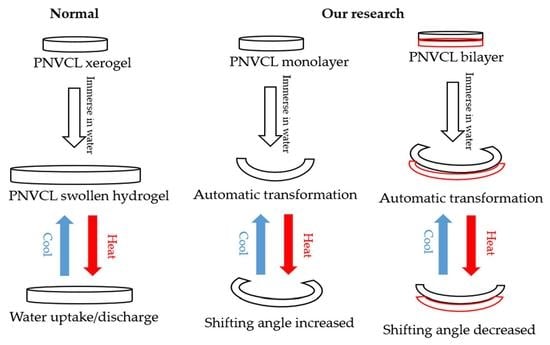
Strategies for Developing Shape-Shifting Behaviours and Potential Applications of Poly (N-vinyl Caprolactam) Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogels Synthesis
2.3. Form 2 SLA 3D Printer
2.4. Shapeshifting Designs
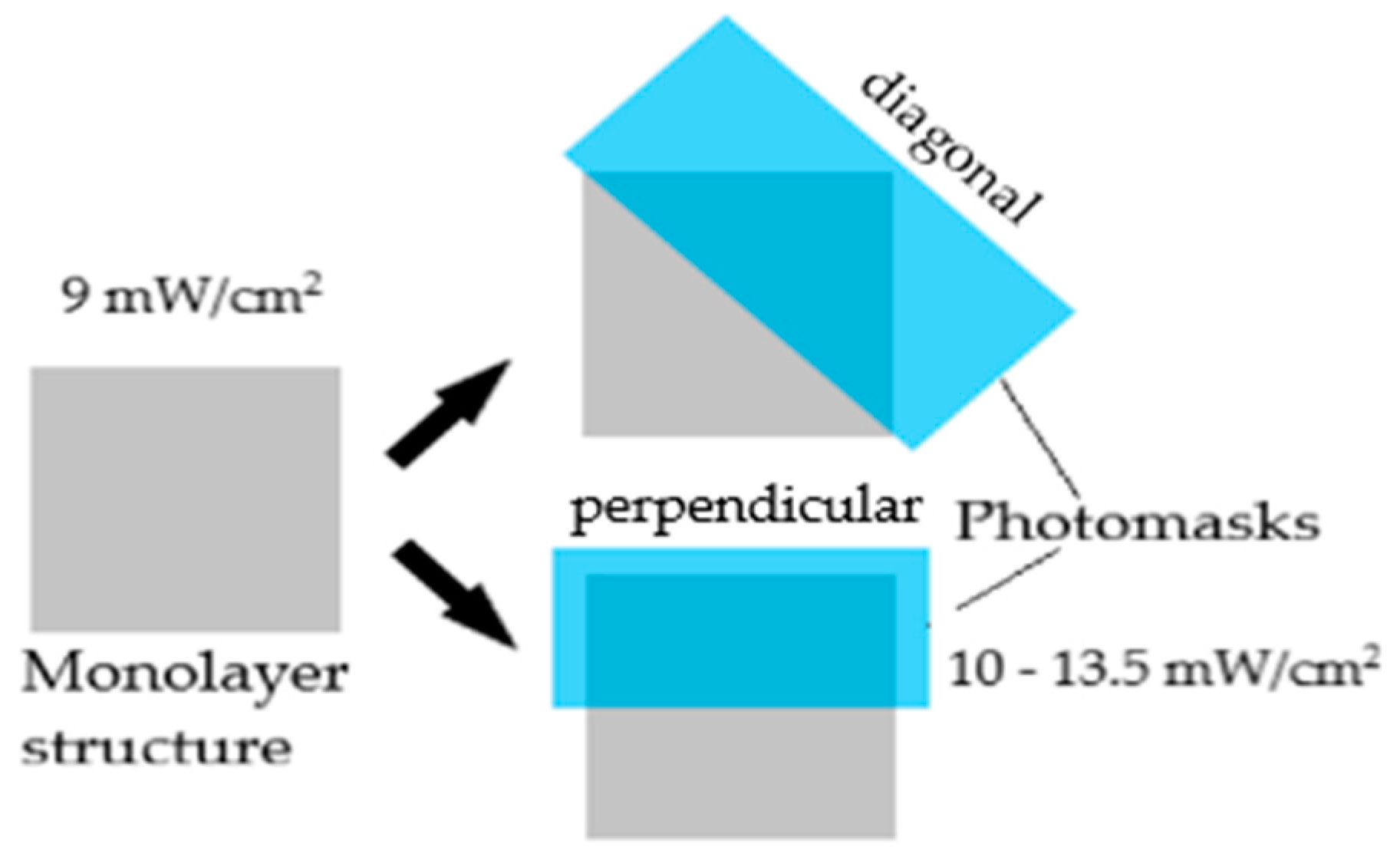
2.4.1. Monolayer
2.4.2. Bilayer
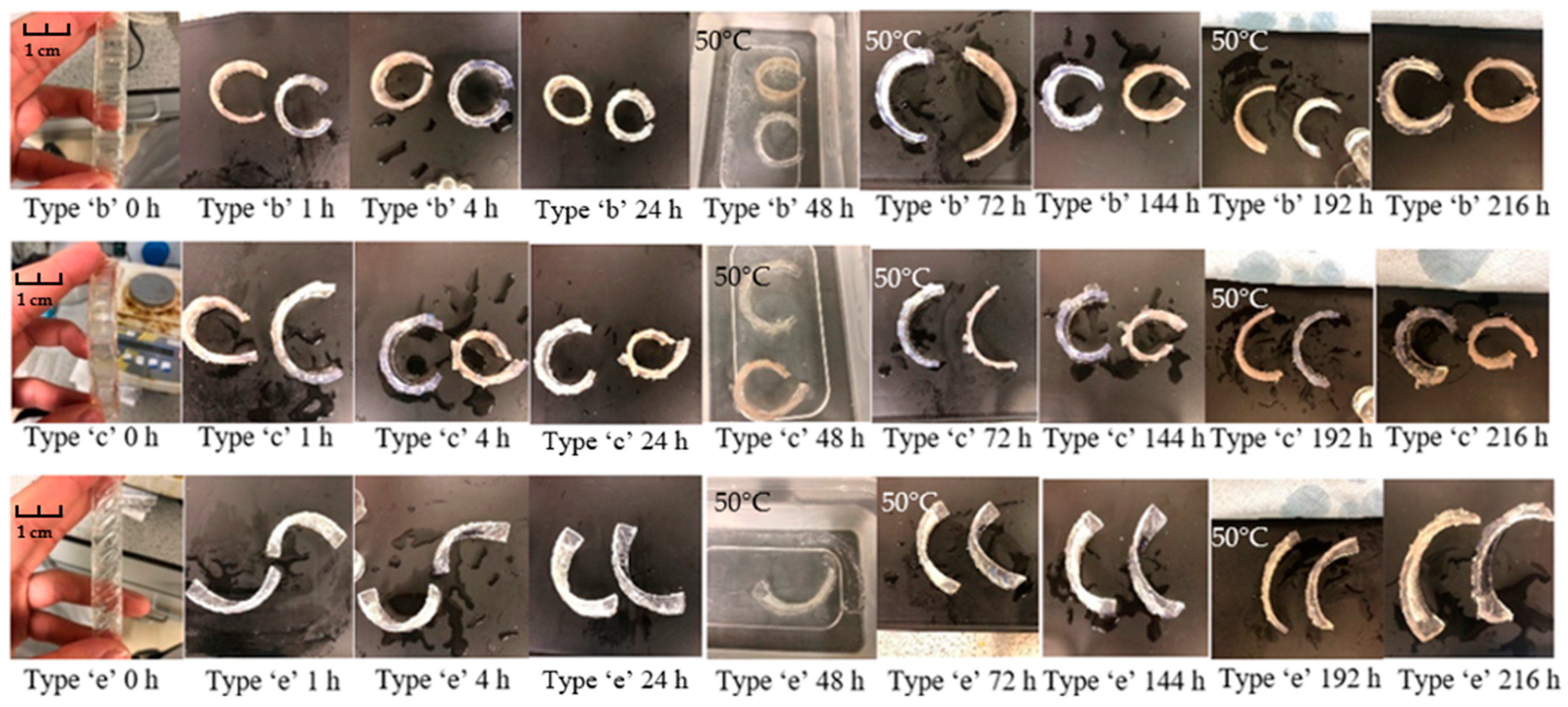
Bilayer Strips
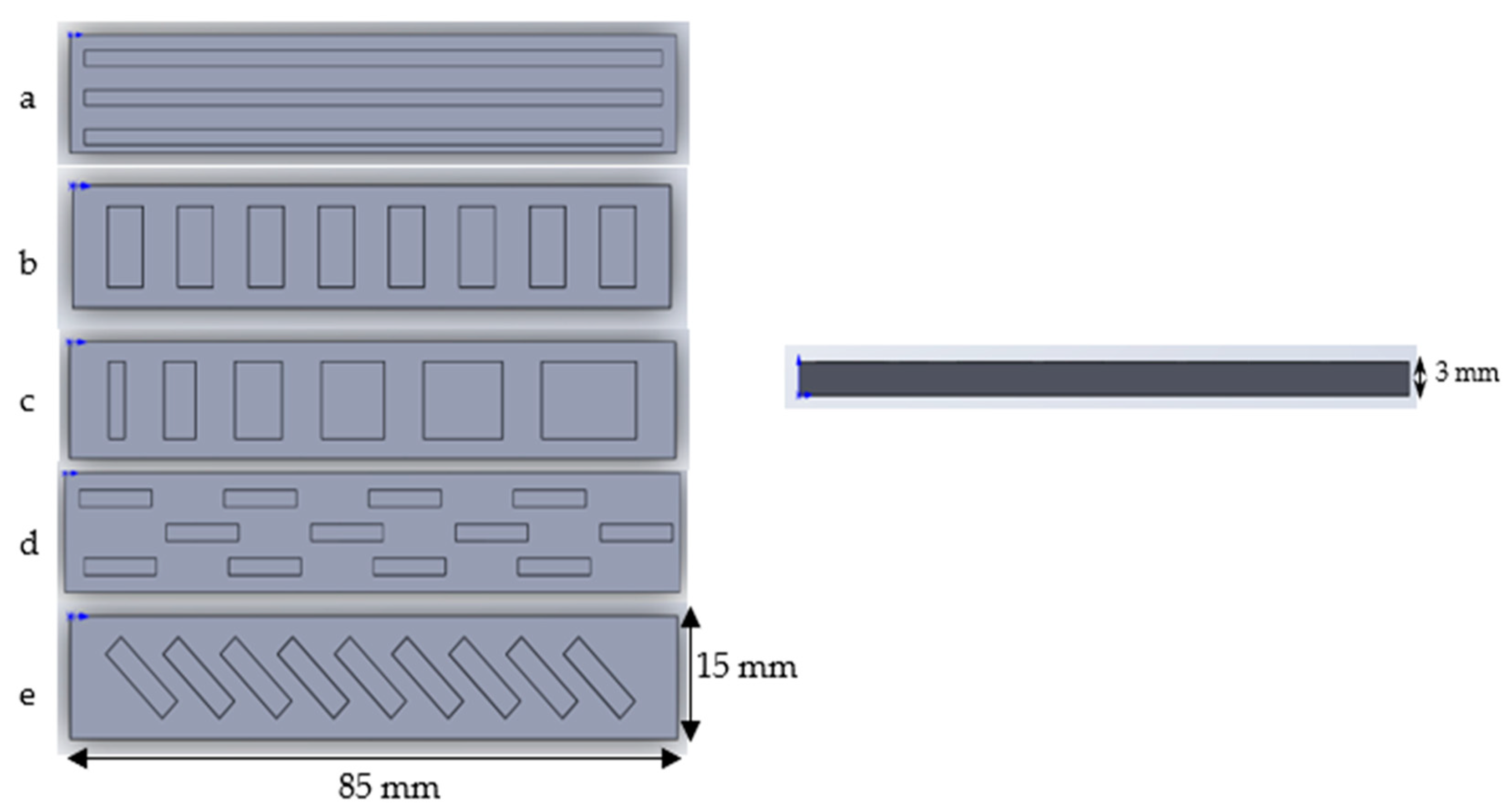
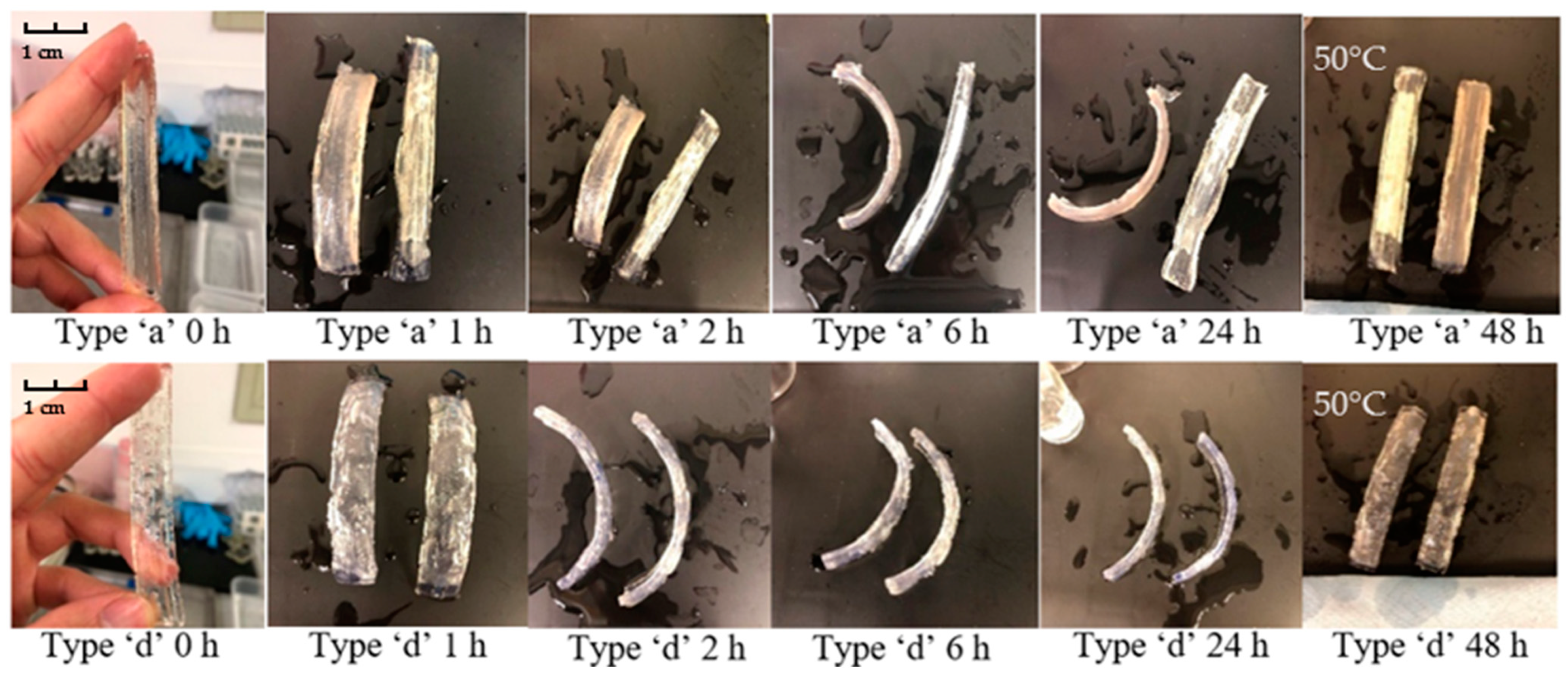
- (a)
- The strip consists of 3 long rectangular recesses, 2 mm in width, 81 mm in length and 1.5 mm in thickness, spaced 5 mm apart from each other.
- (b)
- The strip consists of 8 same size rectangular recesses, 10 mm in width, 5 mm in length and 1.5 mm in thickness, spaced 5 mm apart from each other.
- (c)
- The strip consists of 6 rectangular recesses. The length is increased by 2 mm each, from 2 mm to 12 mm. The width and thickness are maintained at 10 mm and 1.5 mm, respectively.
- (d)
- The strip consists of 12 rectangular recesses, 2 mm in width, 10 mm in length and 1.5 mm deep arranged in three rows. Four recesses are arranged in each row 20 mm apart from each other.
- (e)
- The strip consists of 9 identically sized rectangular recesses placed at a 45-degree angle, 11 mm in width, 3 mm in length and 1.5 mm in thickness, spaced 3 mm apart from each other.
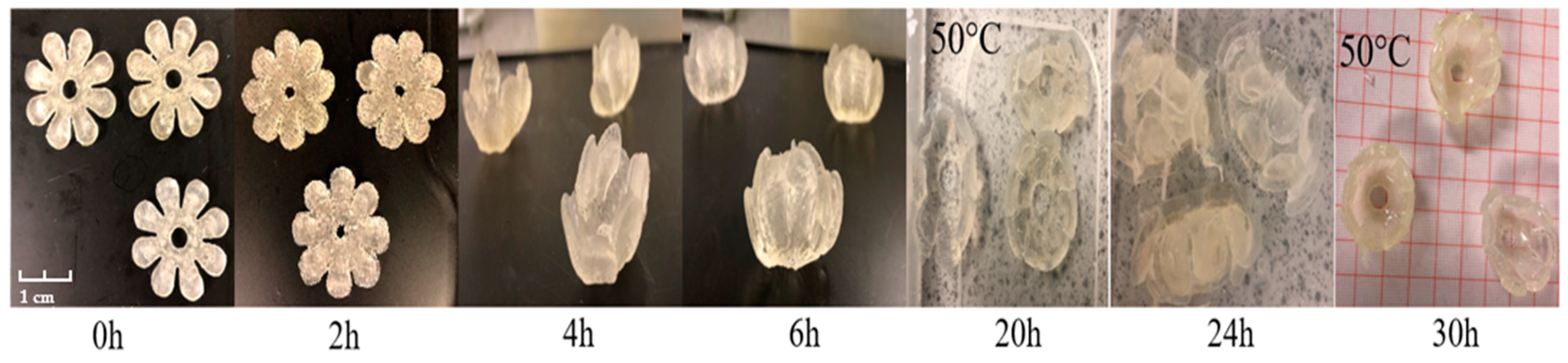
Bilayer Flowers
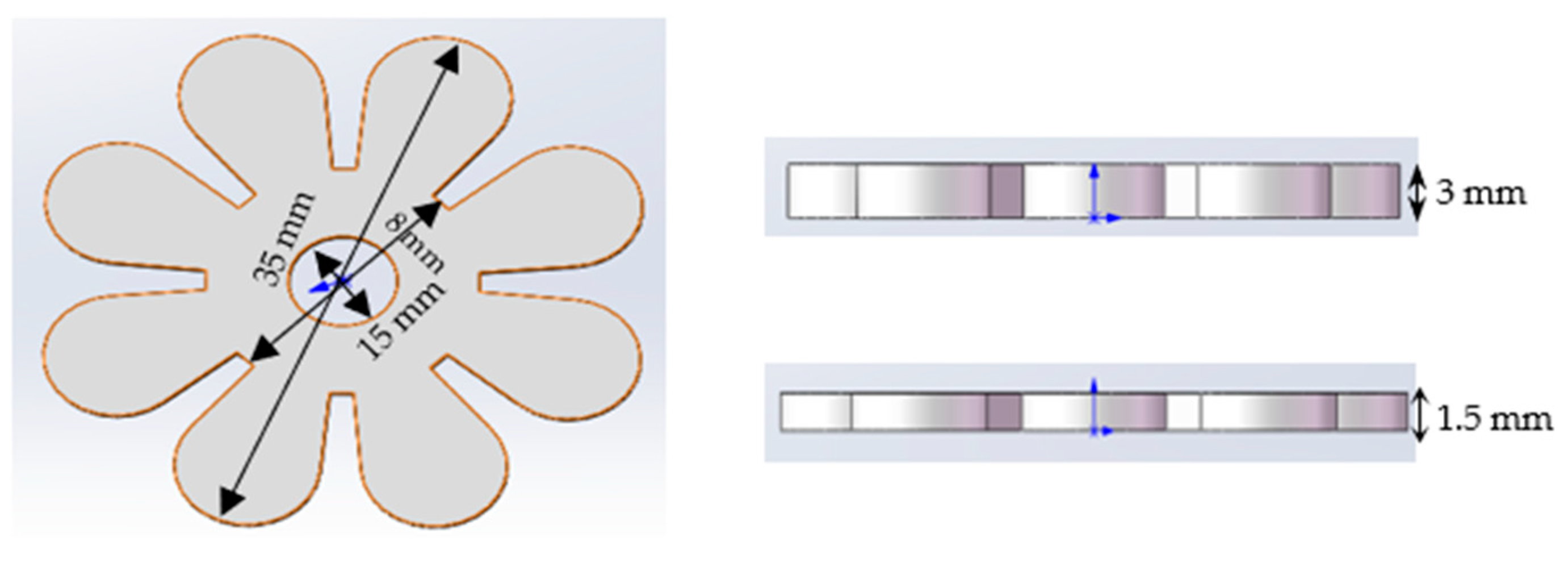
2.5. Silicone Mould Preparation
2.6. Pulsatile Swelling Studies
2.7. Fabrication of the Demonstrators
2.7.1. Automatic Demonstrator
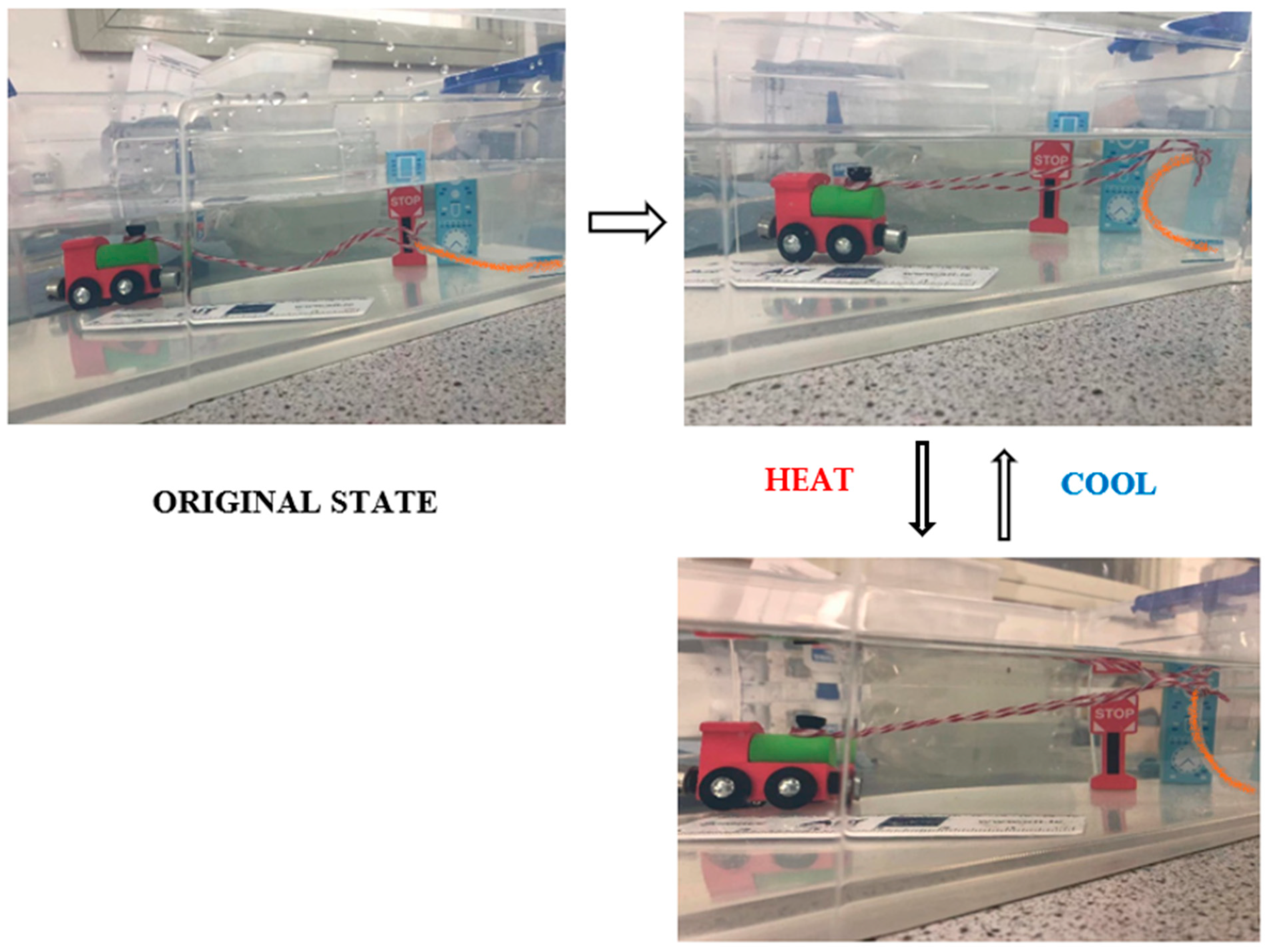
2.7.2. Toy Train Puller
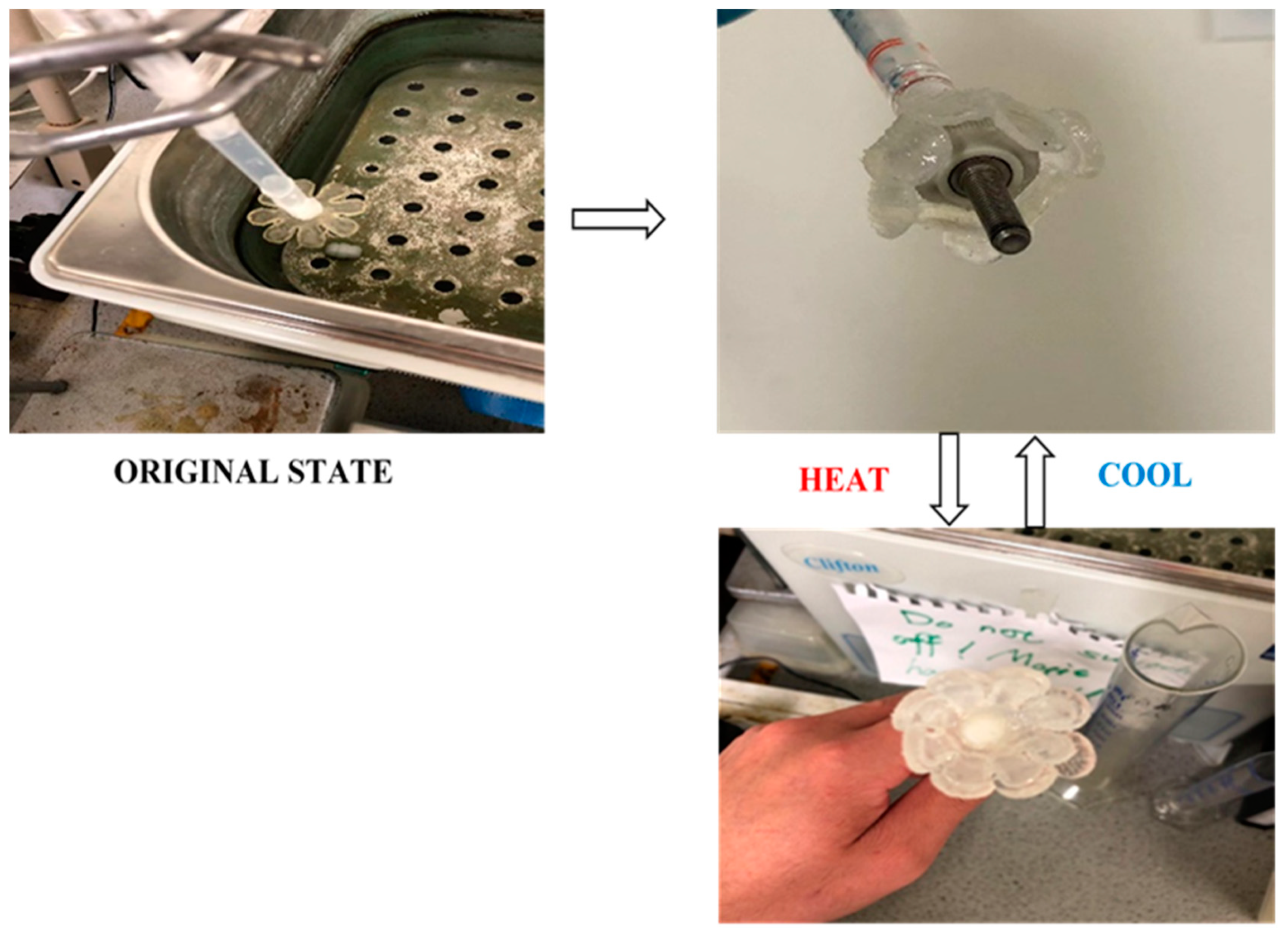
2.7.3. Gripper
3. Results and Discussion
3.1. Monolayer and Bilayer Sample Preparation
3.1.1. Monolayer Preparation
3.1.2. Bilayer Preparation
3.2. Pulsatile Swelling Studies
3.2.1. Monolayers
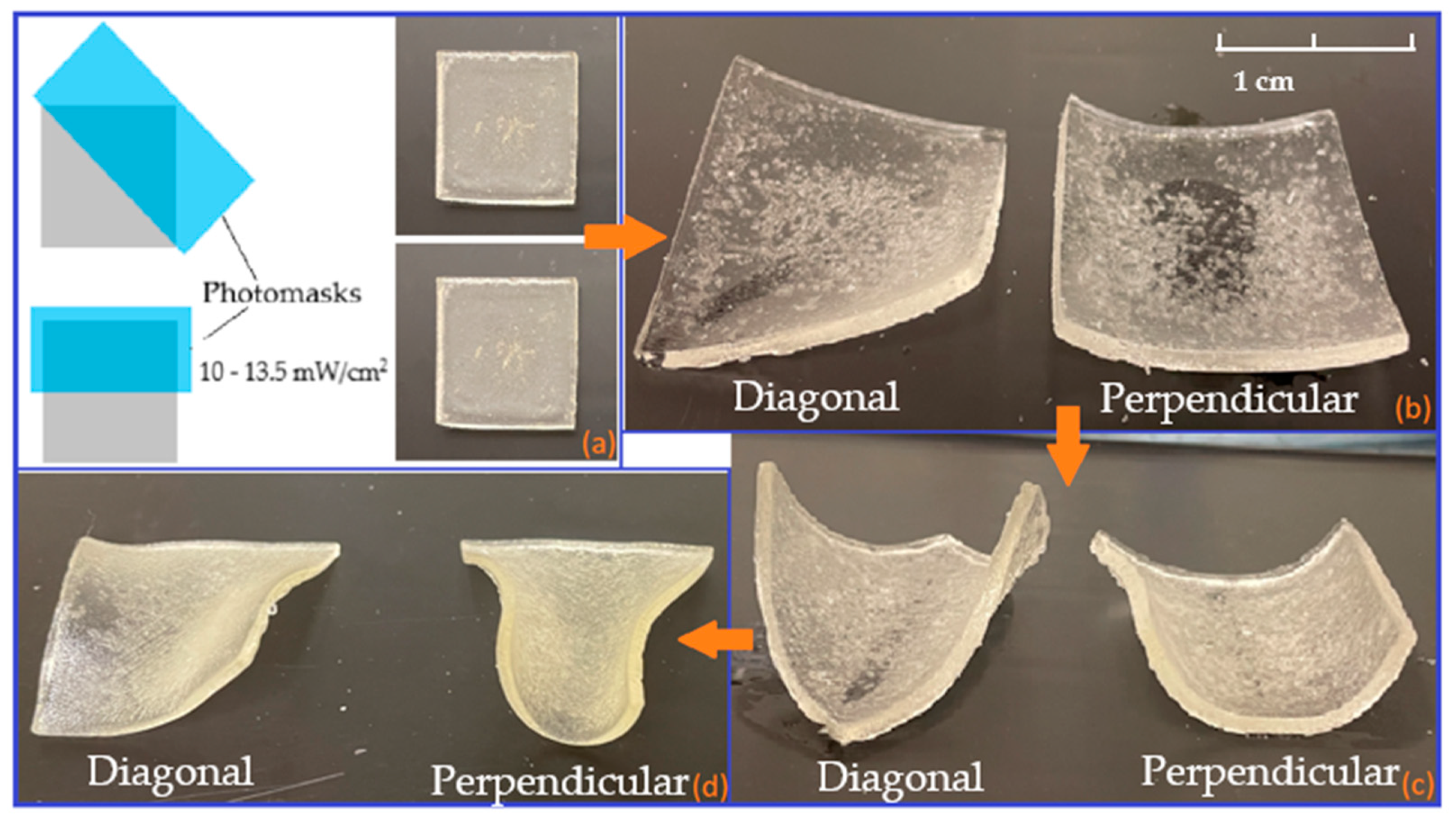
3.2.2. Bilayers Strips
3.2.3. Bilayer Flowers
3.3. Demonstrations
3.3.1. Demonstration I: TUS Demonstration
3.3.2. Demonstration II: Toy Train Puller
3.3.3. Demonstration III: Gripper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Manen, T.; Janbaz, S.; Zadpoor, A.A. Programming the shape-shifting of flat soft matter. Mater. Today 2018, 21, 144–163. [Google Scholar] [CrossRef]
- Studart, A.R. Biologically inspired dynamic material systems. Angew. Chem. Int. Ed. 2015, 54, 3400–3416. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Foster, J.C.; Ashby, T.A.; Walker, R.K.; Johnson, J.A.; Markin, V.S. Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ. 2010, 33, 163–173. [Google Scholar] [CrossRef]
- Oliver, K.; Seddon, A.; Trask, R.S. Morphing in nature and beyond: A review of natural and synthetic shape-changing materials and mechanisms. J. Mater. Sci. 2016, 51, 10663–10689. [Google Scholar] [CrossRef]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef]
- Mokhtar, S.M.A.; Alvarez de Eulate, E.; Yamada, M.; Prow, T.W.; Evans, D.R. Conducting polymers in wearable devices. Med. Devices Sens. 2021, 4, e10160. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymers 2013, 54, 2199–2221. [Google Scholar] [CrossRef]
- Stoychev, G.; Kirillova, A.; Ionov, L. Light-Responsive Shape-Changing Polymers. Adv. Opt. Mater. 2019, 7, 1900067. [Google Scholar] [CrossRef]
- Guenther, M.; Wallmersperger, T.; Keller, K.; Gerlach, G. Swelling Behaviour of Functionalized Hydrogels for Application in Chemical Sensors. In Intelligent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- He, X.; Sun, Y.; Wu, J.; Wang, Y.; Chen, F.; Fan, P.; Zhong, M.; Xiao, S.; Zhang, D.; Yang, J.; et al. Dual-stimulus bilayer hydrogel actuators with rapid, reversible, bidirectional bending behaviors. J. Mater. Chem. C 2019, 7, 4970–4980. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, Y.; Chi, J.; Wang, Y.; Zhao, Y. Bioinspired Bilayer Structural Color Hydrogel Actuator with Multienvironment Responsiveness and Survivability. Small Methods 2019, 3, 1900519. [Google Scholar] [CrossRef]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1282. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, G.; Trase, I.; Han, X.; Mei, Y. Mechanical Self-Assembly of a Strain-Engineered Flexible Layer: Wrinkling, Rolling, and Twisting. Phys. Rev. Appl. 2016, 5, 017001. [Google Scholar] [CrossRef]
- Otero, T.F.; Valero, L. Bending Monolayer Artificial Muscle. ChemElectroChem 2017, 4, 3276–3282. [Google Scholar] [CrossRef]
- Chen, W.; Sun, B.; Biehl, P.; Zhang, K. Cellulose-Based Soft Actuators. Macromol. Mater. Eng. 2022, 307, 2200072. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef]
- Zhuo, S.; Geever, L.M.; Halligan, E.; Shu, B.; Tie, H.; Breheny, C. A Development of New Material for 4D Printing and the Material Properties Comparison between the Conventional and Stereolithography Polymerised NVCL Hydrogels. J. Funct. Biomater. 2022, 13, 262. [Google Scholar] [CrossRef]
- Halligan, S.C.; Dalton, M.B.; Murray, K.A.; Dong, Y.; Wang, W.; Lyons, J.G.; Geever, L.M. Synthesis, characterisation and phase transition behaviour of temperature-responsive physically crosslinked poly (N-vinylcaprolactam) based polymers for biomedical applications. Mater. Sci. Eng. C 2017, 79, 130–139. [Google Scholar] [CrossRef]
- Lee, T.H.; Jho, J.Y. Temperature-Responsive Actuators Fabricated with PVA/PNIPAAm Interpenetrating Polymer Network Bilayers. Macromol. Res. 2018, 26, 659–664. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, P.; Le, X.; Lu, W.; Théato, P.; Ma, C.; Du, B.; Zhang, J.; Huang, Y.; Chen, T. Mimosa inspired bilayer hydrogel actuator functioning in multi-environments. J. Mater. Chem. C 2018, 6, 1320–1327. [Google Scholar] [CrossRef]
- Wu, J.J.; Huang, L.M.; Zhao, Q.; Xie, T. 4D Printing: History and Recent Progress. Chin. J. Polym. Sci. 2018, 36, 563–575. [Google Scholar] [CrossRef]
- Saritha, D.; Boyina, D. A concise review on 4D printing technology. In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; Volume 46, pp. 692–695. [Google Scholar]
- Zhuo, S.; Halligan, E.; Tie, B.S.H.; Breheny, C.; Geever, L.M. Lower Critical Solution Temperature Tuning and Swelling Behaviours of NVCL-Based Hydrogels for Potential 4D Printing Applications. Polymers 2022, 14, 3155. [Google Scholar] [CrossRef]
- Janbaz, S.; Hedayati, R.; Zadpoor, A.A. Programming the shape-shifting of flat soft matter: From self-rolling/self-twisting materials to self-folding origami. Mater. Horiz. 2016, 3, 536–547. [Google Scholar] [CrossRef]
- Kim, J.; Hanna, J.A.; Hayward, R.C.; Santangelo, C.D. Thermally responsive rolling of thin gel strips with discrete variations in swelling. Soft Matter 2012, 8, 2375–2381. [Google Scholar] [CrossRef]
- Li, X.; Cai, X.; Gao, Y.; Serpe, M.J. Reversible bidirectional bending of hydrogel-based bilayer actuators. J. Mater. Chem. B 2017, 5, 2804–2812. [Google Scholar] [CrossRef]




| Sample Codes | Photoinitiators | Monomers | Crosslinker | |
|---|---|---|---|---|
| Irgacure 2959 | NVCL | DMAAm | PEGDMA | |
| (wt %) (365 nm) | (wt %) | (wt %) | (wt %) | |
| S5 | 0.1 | 70 | 30 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, S.; Shu Hieng Tie, B.; Keane, G.; Geever, L.M. Strategies for Developing Shape-Shifting Behaviours and Potential Applications of Poly (N-vinyl Caprolactam) Hydrogels. Polymers 2023, 15, 1511. https://doi.org/10.3390/polym15061511
Zhuo S, Shu Hieng Tie B, Keane G, Geever LM. Strategies for Developing Shape-Shifting Behaviours and Potential Applications of Poly (N-vinyl Caprolactam) Hydrogels. Polymers. 2023; 15(6):1511. https://doi.org/10.3390/polym15061511
Chicago/Turabian StyleZhuo, Shuo, Billy Shu Hieng Tie, Gavin Keane, and Luke M. Geever. 2023. "Strategies for Developing Shape-Shifting Behaviours and Potential Applications of Poly (N-vinyl Caprolactam) Hydrogels" Polymers 15, no. 6: 1511. https://doi.org/10.3390/polym15061511
APA StyleZhuo, S., Shu Hieng Tie, B., Keane, G., & Geever, L. M. (2023). Strategies for Developing Shape-Shifting Behaviours and Potential Applications of Poly (N-vinyl Caprolactam) Hydrogels. Polymers, 15(6), 1511. https://doi.org/10.3390/polym15061511