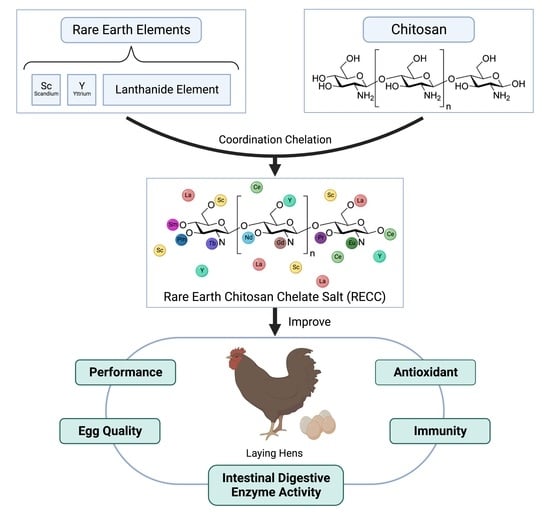
Effects of Dietary Rare Earth Chitosan Chelate on Performance, Egg Quality, Immune and Antioxidant Capacity, and Intestinal Digestive Enzyme Activity of Laying Hens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Diets
2.2. Sample Collection
2.3. Egg Quality Determination
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Performance
3.2. Egg Quality
3.3. Serum Indexes
3.4. Intestinal Immune
3.5. Digestive Enzyme Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yenice, E.; Mızrak, C.; Gültekin, M.; Atik, Z.; Tunca, M. Effects of organic and inorganic forms of manganese, zinc, copper, and chromium on bioavailability of these minerals and calcium in late-phase laying hens. Biol. Trace Elem. Res. 2015, 167, 300–307. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, Q.; Zhu, J.; Lu, X.; Liu, B.; Yu, D.; Lin, G.; Ao, T.; Xu, J. Organic trace minerals improve eggshell quality by improving the eggshell ultrastructure of laying hens during the late laying period. Poult. Sci. 2020, 99, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, H. Dietary Factors Improving Egg Shell Quality in Layer Chicken: A Review. Int. J. Pure Appl. Biosci. 2018, 6, 480–487. [Google Scholar]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Krawczyk, J.; Puchała, M.; Józefiak, D. Dietary factors improving eggshell quality: An updated review with special emphasis on microelements and feed additives. World’s Poult. Sci. J. 2019, 71, 83–94. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; El-Hack, M.A.; Khafaga, A.; Noreldin, A.; Arif, M.; Chaudhry, M.; Losacco, C.; Abdeen, A.; Abdel-Daim, M. Impacts of rare earth elements on animal health and production: Highlights of cerium and lanthanum. Sci. Total Environ. 2019, 672, 1021–1032. [Google Scholar] [CrossRef]
- Tommasi, F.; Thomas, P.J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanesi, M.; Trifuoggi, M. Review of Rare Earth Elements as Fertilizers and Feed Additives: A Knowledge Gap Analysis. Arch. Environ. Contam. Toxicol. 2021, 81, 531–540. [Google Scholar] [CrossRef]
- Tariq, H.; Sharma, A.; Sarkar, S.; Ojha, L.; Pal, R.; Mani, V. Perspectives for rare earth elements as feed additive in livestock—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 373–381. [Google Scholar] [CrossRef]
- Reka, D.; Thavasiappan, V.; Selvaraj, P.; Arivuchelvan, A.; Visha, P.; Preedaa, M. Effect of dietary REE supplementation on intestinal enzyme activities in layer chicken. J. Entomol. Zool. Stud. 2019, 7, 198–201. [Google Scholar]
- Durmuş, O.; Bölükbaşı, Ş. Biological activities of lanthanum oxide in laying hens. J. Appl. Poult. Res. 2015, 24, 481–488. [Google Scholar] [CrossRef]
- Bölükbaşı, S.C.; Al-Sagan, A.A.; Ürüşan, H.; Erhan, M.K.; Durmuş, O.; Kurt, N. Effects of cerium oxide supplementation to laying hen diets on performance, egg quality, some antioxidant enzymes in serum and lipid oxidation in egg yolk. J. Anim. Physiol. Anim. Nutr. 2016, 100, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.L.; Fernandes, A.R.M.; Oliveira, E.R.; Cônsolo, N.R.B.; Marques, O.F.C.; Maciel, T.P.; Pordeus, N.M.; Barbosa, L.C.G.S.; Buarque, V.L.M.; Padilla, A.R.H.; et al. Serum metabolomic fingerprints of lambs fed chitosan and its association with performance and meat quality traits. Animal 2020, 14, 1987–1998. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Chen, S.; Hu, M.; Wang, Z.; Wu, G.; Ma, X.; Chen, Z.; Zheng, C. Low-molecular-weight chitosan supplementation increases the population of prevotella in the cecal contents of weanling pigs. Front. Microbiol. 2017, 8, 2182. [Google Scholar] [CrossRef]
- Ahmad, M.; Manzoor, K.; Ikram, S. Versatile nature of hetero-chitosan based derivatives as biodegradable adsorbent for heavy metal ions; a review. Int. J. Biol. Macromol. 2017, 105, 190–203. [Google Scholar] [CrossRef]
- Zeng, X.H.; Wu, Z.; Liu, Y.C.; Wen, S.M.; Wang, H.M. Preparation and application of chelating multi-metal chitosan selenite. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012018. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Y.; Shi, L.; Xing, Y.; Guo, S.; Gao, Y.; Liu, Z.; Yan, S.; Shi, B. Effects of rare earth-chitosan chelate on growth performance, antioxidative and immune function in broilers. Ital. J. Anim. Sci. 2022, 21, 303–313. [Google Scholar] [CrossRef]
- Li, F.; Zhou, J.; Zhu, Y.; Zhang, X.; Gao, Y.; Meng, L.; Guo, F.; Zhang, Y. Effects of rare earth-chitosan chelate on growth performance, serum biochemical indices, nutrient digestibility and fecal microbial flora of weaned piglets. Chin. J. Anim. Nutr. 2016, 28, 498–506. [Google Scholar]
- Zhou, Q.-L.; Xie, J.; Ge, X.-P.; Habte-Tsion, H.M.; Liu, B.; Ren, M. Growth performance and immune responses of gibel carp, Carassius auratus gibelio, fed with graded level of rare earth-chitosan chelate. Aquac. Int. 2016, 24, 453–463. [Google Scholar] [CrossRef]
- Le, W.; Ting-Ting, L.; Xi-Long, W.; Gui-Ping, Y.; Shi-Bin, Y. Chitosan supplementation may improve the digestive physiology and health of captive Leiothrix lutea. Avian Biol. Res. 2015, 8, 221–226. [Google Scholar] [CrossRef]
- Cai, L.; Nyachoti, C.M.; Hancock, J.D.; Lee, J.Y.; Kim, Y.H.; Lee, D.H.; Kim, I.H. Rare earth element-enriched yeast improved egg production and egg quality in laying hens in the late period of peak egg production. J. Anim. Physiol. Anim. Nutr. 2016, 100, 492–498. [Google Scholar] [CrossRef]
- Xiong, Y.; Pang, J.; Lv, L.; Wu, Y.; Li, N.; Huang, S.; Feng, Z.; Ren, Y.; Wang, J. Effects of maternal supplementation with rare earth elements during late gestation and lactation on performances, health, and fecal microbiota of the sows and their offspring. Animals 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Ladipo, M.; Adu, O.; Oyefeso, D.; Akinmuyisitan, I. Growth performance and blood profile of male rabbits fed dietary cerium oxide. Int. J. Agric. For. 2015, 3, 87–92. [Google Scholar]
- Li, Y.; Zhang, Q.; Feng, Y.; Yan, S.; Shi, B.; Guo, X.; Zhao, Y.; Guo, Y. Dietary Chitosan Supplementation Improved Egg Production and Antioxidative Function in Laying Breeders. Animals 2022, 12, 1225. [Google Scholar] [CrossRef]
- Lim, K.; You, S.; An, B.; Kang, C. Effects of dietary garlic powder and copper on cholesterol content and quality characteristics of chicken eggs. Asian-Australas. J. Anim. Sci. 2006, 19, 582–586. [Google Scholar] [CrossRef]
- Meng, Q.W.; Yan, L.; Ao, X.; Jang, H.D.; Cho, J.H.; Kim, I.H. Effects of chito-oligosaccharide supplementation on egg production, nutrient digestibility, egg quality and blood profiles in laying hens. Asian-Australas. J. Anim. Sci. 2010, 23, 1476–1481. [Google Scholar] [CrossRef]
- Yan, L.; Lee, J.; Meng, Q.; Ao, X.; Kim, I. Evaluation of dietary supplementation of delta-aminolevulinic acid and chito-oligosaccharide on production performance, egg quality and hematological characteristics in laying hens. Asian-Australas. J. Anim. Sci. 2010, 23, 1028–1033. [Google Scholar] [CrossRef]
- Xu, Q.; Azzam, M.M.M.; Zou, X.; Dong, X. Effects of chitooligosaccharide supplementation on laying performance, egg quality, blood biochemistry, antioxidant capacity and immunity of laying hens during the late laying period. Ital. J. Anim. Sci. 2020, 19, 1180–1187. [Google Scholar] [CrossRef]
- Çelik, L.; Küçükgülmez, A.; Serbester, U.; Kutlu, H. Kabuklu Deniz Ürünleri Işleme Atıklarından Farklı Karakterizasyonlarda Üretilmiş Kitosanın, Yumurtacı Tavuk Rasyonlarında Kullanımının Verim, Kalite ve Fonksiyonellik Üzerine Etkisi; Cukurova University: Adana, Turkey, 2014. [Google Scholar]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.-E.; Wang, N.; Liu, X.; An, Q.; Ye, X.-M.; Zhao, Z.-T.; Zhao, M.; Han, Y. Chemical composition and antioxidant activities of polysaccharides from Yingshan cloud mist tea. Oxidative Med. Cell. Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-E.; Wang, W.-J.; Zheng, G.-D.; Li, L.-Y. Physicochemical properties and antioxidant activities of polysaccharides from Gynura procumbens leaves by fractional precipitation. Int. J. Biol. Macromol. 2017, 95, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Thomas, R. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Mei, Q.-X.; Hu, J.-H.; Huang, Z.-H.; Fan, J.-J.; Huang, C.-L.; Lu, Y.-Y.; Wang, X.-P.; Zeng, Y. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol. Sin. 2021, 42, 942–953. [Google Scholar] [CrossRef]
- Pagano, G.; Guida, M.; Tommasi, F.; Oral, R. Health effects and toxicity mechanisms of rare earth elements—Knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 2015, 115, 40–48. [Google Scholar] [CrossRef]
- Kawagoe, M.; Hirasawa, F.; Wang, S.C.; Liu, Y.; Ueno, Y.; Sugiyama, T. Orally administrated rare earth element cerium induces metallothionein synthesis and increases glutathione in the mouse liver. Life Sci. 2005, 77, 922–937. [Google Scholar] [CrossRef]
- Schubert, D.; Dargusch, R.; Raitano, J.; Chan, S.-W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006, 342, 86–91. [Google Scholar] [CrossRef]
- Li, R.; Yu, L.; Qin, Y.; Zhou, Y.; Liu, W.; Li, Y.; Chen, Y.; Xu, Y. Protective effects of rare earth lanthanum on acute ethanol-induced oxidative stress in mice via Keap 1/Nrf2/p62 activation. Sci. Total Environ. 2021, 758, 143626. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Huating, W.; Hao, S.; Ying, C.; Xiaorong, W. The bioaccumulation of rare earth elements in the internal organs of fish and their effect on the activities of enzymes in liver. Zhongguo Huanjing Kexue 1999, 19, 141–144. [Google Scholar]
- Xie, J.; Wang, Z. The effect of organic rare-earth compounds on production performance of chicken. In Proceedings of the 2nd International Symposium on Trace Elements and Food Chain, Wuhan, China, 12–15 November 1998. [Google Scholar]
- Jones, M.P. Avian clinical pathology. Vet. Clin. North Am. Exot. Anim. Pract. 1999, 2, 663–687. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Public Policy Committee of the American Association for the Study of Liver D. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Fudge, A. Avian clinical pathology-hematology and chemistry. Avian Med. Surg. 1997, 142–147. [Google Scholar]
- Filipovic, N.; Stojevic, Z.; Milinkovic-Tur, S.; Ljubic, B.B.; Zdelar-Tuk, M. Changes in concentration and fractions of blood serum proteins of chickens during fattening. Vet. Arh. 2007, 77, 319. [Google Scholar]
- Thomson, C.A. IgG Structure and Function. Encycl. Immunobiol. 2016, 2, 15–22. [Google Scholar]
- Miao, Z.; Zhao, W.; Guo, L.; Wang, S.; Zhang, J. Effects of dietary supplementation of chitosan on immune function in growing Huoyan geese. Poult. Sci. 2020, 99, 95–100. [Google Scholar] [CrossRef]
- Pirzado, S.A.; Arain, M.A.; Huiyi, C.; Fazlani, S.A.; Alagawany, M.; Gouhua, L. Effect of Azomite on growth performance, immune function and tibia breaking strength of broiler chickens during starter period. Anim. Biotechnol. 2021, 33, 1539–1544. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, W.-D.; Liu, J.-S.; Zhang, X.-L. Effect of long-term intake of Y3+ in drinking water on immune function of rats. J. Hyg. Res. 2006, 35, 279–281. [Google Scholar]
- Cheng, J.; Cheng, Z.; Hu, R.; Cui, Y.; Cai, J.; Li, N.; Gui, S.; Sang, X.; Sun, Q.; Wang, L.; et al. Wang. Immune dysfunction and liver damage of mice following exposure to lanthanoids. Environ. Toxicol. 2014, 29, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.S.; Baeten, D.L.P.; Den Dunnen, J. The inflammatory function of human IgA. Cell. Mol. Life Sci. 2019, 76, 1041–1055. [Google Scholar] [CrossRef]
- Cui, L.; Xu, W.; Ai, Q.; Wang, D.; Mai, K. Effects of dietary chitosan oligosaccharide complex with rare earth on growth performance and innate immune response of turbot, Scophthalmus maximus L. Aquac. Res. 2013, 44, 683–690. [Google Scholar] [CrossRef]
- Davydova, V.N.; Kalitnik, A.A.; Markov, P.A.; Volod’Ko, A.V.; Popov, S.V.; Ermak, I.M. Cytokine-inducing and anti-inflammatory activity of chitosan and its low-molecular derivative. Appl. Biochem. Microbiol. 2016, 52, 476–482. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Liu, P.; Yuan, S.; Zang, J.; Li, S.; Piao, X. Effect of chito-oligosaccharide supplementation on immunity in broiler chickens. Asian-Australas. J. Anim. Sci. 2008, 21, 1651–1658. [Google Scholar] [CrossRef]
- Ma, J.Y.; Young, S.-H.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Ma, J.K.; Castranova, V. Interactive effects of cerium oxide and diesel exhaust nanoparticles on inducing pulmonary fibrosis. Toxicol. Appl. Pharmacol. 2014, 278, 135–147. [Google Scholar] [CrossRef]
- Zhang, B. Dietary chitosan oligosaccharides modulate the growth, intestine digestive enzymes, body composition and nonspecific immunity of loach Paramisgurnus dabryanus. Fish Shellfish. Immunol. 2019, 88, 359–363. [Google Scholar] [CrossRef]
- Jiang, Z.; Jia, Z.; Guo, Y.; Zhou, F.; Liu, H. Effect of rare earth elements on digest enzyme and growth of intestinal in common carp (Cyprinus Carpio). Chin. J. Anim. Nutr. 2007, 19, 86–90. [Google Scholar]
- Xinyi, X. Antimicrobial of chitooligosaccharides and its application to food preservation. J. Wuxi Univ. Light Ind. 1998, 17, 10–14. [Google Scholar]
- Redling, K. Rare Earth Elements in Agriculture with Emphasis on Animal Husbandry; LMU: Munich, Germany, 2006. [Google Scholar]
| Items | Content |
|---|---|
| Ingredient (%) | |
| Corn | 62.20 |
| Soybean meal | 26.98 |
| Soybean oil | 0.50 |
| Limestone powder | 8.50 |
| CaHPO4 | 0.90 |
| NaSO4 | 0.25 |
| Salt | 0.15 |
| Choline chloride | 0.12 |
| Vitamin premix 1 | 0.03 |
| Mineral premix 1 | 0.10 |
| DL-methionine (DL-Met) | 0.12 |
| Phytases | 0.05 |
| Zeolite powder | 0.10 |
| Total | 100.00 |
| Nutrients 2 | |
| ME (Mcal/kg) | 2.70 (2.65) |
| Crude protein (CP) (%) | 16.70 (16.50) |
| Calcium (Ca) (%) | 3.37 (3.50) |
| Available phosphorus (AP) (%) | 0.25 (0.32) |
| Lysine (Lys) (%) | 0.85 (0.75) |
| Methionine (Met) (%) | 0.38 (0.34) |
| Met + Cysteine (Cys) (%) | 0.64 (0.65) |
| Items | Dietary RECC Level (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 400 | A | L | Q | ||
| Average daily egg yield (%) | ||||||||
| 1~2 wk | 82.86 | 83.89 | 84.92 | 88.48 | 0.99 | 0.245 | 0.039 | 0.119 |
| 3~4 wk | 85.56 | 87.15 | 86.72 | 89.68 | 1.08 | 0.615 | 0.197 | 0.436 |
| 5~6 wk | 84.80 | 82.48 | 88.30 | 87.32 | 0.98 | 0.140 | 0.155 | 0.361 |
| 7~8 wk | 81.59 | 83.94 | 89.45 | 90.10 | 1.43 | 0.084 | 0.019 | 0.044 |
| 1~4 wk | 84.21 | 85.52 | 85.82 | 90.14 | 0.98 | 0.192 | 0.032 | 0.094 |
| 5~8 wk | 83.19 | 83.21 | 88.87 | 88.02 | 1.09 | 0.113 | 0.018 | 0.055 |
| 1~8 wk | 83.70 | 84.36 | 87.35 | 87.95 | 0.96 | 0.316 | 0.011 | 0.040 |
| ADFI (g) | ||||||||
| 1~2 wk | 108.65 | 107.09 | 107.68 | 107.53 | 0.51 | 0.726 | 0.608 | 0.712 |
| 3~4 wk | 103.04 | 106.04 | 102.85 | 103.23 | 1.25 | 0.802 | 0.806 | 0.930 |
| 5~6 wk | 109.48 | 107.98 | 109.35 | 109.75 | 0.83 | 0.894 | 0.737 | 0.878 |
| 7~8 wk | 105.43 b | 110.09 a | 112.14 a | 111.62 a | 0.80 | 0.005 | 0.008 | 0.001 |
| 1~4 wk | 105.85 | 106.57 | 105.27 | 105.38 | 0.71 | 0.927 | 0.690 | 0.925 |
| 5~8 wk | 106.26 b | 109.04 ab | 110.75 a | 110.69 a | 0.62 | 0.032 | 0.016 | 0.011 |
| 1~8 wk | 106.65 | 108.71 | 108.01 | 108.03 | 0.39 | 0.320 | 0.379 | 0.331 |
| FCR (g/g) | ||||||||
| 1~2 wk | 2.17 | 2.14 | 2.13 | 2.12 | 0.03 | 0.930 | 0.526 | 0.799 |
| 3~4 wk | 1.98 | 2.02 | 1.97 | 1.92 | 0.02 | 0.492 | 0.222 | 0.374 |
| 5~6 wk | 2.13 | 2.18 | 2.05 | 2.12 | 0.03 | 0.624 | 0.719 | 0.830 |
| 7~8 wk | 2.12 | 2.16 | 2.05 | 2.08 | 0.03 | 0.660 | 0.467 | 0.754 |
| 1~4 wk | 2.08 | 2.08 | 2.05 | 2.02 | 0.02 | 0.750 | 0.284 | 0.559 |
| 5~8 wk | 2.13 | 2.17 | 2.05 | 2.10 | 0.03 | 0.535 | 0.557 | 0.771 |
| 1~8 wk | 2.10 | 2.13 | 2.05 | 2.06 | 0.02 | 0.604 | 0.352 | 0.644 |
| Average egg weight (g) | ||||||||
| 1~2 wk | 60.68 | 59.85 | 60.00 | 59.49 | 0.24 | 0.383 | 0.123 | 0.279 |
| 3~4 wk | 61.00 | 60.39 | 60.63 | 60.00 | 0.22 | 0.449 | 0.147 | 0.357 |
| 5~6 wk | 61.22 | 60.85 | 60.79 | 60.19 | 0.20 | 0.366 | 0.073 | 0.209 |
| 7~8 wk | 61.61 | 61.49 | 61.06 | 60.67 | 0.20 | 0.332 | 0.062 | 0.183 |
| 1~4 wk | 60.84 | 60.12 | 60.31 | 59.75 | 0.22 | 0.373 | 0.114 | 0.283 |
| 5~8 wk | 61.42 | 61.17 | 60.93 | 60.43 | 0.19 | 0.329 | 0.058 | 0.172 |
| 1~8 wk | 61.13 | 60.64 | 60.62 | 60.09 | 0.20 | 0.352 | 0.074 | 0.208 |
| Items | Dietary RECC Level (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 400 | A | L | Q | ||
| Week 4 | ||||||||
| Egg weight (g) | 62.31 | 61.71 | 61.04 | 61.60 | 0.27 | 0.454 | 0.375 | 0.280 |
| Eggshell Strength (N/m2) | 41.18 | 38.24 | 39.01 | 38.49 | 0.63 | 0.347 | 0.244 | 0.312 |
| Eggshell Thickness (mm) | 0.453 | 0.447 | 0.444 | 0.440 | 0.003 | 0.556 | 0.160 | 0.348 |
| Egg shape index | 1.358 | 1.368 | 1.367 | 1.363 | 0.003 | 0.745 | 0.772 | 0.601 |
| Haught unit | 70.64 | 71.11 | 73.48 | 73.84 | 0.60 | 0.124 | 0.026 | 0.076 |
| Albumen Height (mm) | 5.48 | 5.50 | 5.72 | 5.80 | 0.06 | 0.186 | 0.036 | 0.113 |
| Week 8 | ||||||||
| Egg weight (g) | 61.75 | 62.37 | 61.42 | 61.96 | 0.32 | 0.790 | 0.712 | 0.935 |
| Eggshell Strength (N/m2) | 35.07 | 36.85 | 37.85 | 35.08 | 0.56 | 0.208 | 0.858 | 0.099 |
| Eggshell Thickness (mm) | 0.430 | 0.443 | 0.442 | 0.432 | 0.003 | 0.439 | 0.437 | 0.431 |
| Egg shape index | 1.356 | 1.359 | 1.364 | 1.355 | 0.003 | 0.765 | 0.890 | 0.598 |
| Haught unit | 77.12 | 75.16 | 77.71 | 76.57 | 0.54 | 0.398 | 0.912 | 0.994 |
| Albumen Height (mm) | 6.30 | 6.36 | 6.35 | 6.24 | 0.06 | 0.883 | 0.630 | 0.716 |
| Items | Dietary RECC Level (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 400 | A | L | Q | ||
| AST (U/L) | 185.67 | 178.00 | 175.67 | 196.17 | 4.46 | 0.379 | 0.320 | 0.207 |
| TP (g/L) | 50.42 b | 45.42 c | 52.77 ab | 55.17 a | 0.98 | <0.001 | 0.004 | 0.009 |
| ALB (g/L) | 14.05 ab | 13.22 b | 13.90 b | 14.87 a | 0.19 | 0.014 | 0.039 | 0.014 |
| GLU (nM) | 8.40 | 9.12 | 8.23 | 9.43 | 0.25 | 0.264 | 0.258 | 0.439 |
| UA (U/L) | 158.20 | 144.40 | 141.50 | 181.25 | 8.56 | 0.412 | 0.355 | 0.228 |
| TG (mM) | 31.64 | 28.02 | 31.37 | 29.55 | 0.70 | 0.967 | 0.900 | 0.989 |
| IgA (g/L) | 0.311 a | 0.262 b | 0.290 ab | 0.291 ab | 0.01 | 0.035 | 0.734 | 0.220 |
| IgG (g/L) | 2.11 a | 2.13 a | 1.94 b | 2.01 ab | 0.03 | 0.029 | 0.062 | 0.099 |
| IgM (g/L) | 1.025 | 0.982 | 1.050 | 1.064 | 0.02 | 0.437 | 0.352 | 0.652 |
| MDA (mM) | 5.48 a | 2.92 b | 3.92 ab | 5.36 a | 0.38 | 0.030 | 0.543 | 0.042 |
| GSH (mg/L) | 17.32 | 19.88 | 7.71 | 10.23 | 2.87 | 0.412 | 0.252 | 0.462 |
| GSH-Px (U/mL) | 3053 | 2568 | 3167 | 3270 | 179 | 0.586 | 0.438 | 0.683 |
| SOD (U/mL) | 85.03 | 74.40 | 83.38 | 84.19 | 1.70 | 0.099 | 0.637 | 0.420 |
| T-AOC (mM) | 0.734 a | 0.723 a | 0.653 ab | 0.587 b | 0.02 | 0.010 | 0.001 | 0.004 |
| Items | Dietary RECC Level (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 400 | A | L | Q | ||
| IL-2 (ng/mL) | 3.857 a | 3.419 b | 3.316 bc | 3.064 c | 0.08 | <0.001 | <0.001 | <0.001 |
| IL-6 (ng/mL) | 0.532 | 0.517 | 0.559 | 0.535 | 0.01 | 0.303 | 0.601 | 0.684 |
| TNF-α (ng/mL) | 1.353 a | 1.168 b | 1.326 a | 1.207 b | 0.02 | 0.001 | 0.132 | 0.302 |
| sIgA (μg/mL) | 22.30 | 22.50 | 23.22 | 21.95 | 0.28 | 0.437 | 0.669 | 0.329 |
| Items | Dietary RECC Level (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 400 | A | L | Q | ||
| AMS (U/mg prot) | 437.77 ab | 459.14 ab | 551.26 a | 296.23 b | 33.34 | 0.036 | 0.116 | 0.022 |
| LPS (U/g prot) | 124.04 b | 173.28 b | 274.47 a | 155.38 b | 18.02 | 0.010 | 0.517 | 0.010 |
| Trypsin (U/mg prot) | 284.68 b | 371.62 b | 571.44 a | 339.55 b | 33.93 | 0.008 | 0.529 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Chang, X.; Zhang, H.; Wang, J.; Qiu, K.; Wu, S. Effects of Dietary Rare Earth Chitosan Chelate on Performance, Egg Quality, Immune and Antioxidant Capacity, and Intestinal Digestive Enzyme Activity of Laying Hens. Polymers 2023, 15, 1600. https://doi.org/10.3390/polym15071600
Lu X, Chang X, Zhang H, Wang J, Qiu K, Wu S. Effects of Dietary Rare Earth Chitosan Chelate on Performance, Egg Quality, Immune and Antioxidant Capacity, and Intestinal Digestive Enzyme Activity of Laying Hens. Polymers. 2023; 15(7):1600. https://doi.org/10.3390/polym15071600
Chicago/Turabian StyleLu, Xinxin, Xinyu Chang, Haijun Zhang, Jing Wang, Kai Qiu, and Shugeng Wu. 2023. "Effects of Dietary Rare Earth Chitosan Chelate on Performance, Egg Quality, Immune and Antioxidant Capacity, and Intestinal Digestive Enzyme Activity of Laying Hens" Polymers 15, no. 7: 1600. https://doi.org/10.3390/polym15071600
APA StyleLu, X., Chang, X., Zhang, H., Wang, J., Qiu, K., & Wu, S. (2023). Effects of Dietary Rare Earth Chitosan Chelate on Performance, Egg Quality, Immune and Antioxidant Capacity, and Intestinal Digestive Enzyme Activity of Laying Hens. Polymers, 15(7), 1600. https://doi.org/10.3390/polym15071600