Micro 3D Printing Elastomeric IP-PDMS Using Two-Photon Polymerisation: A Comparative Analysis of Mechanical and Feature Resolution Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Two-Photon Polymerization Fabrication Protocol
2.3. Fabrication of Specimens for Mechanical and Morphological Characterisation
2.4. Mechanical Nanoindentation
2.5. Scanning Electron Microscopy
3. Results and Discussion
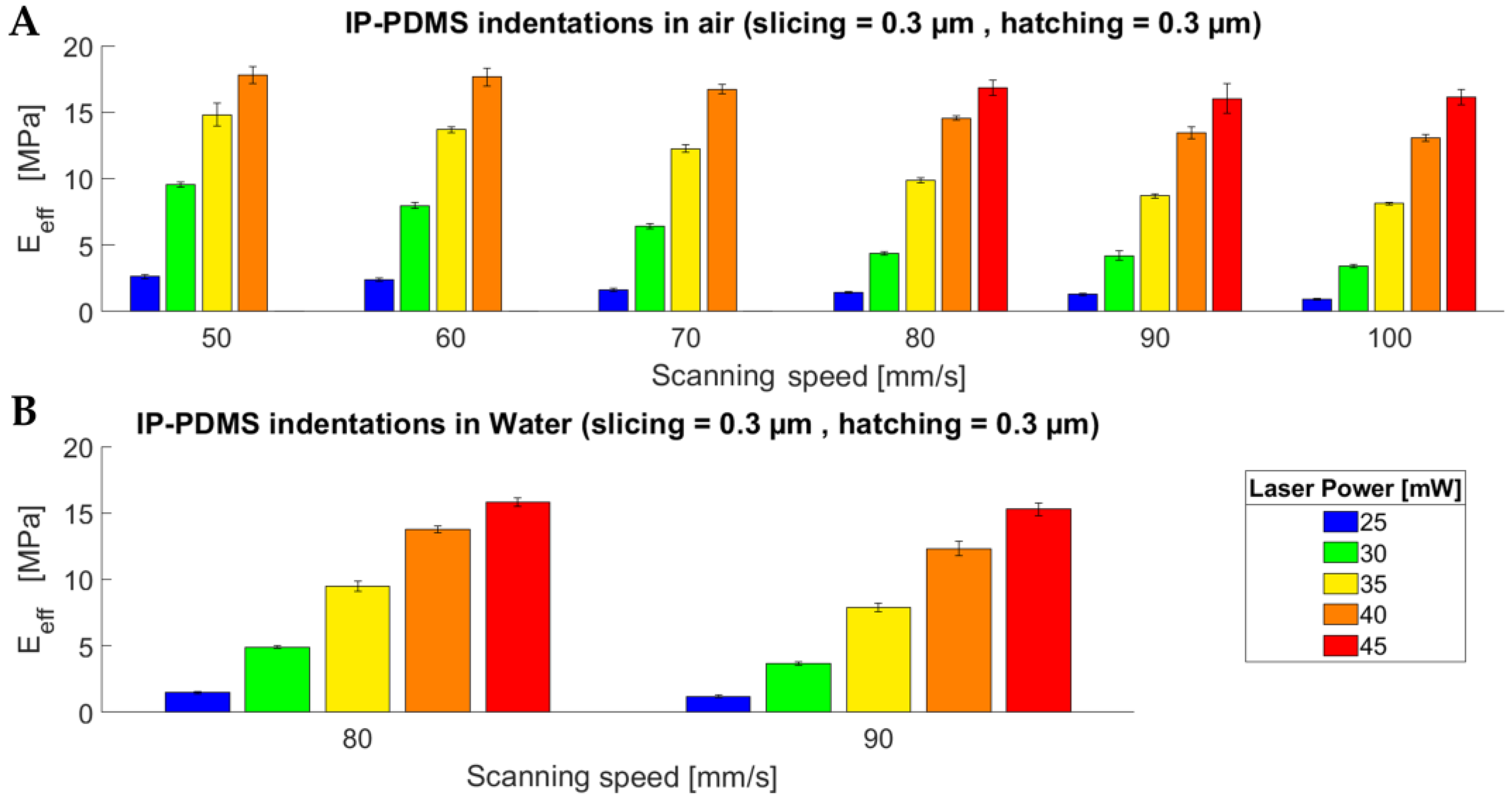
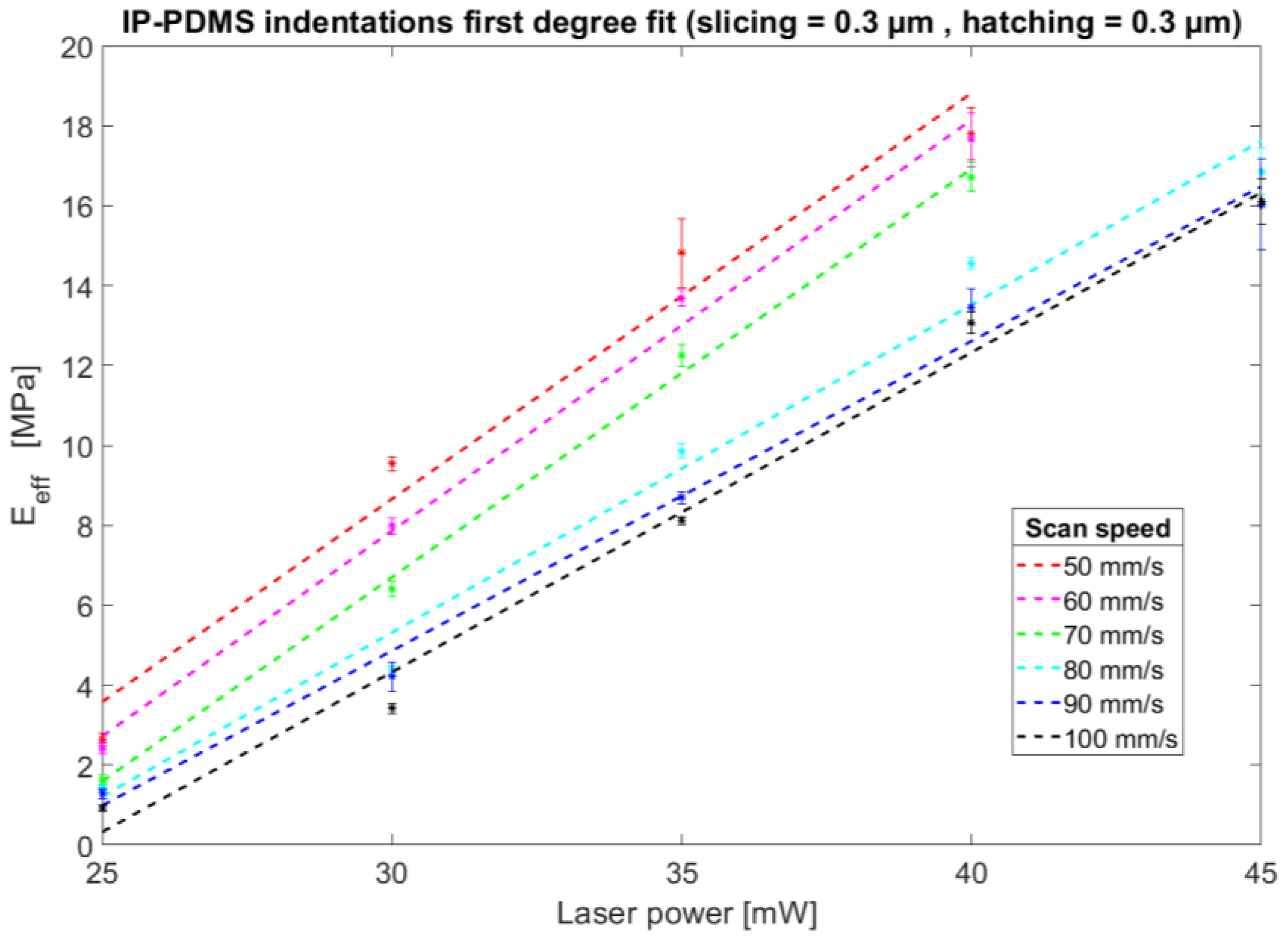
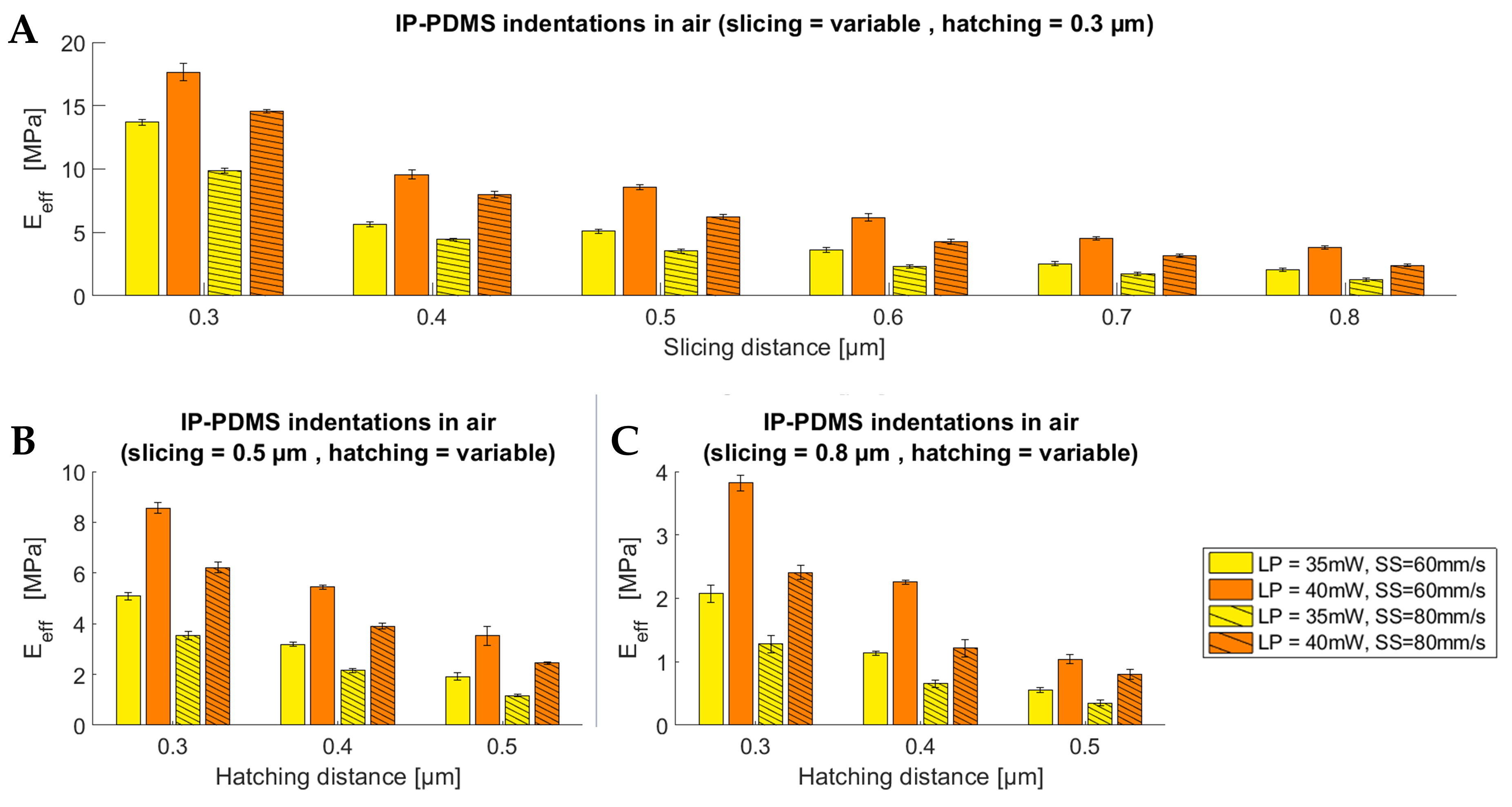
3.1. Mechanical Characterisation of Two-Photon-Polymerised IP-PDMS Microstructures
3.2. Morphological Characterisation of Two-Photon-Polymerised IP-PDMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Hou, Y.; Lin, J. A Review on the Processing Accuracy of Two-Photon Polymerization. AIP Adv. 2015, 5, 030701. [Google Scholar] [CrossRef]
- Harinarayana, V.; Shin, Y.C. Two-Photon Lithography for Three-Dimensional Fabrication in Micro/Nanoscale Regime: A Comprehensive Review. Opt. Laser Technol. 2021, 142, 107180. [Google Scholar] [CrossRef]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. High-Resolution Two-Photon Polymerization: The Most Versatile Technique for the Fabrication of Microneedle Arrays. Microsyst. Nanoeng. 2021, 7, 71. [Google Scholar] [CrossRef]
- Lemma, E.D.; Sergio, S.; Spagnolo, B.; Pisanello, M.; Algieri, L.; Coluccia, M.A.; Maffia, M.; De Vittorio, M.; Pisanello, F. Tunable Mechanical Properties of Stent-like Microscaffolds for Studying Cancer Cell Recognition of Stiffness Gradients. Microelectron. Eng. 2018, 190, 11–18. [Google Scholar] [CrossRef]
- Farsari, M.; Chichkov, B.N. Two-Photon Fabrication. Nat. Photonics 2009, 3, 450–452. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Chichkov, B.N. Three-Dimensional Microfabrication by Two-Photon Polymerization Technique. Methods Mol. Biol. 2012, 868, 311–325. [Google Scholar]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast Laser Processing of Materials: From Science to Industry. Light Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef]
- Bunea, A.-I.; Iniesta, N.d.C.; Droumpali, A.; Wetzel, A.E.; Engay, E.; Taboryski, R. Micro 3D Printing by Two-Photon Polymerization: Configurations and Parameters for the Nanoscribe System. Micro 2021, 1, 164–180. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Mironov, V.; Stampf, J.; Liska, R. Engineering 3D Cell-Culture Matrices: Multiphoton Processing Technologies for Biological and Tissue Engineering Applications. Expert Rev. Med. Devices 2012, 9, 613–633. [Google Scholar] [CrossRef]
- Fan, D.; Staufer, U.; Accardo, A. Engineered 3D Polymer and Hydrogel Microenvironments for Cell Culture Applications. Bioengineering 2019, 6, 113. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Gaidukeviciute, A.; Chichkov, B.N.; Oubaha, M.; MacCraith, B.D.; Sakellari, I.; Giakoumaki, A.; Gray, D.; Vamvakaki, M.; Farsari, M.; et al. Two-Photon Polymerization of Hybrid Sol-Gel Materials for Photonics Applications. Laser Chem. 2008, 2008, 7. [Google Scholar] [CrossRef]
- Qin, X.H.; Ovsianikov, A.; Stampfl, J.; Liska, R. Additive Manufacturing of Photosensitive Hydrogels for Tissue Engineering Applications. BioNanoMaterials 2014, 15, 49–70. [Google Scholar] [CrossRef]
- O’Halloran, S.; Pandit, A.; Heise, A.; Kellett, A. Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies. Adv. Sci. 2022, 10, 2204072. [Google Scholar] [CrossRef] [PubMed]
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Schlie-Wolter, S.; Chichkov, B.N. Hyaluronic Acid Based Materials for Scaffolding via Two-Photon Polymerization. Biomacromolecules 2014, 15, 650–659. [Google Scholar] [CrossRef]
- Costa, B.N.L.; Adão, R.M.R.; Maibohm, C.; Accardo, A.; Cardoso, V.F.; Nieder, J.B. Cellular Interaction of Bone Marrow Mesenchymal Stem Cells with Polymer and Hydrogel 3D Microscaffold Templates. ACS Appl. Mater. Interfaces 2022, 14, 13013–13024. [Google Scholar] [CrossRef] [PubMed]
- Lemma, E.D.; Rizzi, F.; Dattoma, T.; Spagnolo, B.; Sileo, L.; Qualtieri, A.; De Vittorio, M.; Pisanello, F. Mechanical Properties Tunability of Three-Dimensional Polymeric Structures in Two-Photon Lithography. IEEE Trans. Nanotechnol. 2017, 16, 23–31. [Google Scholar] [CrossRef]
- Lemma, E.D.; Spagnolo, B.; De Vittorio, M.; Pisanello, F. Studying Cell Mechanobiology in 3D: The Two-Photon Lithography Approach. Trends Biotechnol. 2019, 37, 358–372. [Google Scholar] [CrossRef]
- Sharaf, A.; Timmerman, R.; Bajramovic, J.; Accardo, A. In Vitro Microglia Models: The Era of Engineered Cell Microenvironments. Neural Regen. Res. 2022, 18, 1709–1710. [Google Scholar] [CrossRef]
- Sharaf, A.; Roos, B.; Timmerman, R.; Kremers, G.-J.; Bajramovic, J.J.; Accardo, A. Two-Photon Polymerization of 2.5D and 3D Microstructures Fostering a Ramified Resting Phenotype in Primary Microglia. Front. Bioeng. Biotechnol. 2022, 10, 926642. [Google Scholar] [CrossRef]
- Akolawala, Q.; Rovituso, M.; Versteeg, H.H.; Rondon, A.M.R.; Accardo, A. Evaluation of Proton-Induced DNA Damage in 3D-Engineered Glioblastoma Microenvironments. ACS Appl. Mater. Interfaces 2022, 14, 20778–20789. [Google Scholar] [CrossRef]
- Barin, N.; Balcioglu, H.E.; de Heer, I.; de Wit, M.; Lamfers, M.L.M.; van Royen, M.E.; French, P.J.; Accardo, A. 3D-Engineered Scaffolds to Study Microtubes and Localization of Epidermal Growth Factor Receptor in Patient-Derived Glioma Cells. Small 2022, 18, 2204485. [Google Scholar] [CrossRef] [PubMed]
- Castillo Ransanz, L.; Van Altena, P.F.J.; Heine, V.M.; Accardo, A. Engineered Cell Culture Microenvironments for Mechanobiology Studies of Brain Neural Cells. Front. Bioeng. Biotechnol. 2022, 10, 1096054. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Zhang, L.; Sun, Y.; Feinberg, A.W. Development of Polydimethylsiloxane Substrates with Tunable Elastic Modulus to Study Cell Mechanobiology in Muscle and Nerve. PLoS ONE 2012, 7, e51499. [Google Scholar] [CrossRef]
- Serino, G.; Lugas, A.T.; Bernava, G.; Ragazzini, S.; Gabetti, S.; Terzini, M.; Morbiducci, U.; Audenino, A.; Pesce, M.; Massai, D. PDMS Substrates with Tunable Stiffness for Cardiac Mechanobiology Investigation: A Nanoindentation Study. Biomed. Sci. Eng. 2021, 5, 105–106. [Google Scholar] [CrossRef]
- Mfoumou, E.; Tango, M.; Kin Wong, P. Tuning Polydimethylsiloxane (PDMS) Properties for Biomedical Applications. Adv. Mater. Lett. 2019, 10, 107–111. [Google Scholar] [CrossRef]
- Sagadevan, S.; Murugasen, P. Novel Analysis on the Influence of Tip Radius and Shape of the Nanoindenter on the Hardness of Materials. Procedia Mater. Sci. 2014, 6, 1871–1878. [Google Scholar] [CrossRef]
- Ebenstein, D.M.; Pruitt, L.A. Nanoindentation of Biological Materials. Nano Today 2006, 1, 26–33. [Google Scholar] [CrossRef]
- Mattei, G.; Looze, N.; Breel, E. Measuring Micro-Mechanical Properties of (Bio)Materials by Nano-Indentation. White Paper. 2017. Available online: https://www.optics11life.com/wp-content/uploads/2020/06/White_Paper_Nanoindentation_Optics11.pdf (accessed on 1 March 2023).
- Schweiger, S.; Schulze, T.; Schlipf, S.; Reinig, P.; Schenk, H. Characterization of Two-Photon-Polymerization Lithography Structures via Raman Spectroscopy and Nanoindentation. J. Opt. Microsyst. 2022, 2, 033501. [Google Scholar] [CrossRef]
- Hadden, W.J.; Young, J.L.; Holle, A.W.; McFetridge, M.L.; Kim, D.Y.; Wijesinghe, P.; Taylor-Weiner, H.; Wen, J.H.; Lee, A.R.; Bieback, K.; et al. Stem Cell Migration and Mechanotransduction on Linear Stiffness Gradient Hydrogels. Proc. Natl. Acad. Sci. USA 2017, 114, 5647–5652. [Google Scholar] [CrossRef]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Crosslinking Effect on Polydimethylsiloxane Elastic Modulus Measured by Custom-Built Compression Instrument. J. Appl. Polym. Sci. 2014, 131, 41050. [Google Scholar] [CrossRef]
- Irawan, V.; Higuchi, A.; Ikoma, T. Physical Cues of Biomaterials Guide Stem Cell Fate of Differentiation: The Effect of Elasticity of Cell Culture Biomaterials. Open Phys. 2018, 16, 943–955. [Google Scholar] [CrossRef]
- Ali, S.; Wall, I.B.; Mason, C.; Pelling, A.E.; Veraitch, F.S. The Effect of Young’s Modulus on the Neuronal Differentiation of Mouse Embryonic Stem Cells. Acta Biomater. 2015, 25, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Kappert, E.J.; Raaijmakers, M.J.T.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 Polymers Commonly Employed for Solvent-Resistant Nanofiltration Membranes: A Comprehensive Dataset. J. Memb. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Liska, R.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. Cross-Linkable Gelatins with Superior Mechanical Properties Through Carboxylic Acid Modification: Increasing the Two-Photon Polymerization Potential. Biomacromolecules 2017, 18, 3260–3272. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Altena, P.F.J.; Accardo, A. Micro 3D Printing Elastomeric IP-PDMS Using Two-Photon Polymerisation: A Comparative Analysis of Mechanical and Feature Resolution Properties. Polymers 2023, 15, 1816. https://doi.org/10.3390/polym15081816
van Altena PFJ, Accardo A. Micro 3D Printing Elastomeric IP-PDMS Using Two-Photon Polymerisation: A Comparative Analysis of Mechanical and Feature Resolution Properties. Polymers. 2023; 15(8):1816. https://doi.org/10.3390/polym15081816
Chicago/Turabian Stylevan Altena, Pieter F. J., and Angelo Accardo. 2023. "Micro 3D Printing Elastomeric IP-PDMS Using Two-Photon Polymerisation: A Comparative Analysis of Mechanical and Feature Resolution Properties" Polymers 15, no. 8: 1816. https://doi.org/10.3390/polym15081816
APA Stylevan Altena, P. F. J., & Accardo, A. (2023). Micro 3D Printing Elastomeric IP-PDMS Using Two-Photon Polymerisation: A Comparative Analysis of Mechanical and Feature Resolution Properties. Polymers, 15(8), 1816. https://doi.org/10.3390/polym15081816







