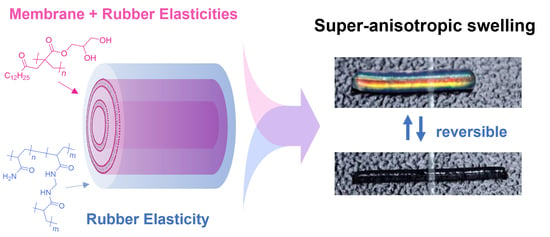
Mechanical Model for Super-Anisotropic Swelling of the Multi-Cylindrical PDGI/PAAm Gels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Dodecyl Glyceryl Itaconate
2.3. Gel Synthesis
2.4. Swelling Ratio Measurements
2.5. Small Angle X-ray Diffraction (SAXD) Measurements
3. Results
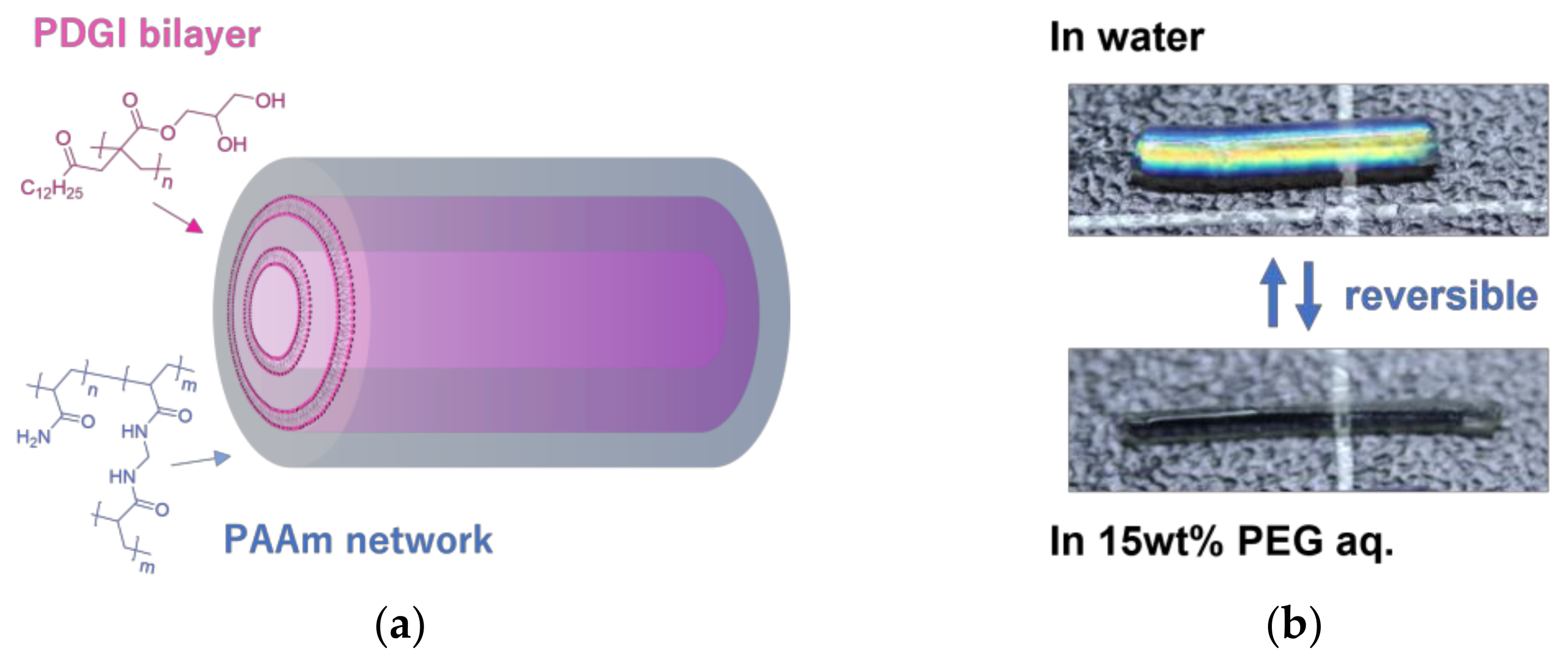
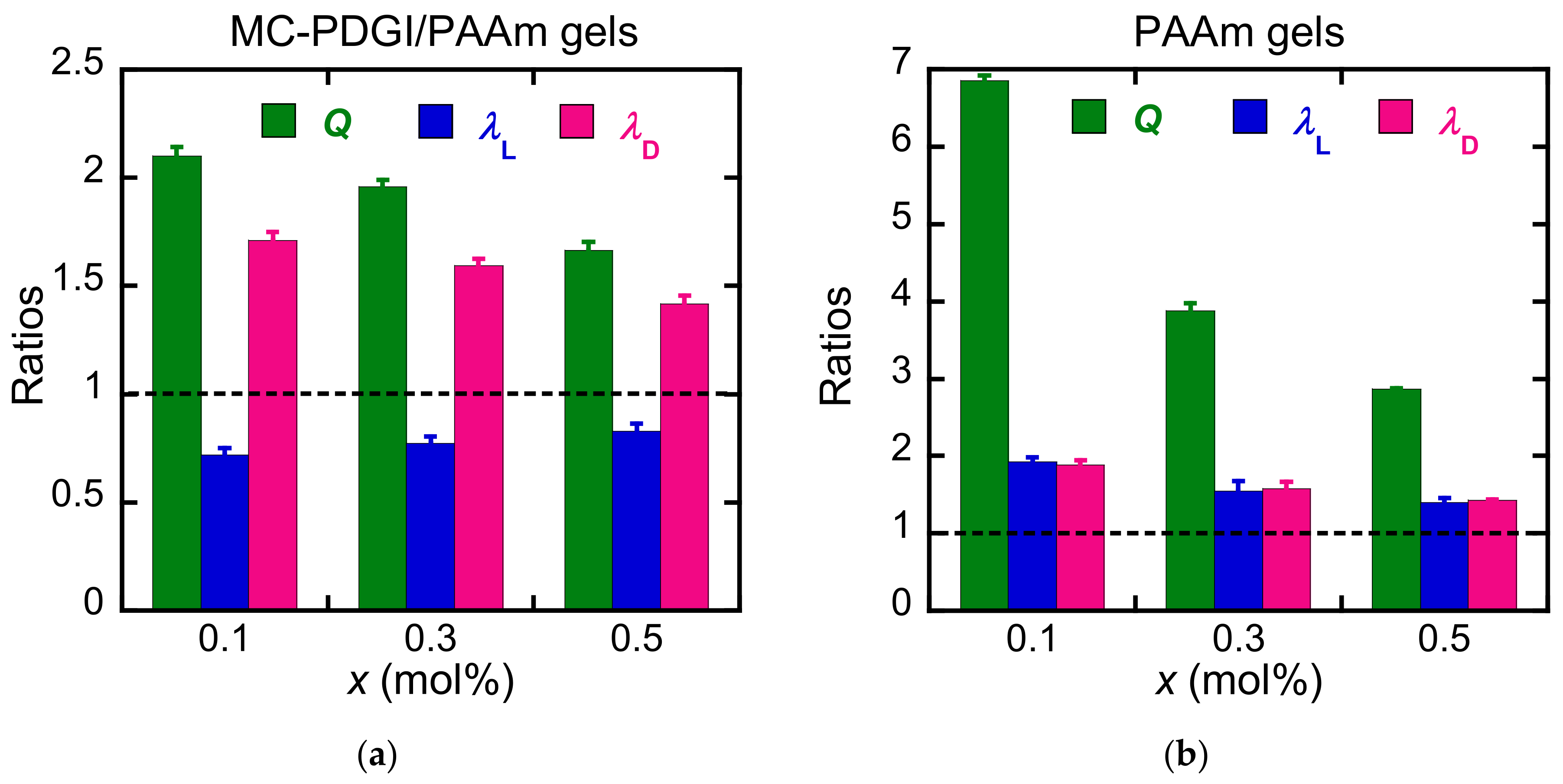
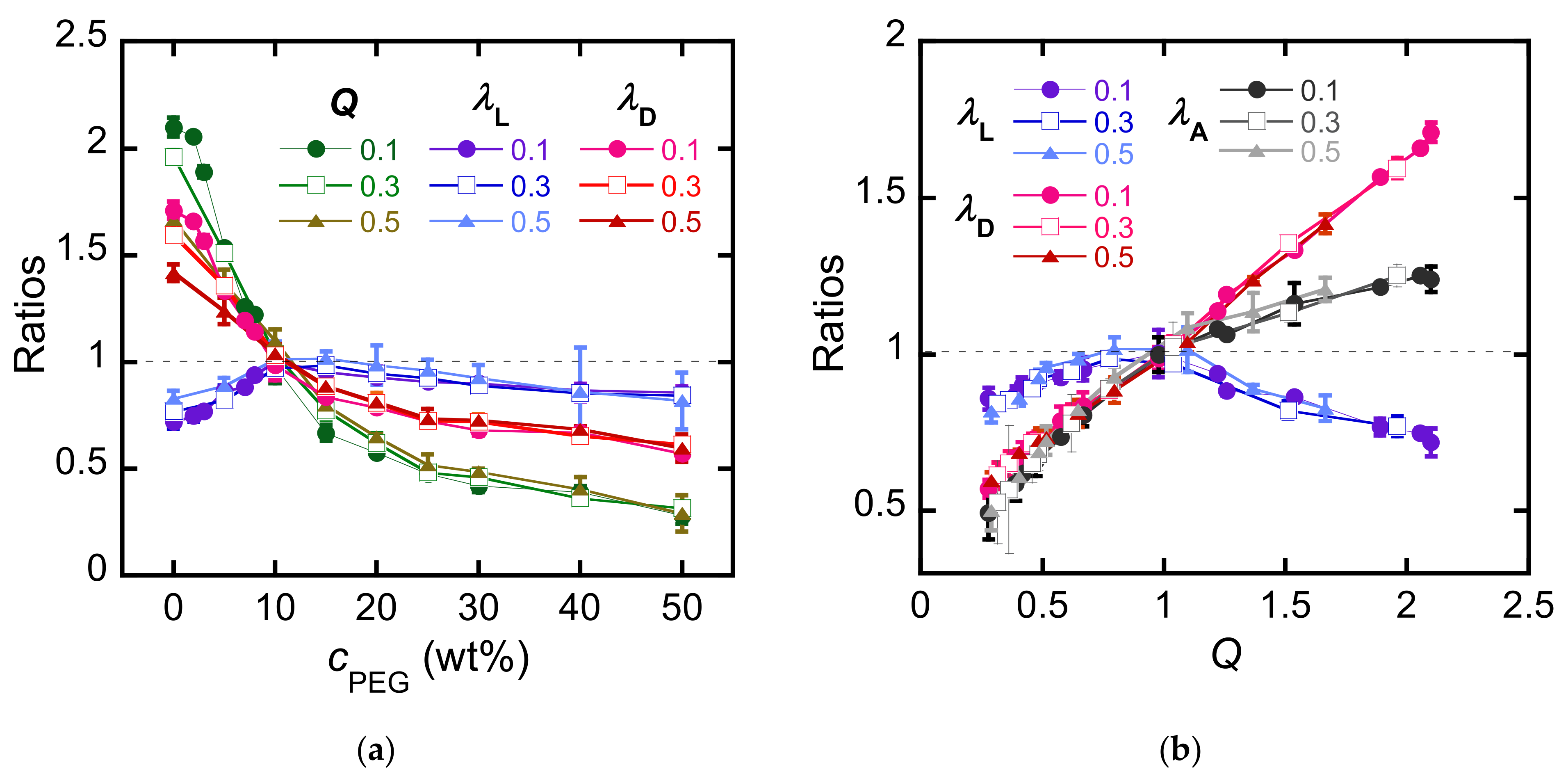
3.1. Anisotropic Swelling
3.2. Structural Analysis
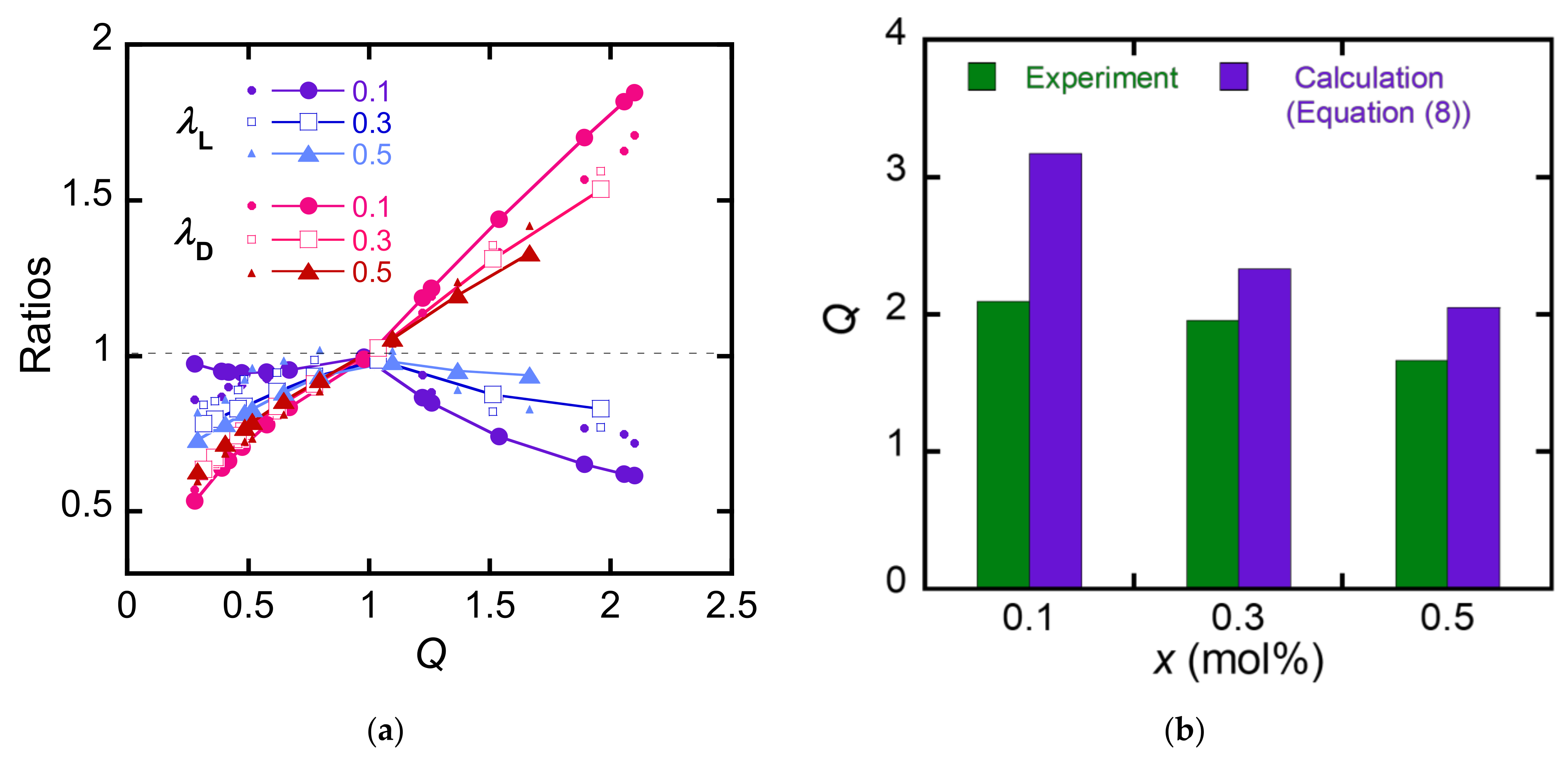
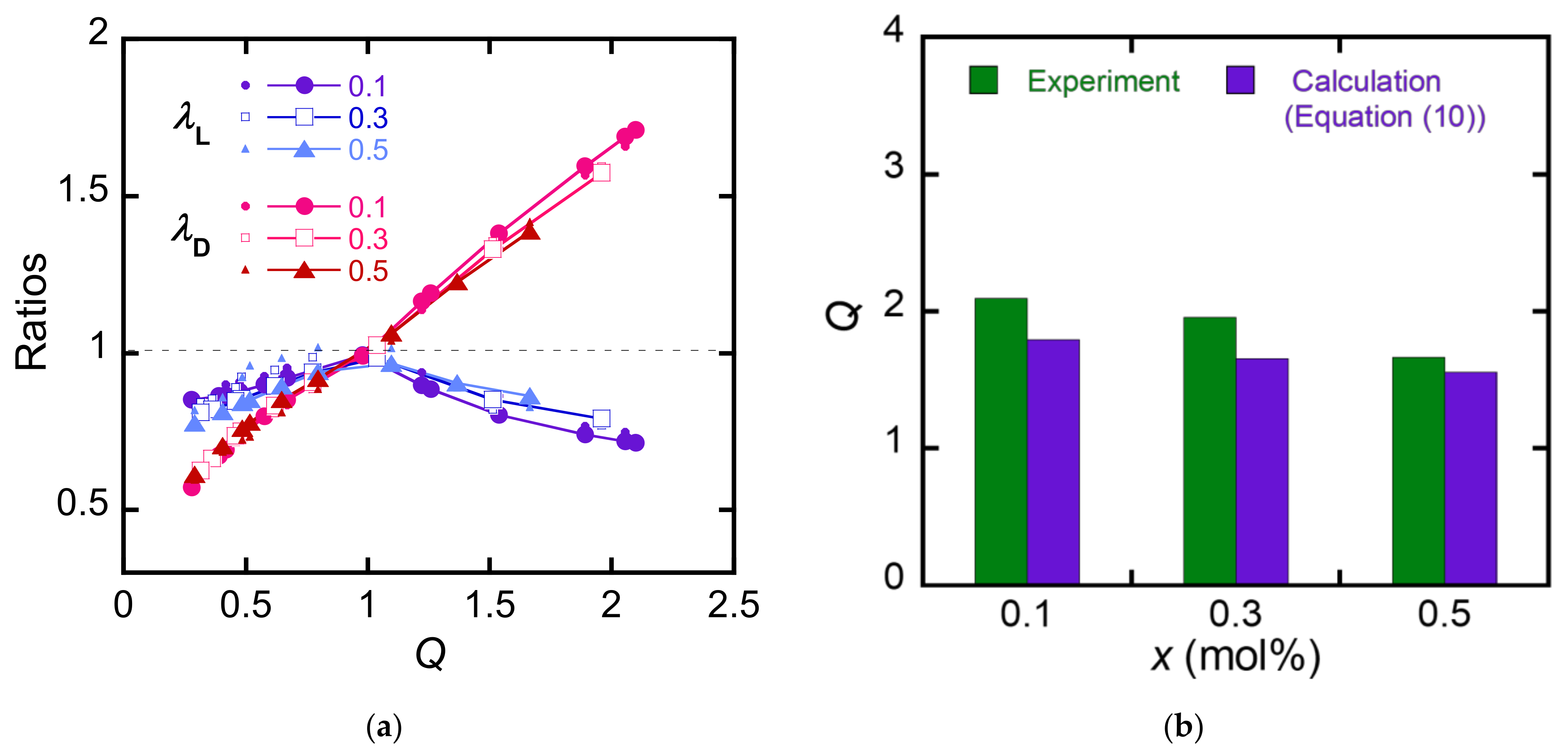
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4, 1900043. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in Sensing Applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Kishi, R.; Sisido, M.; Tazuke, S. Liquid-Crystalline Polymer Gels. 2. Anisotropic Swelling of Poly(γ-Benzyl L-Glutamate) Gel Crosslinked under a Magnetic Field. Macromolecules 1990, 23, 3868–3870. [Google Scholar] [CrossRef]
- Urayama, K.; Arai, Y.O.; Takigawa, T. Anisotropic Swelling and Phase Behavior of Monodomain Nematic Networks in Nematogenic Solvents. Macromolecules 2005, 38, 5721–5728. [Google Scholar] [CrossRef]
- Kleinschmidt, F.; Hickl, M.; Saalwächter, K.; Schmidt, C.; Finkelmann, H. Lamellar Liquid Single Crystal Hydrogels: Synthesis and Investigation of Anisotropic Water Diffusion and Swelling. Macromolecules 2005, 38, 9772–9782. [Google Scholar] [CrossRef]
- Okajima, M.K.; Mishima, R.; Amornwachirabodee, K.; Mitsumata, T.; Okeyoshi, K.; Kaneko, T. Anisotropic Swelling in Hydrogels Formed by Cooperatively Aligned Megamolecules. RSC Adv. 2015, 5, 86723–86729. [Google Scholar] [CrossRef]
- Ishiwata, T.; Kokado, K.; Sada, K. Anisotropically Swelling Gels Attained through Axis-Dependent Crosslinking of MOF Crystals. Angew. Chem. Int. Ed. 2017, 56, 2608–2612. [Google Scholar] [CrossRef]
- Chin, S.M.; Synatschke, C.V.; Liu, S.; Nap, R.J.; Sather, N.A.; Wang, Q.; Álvarez, Z.; Edelbrock, A.N.; Fyrner, T.; Palmer, L.C.; et al. Covalent-Supramolecular Hybrid Polymers as Muscle-Inspired Anisotropic Actuators. Nat. Commun. 2018, 9, 2395. [Google Scholar] [CrossRef]
- Kang, Y.; Walish, J.J.; Gorishnyy, T.; Thomas, E.L. Broad-Wavelength-Range Chemically Tunable Block-Copolymer Photonic Gels. Nat. Mater. 2007, 6, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Shintate, M.; Ikeda, S.; Hoshida, Y.; Yamauchi, Y.; Motokawa, R.; Annaka, M. Liquid Crystalline Inorganic Nanosheets for Facile Synthesis of Polymer Hydrogels with Anisotropies in Structure, Optical Property, Swelling/Deswelling, and Ion Transport/Fixation. Chem. Commun. 2013, 49, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Liu, M.; Ishida, Y.; Ebina, Y.; Osada, M.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. Thermoresponsive Actuation Enabled by Permittivity Switching in an Electrostatically Anisotropic Hydrogel. Nat. Mater. 2015, 14, 1002–1007. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, T.; Li, N.; Cong, H.P.; Yu, S.H. Anisotropic and Self-Healing Hydrogels with Multi-Responsive Actuating Capability. Nat. Commun. 2019, 10, 2202. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Kamita, G.; Kurokawa, T.; Tsujii, K.; Gong, J.P. Unidirectional Alignment of Lamellar Bilayer in Hydrogel: One-Dimensional Swelling, Anisotropic Modulus, and Stress/Strain Tunable Structural Color. Adv. Mater. 2010, 22, 5110–5114. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Gong, J.P. Structure and Unique Functions of Anisotropic Hydrogels Comprising Uniaxially Aligned Lamellar Bilayers. Bull. Chem. Soc. Jpn. 2021, 94, 2221–2234. [Google Scholar] [CrossRef]
- Naitoh, K.; Ishii, Y.; Tsujii, K. Iridescent Phenomena and Polymerization Behaviors of Amphiphilic Monomers in Lamellar Liquid Crystalline Phase. J. Phys. Chem. 1991, 95, 7915–7918. [Google Scholar] [CrossRef]
- Li, X.; Kurokawa, T.; Takahashi, R.; Haque, M.A.; Yue, Y.; Nakajima, T.; Gong, J.P. Polymer Adsorbed Bilayer Membranes Form Self-Healing Hydrogels with Tunable Superstructure. Macromolecules 2015, 48, 2277–2282. [Google Scholar] [CrossRef]
- Mito, K.; Haque, M.A.; Nakajima, T.; Uchiumi, M.; Kurokawa, T.; Nonoyama, T.; Gong, J.P. Supramolecular Hydrogels with Multi-Cylindrical Lamellar Bilayers: Swelling-Induced Contraction and Anisotropic Molecular Diffusion. Polymer 2017, 128, 373–378. [Google Scholar] [CrossRef]
- Nakajima, T.; Durand, C.; Li, X.F.; Haque, M.A.; Kurokawa, T.; Gong, J.P. Quasi-Unidirectional Shrinkage of Gels with Well-Oriented Lipid Bilayers upon Uniaxial Stretching. Soft Matter 2015, 11, 237–240. [Google Scholar] [CrossRef]
- Yue, Y.F.; Haque, M.A.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Lamellar Hydrogels with High Toughness and Ternary Tunable Photonic Stop-Band. Adv. Mater. 2013, 25, 3106–3110. [Google Scholar] [CrossRef]
- Yue, Y.; Kurokawa, T.; Haque, M.A.; Nakajima, T.; Nonoyama, T.; Li, X.; Kajiwara, I.; Gong, J.P. Mechano-Actuated Ultrafast Full-Colour Switching in Layered Photonic Hydrogels. Nat. Commun. 2014, 5, 4659. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Lamellar–Micelle Transition in a Hydrogel Induced by Polyethylene Glycol Grafting. Soft Matter 2013, 9, 5223. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Kamita, G.; Gong, J.P. Lamellar Bilayers as Reversible Sacrificial Bonds to Toughen Hydrogel: Hysteresis, Self-Recovery, Fatigue Resistance, and Crack Blunting. Macromolecules 2011, 44, 8916–8924. [Google Scholar] [CrossRef]
- Hong, W.; Wang, X. Modeling Mechanochromatic Lamellar Gels. Phys. Rev. E 2012, 85, 3934–3947. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Bastide, J.; Candau, S.; Leibler, L. Osmotic Deswelling of Gels by Polymer Solutions. Macromolecules 1981, 14, 719–726. [Google Scholar] [CrossRef]
- Mei, H.; Huang, R.; Chung, J.Y.; Stafford, C.M.; Yu, H.H. Buckling Modes of Elastic Thin Films on Elastic Substrates. Appl. Phys. Lett. 2007, 90, 2720759. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X. Phase Diagrams of Instabilities in Compressed Film-Substrate Systems. J. Appl. Mech. Trans. ASME 2014, 81, 051004. [Google Scholar] [CrossRef]
- Chung, J.Y.; Nolte, A.J.; Stafford, C.M. Surface Wrinkling: A Versatile Platform for Measuring Thin-Film Properties. Adv. Mater. 2011, 23, 349–368. [Google Scholar] [CrossRef]
- Giustiniani, A.; Ilyas, M.; Indei, T.; Gong, J.P. Relaxation Mechanisms in Hydrogels with Uniaxially Oriented Lamellar Bilayers. Polymer 2023, 267, 125686. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; pp. 541–563. [Google Scholar]
- Treloar, L.R.G. The Elasticity of a Network of Long-Chain Molecules—II. Trans. Faraday Soc. 1943, 39, 241–246. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 535–548. [Google Scholar]
- Helfrich, W. Elastic Properties of Lipid Bilayers: Theory and Possible Experiments. Z. Fur Naturforsch. 1973, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Tirumala, V.R.; Lee, S.; Lin, E.K.; Gong, J.P.; Wu, W.L. Thermodynamic Interactions in Double-Network Hydrogels. J. Phys. Chem. B 2008, 112, 3903–3909. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, T.; Mito, K.; Gong, J.P. Mechanical Model for Super-Anisotropic Swelling of the Multi-Cylindrical PDGI/PAAm Gels. Polymers 2023, 15, 1624. https://doi.org/10.3390/polym15071624
Nakajima T, Mito K, Gong JP. Mechanical Model for Super-Anisotropic Swelling of the Multi-Cylindrical PDGI/PAAm Gels. Polymers. 2023; 15(7):1624. https://doi.org/10.3390/polym15071624
Chicago/Turabian StyleNakajima, Tasuku, Kei Mito, and Jian Ping Gong. 2023. "Mechanical Model for Super-Anisotropic Swelling of the Multi-Cylindrical PDGI/PAAm Gels" Polymers 15, no. 7: 1624. https://doi.org/10.3390/polym15071624
APA StyleNakajima, T., Mito, K., & Gong, J. P. (2023). Mechanical Model for Super-Anisotropic Swelling of the Multi-Cylindrical PDGI/PAAm Gels. Polymers, 15(7), 1624. https://doi.org/10.3390/polym15071624