Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Au-NPs
2.3. Characterization of Synthesized Au-NPs
2.3.1. UV–Visible Spectroscopic Determination
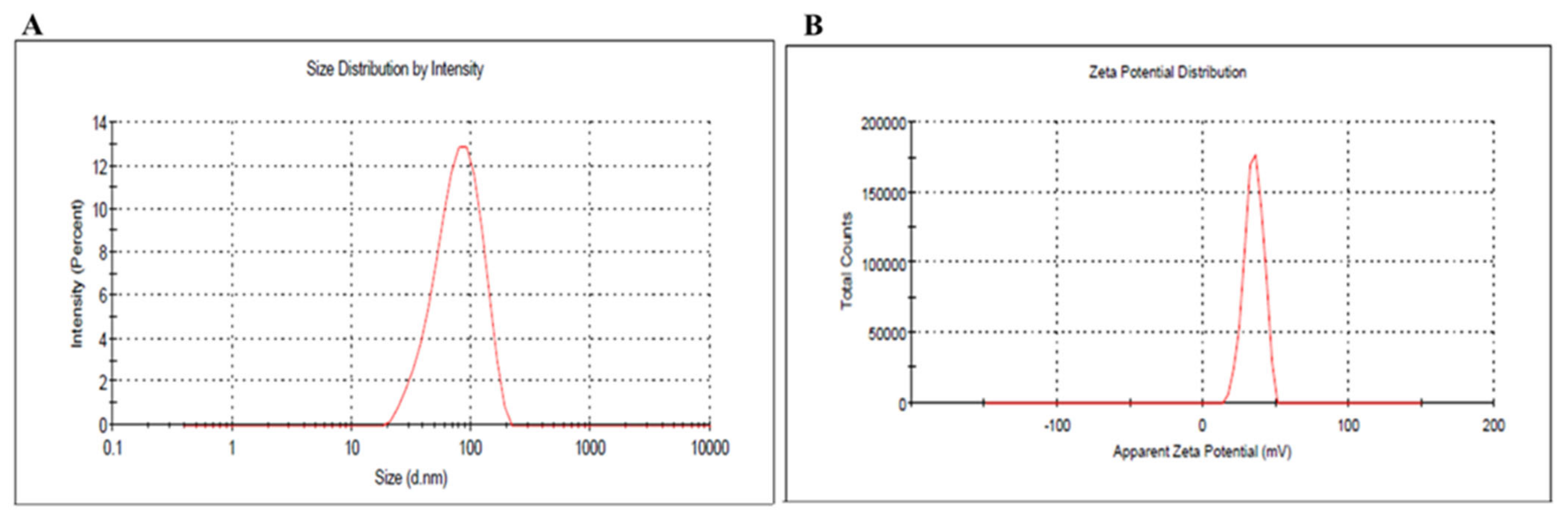
2.3.2. Dynamic Light Scattering Analysis and Zeta Potential
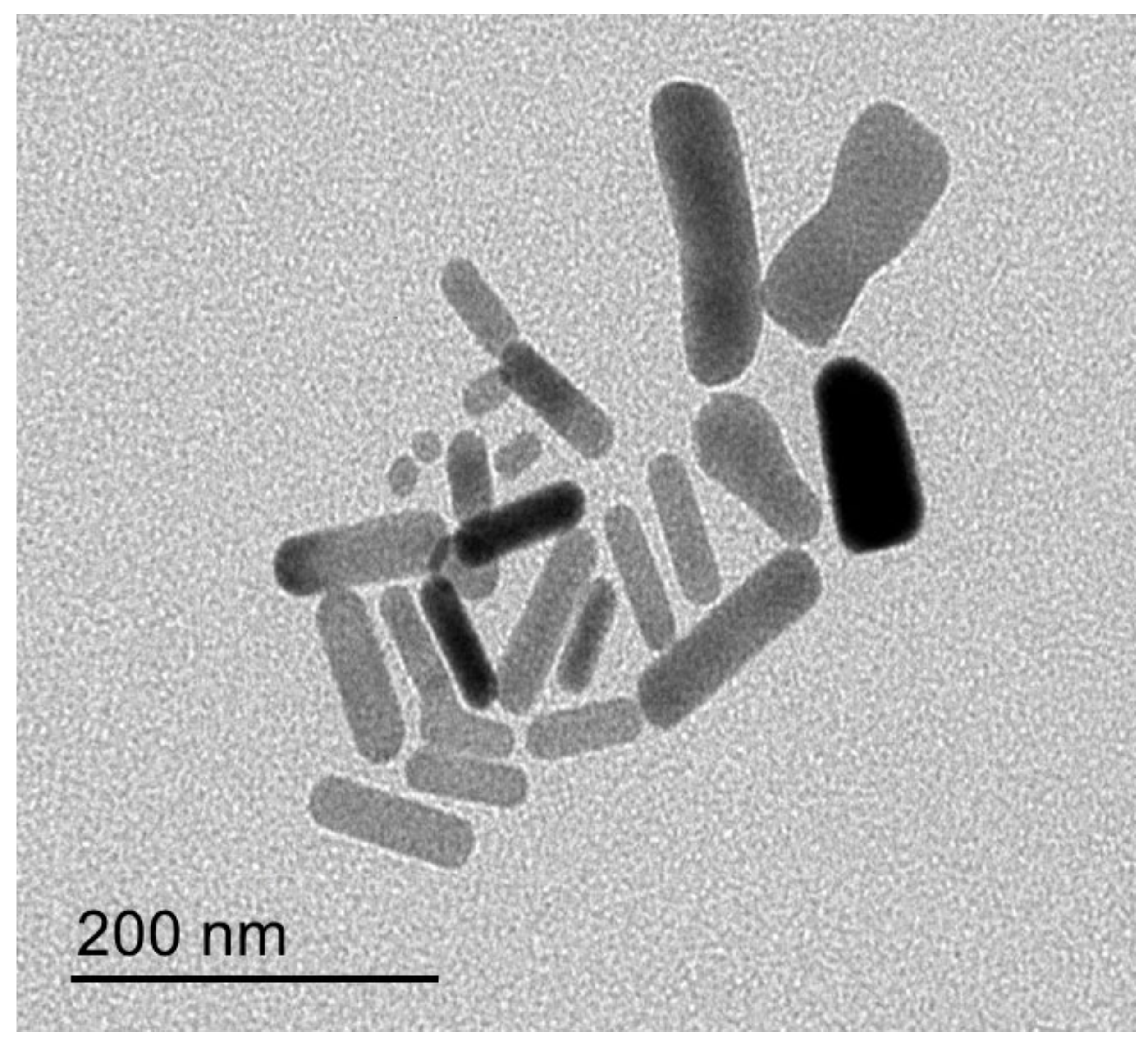
2.3.3. Transmission Electron Microscopy (TEM)
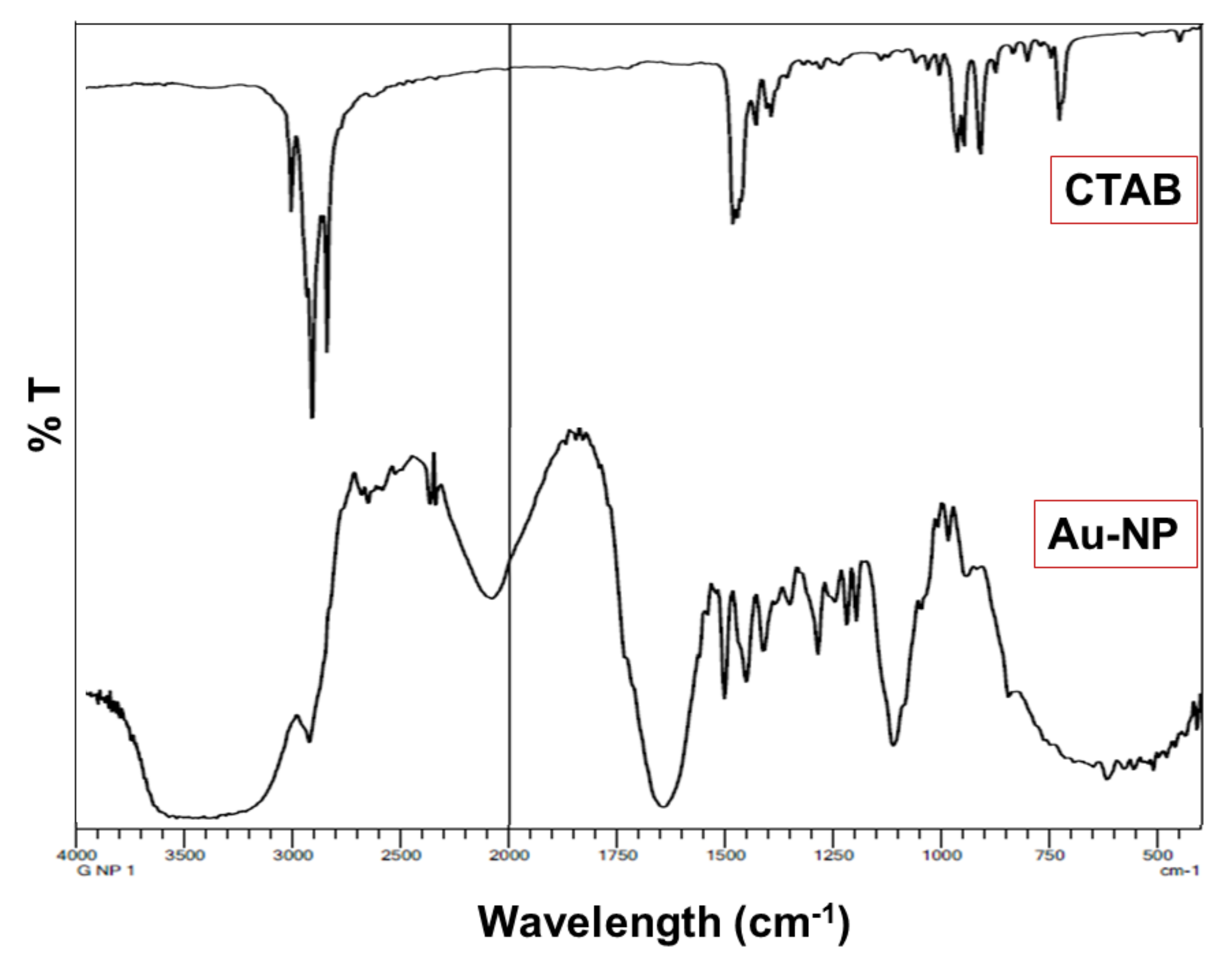
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Fabrication of Topical Au-NPs Gel
2.6. Characterization of the Developed Au-NPs Gel Preparation
2.6.1. Visual Examination
2.6.2. pH Measurement
2.6.3. Viscosity Measurement
2.6.4. Spreadability Determination
2.7. Antimicrobial Studies
2.7.1. Culturing of the Microorganisms
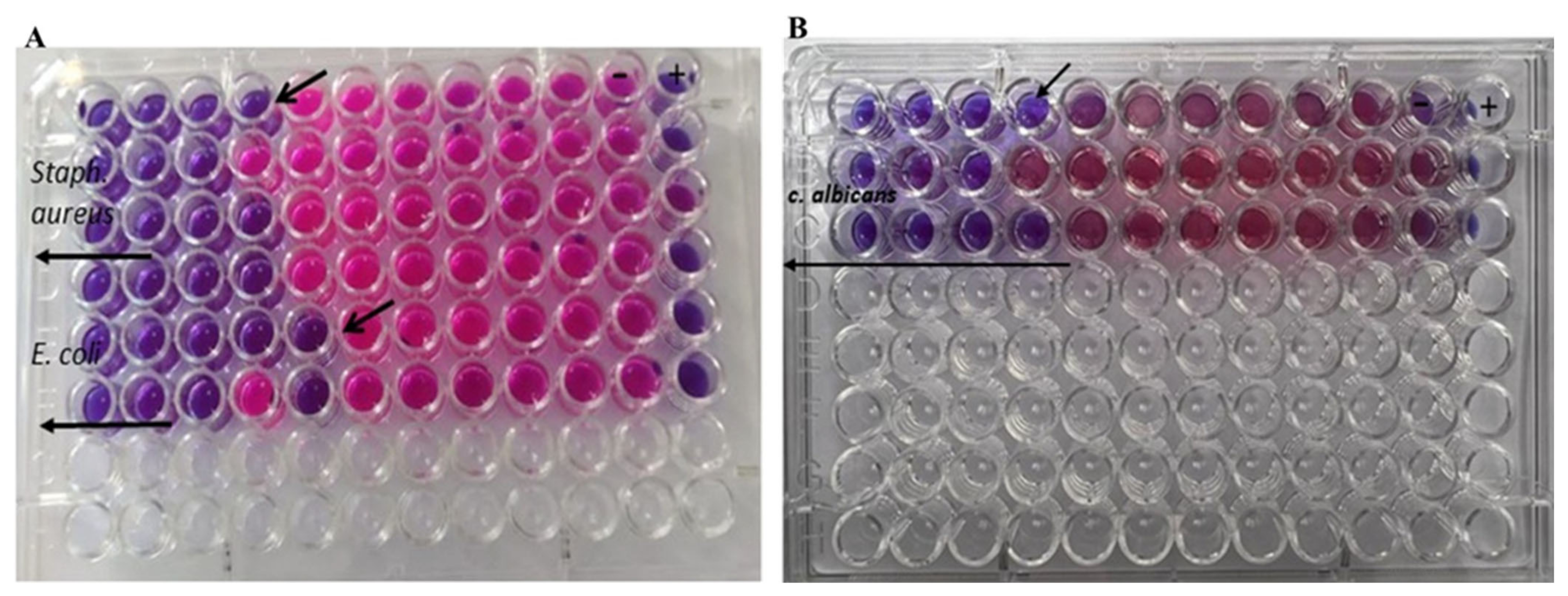
2.7.2. The Antimicrobial Activity of Au-NPs
2.7.3. Minimum Bactericidal and Fungicidal Concentration Determination
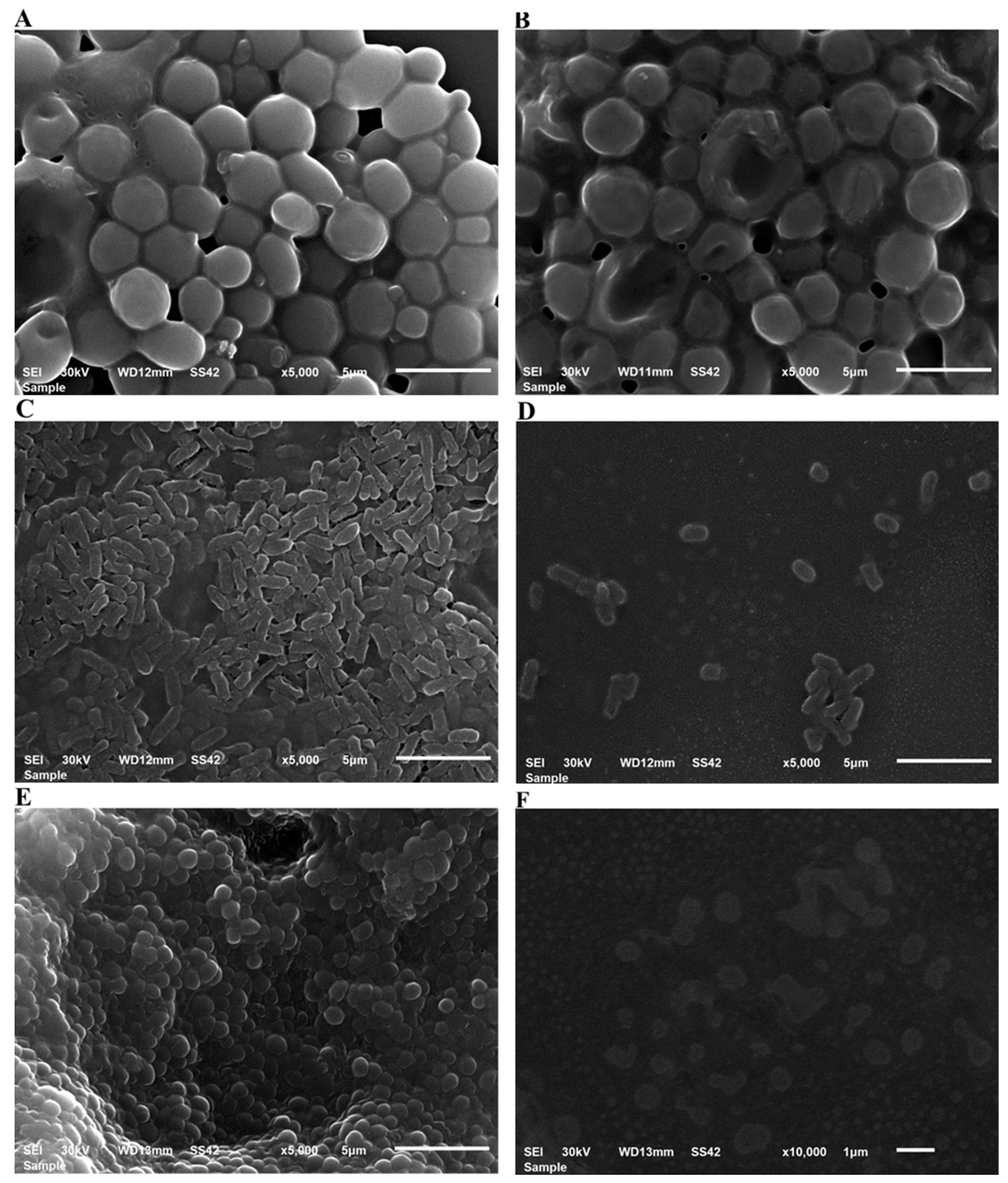
2.7.4. Scanning Electron Microscopy (SEM) Imaging of S. aureus, E. coli and C. albicans treated with Au-NPs
2.8. In Vivo Studies
2.8.1. Animal’s Acquisition
2.8.2. Ethical Approval
2.8.3. Induction of Diabetes
2.8.4. Experimental Design
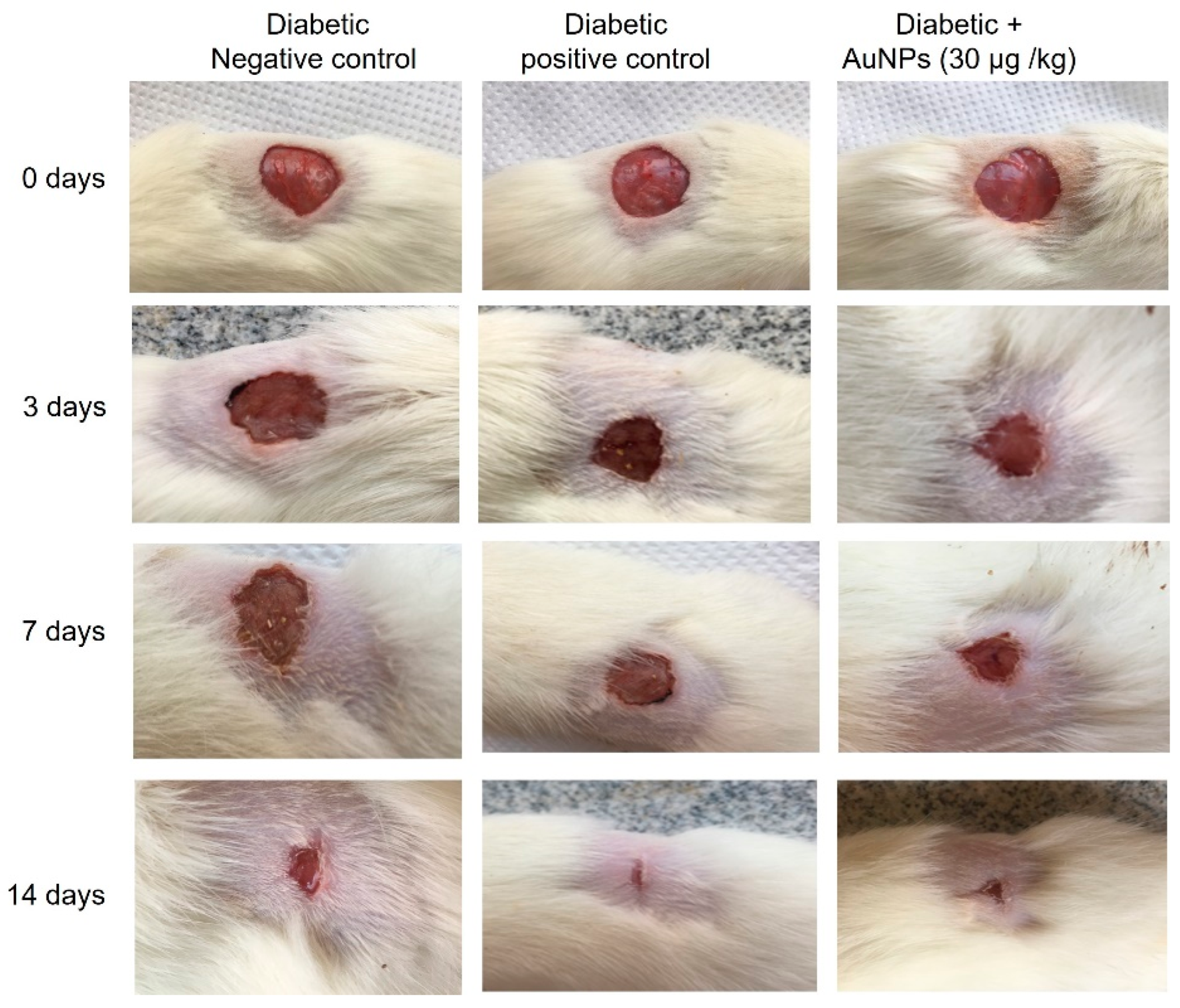
2.8.5. Excision Wound Establishment
2.8.6. Measurement of Wound Area Parameters
Macroscopic Investigation
2.8.7. Skin Tissues Measured Parameters
2.8.8. Measurement of Angiogenesis Related Factors
2.8.9. Measurement of Lipid Peroxidation and Antioxidant Enzymes Activities
2.8.10. Measurement of the Inflammatory Cytokine
2.9. Statistical Analysis
3. Results
3.1. Characterization of Au-NPs
3.1.1. UV–Visible Spectroscopic Determination
3.1.2. Dynamic Light Scattering Analysis and Zeta Potential
3.1.3. Transmission Electron Microscopy (TEM)
3.2. FTIR
3.3. Characterization of the Developed Au-NPs Gel Preparation
3.3.1. Visual Examination
3.3.2. pH Measurement
3.3.3. Viscosity Measurement
3.3.4. Spreadability Determination
3.4. Antibacterial Studies
3.4.1. Determination of the Antibacterial and Antifungal Effects
3.4.2. Antimicrobial and Antibiofilm Potential as Characterized by SEM
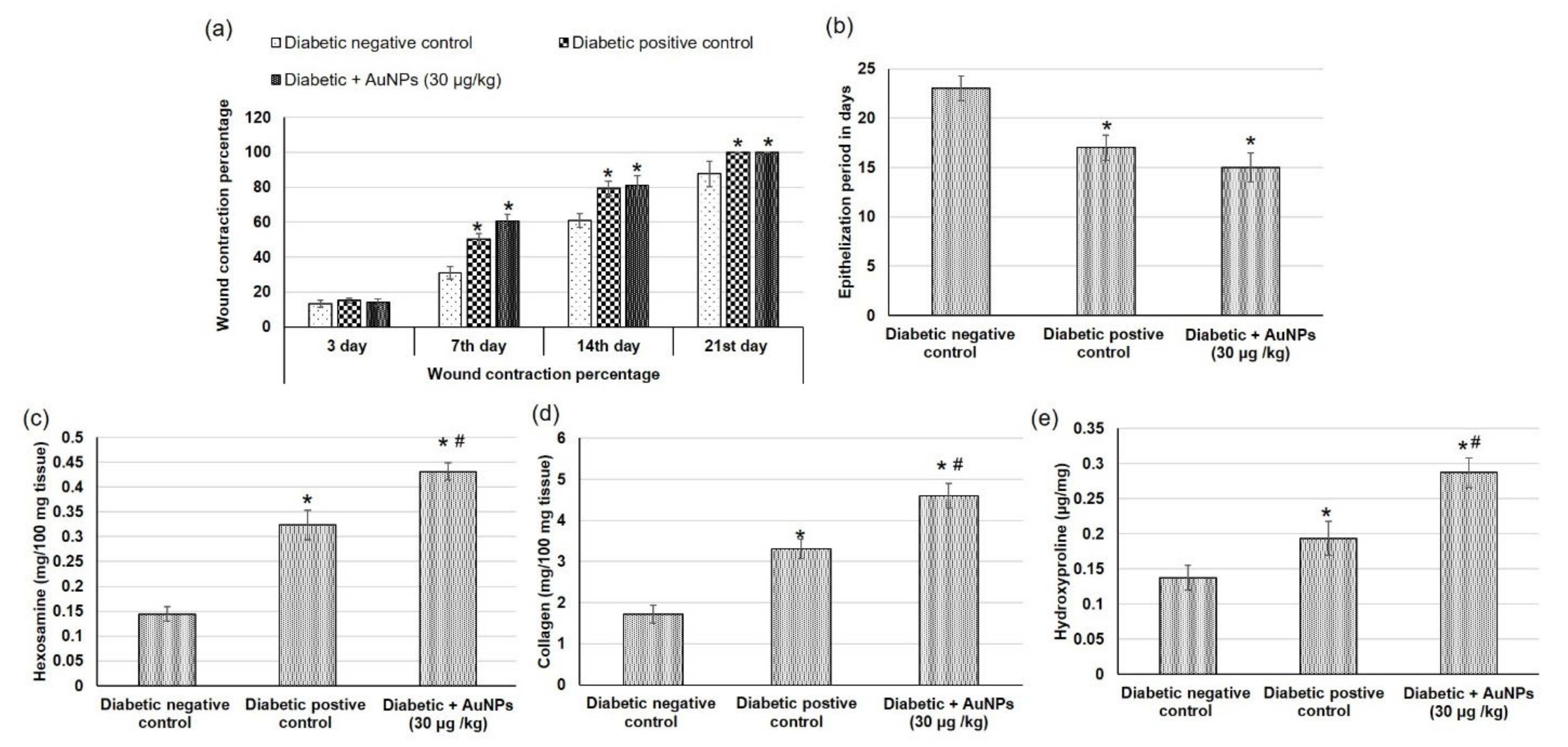
3.5. Assessment of Au-NPs on Excision Wound Healing Parameters in Diabetic Animals
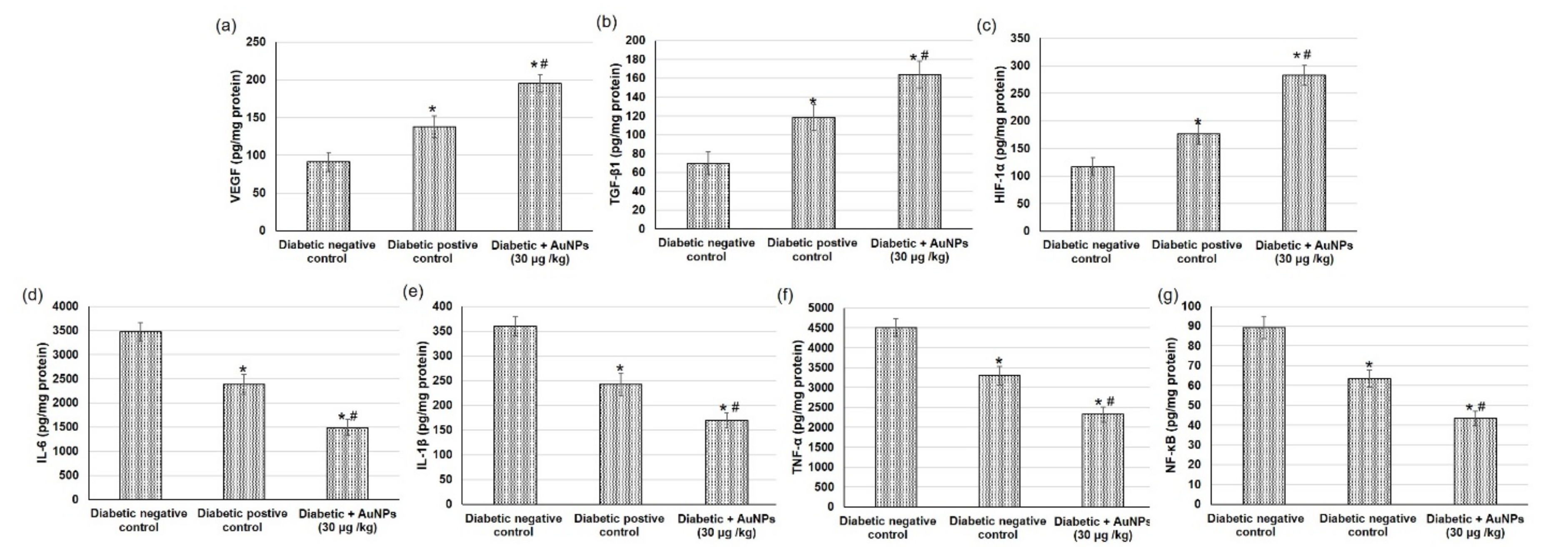
3.6. Assessment of AuNPs on Angiogenesis-Related Factors in Excision Wound Healing in Diabetic Animals
3.7. Assessment of Au-NPs on the Inflammatory Mediators in Excision Wound Healing in Diabetic Animals
3.8. Assessment of AuNPs Antioxidant Activity and Lipid Peroxidation in Excision Wound Healing Parameters in Diabetic Animals
4. Discussion
4.1. Characterization of Au-NPs
4.2. FTIR Study
4.3. Characterization of the Developed Au-NPs Gel Preparation
4.4. Antibacterial Studies
4.5. In Vivo Evaluation of the Wound-Healing Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Welte, T. New antibiotic development: The need versus the costs. Lancet Infect. Dis. 2016, 16, 386–387. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Hamid, A.; Marwa, I.; El-Malt, R.; Azab, D.S.; Albogami, S.; Al-Sanea, M.M.; Soliman, W.E.; Ghoneim, M.M.; Bendary, M.M. Molecular detection of fluoroquinolone resistance among multidrug-, extensively drug-, and pan-drug-resistant Campylobacter species in Egypt. Antibiotics 2021, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Microbiol. Biotechnol. Lett. 2011, 39, 77–85. [Google Scholar]
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and applications. Mater. Biomed. Eng. Inorg. Micro Nanostruct. 2019, 211–240. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef]
- Kandpal, N.; Sah, N.; Loshali, R.; Joshi, R.; Prasad, J. Co-precipitation method of synthesis and characterization of iron oxide nanoparticles. J. Sci. Ind. Res. 2014, 73, 87–90. [Google Scholar]
- Saleh, T.; Alaqad, K. Analytical Toxicology Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Env. Anal. Toxicol. 2016, 6, 4. [Google Scholar]
- Aashritha, S. Synthesis of silver nanoparticles by chemical reduction method and their antifungal activity. Int. Res. J. Pharm. 2013, 4, 111–113. [Google Scholar]
- Rioux, D.; Meunier, M. Seeded growth synthesis of composition and size-controlled gold–silver alloy nanoparticles. J. Phys. Chem. C 2015, 119, 13160–13168. [Google Scholar] [CrossRef]
- Kim, M.; Son, W.-S.; Ahn, K.H.; Kim, D.S.; Lee, H.-s.; Lee, Y.-W. Hydrothermal synthesis of metal nanoparticles using glycerol as a reducing agent. J. Supercrit. Fluids 2014, 90, 53–59. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Kon, K.V.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Coping with antibiotic resistance: Combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev. Anti Infect. Ther. 2011, 9, 1035–1052. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Korani, S.; Rashidi, K.; Hamelian, M.; Jalalvand, A.R.; Tajehmiri, A.; Korani, M.; Sathyapalan, T.; Sahebkar, A. Evaluation of Antimicrobial and Wound Healing Effects of Gold Nanoparticles Containing Abelmoschus esculentus (L.) Aqueous Extract. Bioinorg. Chem. Appl. 2021, 2021, 7019130. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Antibacterial activity of gold nanorods against Staphylococcus aureus and Propionibacterium acnes: Misinterpretations and artifacts. Int. J. Nanomed. 2017, 12, 7311–7322. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Cieśluk, M.; Chmielewska, S.; Durnaś, B.; Król, G.; Wollny, T.; Deptuła, P.; Kochanowicz, J. Rod-shaped gold nanoparticles exert potent candidacidal activity and decrease the adhesion of fungal cells. Nanomedicine 2020, 15, 2733–2752. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-driven therapeutic interventions in wound healing: Potential uses and applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Lau, P.; Bidin, N.; Islam, S.; Shukri, W.N.B.W.M.; Zakaria, N.; Musa, N.; Krishnan, G. Influence of gold nanoparticles on wound healing treatment in rat model: Photobiomodulation therapy. Lasers Surg. Med. 2017, 49, 380–386. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Vishalli, D.; Dheen, S.T.; Bay, B.-H.; Srinivasan, D.K. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials 2020, 10, 1234. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Gao, S.; Cui, Y.; Ji, X.; Zhang, X.; Wang, L. Biomineralized synthesis of palladium nanoflowers for photothermal treatment of cancer and wound healing. Int. J. Pharm. 2022, 615, 121489. [Google Scholar] [CrossRef]
- Volkova, N.; Yukhta, M.; Pavlovich, O.; Goltsev, A. Application of cryopreserved fibroblast culture with Au nanoparticles to treat burns. Nanoscale Res. Lett. 2016, 11, 1–6. [Google Scholar] [CrossRef]
- Leu, J.-G.; Chen, S.-A.; Chen, H.-M.; Wu, W.-M.; Hung, C.-F.; Yao, Y.-D.; Tu, C.-S.; Liang, Y.-J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Al-Kharabsheh, L.M.; Khalil, E.A.; Abu-Dahab, R. Interaction of Gold Nanorods with Human Dermal Fibroblasts: Cytotoxicity, Cellular Uptake, and Wound Healing. Nanomaterials 2019, 9, 1131. [Google Scholar] [CrossRef]
- Hassan, A.; Elebeedy, D.; Matar, E.R.; Fahmy Mohamed Elsayed, A.; Abd El Maksoud, A.I. Investigation of Angiogenesis and Wound Healing Potential Mechanisms of Zinc Oxide Nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Dhubiab, B.E.A.; Mahdy, M.A.; Elnahas, H.M. Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Deliv. 2020, 27, 1134–1146. [Google Scholar] [CrossRef]
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.-I.M.; Emeka, P.M.; Badger-Emeka, L.I.; Matsunami, K.; Shehata, T.M.; Elsewedy, H.S. Date Palm Extract (Phoenix dactylifera) PEGylated Nanoemulsion: Development, Optimization and Cytotoxicity Evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Elsewedy, H.S.; AbuLila, A.S.; Almansour, K.; Unissa, R.; Elghamry, H.A.; Soliman, M.S. Quality by Design for Optimizing a Novel Liposomal Jojoba Oil-Based Emulgel to Ameliorate the Anti-Inflammatory Effect of Brucine. Gels 2021, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.M.; Ibrahim, M.M.; Abdallah, M.H.; Mahdy, M.A. Sulpiride microemulsions as antipsychotic nasal drug delivery systems: In-vitro and pharmacodynamic study. J. Drug Delivery Sci. Technol. 2016, 36, 10–22. [Google Scholar] [CrossRef]
- Vowden, P.; Bond, E.; Meuleneire, F. Managing high viscosity exudate. Wounds UK 2015, 11, 56–60. [Google Scholar]
- Elsewedy, H.S.; Aldhubiab, B.E.; Mahdy, M.A.; Elnahas, H.M. Brucine PEGylated nanoemulsion: In vitro and in vivo evaluation. Colloids Surf. A 2021, 608, 125618. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. [Google Scholar] [CrossRef]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef]
- Das, S.; Pramanik, T.; Jethwa, M.; Roy, P. Flavonoid-Decorated Nano-gold for Antimicrobial Therapy Against Gram-negative Bacteria Escherichia coli. Appl. Biochem. Biotechnol. 2021, 193, 1727–1743. [Google Scholar] [CrossRef]
- Prasad, S.K.; Kumar, R.; Patel, D.K.; Hemalatha, S. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm. Biol. 2010, 48, 1397–1404. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Green Synthesis of Silver Nanoparticles by the Cyanobacteria Synechocystis sp.: Characterization, Antimicrobial and Diabetic Wound-Healing Actions. Mar. Drugs 2022, 20, 56. [Google Scholar] [CrossRef]
- Ren, J.; Yang, M.; Xu, F.; Chen, J.; Ma, S. Acceleration of wound healing activity with syringic acid in streptozotocin induced diabetic rats. Life Sci. 2019, 233, 116728. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Guo, J.; Armstrong, M.J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Positively charged, surfactant-free gold nanoparticles for nucleic acid delivery. Rsc Adv. 2015, 5, 17862–17871. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Su, G.; Yang, C.; Zhu, J.-J. Fabrication of Gold Nanorods with Tunable Longitudinal Surface Plasmon Resonance Peaks by Reductive Dopamine. Langmuir 2014, 31, 817–823. [Google Scholar] [CrossRef]
- Castillo-Martínez, J.C.; Martínez-Castañón, G.A.; Martínez-Gutierrez, F.; Zavala-Alonso, N.V.; Patiño-Marín, N.; Niño-Martinez, N.; Zaragoza-Magaña, V.; Cabral-Romero, C. Antibacterial and antibiofilm activities of the photothermal therapy using gold nanorods against seven different bacterial strains. J. Nanomater. 2015, 2015, 783671. [Google Scholar] [CrossRef]
- Kallis, P.J.; Friedman, A.J. Collagen Powder in Wound Healing. J. Drugs Dermatol. JDD 2018, 17, 403–408. [Google Scholar]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, M. Fast loading of PEG–SH on CTAB-protected gold nanorods. RSC Adv. 2014, 4, 17760–17767. [Google Scholar] [CrossRef]
- Clogston, J.; Patri, A. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards; Barry, A.L. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; Volume 19. [Google Scholar]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Reddy, G.B.; Madhusudhan, A.; Ramakrishna, D.; Ayodhya, D.; Venkatesham, M.; Veerabhadram, G. Green chemistry approach for the synthesis of gold nanoparticles with gum kondagogu: Characterization, catalytic and antibacterial activity. J. Nanostruct. Chem. 2015, 5, 185–193. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Shanmugam, C.; Sivasubramanian, G.; Parthasarathi, B.; Baskaran, K.; Balachander, R.; Parameswaran, V. Antimicrobial, free radical scavenging activities and catalytic oxidation of benzyl alcohol by nano-silver synthesized from the leaf extract of Aristolochia indica L.: A promenade towards sustainability. Appl. Nanosci. 2016, 6, 711–723. [Google Scholar] [CrossRef]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Onwudiwe, D.C.; Fayemi, O.E.; Ekennia, A.C.; Ebenso, E.E.; Tiedt, L.R. Biosynthesis, electrochemical, antimicrobial and antioxidant studies of silver nanoparticles mediated by Talinum triangulare aqueous leaf extract. J. Cluster Sci. 2017, 28, 309–330. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Eco-friendly synthesis of silver and gold nanoparticles with enhanced antimicrobial, antioxidant, and catalytic activities. IET Nanobiotechnol. 2018, 12, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Janis, J.; Harrison, B. Wound healing: Part II. Clinical applications. Plast. Reconstr. Surg. 2014, 133, 383e–392e. [Google Scholar] [CrossRef]
- Lian, N.; Li, T. Growth factor pathways in hypertrophic scars: Molecular pathogenesis and therapeutic implications. Biomed. Pharmacother. 2016, 84, 42–50. [Google Scholar] [CrossRef]
- Raghuwanshi, N.; Kumari, P.; Srivastava, A.K.; Vashisth, P.; Yadav, T.C.; Prasad, R.; Pruthi, V. Synergistic effects of Woodfordia fruticosa gold nanoparticles in preventing microbial adhesion and accelerating wound healing in Wistar albino rats in vivo. Mater. Sci. Eng. C. 2017, 80, 252–262. [Google Scholar] [CrossRef]
- Pivodová, V.; Franková, J.; Galandáková, A.; Ulrichová, J. In vitro AuNPs’ cytotoxicity and their effect on wound healing. Nanobiomedicine 2015, 2, 7. [Google Scholar] [CrossRef]


| Strains | MIC/MBC |
|---|---|
| Staphy. aureus | 0.25/0.1 nmole/mL |
| E. coli | 0.125/0.125 nmole/mL |
| C. albicans | 0.25/0.5 nmole/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, W.E.; Elsewedy, H.S.; Younis, N.S.; Shinu, P.; Elsawy, L.E.; Ramadan, H.A. Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles. Polymers 2022, 14, 2637. https://doi.org/10.3390/polym14132637
Soliman WE, Elsewedy HS, Younis NS, Shinu P, Elsawy LE, Ramadan HA. Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles. Polymers. 2022; 14(13):2637. https://doi.org/10.3390/polym14132637
Chicago/Turabian StyleSoliman, Wafaa E., Heba S. Elsewedy, Nancy S. Younis, Pottathil Shinu, Lamis E. Elsawy, and Heba A. Ramadan. 2022. "Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles" Polymers 14, no. 13: 2637. https://doi.org/10.3390/polym14132637
APA StyleSoliman, W. E., Elsewedy, H. S., Younis, N. S., Shinu, P., Elsawy, L. E., & Ramadan, H. A. (2022). Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles. Polymers, 14(13), 2637. https://doi.org/10.3390/polym14132637






