2.1. Materials
The following materials and solvents were used: melamine (99%), terephthalaldehyde (99%), 4-ethylphenol (97%), 4-hydroxybenzoic acid (99%), 2-nitrobenzaldehyde (98%), 3-nitrobenzaldehyde (99%), 2-chlorobenzaldehyde (99%), 2-methylbenzaldehyde (97%), 2-bromobenzaldehyde (98%), 4-methylbenzaldehyde (97%), 4-bromobenzaldehyde (99%), 1-naphthaldehyde (95%), and 4-carboxybenzaldehyde (97%) were purchased from Sigma-Aldrich (Schnelldorf, Germany); glacial acetic acid (≥99%), potassium hydrogen phthalate (≥99.9%), and methyl orange were supplied by Merck (Darmstadt, Germany); hexamethylenetetramine (≥99%), nitromethane (99%), and methanol (≥99.8%) were purchased from Riedel-de Haën (Seelze, Germany); standard solution 1000 mg L−1 Cu(II) in 0.5 M nitric acid and methylene blue were purchased from PanReac (Barcelona, Spain); anhydrous sodium sulphate (99.7%), sodium chloride (>99%), sulfuric acid (96%), acetone (>99.6%), and sodium hydroxide (99.4%) were acquired from José Manuel Gomes dos Santos (Lisboa, Portugal); nitric acid (65%) and hydrochloric acid (37%) were purchased from Chem-Lab (Zedelgem, Belgium); 3-chlorobenzaldehyde, 3-methylbenzaldehyde, 2-naphthaldehyde were provided by Fluorochem (Hadfield, UK); tetrahydrofuran (≥99.9%) and cyclohexane (≥99.8%) were received from Carlo Erba (Val de Reuil, France); and deuterated chloroform (CDCl3, 99.8%D) and dimethylsulfoxide (DMSO-d6, 99.8%D) were purchased from Eurisotop (Saint-Aubin, France). Other reagents and solvents were used: 4-nitrobenzaldehyde (99%, Alfa Aesar, Karlsruhe, Germany), benzaldehyde (98%, Thermo Fisher Scientific, Geel, Belgium), trifluoroacetic acid (99.9%, Fluorochem, Hadfield, UK), dimethylsulfoxide (≥99.5%, Riedel-de Haën, Seelze, Germany), and copper(II) acetate monohydrate (≥98%, J.T. Baker, Center Vally, PA, USA).
All materials and solvents, with the exception of terephthalaldehyde, were used as received.
2.2. Synthesis of the Porous Organic Polymers (T-POPs)
The porous organic polymers with aminal structure and based on triazine rings (T-POPs) were obtained by reaction of melamine with a dialdehyde. Among the dialdehydes, a commercially available non-functionalized derivative (terephthalaldehyde) and functionalized monomers (4-ethyl-2,6-diformylphenol (EDP) and 3,5-diformyl-4-hydroxybenzoic acid (DHA)) were used for obtaining T-POP1, T-POP2, and T-POP3, respectively.
(1) Terephthalaldehyde: High-purity terephthalaldehyde was obtained by recrystallization using the method described by Yoon et al. [
26]. Briefly, terephthalaldehyde (5.00 g) was dissolved in methanol (40 mL) under stirring and slight heating, and the undissolved acid fraction was filtered. Then, water (200 mL) was added to precipitate the aldehyde, which was filtered off and vacuum dried.
1H-NMR (400 MHz, CDCl
3, ppm): 8.05 (s, 4H, H–Ar); 10.13 (s, 2H, CHO). IR (cm
−1): 769, 813, 1009, 1090, 1098, 1196, 1299, 1334, 1367, 1385, 1431, 1498, 1687, 2758, 2806, 2865.
The functionalized monomers were prepared by diformylation of phenol derivatives through Duff reaction:
(2) 4-ethyl-2,6-diformylphenol (EDP): 4-ethylphenol (4.18 g, 34.2 mmol) and hexamethylenetetramine (9.60 g, 68.5 mmol) were dissolved in trifluoroacetic acid (60 mL). The resulting solution was refluxed (100 °C) with magnetic stirring and under an inert atmosphere for 24 h. After this time, the cooled reaction mixture was poured into an Erlenmeyer flask containing hydrochloric acid 4 M (50 mL) and stirred for 10 min, and then it was extracted using dichloromethane (2 × 150 mL). The combined organic phases were extracted with hydrochloric acid 4 M (2 × 100 mL), distilled water (200 mL), and finally, a saturated solution of sodium chloride (200 mL). The final organic fraction was dried with anhydrous sodium sulfate, filtered under reduced pressure, and evaporated, yielding a yellow-brown solid residue [
27]. The pure product was collected as a fine yellow powder, after recrystallization with cyclohexane, in 21% yield.
1H-NMR (400 MHz, CDCl
3, ppm): 1.27 (t, 3H, CH
3,
= 7.6 Hz); 2.69 (q, 2H, CH
2,
= 7.6 Hz); 7.80 (s, 2H, H–Ar); 10.23 (s, 2H, CHO); 11.47 (s, 1H, OH). IR (cm
−1): 744, 789, 908, 972, 1003, 1066, 1154, 1201, 1267, 1298, 1326, 1377, 1403, 1444, 1456, 1596, 1661, 1679, 2777, 2868, 2932, 2967, 3142.
(3) 3,5-diformyl-4-hydroxybenzoic acid (DHA): 4-hydroxybenzoic acid (1.10 g, 8.0 mmol) and hexamethylenetetramine (8.97 g, 64.0 mmol) were dissolved in trifluoroacetic acid (40 mL), and the reaction mixture was maintained at reflux (110 °C) with magnetic stirring and under an inert atmosphere for 72 h. After cooling, the solution was poured into an Erlenmeyer flask containing hydrochloric acid 4 M (200 mL) and stirred vigorously for 30 min. Thereafter, the mixture was allowed to stand for 72 h to allow a precipitate to form [
28]. The solid product formed was filtered under reduced pressure, washed with distilled water (3 × 20 mL), and dried in vacuo. A pure yellow solid was obtained with a yield of 66%.
1H-NMR (400 MHz, DMSO-d
6, ppm): 8.55 (s, 2H, H–Ar); 10.30 (s, 2H, CHO). IR (cm
−1): 727, 771, 796, 810, 931, 942, 1002, 1124, 1166, 1204, 1264, 1295, 1348, 1365, 1388, 1587, 1647, 1685, 1717, 2765, 2874, 3040, 3063, 3308.
The synthesis of porous polymers T-POP1, T-POP2, and T-POP3 was carried out by reaction between melamine (0.3847 g; 3.05 mmol) and the respective dialdehyde (4.58 mmol): terephthalaldehyde, 4-ethyl-2,6-diformylphenol (EDP), or 3,5-diformyl-4-hydroxybenzoic acid (DHA), dissolved in 20 mL of DMSO and 7.50 mL of 3 M acetic acid [
29]. A reflux condenser was attached to the round bottom flask, and the mixture was heated to 140 °C, in an inert atmosphere and under magnetic stirring for 48 h (T-POP1 and T-POP3) or 72 h (T-POP2). In each case, the formed precipitate was filtered under gravity, washed with THF, acetone, and methanol, and dried in an oven at 40 °C. The final products were obtained in the form of fine powders of white (T-POP1), beige (T-POP2), and yellow (T-POP3) colors, in 61%, 68%, and 53% yield, respectively.
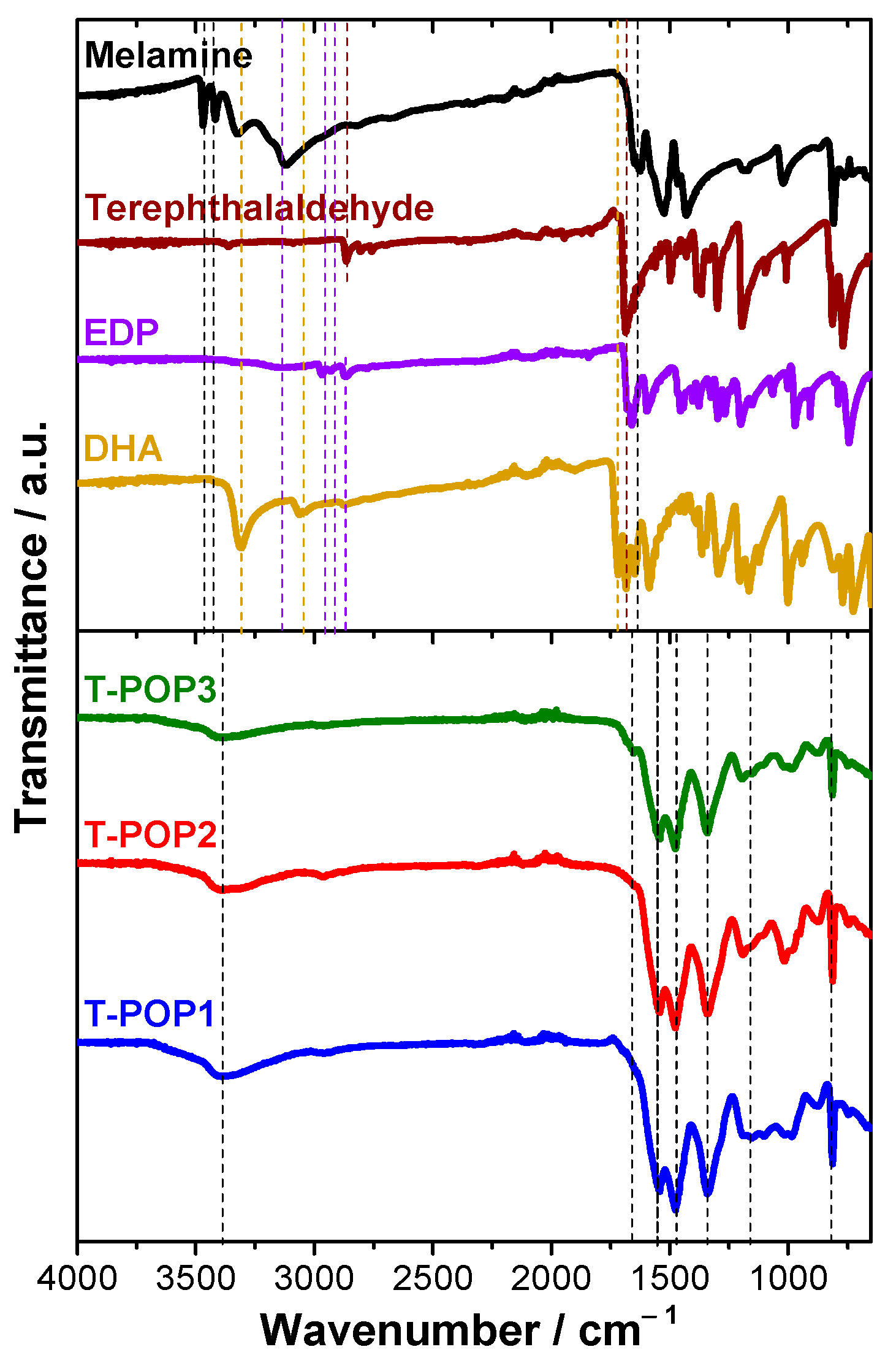
IRT-POP1 (cm−1): 669, 747, 813, 875, 986, 1016, 1103, 1153, 1189, 1342, 1475, 1542, 2960, 3391.
IRT-POP2 (cm−1): 669, 745, 813, 875, 952, 988, 1016, 1113, 1153, 1192, 1342, 1475, 1542, 2960, 3384.
IRT-POP3 (cm−1): 672, 750, 813, 876, 988, 1014, 1107, 1156, 1196, 1342, 1475, 1542, 1653, 2960, 3393.
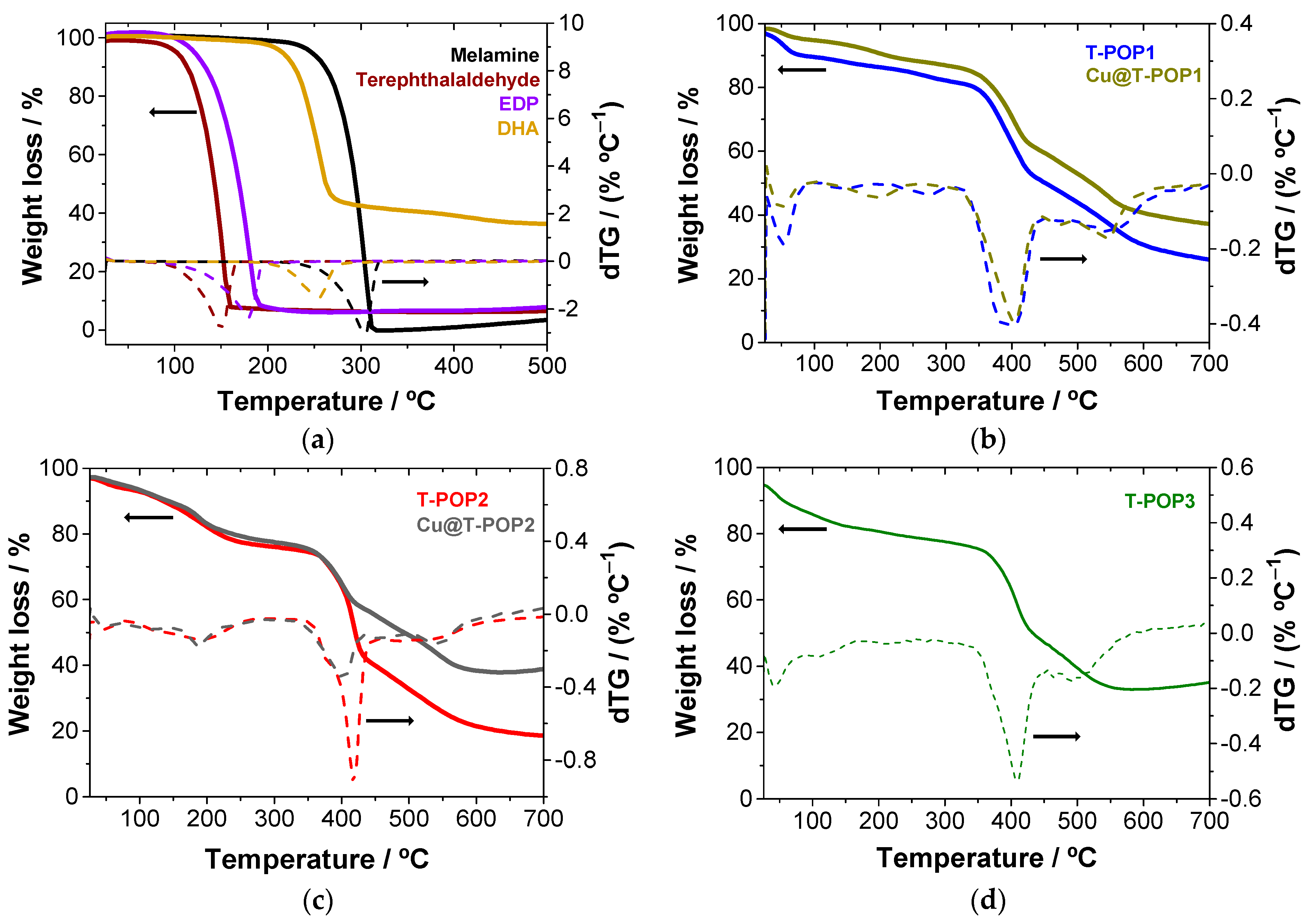
For catalytic purposes, T-POP1 or T-POP2 (200 mg) were metalated using a solution of Cu(OAc)2∙H2O (159.7 mg; 0.80 mmol) in 60 mL of methanol, and then the suspension was heated at 60 °C under magnetic stirring for 24 h. After reaction, the metalated polymer (Cu@T-POP) was filtered under reduced pressure, washed with methanol and acetone, and dried in an oven at 40 °C. This strategy allowed a retention of Cu(II) equal to (217 ± 10) and (178 ± 10) mg g−1 for T-POP1 and T-POP2, respectively, corresponding to (682 ± 30) and (559 ± 32) mg g−1 in Cu(OAc)2∙H2O and adsorption efficiencies of (85 ± 4)% and (70 ± 4)%.
2.3. Characterisation of Monomers and POPs
(1) Infrared spectroscopy: Attenuated total reflectance Fourier transform infrared spectra (FTIR-ATR) were recorded at room temperature, in the 4000–650 cm−1 wavenumber range, using an Agilent Technologies Cary 630 FTIR spectrometer.
(2) Thermogravimetric Analysis (TGA): Thermogravimetric analysis was carried out by using a Nietzsch Tarsus TG 209 F3 analyzer, in a 25–700 °C temperature range, at a heating rate of 10 °C min−1 and a nitrogen purge flow rate of 50 mL min−1.
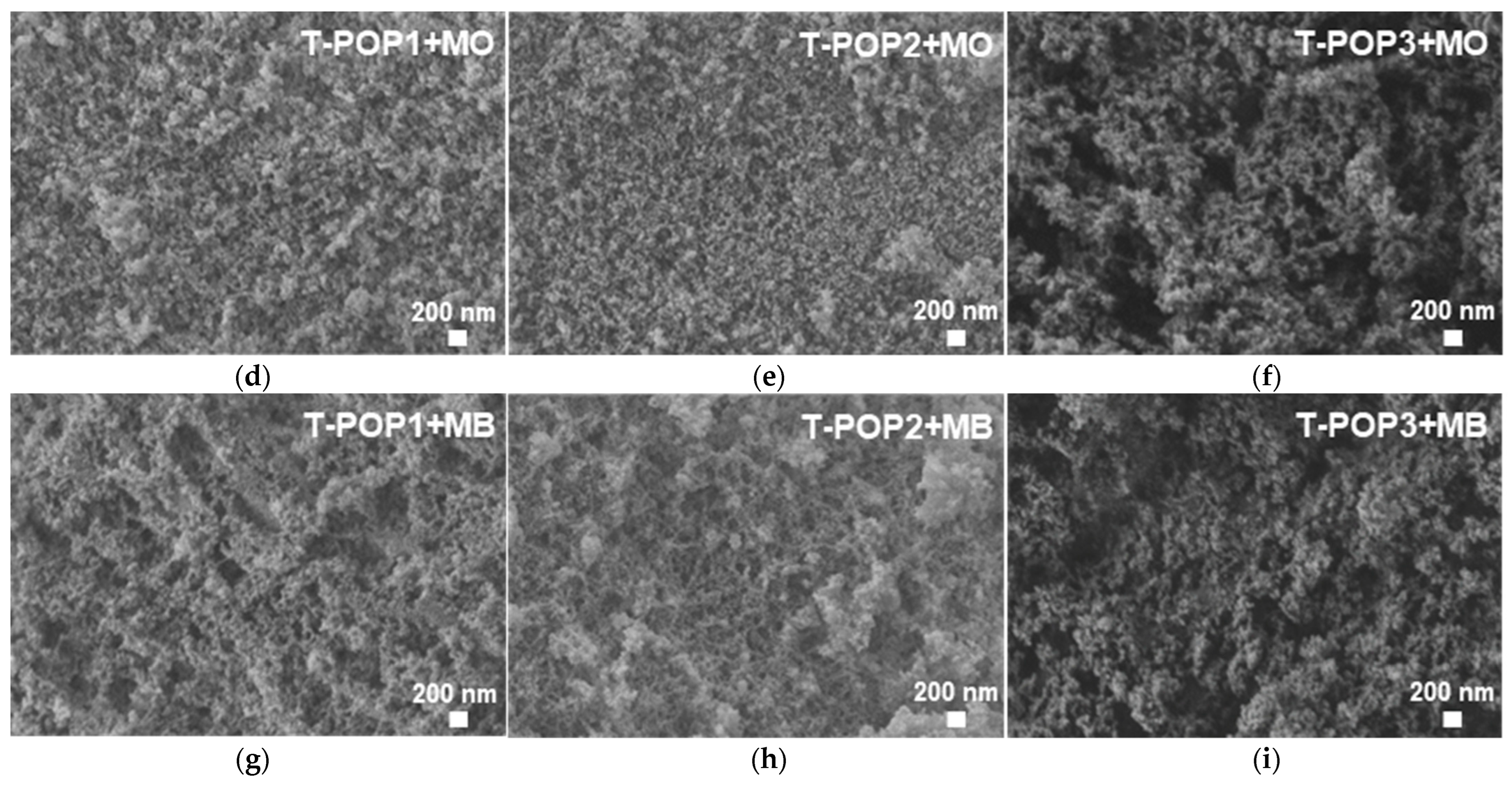
(3) Scanning electron microscopy (SEM): For an evaluation of the surface morphology, SEM micrographs were taken at 1 or 2 kV by using a field emission scanning electron microscope FE-SEM Zeiss Merlin Gemini 2. Before that, samples were frozen in liquid nitrogen, freeze-dried for 24 h on a Labconco Freezone 4.5 device, and coated with a thin gold film.
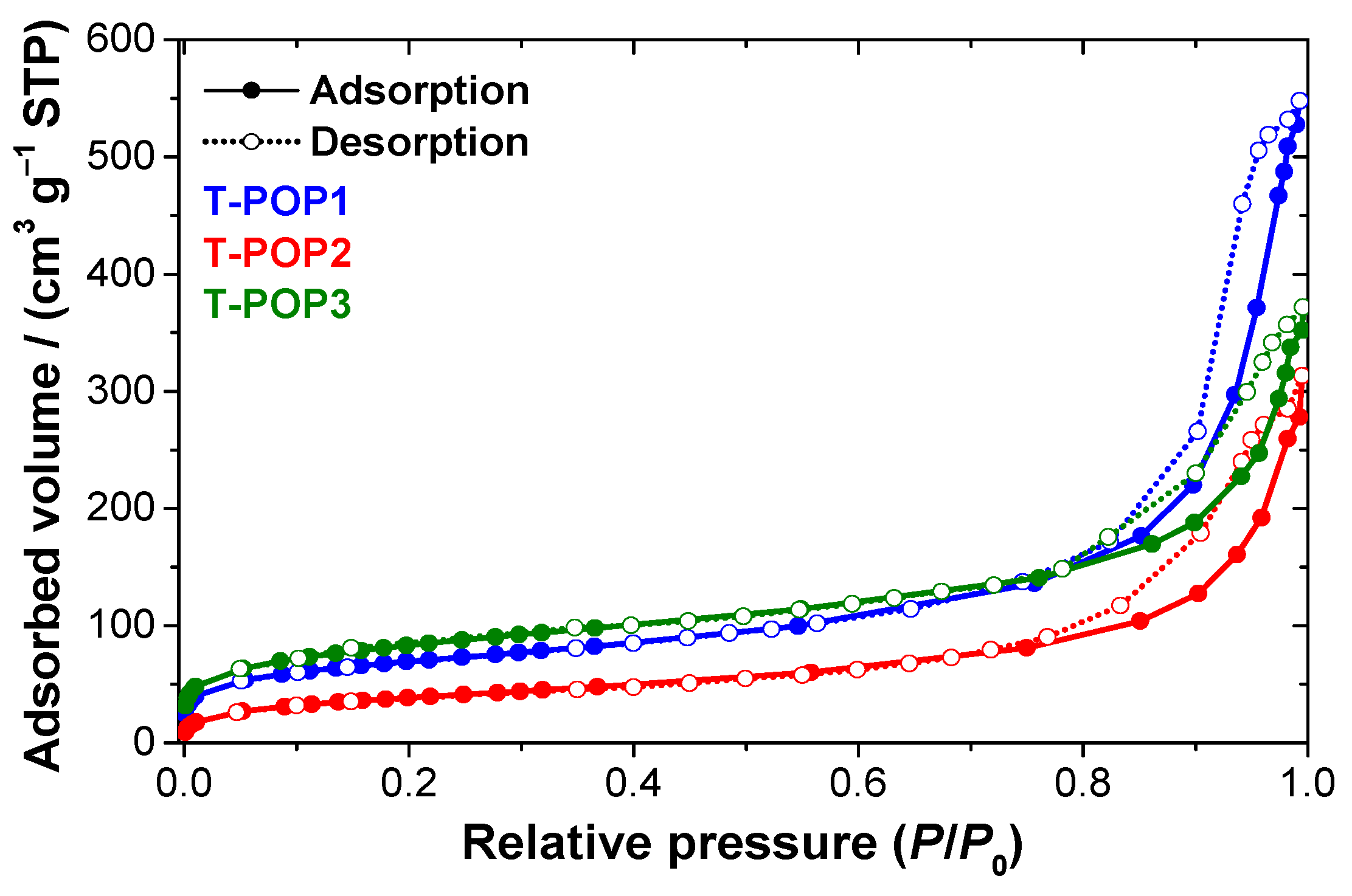
(4) Surface area and porosimetry: The nitrogen adsorption–desorption isotherms of polymers were performed in a Micromeritics ASAP 2000 apparatus to determine the specific surface area (SBET), the specific volume of pores (Vp) and the average size of each pore (dp). The pore size was computed by using the Brunauer–Emmett–Teller (BET) model through the following equation: ().
(5) Dynamic and electrophoretic light scattering: Dynamic light scattering (DLS) and ζ-potential measurements were performed by using a Malvern Zetasizer NanoZS; the measurements were carried out by using 1 mL of polymer dispersion in milli-Q water at 25 °C.
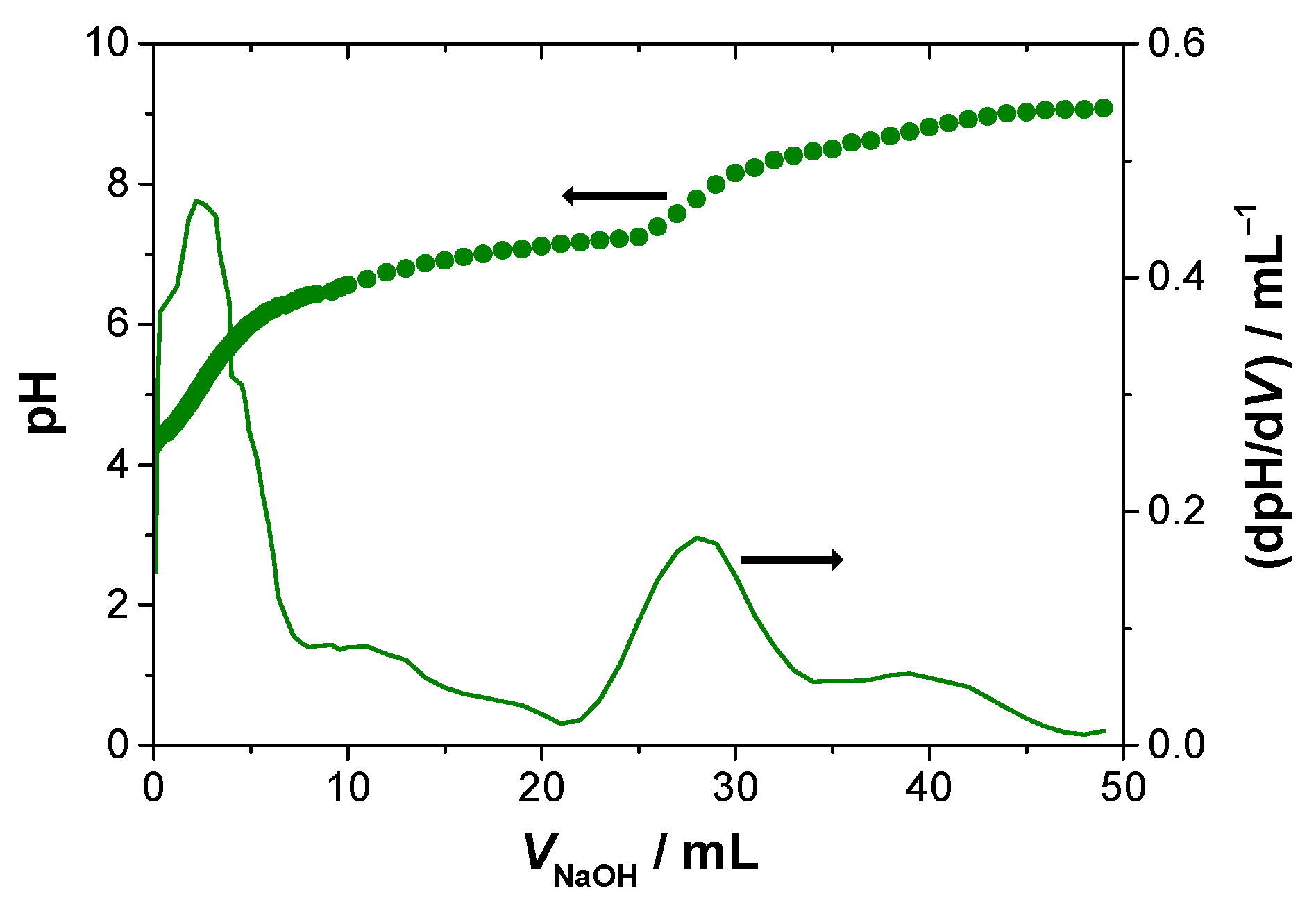
(6) Potentiometry: For the assessment of carboxyl groups content in the T-POP3, potentiometric titration was carried out using the following experimental conditions: 15.2 mg of T-POP3 was suspended in 25 mL of water and titrated with a 0.584 mM NaOH solution, previously standardized with a 5 mM potassium hydrogen phthalate solution. A pH meter from Radiometer Copenhagen MeterLab PHM240 (Radiometer, Copenhagen, Denmark), coupled with an Ingold U457-K7pH conjugated electrode, was used.
(7) Flame atomization atomic absorption spectroscopy (F-AAS): Cu@T-POP1 and Cu@T-POP2 (20.0 mg) were subjected to digestion in 65% nitric acid (5 mL), refluxing the mixture at 90 °C until it becomes translucent (4 h). After appropriate dilution of the resulting solutions, Cu(II) concentration was quantified by using an atomic absorption spectrometer Unicam Solaar 939, equipped with a copper hollow cathode lamp (λ = 325 nm; slit = 37 mm) and an air/acetylene flame (optical path~10 cm).
(8) Nuclear magnetic resonance spectroscopy: Proton nuclear magnetic resonance (1H-NMR) spectra were performed on a Bruker Avance III spectrometer, 400 MHz. CDCl3 or DMSO-d6 were used as deuterated solvents and tetramethylsilane (TMS) as internal standard, with chemical shifts expressed in ppm and coupling constants (J) in Hz.
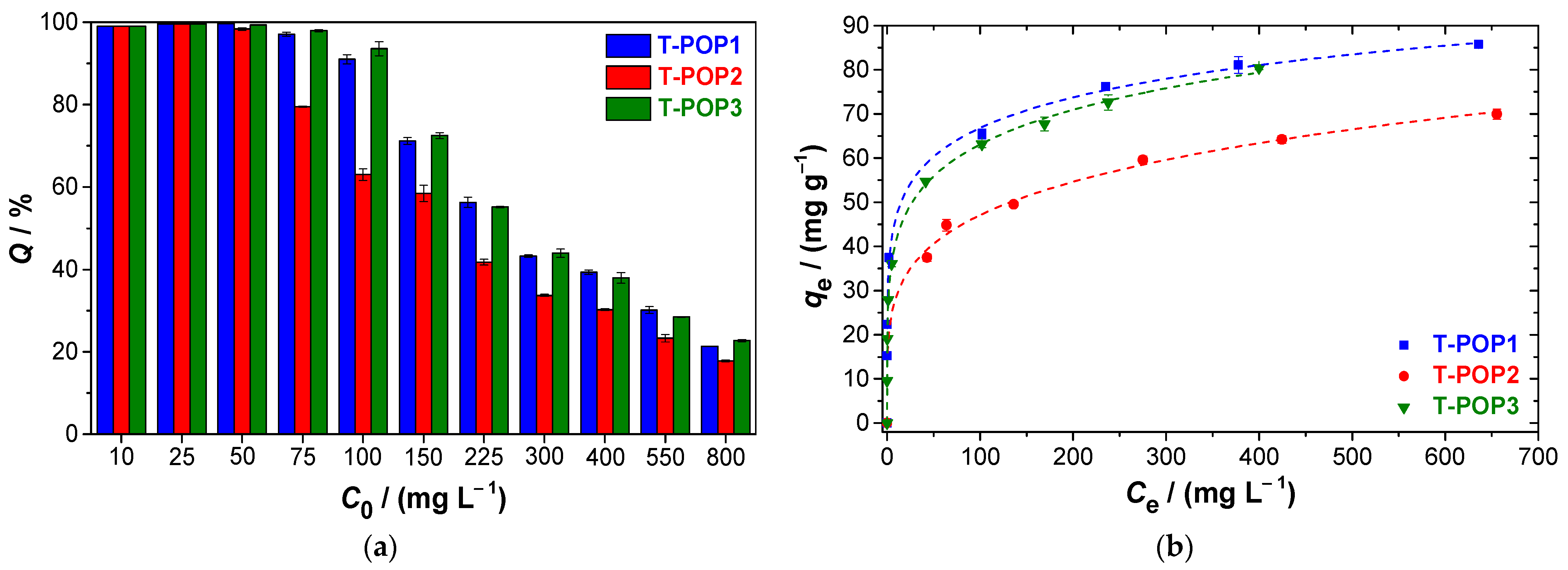
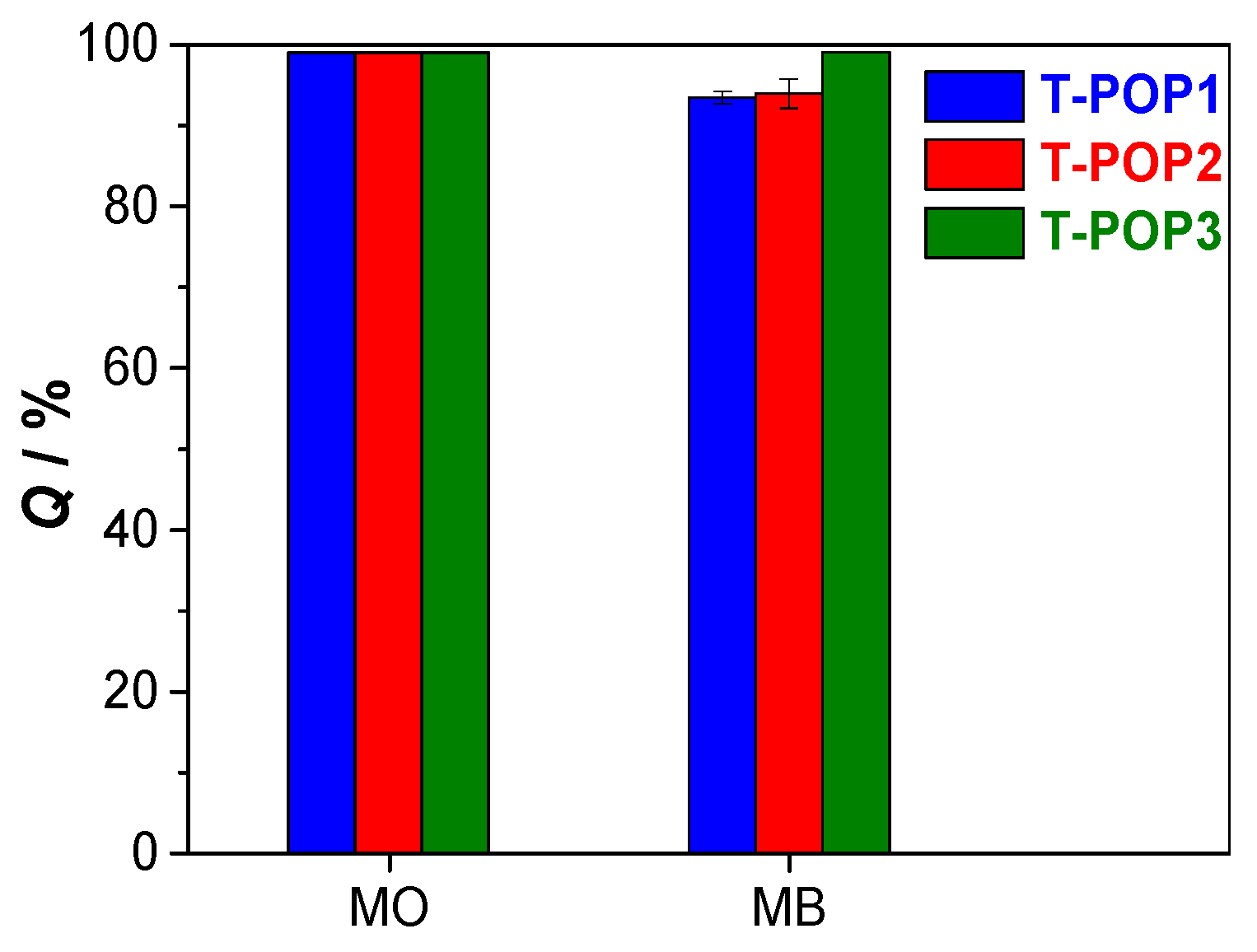
2.4. Adsorption Isotherms and Kinetics
The sorption analyses for polymers were performed in aqueous solution of dye, shaken at 120 rpm and at 25 °C, using a solid–liquid ratio (R
S-L) of 2 mg mL
−1. All experiments were carried out in duplicate, and dye quantification was performed by UV-vis spectroscopy (Shimadzu UV-2600i), using quartz cuvette with a 1 cm optical path, from the maximum absorbance at 463 nm for methyl orange (MO) and 663 nm for methylene blue (MB). The amount of dye adsorbed per unit mass of adsorbent at equilibrium (
, mg g
−1) and the removal efficiency (
Q(%)) was assessed using Equations (1) and (2), where
and
(mg L
−1) are the initial and equilibrium dye concentrations, respectively,
(L) is the volume of solution, and
m (g) is the mass of adsorbent.
Sorption isotherms were obtained in the dye concentration range of 0–800 mg L
−1 and after an equilibrium time equal to 24 h. Different models were investigated to fit the experimental data, namely the Langmuir (Equation (3)), Freundlich (Equation (4)), and Hill (Equation (5)) equations:
where
(mg g
−1) and
(L mg
−1) define, respectively, the maximum adsorption capacity and the equilibrium constant, both given by Langmuir model [
30];
(
) and (
) are the Freundlich constant and the surface heterogeneity factor [
31], and
(mg g
−1),
and
((mg L
−1)
are the maximum adsorption capacity calculated by the Hill isotherm, the Hill cooperativity coefficient, and the Hill constant, respectively [
32,
33].
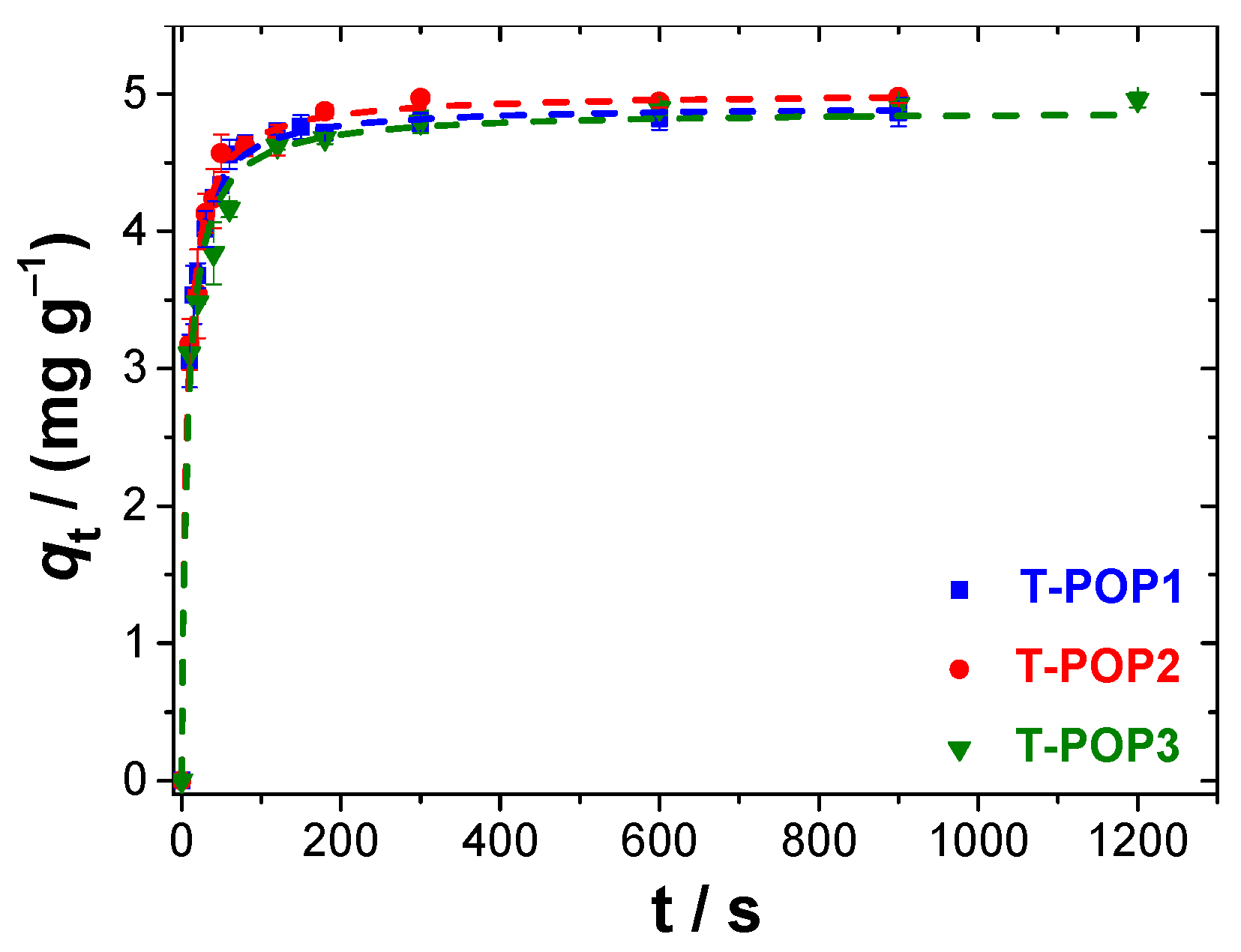
Sorption kinetics were performed using dye aqueous solution of 10 mg L
−1, and the pseudo-first (Equation (6)) and second (Equation (7)) order kinetic equations were used to evaluate the mechanism [
31,
34,
35]:
where
(mg g
−1) is the amount of analyte adsorbed at time
t (min), and
(min
−1) and
(g mg
−1 min
−1) are the pseudo-first-order and pseudo-second-order rate constants, respectively.
The goodness of different fit models was evaluated through the coefficient of determination (R
2) and the Akaike information criterion (AIC), Equation (8) [
36].
where
is the residual sum of squares,
is the number of experimental data points, and
is the number of model parameters.
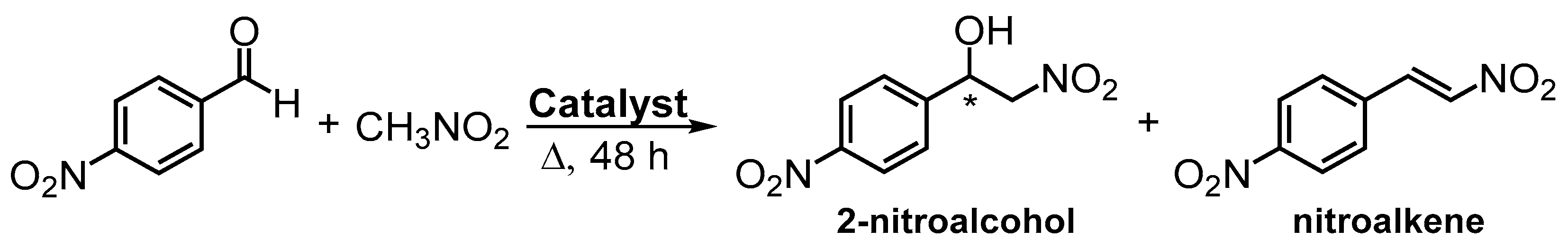
2.5. General Procedure for Henry Reactions
In general, the aldehyde substrate (0.80 mmol), an excess of nitromethane (2 mL), used as solvent and reagent, and the synthesized catalysts (20.0 mg) were added in a glass vial. Then, the reaction mixture was stirred for 48 h at 40 °C or 60 °C. A blank reaction (without catalyst) was made at 60 °C using 4-nitrobenzaldehyde as substrate, and a control reaction was performed using 4-nitrobenzaldehyde, and Cu(OAc)2∙H2O (8.3 mg) as catalyst.
After the reaction, the catalyst (when used) was recovered by filtration, followed by washing with dichloromethane. The solution was evaporated under reduced pressure, and the residue was dissolved in CDCl
3 and analyzed by
1H-NMR spectroscopy to quantify the reaction conversion (Equation (9)) and 2-nitroalcohol selectivity (Equation (10)). The peaks areas corresponding to the CHO resonance (A
CHO), the
1H resonance of the hydrogen linked to the carbon containing the hydroxyl group of the 2-nitroalcohol (A
CH), and the proton with the highest chemical shift of the R=C–H function of the nitroalkene (A
R=C–H), were used to calculate the conversions and selectivities.
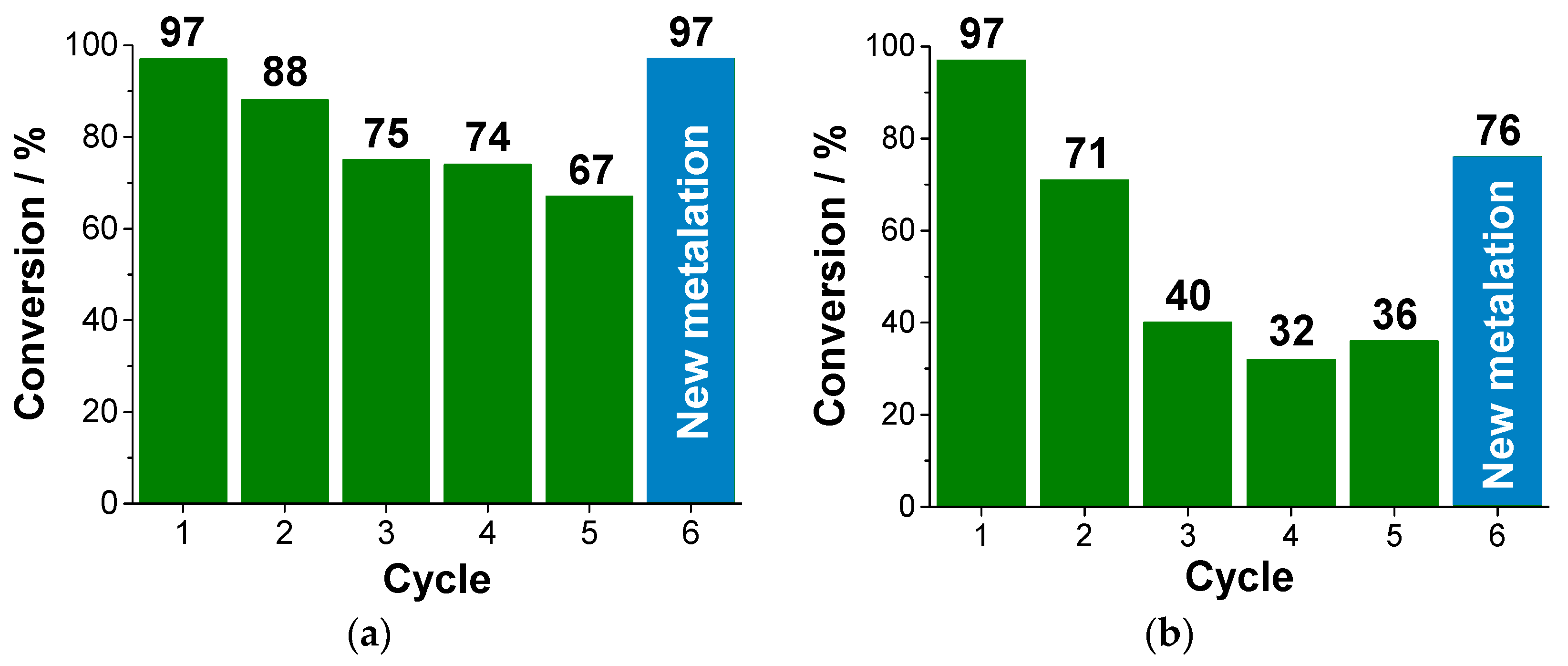
The reuse cycles of heterogeneous catalysts (Cu@T-POP1 and Cu@T-POP2) were carried out under the optimized conditions for the Henry reaction between 4-nitrobenzaldehyde and nitromethane, i.e., conventional heating at 40 °C for 48 h. 4-nitrobenzaldehyde was selected as the aldehyde substrate, given the best results obtained using this compound. For each catalyst, 5–6 reuse cycles were performed. After each reaction, the mixture was subjected to centrifugation for 10 min at 8000 rpm, followed by liquid-phase decantation. The remaining solid (catalyst) was washed three times using dichloromethane and subjected to centrifugation and decantation each time. The remaining catalyst was then dried in an oven at 40 °C, before the addition of a new reactant fraction (4-nitrobenzaldehyde and nitromethane) to carry out a new catalytic cycle. The percentages of conversion and selectivity in each recycling step were determined as above.