Physical, Mechanical, and Structural Properties of the Polylactide and Polybutylene Adipate Terephthalate (PBAT)-Based Biodegradable Polymer during Compost Storage
Abstract
1. Introduction
2. Materials and Methods
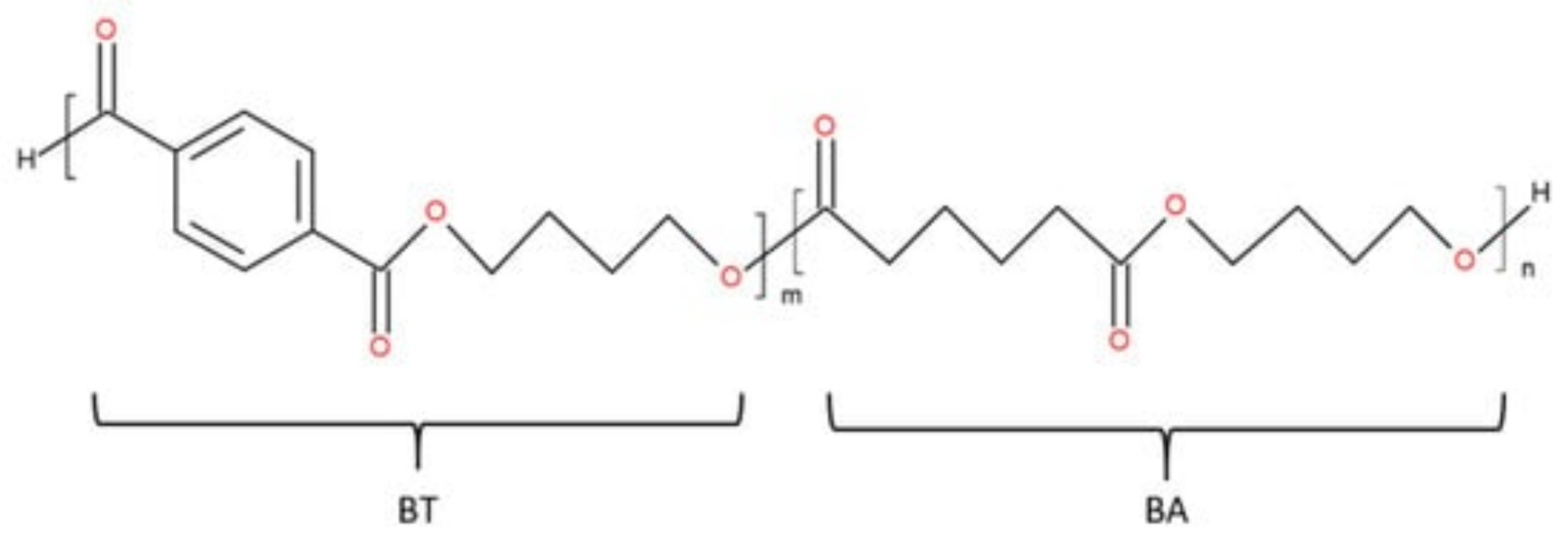
2.1. Polybutylene Adipate Terephthalate
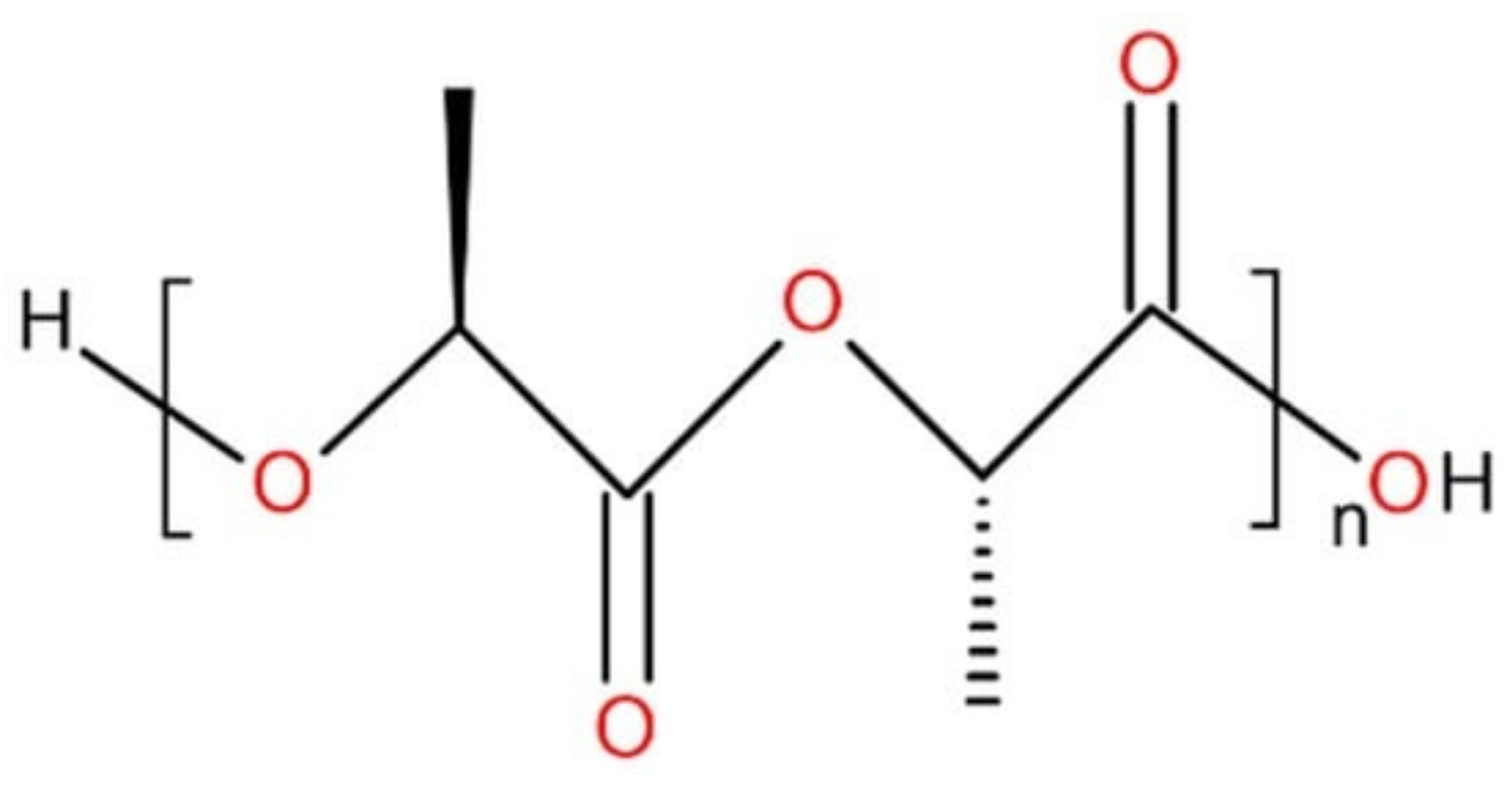
2.2. Polylactic Acid (Polylactide)
2.3. PLA/PBAT Mixture-Based Biodegradable Films
2.4. Composting Conditions
2.5. Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Take Action for the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 11 May 2022).
- Impact through Research: Applied Research for Europe’s Future. Available online: https://www.fraunhofer.de/en/press/research-news/2018/november/the-rto-summit.html (accessed on 11 May 2022).
- European Bioplastic. The Plastics Strategy—The Contribution of Bioplastics to a Sustainable Plastics Circular Economy; European Bioplastic: Berlin, Germany, 2018. [Google Scholar]
- Post, W.; Kuijpers, L.J.; Zijlstra, M.; Van Der Zee, M.; Molenveld, K. Effect of Mineral Fillers on the Mechanical Properties of Commercially Available Biodegradable Polymers. Polymers 2021, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)—PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Garifullina, L.I.; Li, N.I.; Garipov, R.M.; Minnakhmetova, A.K. Biodegradation of polymer film materials (review). Bull. Technol. Univ. 2019, 22, 47–53. [Google Scholar]
- Van Velzen, E.T.; Jansen, M.; Brower, M.; Feil, A.; Molenveld, K.P. Recycling Efficiency of Used Plastic Packaging. In Proceedings of the 32nd International Conference of the Society for the Processing of Polymers, Lyon, France, 25–29 July 2016; pp. 25–29. [Google Scholar]
- Olkhov, A.A.; Goldstrakh, M.A.; Shibryaeva, L.S.; Tertyshnaya, Y.V.; Jordansky, A.L. Promising biomaterials based on polyhydroxybutyrate and double ethylene propylene phosphopolymer for the transport of physiological media: Phase structure. Perspect. Mater. 2015, 10, 56–63. [Google Scholar]
- Salikov, P.Y. Pyrolysis utilization of used polyethylene terephthalate products. Ecol. Ind. Russ. 2014, 3, 16–20. [Google Scholar]
- Pryanichnikova, N.S. Edible packaging: Transport for functional and bioactive compounds. Milk River 2020, 4, 32–34. [Google Scholar]
- Van den Over, M.; Molenveld, K. Replacing fossil fuel-based plastic products with bio-based plastic products—Technical feasibility. New Biotechnol. 2017, 37, 48–59. [Google Scholar] [CrossRef]
- Ailes, A.; Martin, A.N. Expanding Bioplastics: Sustainable Business Innovation in the Chemical Industry. J. Clean. Prod. 2013, 45, 38–49. [Google Scholar] [CrossRef]
- Kiruthika, A.V. PHBV based blends and composites. In Biodegradable Polymers, Blends and Composites; Woodhead Publishing: Cambridge, UK, 2022; pp. 283–308. [Google Scholar]
- Brdlík, P.; Borůvka, M.; Běhálek, L.; Lenfeld, P. The Influence of Additives and Environment on Biodegradation of PHBV Biocomposites. Polymers 2022, 14, 838. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef]
- Barron, A.; Sparks, T.D. Commercial Marine-Degradable Polymers for Flexible Packaging. iScience 2020, 23, 101353. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef]
- Ponjavic, M.; Malagurski, I.; Lazic, J.; Jeremic, S.; Pavlovic, V.; Prlainovic, N.; Maksimovic, V.; Cosovic, V.; Atanase, L.I.; Freitas, F.; et al. Advancing PHBV Biomedical Potential with the Incorporation of Bacterial Biopigment Prodigiosin. Int. J. Mol. Sci. 2023, 24, 1906. [Google Scholar] [CrossRef]
- Xu, J.; Lei, Z.; Liu, S.; Chen, J.; Gong, G.; Cai, X. Preparation and characterization of biodegradable electrospinning PHBV/PBAT/TiO2 antibacterial nanofiber membranes. J. Eng. Fibers Fabr. 2022, 17, 15589250221136566. [Google Scholar] [CrossRef]
- Hobbs, C.E. Recent Advances in Bio-Based Flame Retardant Additives for Synthetic Polymeric Materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef]
- Maraveas, C. Production of Sustainable and Biodegradable Polymers from Agricultural Waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Khajavi, M.Z.; Ebrahimi, A.; Yousefi, M.; Ahmadi, S.; Farhoudi, M.; Alizade, A.M.; Taslich, M. Strategies for the production of improved oxygen barrier materials suitable for the food packaging sector. Food Eng. 2020, 12, 346–363. [Google Scholar] [CrossRef]
- Van Der Zee, M. Methods for Evaluating the Biodegradability of Environmentally Degradable Polymers; Smither Rapra: Shawburry, UK, 2014; pp. 1–28. [Google Scholar]
- Quecholac-Piña, X.; Hernández-Berriel, M.D.C.; Mañón-Salas, M.D.C.; Espinosa-Valdemar, R.M.; Vázquez-Morillas, A. Degradation of plastics under anaerobic conditions: A short review. Polymers 2020, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Loong-Tak, L. Biodegradable food packaging. Milk Process. 2011, 6, 61–67. [Google Scholar]
- Kirsh, I.; Frolova, Y.; Bannikova, O.; Beznaeva, O.; Tveritnikova, I.; Myalenko, D.; Romanova, V.; Zagrebina, D. Research of the Influence of the Ultrasonic Treatment on the Melts of the Polymeric Compositions for the Creation of Packaging Materials with Antimicrobial Properties and Biodegrability. Polymers 2020, 12, 275. [Google Scholar] [CrossRef]
- Prudnikova, S.V.; Volkova, T.G. The Ecological Role of Polyhydroxyalkanoates, An Analogue of Synthetic Plastics: Patterns of Biodegradation in the Natural Environment and Interaction with Microorganisms; Krasnoyarsk Writer: Krasnoyarsk, Russia, 2012; p. 184. [Google Scholar]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Biopolymers for food packaging applications. In Smart Polymers and Their Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 476–509. [Google Scholar]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Véronique, C. Bioactive packaging technologies for extended shelf life of meat-based products. Meat Sci. 2008, 78, 90–103. [Google Scholar]
- Othman, S.H. Bio-nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Mahalik, N.P. Advances in Packaging Methods, Processes and Systems. Challenges 2014, 5, 374–389. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Rydz, J.; Musioł, M.; Zawidlak-Węgrzyńska, B.; Sikorska, W. Present and future of biodegradable polymers for food packaging applications. In Biopolymers for Food Design; Academic Press: Waltham, MA, USA, 2018; pp. 431–467. [Google Scholar]
- Fedotova, O.B. About biodegradable packaging and prospects for its use. Dairy Ind. 2020, 1, 10–12. [Google Scholar]
- Radaeva, I.A.; Illarionova, E.E.; Turovskaya, S.N.; Ryabova, A.E.; Galstyan, A.G. Principles of quality assurance of domestic milk powder. Food Ind. 2019, 9, 54–57. [Google Scholar]
- Pryanichnikova, N.S. Protective coatings for food products. Modern achievements of biotechnology. Equipment, technologies and packaging for the implementation of innovative projects in the food and biotechnology industry. In Proceedings of the Materials of the VII International Scientific and Practical Conference, Pyatigorsk, Russia, 20–24 October 2020; pp. 86–89. [Google Scholar]
- Pryanichnikova, N.S.; Fedotova, O.B. The use of qualimetric design techniques in the creation of functional coatings on food products. Modern biotechnology: Current issues, innovations and achievements. In Proceedings of the Collection of Abstracts of the All-Russian, Online Conference with International Participation. Online, 21 October 2020; pp. 141–143. [Google Scholar]
- Khurshudyan, S.A.; Pryanichnikova, N.S.; Ryabova, A.E. Food quality and safety. Transformation of concepts. Food Ind. 2022, 3, 8–10. [Google Scholar]
- Yurova, E.A.; Filchakova, S.A. Evaluation of the quality and storage capacity of functional dairy products. Milk Process. 2019, 10, 6–11. [Google Scholar]
- Fedotova, O.B. Specific features of the evaluation of the organoleptic properties of packaging. Milk Process. 2017, 6, 6–8. [Google Scholar]
- Preservation of Soy Protein-Based Meat Analogues by Using PLA/PBAT Antimicrobial Packaging Film. Available online: https://www.sciencedirect.com/science/article/pii/S0308814621030284 (accessed on 17 March 2022).
- Christina, C.C.; Trinh, B.M.; Mekonnen, T.H. Robust multiphase and multilayer starch/polymer (TPS/PBAT) film with simultaneous oxygen/moisture barrier properties. J. Colloid Interface Sci. 2021, 593, 290–303. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Kobzeva, T.V.; Yurova, E.A. Evaluation of quality indicators and identification characteristics of milk powder. Dairy Ind. 2016, 3, 32–35. [Google Scholar]
- Kruchinin, A.G.; Illarionova, E.E.; Turovskaya, S.N.; Bigaeva, A.V. A study of the effect of protein profile on structural and mechanical parameters of dairy biosystems with intermediate moisture content. Food Process. Ind. 2023, 1, 59–62. [Google Scholar]
- Strizhko, M.N. Technological aspects of analogues dairy systems based on vegetable raw materials. Food Process. Ind. 2023, 1, 36–40. [Google Scholar]
- Zobkova, Z.S.; Fursova, T.P.; Zenina, D.V. Protein ingredients selection, enriching and modifying the oxidum drinks structure. Top. Issues Beverage Ind. 2018, 2, 64–69. [Google Scholar]
- Galstyan, A.G.; Aksenova, L.M.; Lisitsyn, A.B.; Oganesyants, L.A.; Petrov, A.N. Modern approaches to storage and effective processing of agricultural products for obtaining high-quality food products. Bull. Russ. Acad. Sci. 2019, 89, 539–542. [Google Scholar]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and hydrolysis rate of aliphatic aromatic polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and its blends with poly (butylene succinate) (PBS): A brief review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Zhang, Y.; Wan, C.; Ma, P. Toughening modification of PLLA/PBS blends via in situ compatibilization. Polym. Eng. Sci. 2009, 49, 26–33. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- D’Ambrosio, R.M.; Michell, R.M.; Mincheva, R.; Hernández, R.; Mijangos, C.; Dubois, P.; Müller, A.J. Crystallization and Stereocomplexation of PLA-mb-PBS Multi-Block Copolymers. Polymers 2018, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, O.O. Miscibility of biodegradability and mixtures of poly(butylene succinate)/poly(butylene terephthalate). Polym. Environ. 1999, 7, 53–66. [Google Scholar] [CrossRef]
- Leejarkpai, T.; Suwanmanee, U.; Rudeekit, Y.; Mungcharoen, T. Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag. 2011, 31, 1153–1161. [Google Scholar] [CrossRef]
- Stloukal, P.; Pekařová, S.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Chitu, L.; Bodner, S.; Maier, G.; Slouf, M.; et al. Kinetics and mechanism of the biodegradation of PLA/clay nanocomposites during thermophilic phase of composting process. Waste Manag. 2015, 42, 31–40. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Ghorpade, V.M.; Gennadios, A.; Hanna, M.A. Laboratory composting of extruded poly(lactic acid) sheets. Bioresour. Technol. 2001, 76, 57–61. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Jarerat, A. Biodegradation of poly(l-lactide). Biotechnol. Lett. 2004, 26, 771–777. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Mastalygina, E.E.; Ovchinnikov, V.A.; Monakhova, T.V.; Vetcher, A.A.; Iordanskii, A.L. Thermo-Oxidative destruction and biodegradation of nanomaterials from composites of Poly (3-hydroxybutyrate) and Chitosan. Polymers 2021, 13, 3528. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Iovino, R.; Zullo, R.; Rao, M.A.; Cassar, L.; Gianfreda, L. Biodegradation of poly (lactic acid)/starch/coir biocomposites under controlled composting conditions. Polym. Degrad. Stab. 2008, 93, 147–157. [Google Scholar] [CrossRef]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly (butylene adipate-co-terephthalate)(PBAT), poly (lactic acid)(PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Hernández-López, M.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Zavaleta-Avejar, L.; Benítez-Jiménez, J.J.; Sabino-Gutiérrez, M.A.; Ortega-Gudiño, P. Bio-based composite fibers from pine essential oil and PLA/PBAT polymer blend. Morphological, physicochemical, thermal and mechanical characterization. Mater. Chem. Phys. 2019, 234, 345–353. [Google Scholar] [CrossRef]
- Chen, W.; Qi, C.; Li, Y.; Tao, H. The degradation investigation of biodegradable PLA/PBAT blend: Thermal stability, mechanical properties and PALS analysis. Radiat. Phys. Chem. 2021, 180, 109239. [Google Scholar] [CrossRef]
- Zheng, Y.; Jia, X.; Zhao, Z.; Ran, Y.; Du, M.; Ji, H.; Pan, Y.; Li, Z.; Ma, X.; Liu, Y.; et al. Innovative natural antimicrobial natamycin incorporated titanium dioxide (nano-TiO2)/poly (butylene adipate-co-terephthalate)(PBAT)/poly (lactic acid)(PLA) biodegradable active film (NTP@ PLA) and application in grape preservation. Food Chem. 2023, 400, 134100. [Google Scholar] [CrossRef]
- Kalita, N.K.; Bhasney, S.M.; Mudenur, C.; Kalamdhad, A.; Katiyar, V. End-of-life evaluation and biodegradation of Poly (lactic acid)(PLA)/Polycaprolactone (PCL)/Microcrystalline cellulose (MCC) polyblends under composting conditions. Chemosphere 2020, 247, 125875. [Google Scholar] [CrossRef]
- Fellows, P.J. Packaging. In Food Processing Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 713–781. [Google Scholar]
- Selke, S.; Hernandez, R.J. Packaging: Polymers for Containers. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wypych, G. HDPE high density polyethylene. In Handbook of Polymers; ChemTec Publishing: Scarborough, ON, Canada, 2012; pp. 150–156. [Google Scholar]
- Abdel-Bari, E.M.; Zaikov, G.E. Polymer films. In Production Technologies, Destruction and Stabilization, Application, Recycling; Profession: St. Petersburg, FL, USA, 2010. [Google Scholar]
- Zaikov, G.E. Why polymers age. Soros Educ. J. 2000, 12, 48–55.84. [Google Scholar]
- Zaikov, T.E. Destruction and Stabilization of Polymers: Tutorial; House of the Moscow Institute of Fine Chemical Technology Named after M.V. Lomonosov: Moscow, Russia, 1990; p. 151. [Google Scholar]
- White, D.L. Polypropylene and Other Polyolefins; Translated from English; Profession: St. Petersburg, FL, USA, 2006; p. 256. ISBN 5939131050. [Google Scholar]
- Fedotova, O.B.; Pryanichnikova, N.S. Research of the polyethylene packaging layer structure change in contact with a food product at exposure to ultraviolet radiation. Food Syst. 2021, 4, 56–61. [Google Scholar] [CrossRef]
- Fedotova, O.B.; Myalenko, D.M. The research of physical and mechanical indicators of filled food soot of polyethylene film for dairy and food products after exposingto its pulse uv radiation. Bull. KSAU 2020, 7, 166–172. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Shatalova, O.V.; Kolesnikova, N.N.; Popov, A.A.; Krivandin, A.V. Modification of isotactic polypropylene with additives of low-density polyethylene and powdered cellulose. Mater. Sci. 2015, 7, 34–42. [Google Scholar] [CrossRef]
- Kryzhanovsky, V.K. Engineering Selection and Identification of Plastics; Scientific Foundations and Technologies: Moscow, Russia, 2009; p. 234. ISBN 978-5-91703-012-8. [Google Scholar]
- Coles, R.; McDowell, D.; Kirwan, M.D. Food Packaging; Makhotina, L.G., Ed.; Translated from English; Profession: St. Petersburg, FL, USA, 2008; p. 416. [Google Scholar]
- Dammak, M.; Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Blends of PBAT with plasticized starch for packaging applications: Mechanical properties, rheological behaviour and biodegradability. Ind. Crops Prod. 2020, 144, 112061. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Cappiello, G.; Gisario, A. Compatibilization strategies and analysis of morphological features of poly (butylene adipate-co-terephthalate)(PBAT)/poly (lactic acid) PLA blends: A state-of-art review. Eur. Polym. J. 2022, 173, 111304. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P. Comparison of the degradability of poly(lactide) packages in composting and ambient exposure conditions. Packag. Technol. Sci. 2007, 20, 49–70. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Janczak, K.; Dąbrowska, G.B.; Raszkowska-Kaczor, A.; Kaczor, D.; Hrynkiewicz, K.; Richert, A. Biodegradation of the plastics PLA and PET in cultivated soil with the participation of microorganisms and plants. Int. Biodeterior. Biodegrad. 2020, 155, 105087. [Google Scholar] [CrossRef]
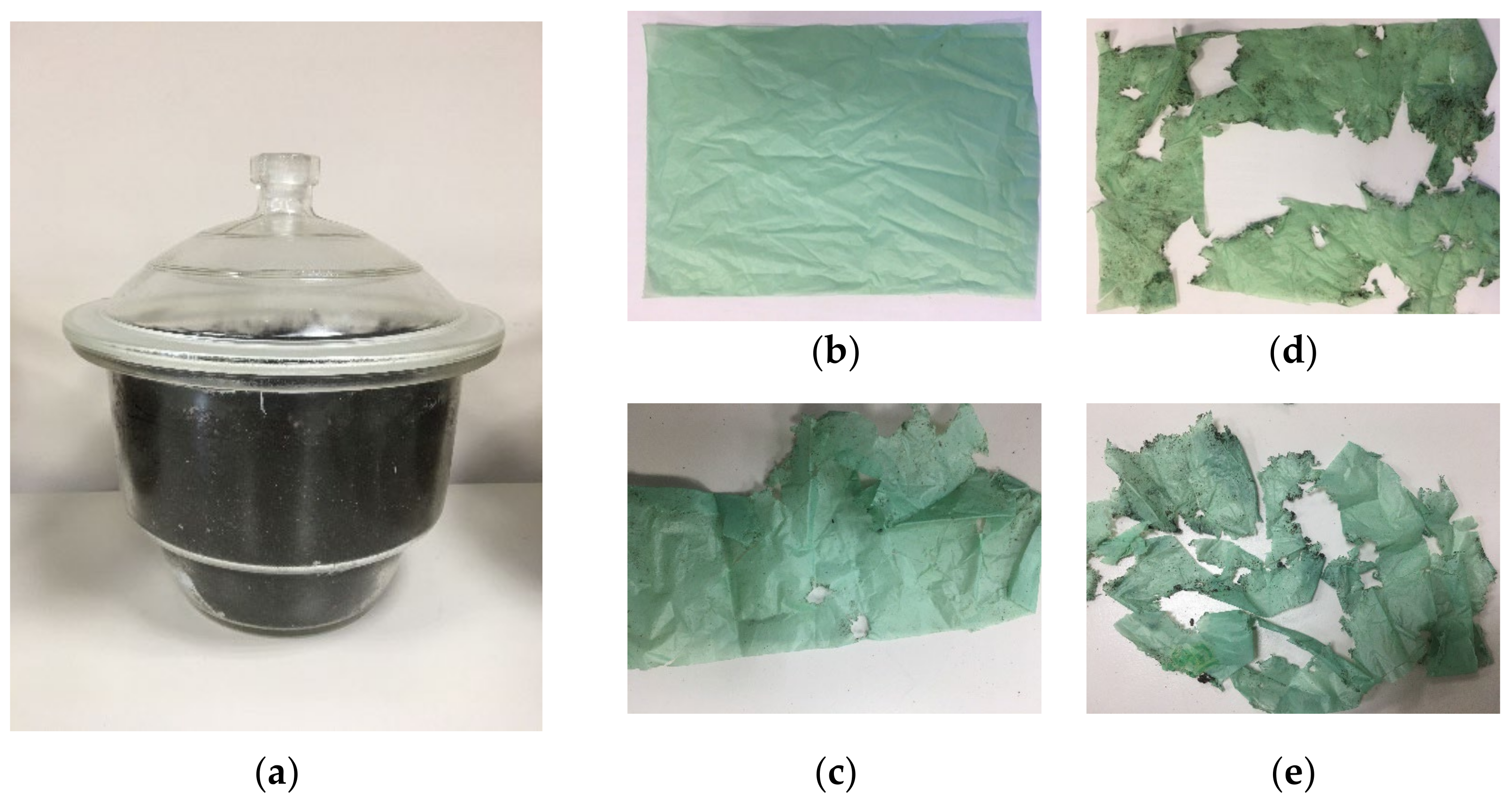

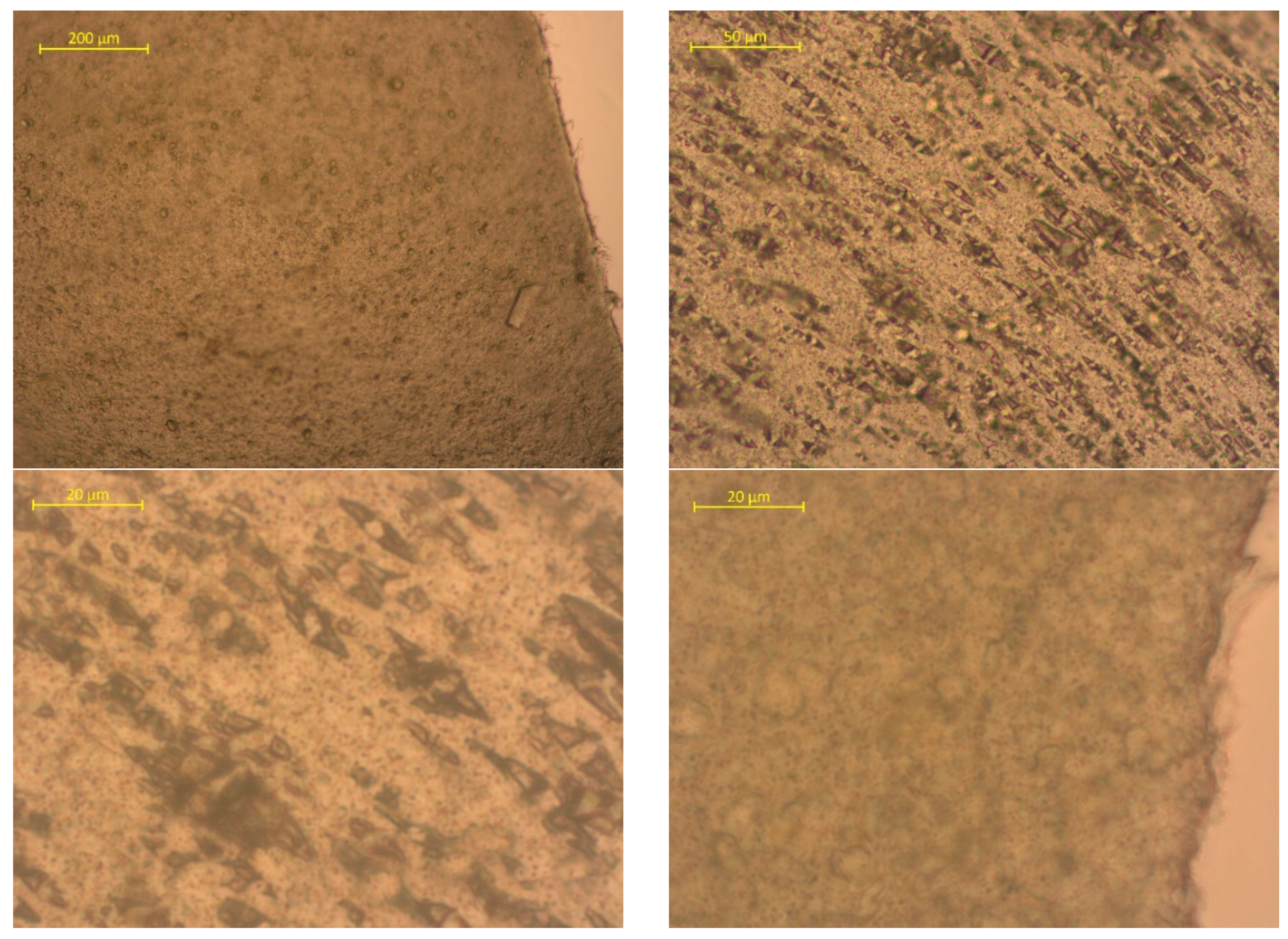
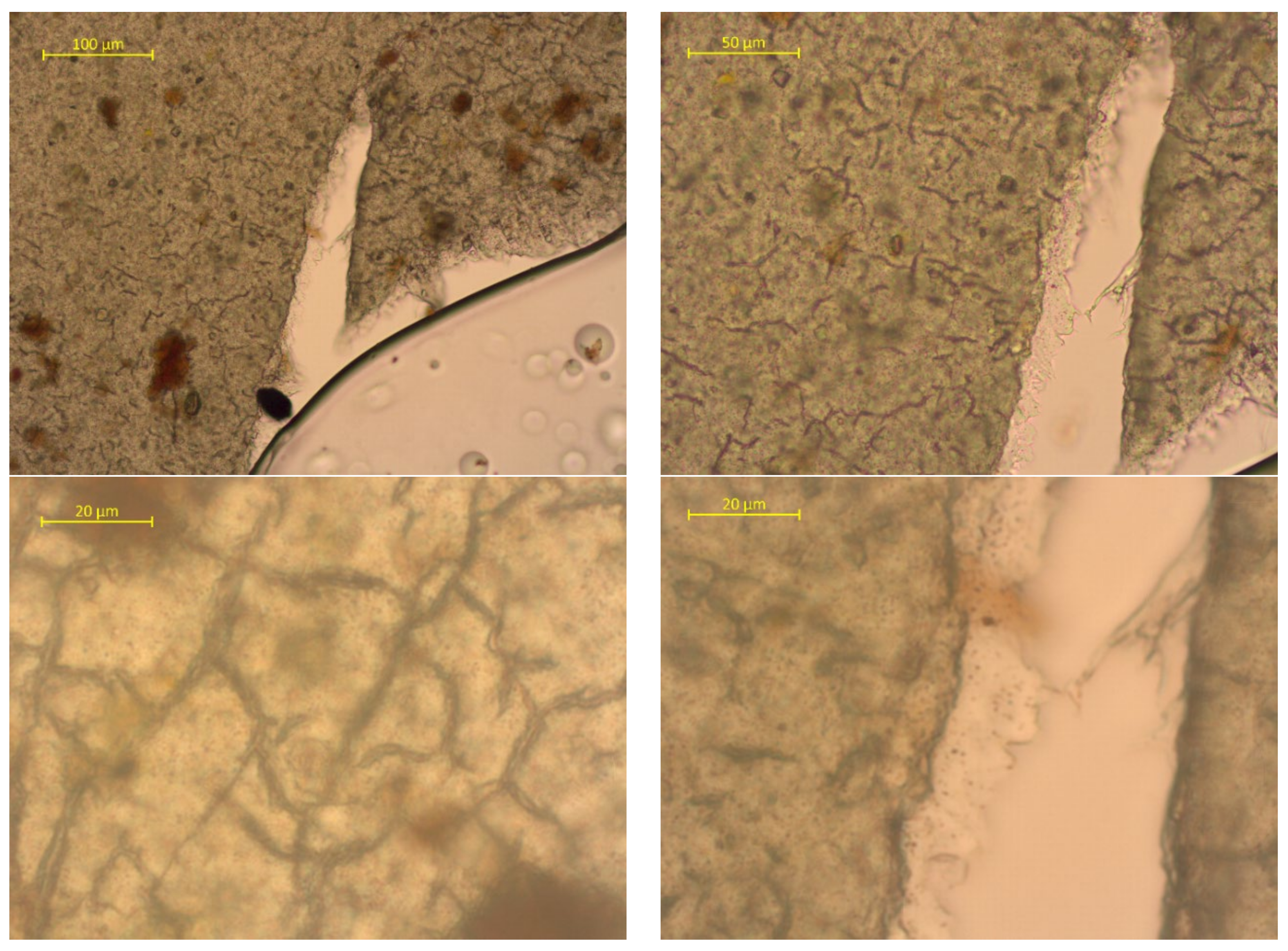
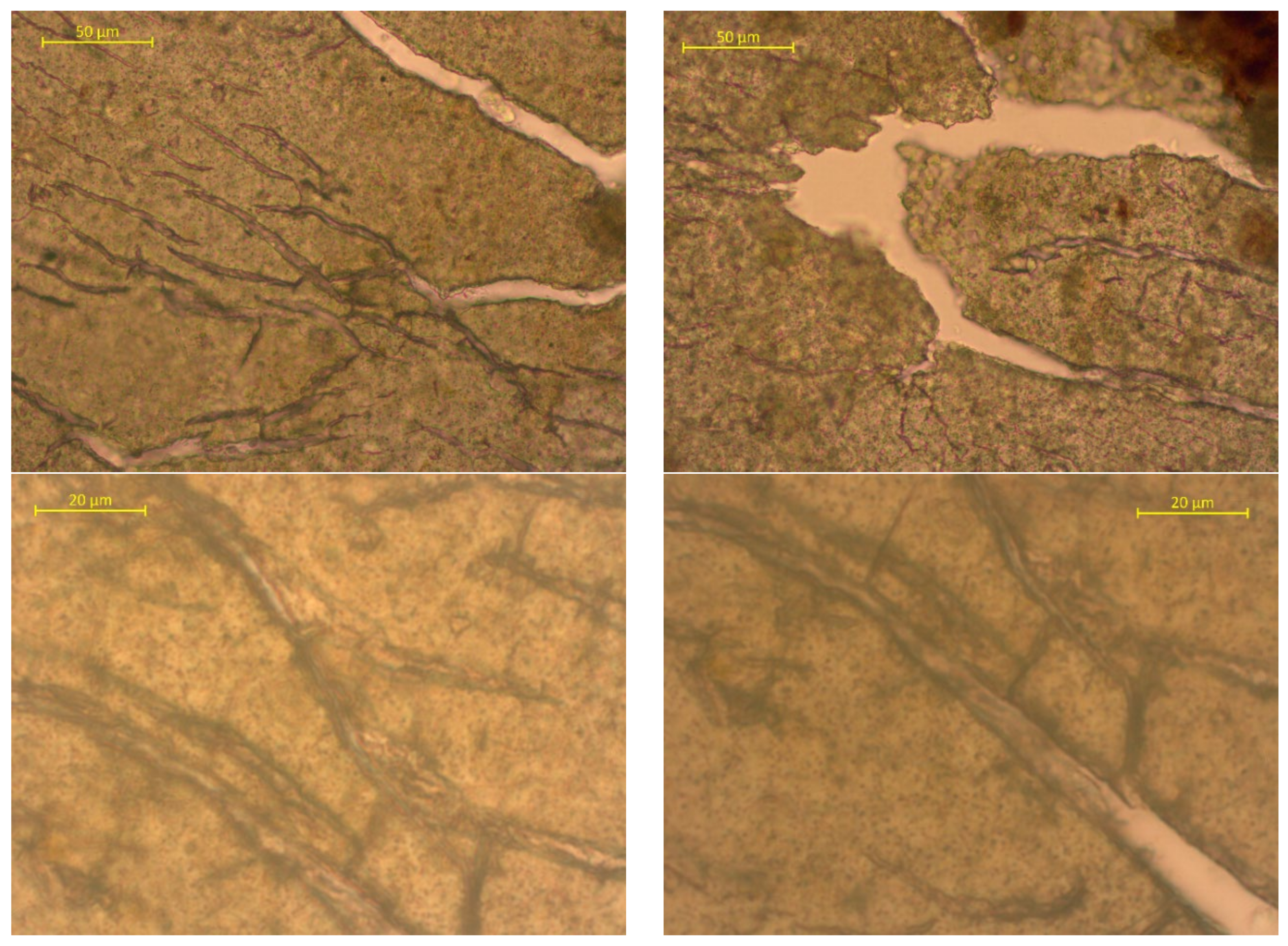


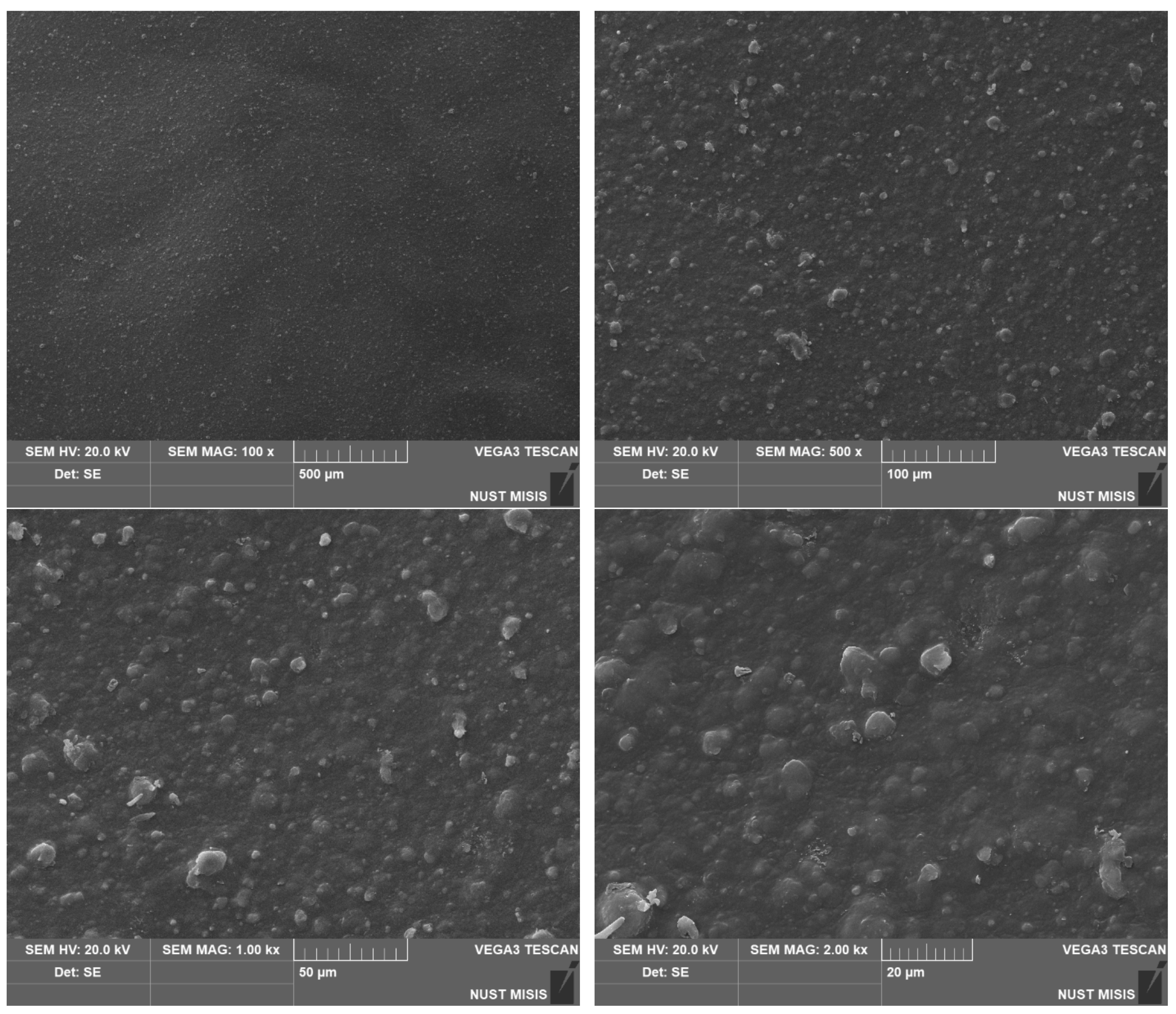

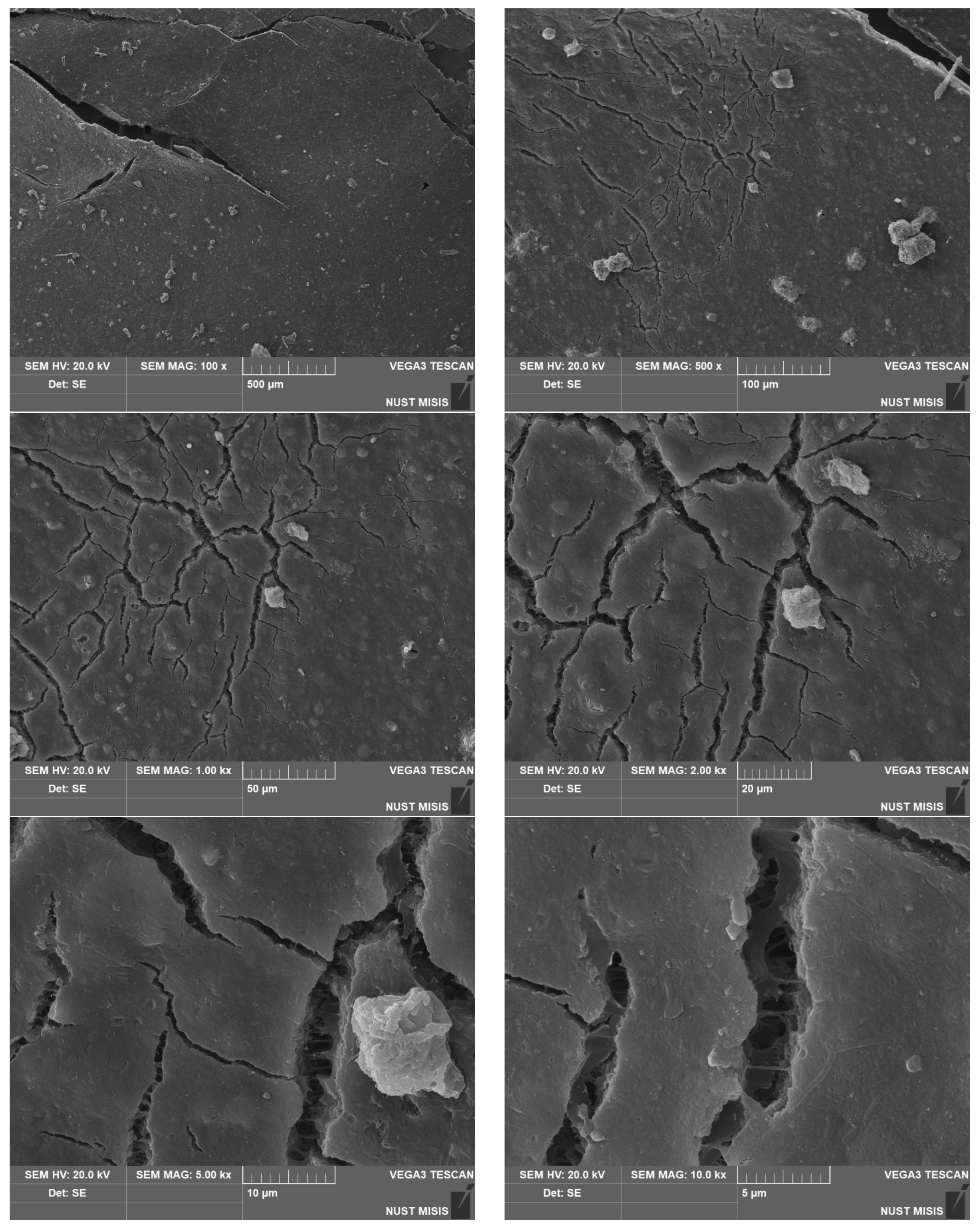
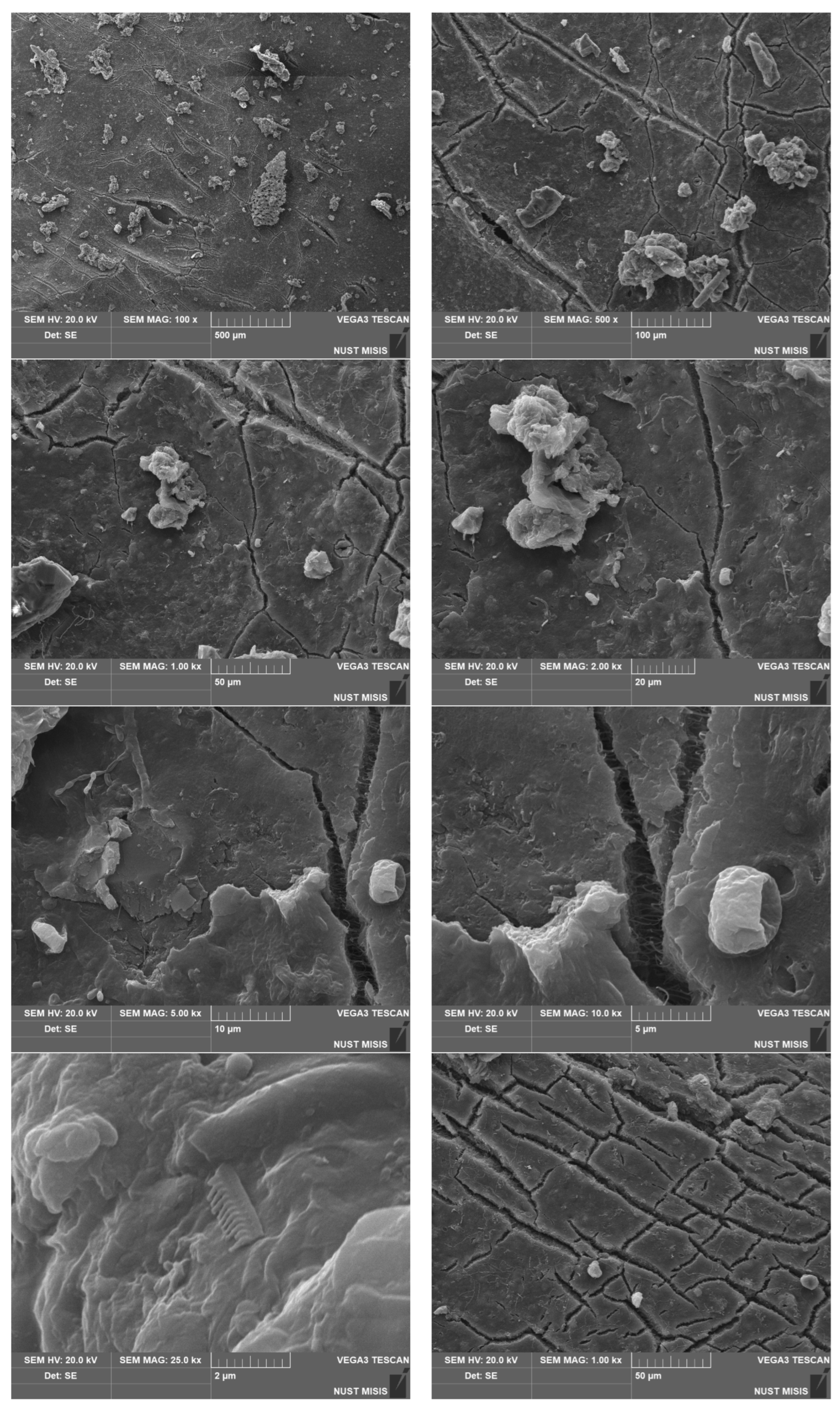
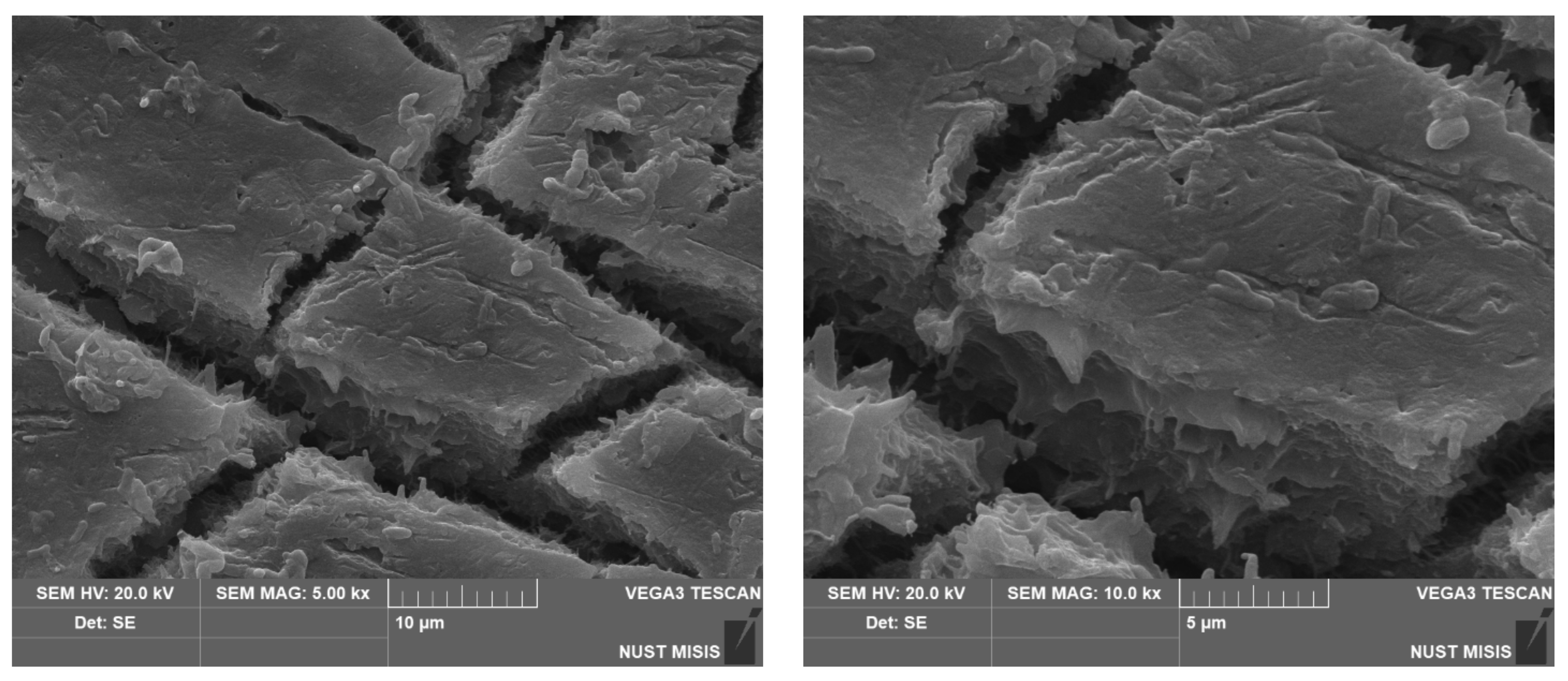
| Sample No. | Experimental Results: 1 m2; Weight, g | |||
|---|---|---|---|---|
| 0 Days | 30 Days | 60 Days | 90 Days | |
| Average | 2.7 | 2.5 | 2.3 | 1.9 |
| Weight drop, % | 0 | 7.4 | 14.8 | 29.6 |
| Actual Results | ||||
|---|---|---|---|---|
| PE Film | PLA/PBAT Film | |||
| Longitudinal | Transverse | Longitudinal | Transverse | |
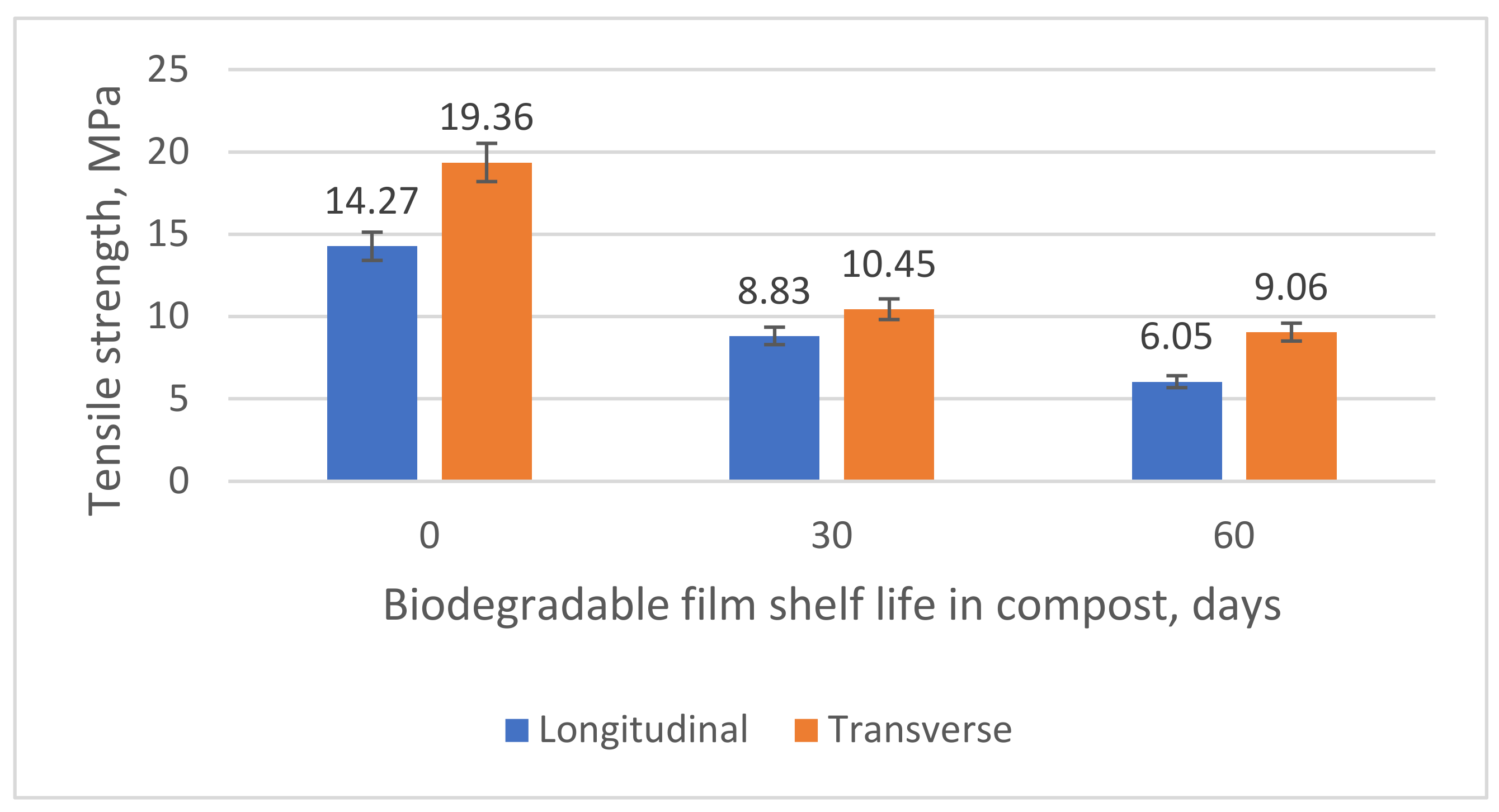
| δ, MPa | 21.17 | 16.97 | 14.27 | 19.36 |
| ε, % | 466.60 | 817.63 | 507.8 | 331.9 |
| Property | Soil Storage Period, days | |
|---|---|---|
| 30 | 60 | |
| Reduction in δ (MPa) relative to the reference sample, %: | ||
| Longitudinal | 38.12 | 57.60 |
| Transverse | 46.02 | 53.26 |
| Reduction in ε (%) relative to the reference sample, %: | ||
| Longitudinal | 95.55 | 93.28 |
| Transverse | 91.47 | 88.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myalenko, D.; Fedotova, O. Physical, Mechanical, and Structural Properties of the Polylactide and Polybutylene Adipate Terephthalate (PBAT)-Based Biodegradable Polymer during Compost Storage. Polymers 2023, 15, 1619. https://doi.org/10.3390/polym15071619
Myalenko D, Fedotova O. Physical, Mechanical, and Structural Properties of the Polylactide and Polybutylene Adipate Terephthalate (PBAT)-Based Biodegradable Polymer during Compost Storage. Polymers. 2023; 15(7):1619. https://doi.org/10.3390/polym15071619
Chicago/Turabian StyleMyalenko, Dmitry, and Olga Fedotova. 2023. "Physical, Mechanical, and Structural Properties of the Polylactide and Polybutylene Adipate Terephthalate (PBAT)-Based Biodegradable Polymer during Compost Storage" Polymers 15, no. 7: 1619. https://doi.org/10.3390/polym15071619
APA StyleMyalenko, D., & Fedotova, O. (2023). Physical, Mechanical, and Structural Properties of the Polylactide and Polybutylene Adipate Terephthalate (PBAT)-Based Biodegradable Polymer during Compost Storage. Polymers, 15(7), 1619. https://doi.org/10.3390/polym15071619







