., Zhang, S., & Liu, X. (2023). Hierarchical Emulsion-Templated Monoliths (polyHIPEs) as Scaffolds for Covalent Immobilization of P. acidilactici. Polymers, 15(8), 1862. https://doi.org/10.3390/polym15081862







