Viscosity Factor (VF) Complementary to the Statistical Indicators Associated with the Rheological Behavior of Aqueous Solutions of Polyvinyl Alcohol
Abstract
1. Introduction
1.1. Properties and Applications of PVA
1.2. Viscoelasticity
1.3. Shear and Extensional Flows
1.4. Yielding Effort
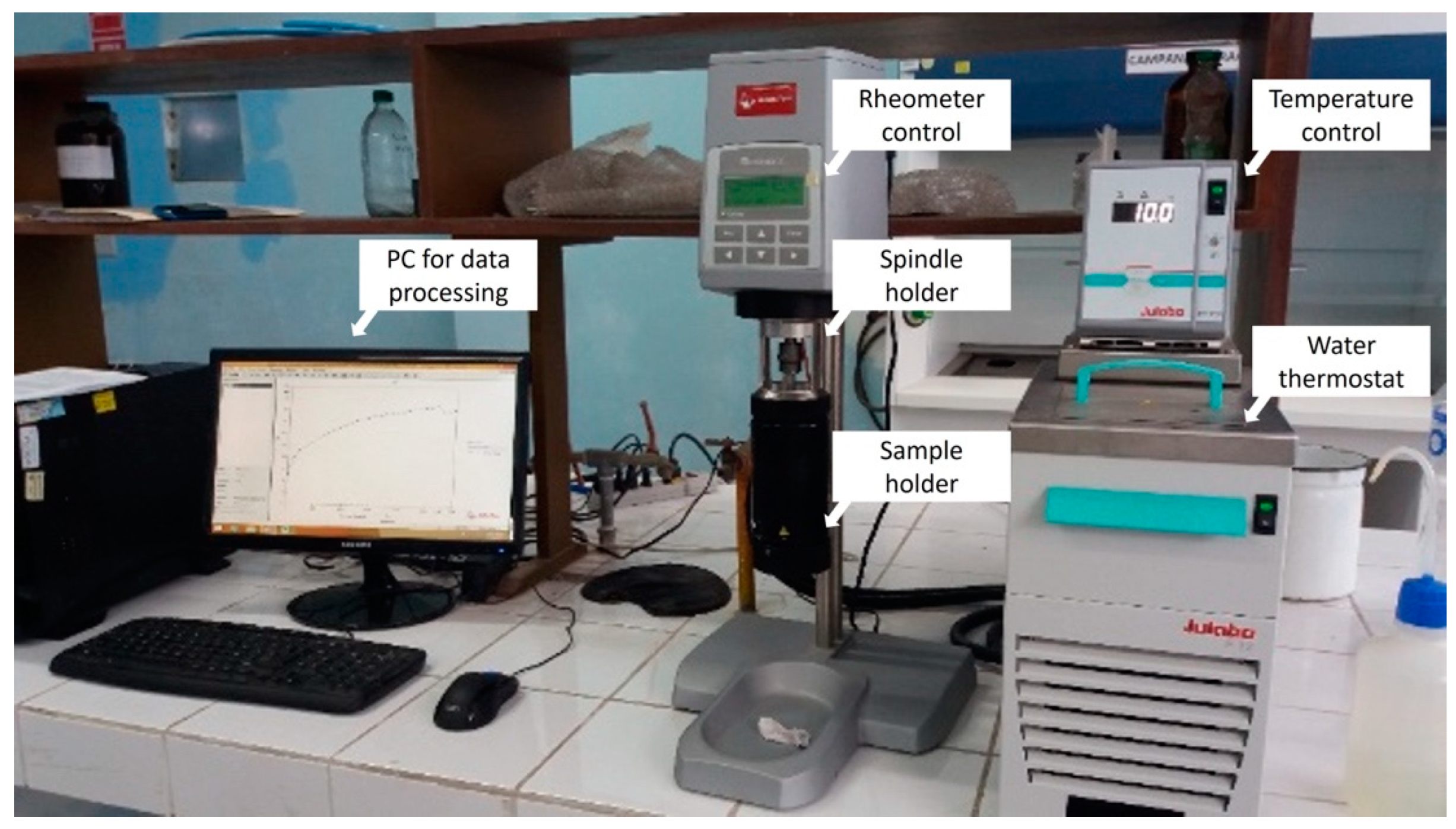
2. Materials and Methods
2.1. Rheological Methods Evaluated
2.1.1. Nonlinear Least Squares Regression
2.1.2. Rheological Models
2.2. Evaluation of the Regression and Sensitivity of the VF Criterion
2.3. Procedure for the Preparation of Experimental Solutions
2.4. Computing Environment: Polymath® v6.2 Software
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Kumar, N.; Mehrotra, T.; Bisaria, K.; Sinha, S. Environmental Hazards and Biodegradation of Plastic Waste: Challenges and Future Prospects. In Bioremediation for Environmental Sustainability: Toxicity, Mechanisms of Contaminants Degradation, Detoxification and Challenges; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–214. ISBN 9780128205242. [Google Scholar]
- Arango, M.C.; Santana, W.; Jaramillo-Quinceno, N.; Rueda-Mira, S.; Álvarez-López, C. Síntesis y Propiedades de Hidrogeles a Base de Sericina y Alcohol Polivinílico Reticulados Térmicamente. In Ciencia Transdisciplinar en la Nueva era; Instituto Antioqueño de Investigación: Medellín, Colombia, 2022; pp. 306–316. [Google Scholar]
- Carhuapoma Bernabé, W.; Santiago Contreras, J. Caracterización de Hidrogeles de Quitosano-Alcohol Polivinílico Obtenidos Por Radiación Gamma. Rev. Iberoam. De Polímeros 2005, 6, 333–346. [Google Scholar]
- Rogers, S.A.; Park, J.D.; Lee, C.W.J. Instantaneous Dimensionless Numbers for Transient Nonlinear Rheology. Rheol. Acta 2019, 58, 539–556. [Google Scholar] [CrossRef]
- Reiner, M. The Deborah Number. Phys. Today 1964, 17, 62. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Li, W.H.; Gong, X.L. The Rheology of Shear Thickening Fluid (STF) and the Dynamic Performance of an STF-Filled. Smart Mater. Struct. 2008, 17, 035027. [Google Scholar] [CrossRef]
- Ramírez Navas, J.S. Fundamentos de Reología de Alimentos; JSR e-books: Cali, Colombia, 2006. [Google Scholar]
- Zargaraan, A.; Omaraee, Y.; Rastmanesh, R.; Taheri, N.; Fadavi, G.; Fadaei, M.; Mohammadifar, M.A. Rheological Characterization and Cluster Classification of Iranian Commercial Foods, Drinks and Desserts to Recommend for Esophageal Dysphagia Diets. Iran J. Public Health 2013, 42, 1446. [Google Scholar]
- Pérez-Trejo, L.; Méndez Sánchez, A.F.; Paniagua Mercado, A.M. Determinación de La Viscosidad de Fluidos Newtonianos y No Newtonianos (Una Revisión Del Viscosímetro de Couette). Lat.-Am. J. Phys. Educ. 2010, 4, 36. [Google Scholar]
- Laurencio Alfonso, H.; Gilbert Hernández, A.; Retirado Mediaceja, Y.; Laurencio Alfonso, H.; Gilbert Hernández, A.; Retirado Mediaceja, Y. Modelado de La Viscosidad Aparente de Un Petróleo Crudo de 11°API Con Comportamiento No Newtoniano. Ingeniare Rev. Chil. De Ing. 2017, 25, 674–680. [Google Scholar] [CrossRef]
- Carrasco Venegas, L.; Castañeda Pérez, L.; Altamirano Oncoy, K. Determination of Rheological Parameters of Shampoo with the Carreu-Yasuda Model. Campus 2015, 20, 21–38. [Google Scholar] [CrossRef]
- Martínez-Rojas, R.; Hernández-Ramírez, G. Caracterización Reológica de Pulpas de Cieno Carbonatado. Min. Y Geol. 2015, 31, 70–83. [Google Scholar]
- Martínez-Padilla, L.P.; Rivera-Vargas, C. Flow Behavior of Mexican Sauces Using a Vane-in-a-Large Cup Rheometer. J. Food Eng. 2006, 72, 189–196. [Google Scholar] [CrossRef]
- Dahdouh, L.; Delalonde, M.; Ricci, J.; Ruiz, E.; Wisnewski, C. Influence of High Shear Rate on Particles Size, Rheological Behavior and Fouling Propensity of Fruit Juices during Crossflow Microfiltration: Case of Orange Juice. Innov. Food Sci. Emerg. Technol. 2018, 48, 304–312. [Google Scholar] [CrossRef]
- Yang, R.; Xu, A.; Chen, Y.; Sun, N.; Zhang, J.; Jia, R.; Huang, T.; Yang, W. Effect of Laver Powder on Textual, Rheological Properties and Water Distribution of Squid (Dosidicus gigas) Surimi Gel. J. Texture Stud. 2020, 51, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos da Silva Pereira, G.; Vasconcelos da Silva Pereira, G.; Paixão Xavier Neves, E.M.; Caldas de Oliveira, L.; Da Silva Pena, R.; Calado, V.; Henriques Lourenço, L. de F. Biodegradable Films from Fishing Industry Waste: Technological Properties and Hygroscopic Behavior. J. Compos. Mater. 2021, 55, 4169–4181. [Google Scholar] [CrossRef]
- Takeda, S.; Ohashi, M.; Kuwano, O.; Kameda, M.; Ichihara, M. Rheological Tests of Polyurethane Foam Undergoing Vesiculation-Deformation-Solidification as a Magma Analogue. J. Volcanol. Geotherm. Res. 2020, 393, 106771. [Google Scholar] [CrossRef]
- Vaish, A.; Yang Lee, S.; Valdivia y Alvarado, P. Viscosity Control of Pseudo-Plastic Polymer Mixtures for Applications in Additive Manufacturing. In Proceedings of the Solid Freeform Fabrication 2018: Proceedings of the 29th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 13–15 August 2018. [Google Scholar]
- Delmar Salomón, C.; Huachum Zhai, C. Determinación de La Energía de Activación de Flujo En Ligantes Asfálticos. In Proceedings of the 12th Ibero-Latin American Asphalt Congress, Antigua Guatemala, Guatemala, 10–13 November 2003. [Google Scholar]
- Carrasco Venegas, L. Modelamiento de Los Fenómenos de Transporte, 1st ed.; Sánchez Solís, A., Palomino Infante, A., Eds.; Macro EIRL: Lima, Peru, 2018. [Google Scholar]
- Raharja, D.C.; Prasad, D.; Mittelbach, C. A Scientific Approach for Generating a Rheology Model to Simulate Polymer Injectivity. In Proceedings of the IOR 2019—20th European Symposium on Improved Oil Recovery; European Association of Geoscientists and Engineers, EAGE. Pau, France, 8–11 April 2019; Volume 2019, pp. 1–12. [Google Scholar]
- Wang, X.; Zhou, D.; Zhu, G.; Guo, C. Rheological Properties of Two High Polymers Suspended in an Abrasive Slurry Jet. E-Polymers 2021, 21, 186–193. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales: Aberystwyth, UK, 2000; ISBN 0953803201. [Google Scholar]
- Ferguson, J.; Kembłowski, Z. Applied Fluid Rheology; Elsevier Applied Science: Amsterdam, The Netherlands, 1991; ISBN 9781851665884. [Google Scholar]
- Hurst, G.A.; Bella, M.; Salzmann, C.G. The Rheological Properties of Poly(Vinyl Alcohol) Gels from Rotational Viscometry. J. Chem. Educ. 2015, 92, 940–945. [Google Scholar] [CrossRef]
- Fischer, P.; Windhab, E.J. Rheology of Food Materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 36–40. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Meiselman, H.J.; Baskurt, O.K. Blood Rheology and Aging. J. Geriatr. Cardiol. 2013, 10, 291. [Google Scholar] [CrossRef]
- Chen, D.T.N.; Wen, Q.; Janmey, P.A.; Crocker, J.C.; Yodh, A.G. Rheology of Soft Materials. Annu. Rev. Condens. Matter Phys. 2010, 1, 301–322. [Google Scholar] [CrossRef]
- Urakov, A.; Urakova, N. Rheology and Physical-Chemical Characteristics of the Solutions of the Medicines. J. Phys. Conf. Ser. 2015, 602, 012043. [Google Scholar] [CrossRef]
- Hassan, C.M.; Trakampan, P.; Peppas, N.A. Water Solubility Characteristics of Poly(Vinyl Alcohol) and Gels Prepared by Freezing/Thawing Processes. In Water Soluble Polymers; Springer: Boston, MA, USA, 2002; pp. 31–40. [Google Scholar] [CrossRef]
- Parisi, D.; Ditillo, C.D.; Han, A.; Lindberg, S.; Hamersky, M.W.; Colby, R.H. Rheological Investigation on the Associative Properties of Poly(Vinyl Alcohol) Solutions. J. Rheol. 2022, 66, 1141. [Google Scholar] [CrossRef]
- Babayevskii, P.G.; Kozlov, N.A.; Trostyanskaya, Y.B. Effect of Water on Physical Transformations and Viscoelastic Properties of Sparse-Network Polyvinyl Alcohol. Polym. Sci. U.S.S.R. 1986, 28, 475–482. [Google Scholar] [CrossRef]
- Waheed, S.; Butcher, A.L.; Oyen, M.L. The Viscoelastic Response of Electrospun Poly(Vinyl Alcohol) Mats. J. Mech. Behav. Biomed. Mater. 2018, 77, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Wu, D. Mapping Hierarchical Networks of Poly(Vinyl Alcohol)/Cellulose Nanofiber Composite Hydrogels via Viscoelastic Probes. Carbohydr. Polym. 2022, 288, 119372. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Kobayashi, Y.; Ito, H. Structural, Rheological, and Mechanical Properties of Polyvinyl Alcohol Composites Reinforced with Cellulose Nanofiber Treated by Ultrahigh-Pressure Homogenizer. Mater. Today Commun. 2022, 33, 104316. [Google Scholar] [CrossRef]
- Abraham, B.M.; Miyano, K.; Ketterson, J.B. Static Shear Modulus of a Methyl Cellulose Solution and Viscoelasticity of a Polyvinyl Alcohol Solution at the Air/Water Interface. J. Colloid Interface Sci. 1985, 107, 264–266. [Google Scholar] [CrossRef]
- Bian, H.; Jiao, L.; Wang, R.; Wang, X.; Zhu, W.; Dai, H. Lignin Nanoparticles as Nano-Spacers for Tuning the Viscoelasticity of Cellulose Nanofibril Reinforced Polyvinyl Alcohol-Borax Hydrogel. Eur. Polym. J. 2018, 107, 267–274. [Google Scholar] [CrossRef]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Viscoelastic Properties of Poly (Vinyl Alcohol) Hydrogels with Cellulose Nanocrystals Fabricated through Sodium Chloride Addition: Rheological Evidence of Double Network Formation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125577. [Google Scholar] [CrossRef]
- Osswald, T.; Rudolph, N. Polymer Rheology, Fundamentals and Applications; Carl Hanser Verlag GmbH & Co., KG: München, Germany, 2015; ISBN 978-1-56990-517-3. [Google Scholar]
- Reiner, M. Deformation, Strain and Flow: An Elementary Introduction to Rheology; H.K. Lewis: London, UK, 1960; ISBN 0718601629. [Google Scholar]
- Bustamante Rúa, M.O.; Aguilera, G. An Approach to the Modeling Yield Stress in Mineral Suspensions. Dyna 2005, 72, 13–26. [Google Scholar]
- Maestro Garriga, A. Reología de Espesantes Celulósicos Para Pinturas al Agua: Modelización y Mecanismo de Espesamiento Asociativo. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2002. [Google Scholar]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-Forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Münstedt, H. Viscoelasticity of Polystyrene Melts in Tensile Creep Experiments. Rheol. Acta 1975, 14, 1077–1088. [Google Scholar] [CrossRef]
- Powell, P.C.; Housz, A.J.I. Engineering with Polymers; CRC Press: Cheltenham, UK, 1998. [Google Scholar]
- Frankel, N.A.; Acrivos, A. On the Viscosity of a Concentrated Suspension of Solid Spheres. Chem. Eng. Sci. 1967, 22, 847–853. [Google Scholar] [CrossRef]
- Wildemuth, C.R.; Williams, M.C. A New Interpretation of Viscosity and Yield Stress in Dense Slurries: Coal and Other Irregular Particles. Rheol. Acta 1985, 24, 75–91. [Google Scholar] [CrossRef]
- Frith, W.J.; Mewis, J.; Strivens, T.A. Rheology of Concentrated Suspensions: Experimental Investigations. Powder Technol. 1987, 51, 27–34. [Google Scholar] [CrossRef]
- Johnson, S.B.; Franks, G.V.; Scales, P.J.; Boger, D.V.; Healy, T.W. Surface Chemistry–Rheology Relationships in Concentrated Mineral Suspensions. Int. J. Miner. Process. 2000, 58, 267–304. [Google Scholar] [CrossRef]
- James, D.F. A Review on Extensional Viscosity. In The Influence of Polymer Additives on Velocity and Temperature Fields; Symposium Universität-GH-Essen: Essen, Germany, 1984; pp. 25–28. [Google Scholar]



| Model | Ferrys | Robertson-Stiff | Ellis de Haven | Williamson | Sisko |
|---|---|---|---|---|---|
| Equation | |||||
| Apparent viscosity | |||||
| Differential viscosity |
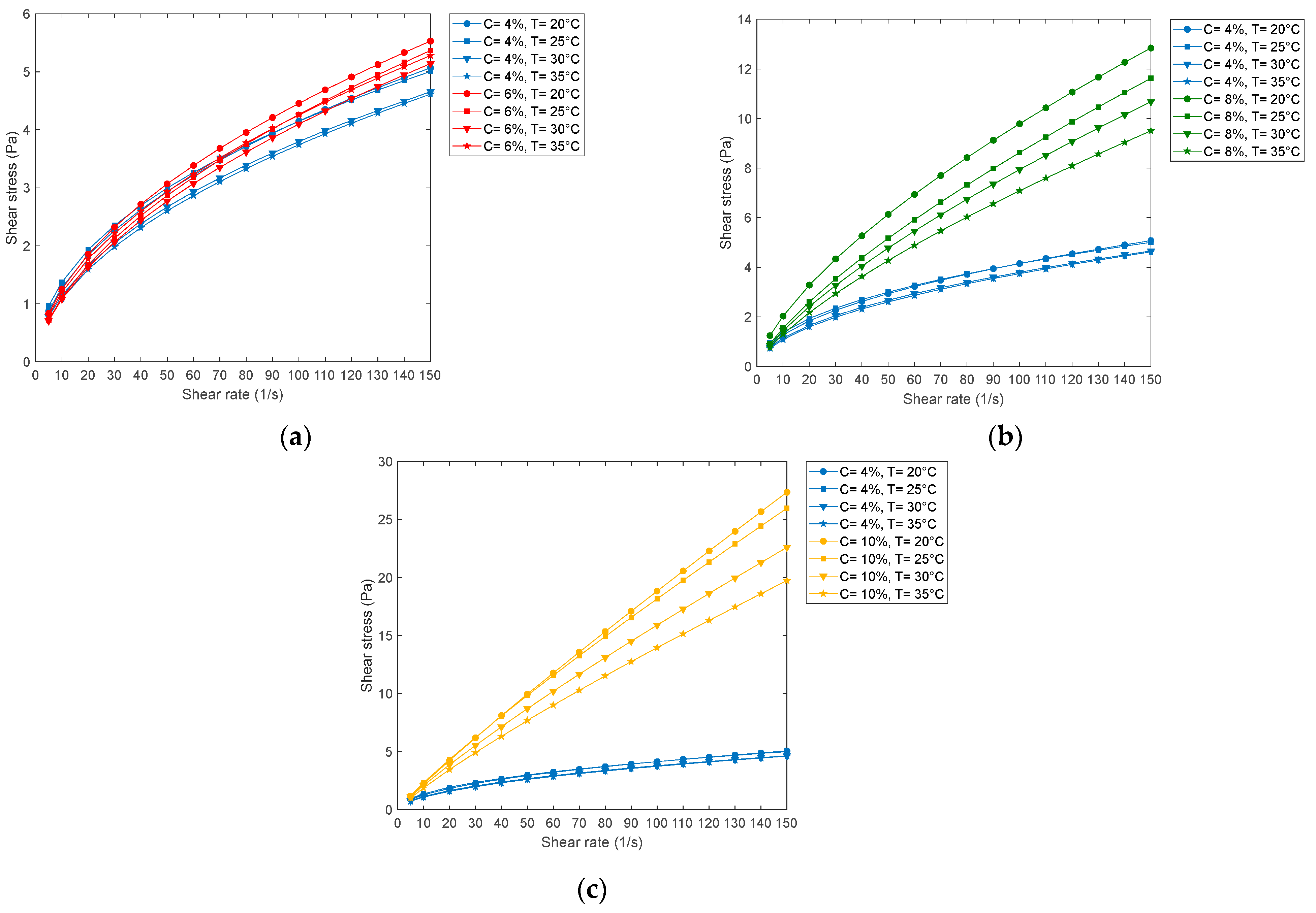
| T 20 °C | T 25 °C | T 30 °C | T 35 °C | |||||
|---|---|---|---|---|---|---|---|---|
| (1/s) | (Pa) | (1/s) | (Pa) | (1/s) | (Pa) | (1/s) | (Pa) | |
| 4% of PVA in water | 4.63 | 1 | 4.47 | 1 | 6.61 | 1 | 6.91 | 1 |
| 12.8 | 1.5 | 10.6 | 1.5 | 15.7 | 1.5 | 17.4 | 1.5 | |
| 23.3 | 1.99 | 21.4 | 1.99 | 28.8 | 1.99 | 30.8 | 1.99 | |
| 36.7 | 2.49 | 34.5 | 2.49 | 44.5 | 2.49 | 46.7 | 2.49 | |
| 52.6 | 2.99 | 50.5 | 2.99 | 62.8 | 2.99 | 65.5 | 2.99 | |
| 70.8 | 3.49 | 69.2 | 3.49 | 84.1 | 3.49 | 86.9 | 3.49 | |
| 91.4 | 3.98 | 91.2 | 3.98 | - | - | - | - | |
| 6% of PVA in water | 3.23 | 1 | 6.41 | 1 | 5.32 | 1 | 5.99 | 1 |
| 12.5 | 1.5 | 15.6 | 1.5 | 15.7 | 1.5 | 14.6 | 1.5 | |
| 22.5 | 1.99 | 27.1 | 1.99 | 28.4 | 1.99 | 25.1 | 1.99 | |
| 34.9 | 2.49 | 40.2 | 2.49 | 43 | 2.49 | 37.4 | 2.49 | |
| 48.8 | 2.99 | 54.5 | 2.99 | 58.8 | 2.99 | 52.2 | 2.99 | |
| 64.5 | 3.49 | 70.7 | 3.49 | 75.7 | 3.49 | 69 | 3.49 | |
| 81.3 | 3.98 | 87.8 | 3.98 | 93.5 | 3.98 | 88.1 | 3.98 | |
| 99.9 | 4.48 | - | - | - | - | - | - | |
| 8% of PVA in water | 2.78 | 1 | 3.88 | 1 | 3.75 | 1 | 3.48 | 1 |
| 6.45 | 1.745 | 6.49 | 1.5 | 7.51 | 1.5 | 9.45 | 1.5 | |
| 12.1 | 2.49 | 12.4 | 1.99 | 14.1 | 1.99 | 16.4 | 1.99 | |
| 16.7 | 2.99 | 17.8 | 2.49 | 20.2 | 2.49 | 23.8 | 2.49 | |
| 21.5 | 3.49 | 23.6 | 2.99 | 26.4 | 2.99 | 31.1 | 2.99 | |
| 26.5 | 3.98 | 29.5 | 3.49 | 33.1 | 3.49 | 38.6 | 3.49 | |
| 31.8 | 4.48 | 35.6 | 3.98 | 40.3 | 3.98 | 46.6 | 3.98 | |
| 37.0 | 4.98 | 41.9 | 4.48 | 47.1 | 4.48 | 54.7 | 4.48 | |
| 42.6 | 5.48 | 48.8 | 4.98 | 53.9 | 4.98 | 62.9 | 4.98 | |
| 48.3 | 5.97 | 55.6 | 5.48 | 61.2 | 5.48 | 71.0 | 5.48 | |
| 54.1 | 6.47 | 61.9 | 5.97 | 68.5 | 5.97 | 79.5 | 5.97 | |
| 60.3 | 6.97 | 68.4 | 6.47 | 76.0 | 6.47 | 87.7 | 6.47 | |
| 66.9 | 7.47 | 75.4 | 6.97 | 83.6 | 6.97 | 96.0 | 6.97 | |
| 73.4 | 7.96 | 82.1 | 7.47 | 91.4 | 7.47 | - | - | |
| 80.1 | 8.46 | 88.8 | 7.96 | 99.1 | 7.96 | - | - | |
| 86.9 | 8.96 | 95.9 | 8.46 | - | - | - | - | |
| 93.7 | 9.46 | - | - | - | - | - | - | |
| 10% of PVA in water | 2.7 | 1 | 1.94 | 1 | 2.07 | 1 | 4.5 | 1 |
| 7.1 | 1.745 | 4.78 | 1.5 | 5.43 | 1.5 | 7.01 | 1.5 | |
| 10.9 | 2.49 | 8.2 | 1.99 | 9.13 | 1.99 | 10.4 | 1.99 | |
| 13.3 | 2.99 | 10.4 | 2.49 | 12 | 2.49 | 13.0 | 2.49 | |
| 15.7 | 3.49 | 12.3 | 2.99 | 14.5 | 2.99 | 16.7 | 2.99 | |
| 18.1 | 3.98 | 15.1 | 3.49 | 17.2 | 3.49 | 19.9 | 3.49 | |
| 20.9 | 4.48 | 18.1 | 3.98 | 20.2 | 3.98 | 23.3 | 3.98 | |
| 23.7 | 4.98 | 20.7 | 4.48 | 23.4 | 4.48 | 26.9 | 4.48 | |
| 26.4 | 5.48 | 23.4 | 4.98 | 26.2 | 4.98 | 30.7 | 4.98 | |
| 29 | 5.97 | 26.0 | 5.48 | 29.3 | 5.48 | 34.3 | 5.48 | |
| 31.7 | 6.47 | 28.6 | 5.97 | 32.6 | 5.97 | 37.3 | 5.97 | |
| 34.4 | 6.97 | 31.2 | 6.47 | 35.9 | 6.47 | 40.8 | 6.47 | |
| 37.1 | 7.47 | 34.0 | 6.97 | 39.2 | 6.97 | 44.9 | 6.97 | |
| 39.7 | 7.96 | 36.8 | 7.47 | 42.3 | 7.47 | 48.6 | 7.47 | |
| 42.3 | 8.46 | 39.5 | 7.96 | 45.7 | 7.96 | 52.7 | 7.96 | |
| 45.0 | 8.96 | 42.7 | 8.46 | 49 | 8.46 | 55.8 | 8.46 | |
| 47.7 | 9.46 | 45.5 | 8.96 | 52.4 | 8.96 | 59.8 | 8.96 | |
| 50.1 | 9.95 | 48.1 | 9.46 | 55.6 | 9.46 | 64.3 | 9.46 | |
| 52.8 | 10.5 | 51.1 | 9.95 | 58.9 | 9.95 | 67.7 | 9.95 | |
| 55.5 | 10.9 | 53.7 | 10.5 | 62.2 | 10.5 | 71.5 | 10.5 | |
| 58.3 | 11.4 | 56.9 | 10.9 | 65.4 | 10.9 | 76.1 | 10.9 | |
| 60.9 | 11.9 | 59.5 | 11.4 | 68.7 | 11.4 | 79.4 | 11.4 | |
| 63.7 | 12.4 | 62.5 | 11.9 | 72.1 | 11.9 | 83.3 | 11.9 | |
| 66.4 | 12.9 | 65.6 | 12.4 | 75.5 | 12.4 | 87.6 | 12.4 | |
| 69.1 | 13.4 | 68.4 | 12.9 | 78.9 | 12.9 | 90.8 | 12.9 | |
| 71.8 | 13.9 | 71.2 | 13.4 | 82.4 | 13.4 | 95.2 | 13.4 | |
| 74.8 | 14.4 | 74.4 | 13.9 | 85.8 | 13.9 | 98.9 | 13.9 | |
| 77.5 | 14.9 | 77.1 | 14.4 | 89.2 | 14.4 | - | - | |
| 80.4 | 15.4 | 79.9 | 14.9 | 92.5 | 14.9 | - | - | |
| 83.3 | 15.9 | 82.9 | 15.4 | 95.8 | 15.4 | - | - | |
| 86.0 | 16.4 | 85.7 | 15.9 | 99.3 | 15.9 | - | - | |
| 88.9 | 16.9 | 88.8 | 16.4 | - | - | - | - | |
| 91.7 | 17.4 | 91.9 | 16.9 | - | - | - | - | |
| 94.5 | 17.9 | 94.9 | 17.4 | - | - | - | - | |
| 97.3 | 18.4 | 98 | 17.9 | - | - | - | - | |
| 4% of PVA in Water | 6% of PVA in Water | 8% of PVA in Water | 10% of PVA in Water | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T 20 °C | T 25 °C | T 30 °C | T 35 °C | T 20 °C | T 25 °C | T 30 °C | T 35 °C | T 20 °C | T 25 °C | T 30 °C | T 35 °C | T 20 °C | T 25 °C | T 30 °C | T 35 °C | ||
| Ferrys parameters | A | 1.664 | 1.948 | 1.254 | 1.318 | 0.460 | 0.371 | 0.255 | 0.410 | 0.242 | 0.159 | 0.148 | 0.135 | 0.247 | 0.240 | 0.205 | 0.180 |
| B | 0.107 | 0.093 | 0.119 | 0.108 | 0.475 | 0.536 | 0.776 | 0.491 | 6.560 | 9.963 | 9.042 | 7.636 | 51.500 | 51.500 | 51.500 | 46.505 | |
| 0.999 | 0.998 | 0.999 | 0.999 | 0.997 | 0.996 | 0.996 | 0.999 | 0.998 | 0.995 | 0.996 | 0.994 | 0.996 | 0.998 | 0.999 | 0.999 | ||
| 0.711 | 3.081 | 0.668 | 1.674 | 3.486 | 3.862 | 5.631 | 0.597 | 1.834 | 4.443 | 4.582 | 6.035 | 3.493 | 1.702 | 1.207 | 0.738 | ||
| Robertson-Stiff parameters | A | 0.3457 | 0.441 | 0.309 | 0.260 | 0.248 | 0.217 | 0.175 | 0.3005 | 0.384 | 0.186 | 0.168 | 0.128 | 0.228 | 0.246 | 0.224 | 0.241 |
| B | 2.5550 | 1.103 | 2.150 | 3.402 | 6.036 | 4.389 | 7.989 | 2.0124 | 1.621 | 4.940 | 5.565 | 7.674 | 1.535 | 2.025 | 2.339 | 0.795 | |
| n | 0.5380 | 0.485 | 0.543 | 0.576 | 0.620 | 0.642 | 0.676 | 0.5746 | 0.702 | 0.826 | 0.829 | 0.859 | 0.955 | 0.930 | 0.921 | 0.879 | |
| 1.0000 | 0.999 | 0.999 | 1.000 | 0.999 | 1.000 | 1.000 | 0.9999 | 0.999 | 0.999 | 0.999 | 0.999 | 1.000 | 0.999 | 0.999 | 0.999 | ||
| - | 0.0006 | 0.0002 | - | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.001 | 0.002 | 0.001 | 0.0002 | 0.001 | 0.002 | 0.002 | 0.002 | ||
| Williamson parameters | A | 1.687 | 1.897 | 1.590 | 1.473 | 1.445 | 1.412 | 1.273 | 1.684 | 2.562 | 1.485 | 1.371 | 1.136 | 0.873 | 1.275 | 1.266 | 1.930 |
| B | 4.774 | 5.378 | 6.131 | 5.667 | 2.271 | 5.181 | 2.890 | 6.807 | 7.278 | 3.864 | 3.304 | 1.750 | 4.973 | 5.350 | 5.073 | 16.85 | |
| C | 0.027 | 0.024 | 0.024 | 0.025 | 0.032 | 0.031 | 0.030 | 0.028 | 0.076 | 0.074 | 0.068 | 0.061 | 0.181 | 0.171 | 0.149 | 0.124 | |
| 0.998 | 0.999 | 0.999 | 0.999 | 0.997 | 0.999 | 0.999 | 0.998 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| 0.003 | 0.002 | 0.001 | 0.001 | 0.007 | 0.001 | 0.001 | 0.003 | 0.004 | 0.001 | 0.002 | 0.001 | 0.004 | 0.008 | 0.008 | 0.002 | ||
| Sisko parameters | A | 0.012 | 0.007 | 0.010 | 0.013 | 0.023 | 0.020 | 0.023 | 0.010 | 0.044 | 0.064 | 0.059 | 0.057 | 0.174 | 0.161 | 0.138 | 0.087 |
| B | 0.515 | 0.533 | 0.431 | 0.429 | 0.642 | 0.436 | 0.564 | 0.409 | 0.553 | 0.539 | 0.541 | 0.613 | 0.260 | 0.372 | 0.383 | 0.226 | |
| n | 0.384 | 0.408 | 0.412 | 0.387 | 0.274 | 0.369 | 0.258 | 0.451 | 0.500 | 0.321 | 0.297 | 0.203 | 0.390 | 0.390 | 0.379 | 0.683 | |
| 1.000 | 1.000 | 1.000 | 1.000 | 0.999 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.001 | 0.000 | 0.002 | 0.004 | 0.004 | 0.002 | ||
| Ellis de Haven parameters | A | 0.631 | 0.744 | 0.511 | 0.463 | 0.592 | 0.473 | 0.456 | 0.548 | 0.946 | 0.712 | 0.648 | 0.567 | 0.437 | 0.461 | 0.452 | 0.351 |
| B | 3.020 | 3.020 | 3.020 | 3.020 | 3.020 | 3.020 | 3.020 | 3.020 | 2.497 | 3.020 | 3.020 | 3.020 | 0.851 | 0.822 | 0.974 | 0.735 | |
| n | 2.089 | 2.213 | 2.062 | 2.006 | 1.939 | 1.831 | 1.859 | 1.945 | 1.546 | 1.407 | 1.417 | 1.430 | 1.150 | 1.216 | 1.231 | 1.276 | |
| 0.999 | 0.999 | 0.999 | 0.999 | 0.998 | 0.999 | 0.997 | 1.000 | 0.999 | 0.998 | 0.998 | 0.997 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| 1.198 | 0.935 | 1.239 | 1.590 | 3.681 | 1.910 | 5.494 | 0.581 | 0.645 | 1.981 | 2.057 | 3.183 | 0.142 | 0.321 | 0.405 | 0.215 | ||
| Parameter | Source | DF | SS | MS | F | p-Value |
|---|---|---|---|---|---|---|
| ANOVA for | Models | 4 | 0.000067 | 0.000017 | 17.28 | 0.000 |
| Error | 75 | 0.000073 | 0.000001 | |||
| Total | 79 | 0.000139 | ||||
| ANOVA for | Models | 4 | 100.26 | 25.066 | 22.75 | 0.000 |
| Error | 75 | 82.65 | 1.102 | |||
| Total | 79 | 182.91 |
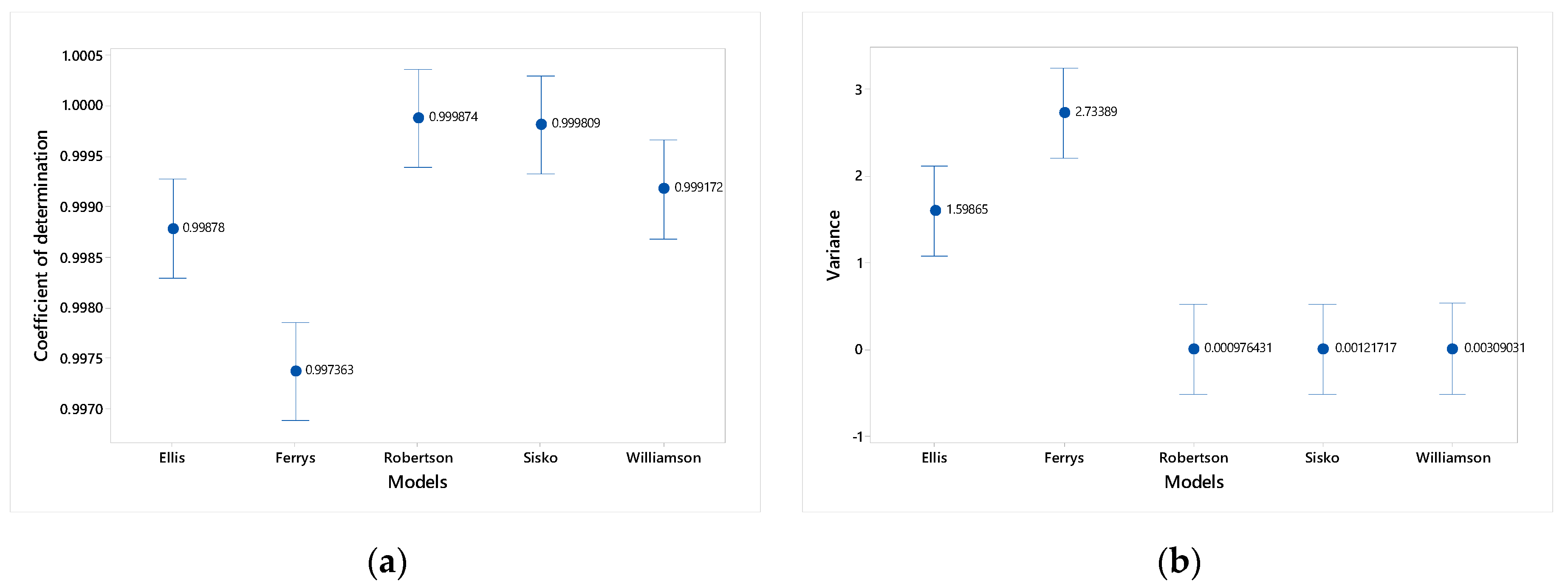
| Model | %CV Minimum | %CV Average | %CV Maximum | Stability | Order |
|---|---|---|---|---|---|
| Ferrys | 1.04 | 6.12 | 10.49 | NO | 2 |
| Ellis | 0.47 | 2.26 | 3.65 | YES | 1 |
| Robertson-Stiff | 3.90 | 15.26 | 31.82 | NO | 5 |
| Sisko | 2.89 | 12.78 | 26.07 | NO | 3 |
| Williamson | 3.25 | 14.95 | 27.57 | NO | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Venegas, L.A.; González-Fernández, J.V.; Castañeda-Pérez, L.G.; Palomino-Hernández, G.; Dueñas-Dávila, F.A.; Trujillo-Pérez, S.A. Viscosity Factor (VF) Complementary to the Statistical Indicators Associated with the Rheological Behavior of Aqueous Solutions of Polyvinyl Alcohol. Polymers 2023, 15, 1743. https://doi.org/10.3390/polym15071743
Carrasco-Venegas LA, González-Fernández JV, Castañeda-Pérez LG, Palomino-Hernández G, Dueñas-Dávila FA, Trujillo-Pérez SA. Viscosity Factor (VF) Complementary to the Statistical Indicators Associated with the Rheological Behavior of Aqueous Solutions of Polyvinyl Alcohol. Polymers. 2023; 15(7):1743. https://doi.org/10.3390/polym15071743
Chicago/Turabian StyleCarrasco-Venegas, Luis Américo, José Vulfrano González-Fernández, Luz Genara Castañeda-Pérez, Guido Palomino-Hernández, Federico Alexis Dueñas-Dávila, and Salvador Apolinar Trujillo-Pérez. 2023. "Viscosity Factor (VF) Complementary to the Statistical Indicators Associated with the Rheological Behavior of Aqueous Solutions of Polyvinyl Alcohol" Polymers 15, no. 7: 1743. https://doi.org/10.3390/polym15071743
APA StyleCarrasco-Venegas, L. A., González-Fernández, J. V., Castañeda-Pérez, L. G., Palomino-Hernández, G., Dueñas-Dávila, F. A., & Trujillo-Pérez, S. A. (2023). Viscosity Factor (VF) Complementary to the Statistical Indicators Associated with the Rheological Behavior of Aqueous Solutions of Polyvinyl Alcohol. Polymers, 15(7), 1743. https://doi.org/10.3390/polym15071743





