Eco-Friendly Catalytic Synthesis of Top Value Chemicals from Valorization of Cellulose Waste
Abstract
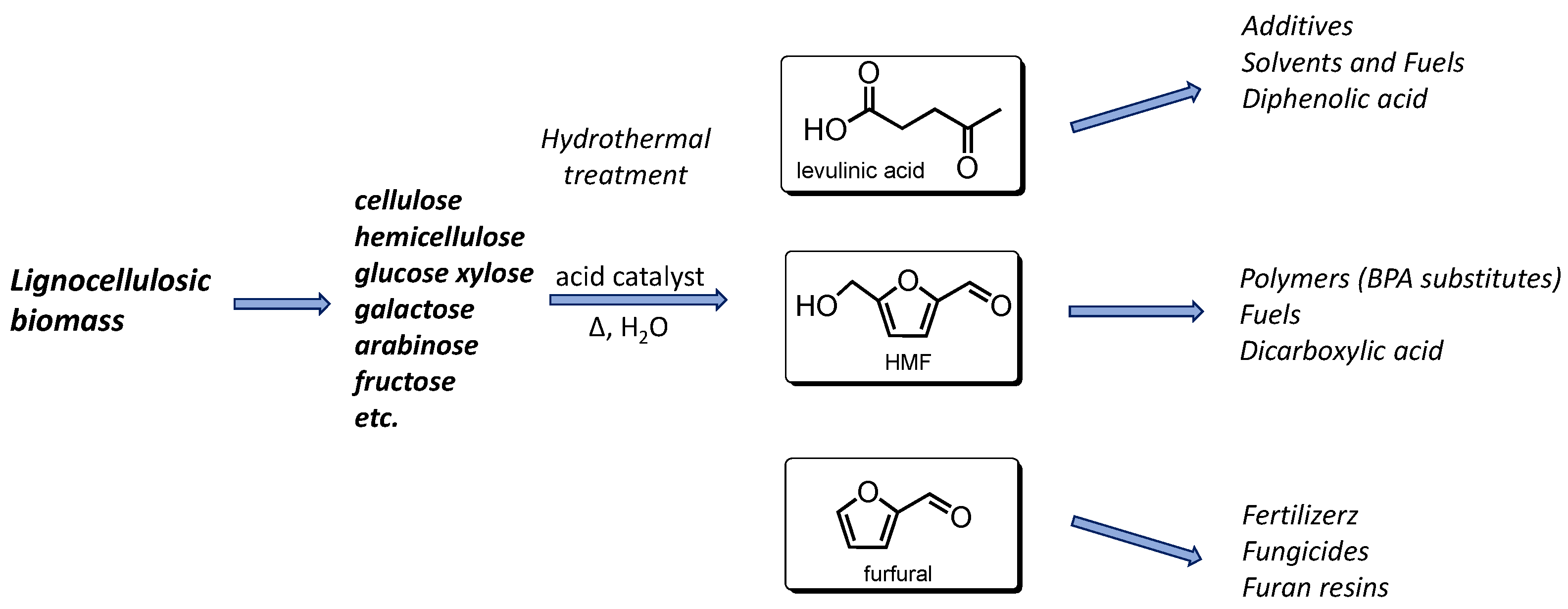
1. Introduction
2. Materials and Methods
2.1. E-Factor of the Reaction Listed in Table 4, Entry 2
2.2. Determination of E-Factor of the Reaction Listed in Table 4, Entry 5
2.3. Determination of E-Factor of the Reaction Listed in Table 5, Entry 1
2.4. Determination of E-Factor of the Reaction Listed in Table 6, Entry 7 [14]
2.5. Determination of E-Factor of the Reaction Listed Table 6, Entry 4 [15]
2.6. Determination of E-Factor of the Reaction Listed in Table 6, Entry 5 [17]
2.7. Determination of E-Factor of the Reaction Listed in Table 6, Entry 6 [17]
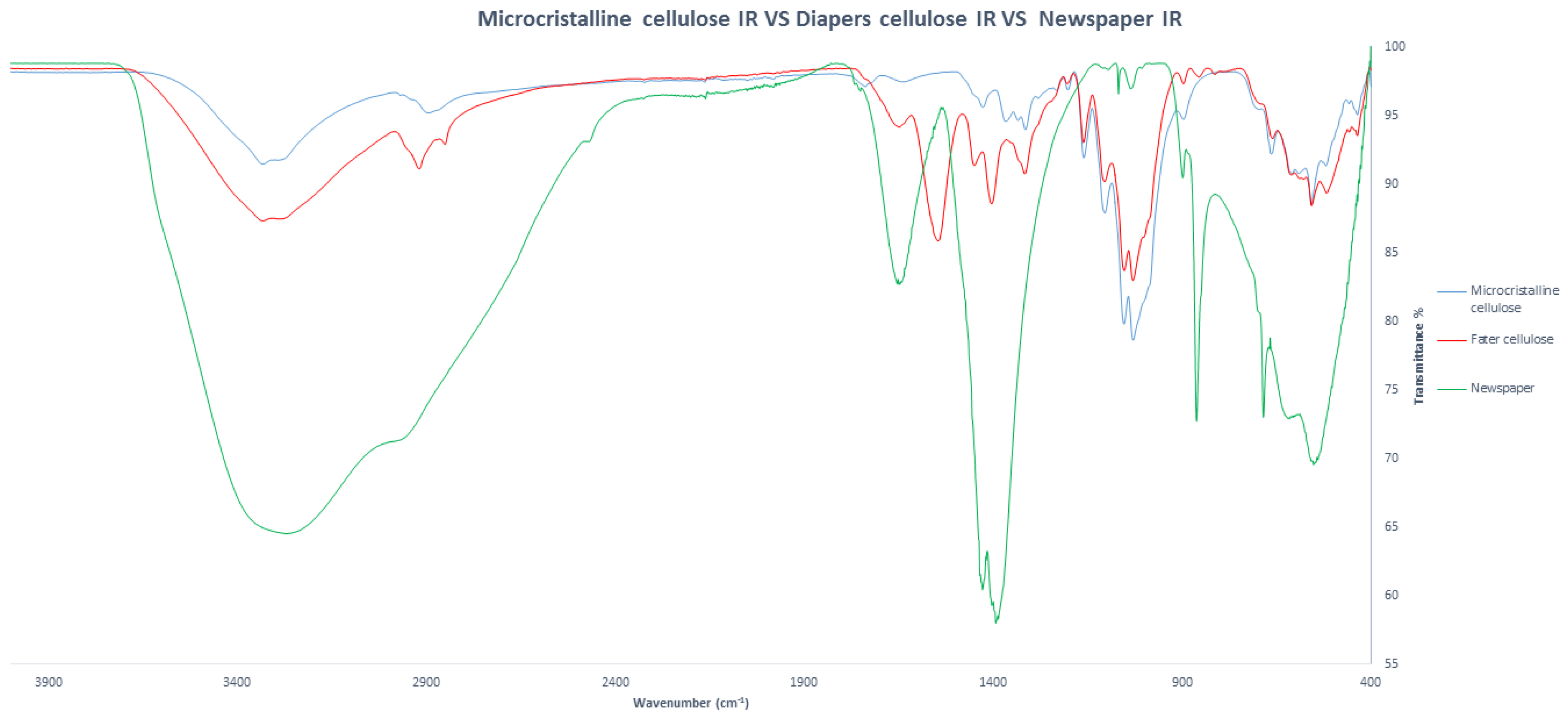
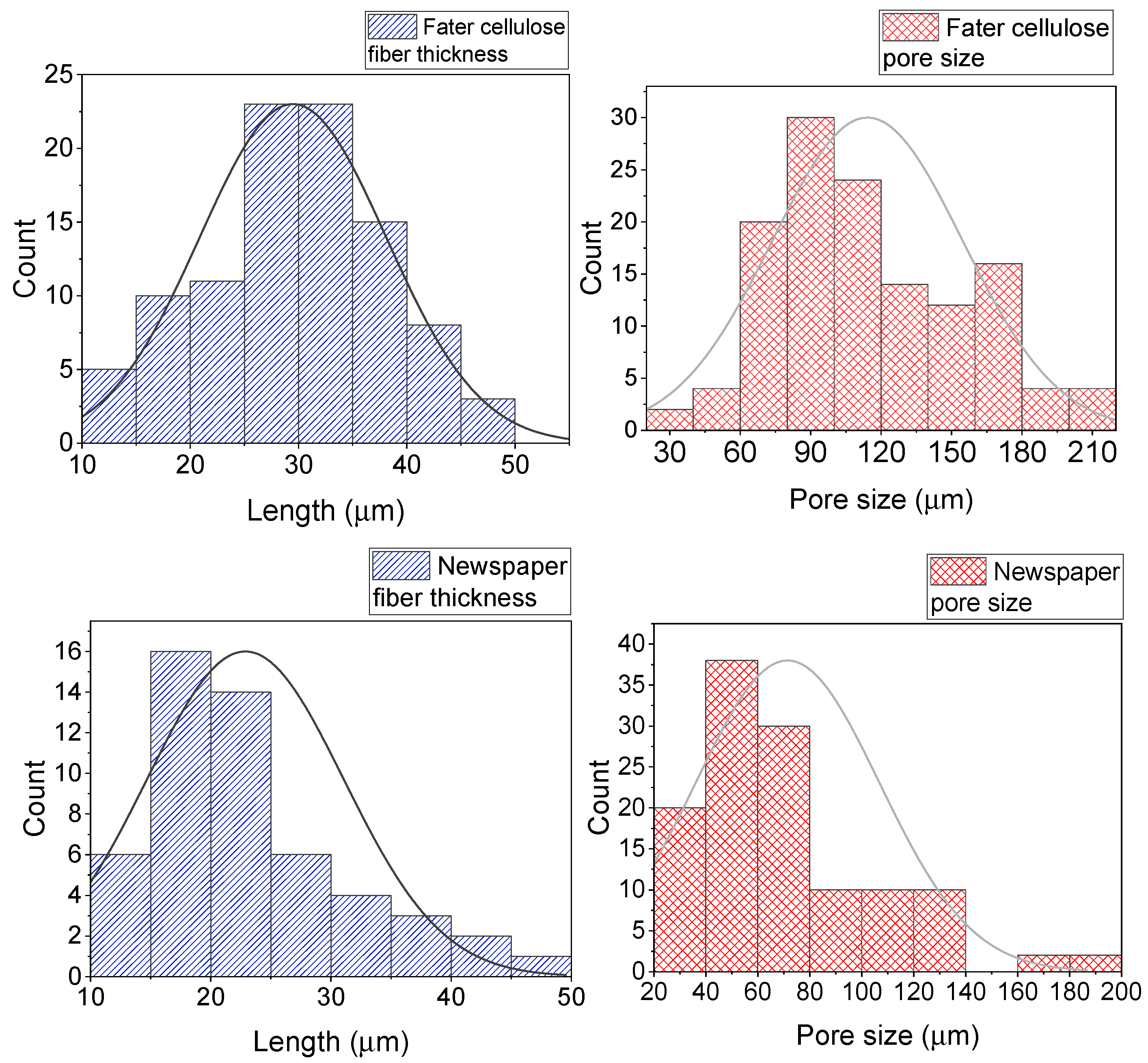
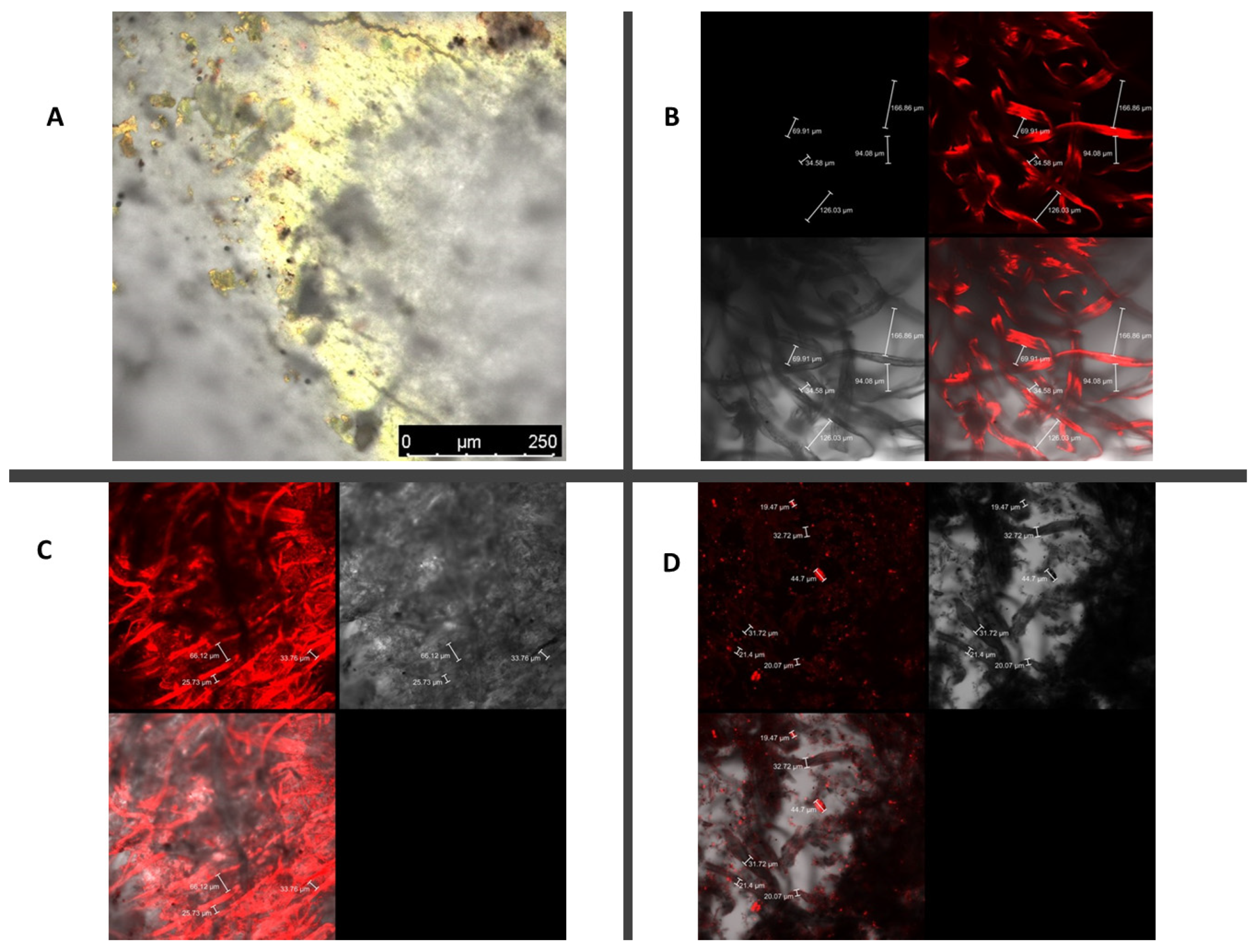
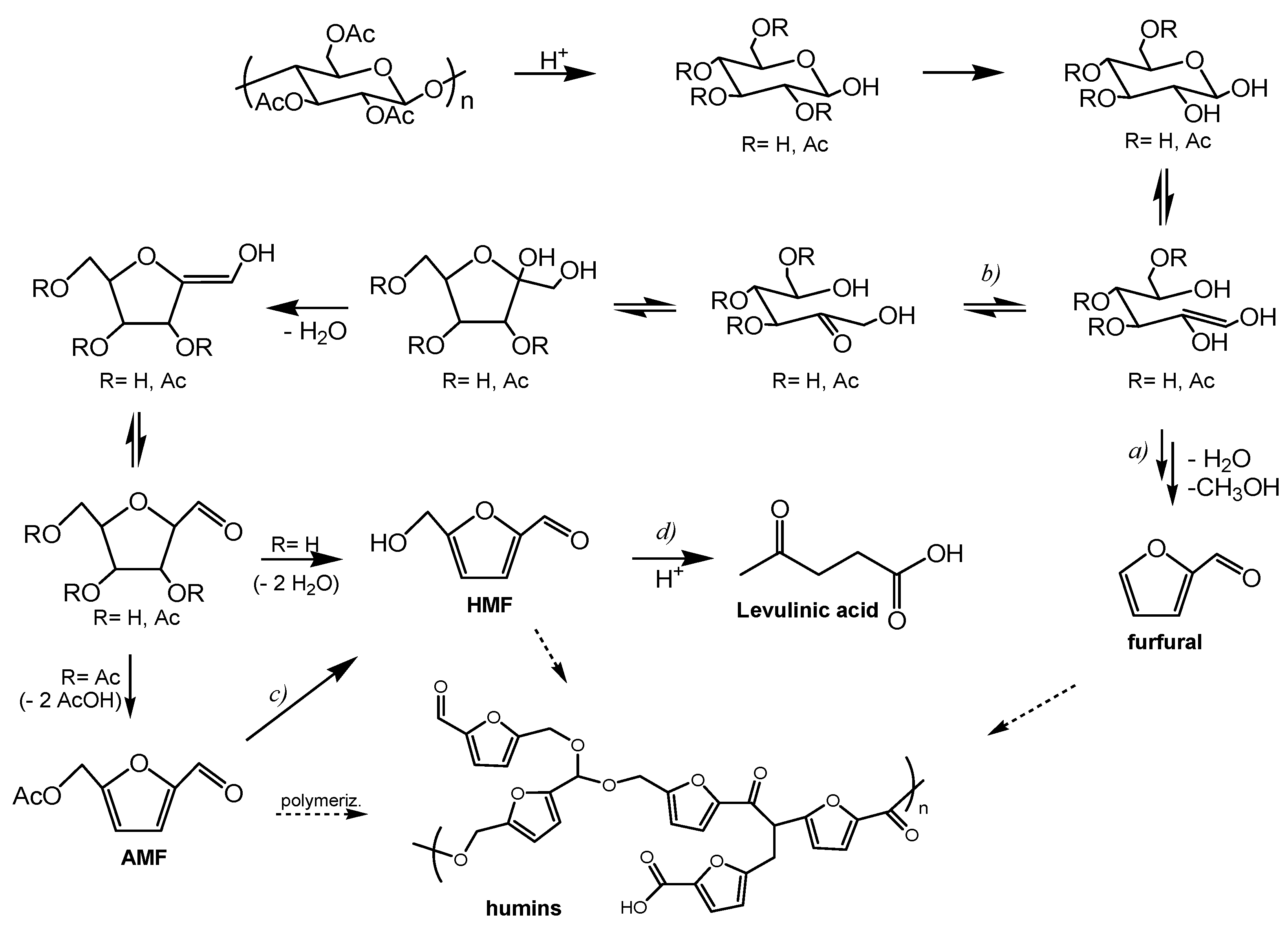
3. Results and Discussion
Hydrothermal Tests: Synthesis of Levulinic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sankaran, R.; Markandan, K.; Khoo, K.S.; Cheng, C.K.; Ashokkumar, V.; Deepanraj, B.; Show, P.L. The Expansion of Lignocellulose Biomass Conversion into Bioenergy via Nanobiotechnology. Front. Nanotechnol. 2021, 3, 793528. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of Xylose to Furfural. Using Lewis and Bronsted Acid Catalyst in Aqueous Media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; He, F.; Yue, W.; Hu, G.; Gan, J.; Xie, H. Pretreatment of Corn Stover by Levulinic Acid-Based Protic Ionic Liquids for Enhanced Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2022, 10, 7134–7148. [Google Scholar] [CrossRef]
- Nguyen, X.P.; Hoang, A.T.; Olçer, A.I.; Engel, D.; Pham, V.V.; Nayak, S.-K. Biomass-derived 2,5-dimethylfuran as a promising alternative fuel: An application review on the compression and spark ignition engine. Fuel Process. Technol. 2021, 214, 106687. [Google Scholar] [CrossRef]
- Casiello, M.; Catucci, L.; Fracassi, F.; Fusco, C.; Laurenza, A.G.; di Bitonto, L.; Pastore, C.; D’Accolti, L.; Nacci, A. ZnO/Ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks. Catalysts 2019, 9, 71. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Department of Energy: Washington, DC, USA, 2004; pp. 1–76.
- Mennino, S. Valorization of Waste: Sustainable Organocatalysts from Renewable Resources. ChemSusChem 2020, 13, 439–468. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang., D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef]
- Laurenza, A.G.; Losito, O.; Casiello, M.; Fusco, C.; Nacci, A.; Pantone, V.; D’Accolti, L. Valorization of cigarette butts for synthesis of levulinic acid as top value-added chemicals. Sci. Rep. 2021, 11, 15775. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed]
- Alleima Technical Center. 2023. Available online: https://www.alleima.com/en/technical-center/corrosion-tables/acetic-acid/ (accessed on 21 January 2021).
- Wang, F.; Liu, C.L.; Dong, W.-S. Highly efficient production of lactic acid from cellulose using lanthanide triflate catalysts. Green Chem. 2013, 15, 2091–2095. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Essayem, N.; Lopez De souza, R.; Rataboul, F.; Doiseau, A.C. Method for Preparing Furfural. U.S. Patent 2014309440A1, 2014. [Google Scholar]
- Dumesic, J.A.; Riberio Gallo, J.M.; Alonso, D. Method to Convert Biomass To 5-(Hydroxymethyl)-Furfural (Hmf) and Furfural Using Lactones, Furans, and Pyransas Solvents. U.S. Patent 8772515, 2014. [Google Scholar]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Wang, F.; Shi, A.-W.; Qin, X.; Liu, C.L.; Dong, W.-S. Dehydration of fructose to 5-hydroxymethylfurfural by rare earth metal trifluoromethanesulfonates in organic solvents. Carbohydr. Res. 2011, 346, 982–985. [Google Scholar] [CrossRef]
- Caputo, D.; Casiello, M.; Milella, A.; Oberhauser, W.; Maffezzoli, A.; Nacci, A.; Fusco, C.; D’Accolti, L. Deep control of linear oligomerization of glycerol using lanthanum catalyst on mesoporous silica gel. Catalysts 2020, 10, 1170. [Google Scholar] [CrossRef]
- Searle, S.; Malins, C. Availability of Cellulosic Residues and Wastes in the EU; European Climate Foundation (ECF): The Hague, The Netherlands, 2013. [Google Scholar]
- D’Accolti, L.; Annese, C.; De Riccardis, A.; De Giglio, E.; Cafagna, D.; Fanelli, F.; Fusco, C. Dioxirane-Mediated Heterogeneous Epoxidations with Potassium Caroate: A Solid Catalyst Bearing Anchored Ketone Moieties. Eur. J. Org. Chem. 2012, 2012, 4616–4621. [Google Scholar] [CrossRef]
- Mehner, A.; Montero, A.L.; Martinez, R.; Spange, S. Synthesis of 5-Acetoxymethyl- and 5-Hydroxymethyl-2-vinylfuran. Molecules 2007, 12, 634–640. [Google Scholar] [CrossRef]
- Coelho, J.A.S.; Trindade, A.F.; André, V.; Duarte, M.T.; Veiros, L.F.; Afonso, C.A.M. Trienamines derived from 5-substituted furfurals: Remote ε-functionalization of 2,4-dienals. Org. Biomol. Chem. 2014, 12, 9324–9328. [Google Scholar] [CrossRef]
- You, S.; Zhang, R.; Caia, M. A Magnetically Recyclable Palladium-Catalyzed Formylation of Aryl Iodides with Formic Acid as CO Source: A Practical Access to Aromatic Aldehydes. Synthesis 2021, 53, 1962–1970. [Google Scholar] [CrossRef]
- Pantone, V.; Annese, C.; Fusco, C.; Fini, P.; Nacci, A.; Russo, A. D’Accolti One-pot conversion of epoxidized soybean oil (ESO) into soy-based polyurethanes by MoCl2O2 catalysis. Molecules 2017, 22, 333. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Songa, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Grabowska, B.; Holtzer, M. Structural Examination of the Cross-Linking Reaction Mechanism of Polyacrylate Binding Agents. Arch. Metall. Mater. 2009, 54, 428–437. [Google Scholar]
- Fitzpatrick, S.W. Production of Levulinic Acid from Carbohydrate-Containing Materials. U.S. Patent US005608105A, 1997. [Google Scholar]
- Körner, P.; Junga, D.; Kruse, A. The effect of different Brønsted acids on the hydrothermal conversion of fructose to HMF. Green Chem. 2018, 20, 2231–2241. [Google Scholar] [CrossRef]
- Zuo, M.; Jia, W.; Feng, Y.; Zeng, X.; Tang, X.; Yong Sun, Y.; Lin, L. Effective selectivity conversion of glucose to furan chemicals in the aqueous deep eutectic solvent. Renew. Energy 2021, 164, 23–33. [Google Scholar] [CrossRef]
- Gavilà, L.; Esposito, D. Cellulose acetate as a convenient intermediate for the preparation of 5-acetoxymethylfurfural from biomass. Green Chem. 2017, 19, 2496–2500. [Google Scholar] [CrossRef]
- He, O.; Zhang, Y.; Wang, P.; Liu, L.; Wang, Q.; Yang, N.; Wenjie Li, W.; Champagne, P.; Yu, H. Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose. Catalysts 2021, 11, 11. [Google Scholar] [CrossRef]

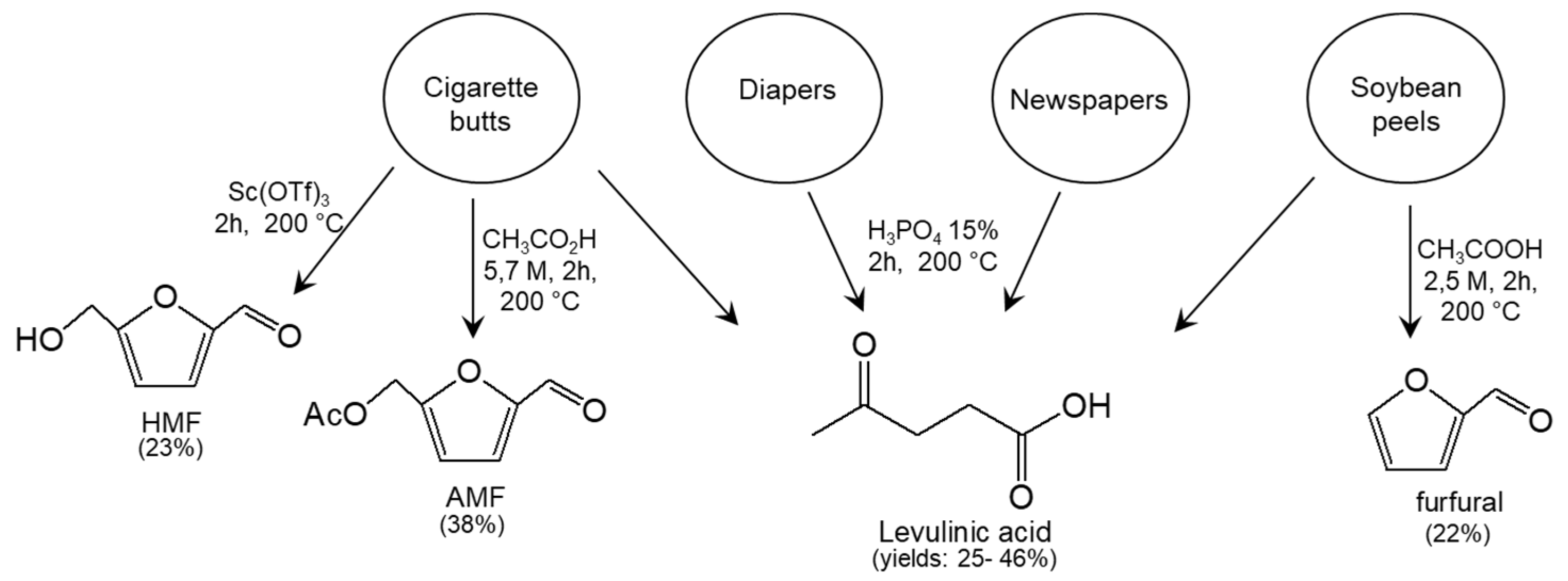
| Entry | Matrix | Catalyst | Product | Yield | Ref. |
|---|---|---|---|---|---|
| 1 | xylose | CH3COOH | furfural | 80% molar yield | [16] |
| 2 | cellulose | AlCl3 | 5-HMF | 31% molar yield | [17] |
| 3 | fructose | HCl | 5-HMF | 25.5% wt | [18] |
| 4 | fructose | Sc(OTf)3 | 5-HMF | 38% molar yield | [19] |
| Cigarette Butts | Newspapers | Diaper Cellulose | Soybean Peels |
|---|---|---|---|
 |  |  |  |
| Entry | Matrix | Catalyst b | Time (h) | Temperature (°C) | Levulinic Acid c Yield% (w/w) |
|---|---|---|---|---|---|
| 1 d | Cigarette butts | Phosphoric acid 15% w/w | 2 | 200 | 17.3 |
| 2 | Diapers cellulose | Phosphoric acid 15% w/w | 2 | 200 | 14.8 |
| 3 | Newspapers | Phosphoric acid 15% w/w | 2 | 200 | 10.0 |
| 4 | Newspapers | Phosphoric acid15% w/w | 2 | 200 | 25.6 e |
| 5 | Soybean peels | Phosphoric acid15% w/w | 2 | 200 | 46.0 |
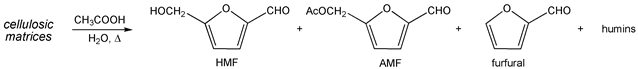 | |||||
| Entry | Matrix | Catalyst CH3COOH (M) | Time | T (°C) | Product Yield% b (w/w) |
|---|---|---|---|---|---|
| 1 | Cigarette butts | 4 | 2 h | 200 | HMF 21.3AMF 18.5 |
| 2 | Cigarette butts | 5.7 | 2 h | 200 | AMF 38.3 |
| 3 | Cigarette butts | 4 | 2 h | 220 | humins |
| 4 | Cigarette butts | 5.7 | 16 h | 160 | n.r. |
| 5 | Soybean peels | 2.5 | 2 h | 200 | furfural 21.8 |
| 6 | Soybean peels | 4 | 2 h | 200 | furfural 21.6 |
| 7 | Soybean peels | 5.7 | 3 h | 200 | humins |
| 8 | Newspapers | 4 | 2 h | 200 | n.r. |
| 9 | Newspapers | 5.7 | 2 h | 200 | humins |
| 10 | Diapers cellulose | 4 | 2 h | 200 | n.r. |
| 11 | Diapers cellulose | 5.7 | 2 h | 200 | humins |
| Entry | Substrate | Time (h) | Temperature | Products Yield% (w/w) b |
|---|---|---|---|---|
| 1 | Cigarette butts | 2 h | 200 °C | HMF 23.2 |
| 2 | Newspapers | 2 h | 200 °C | n.r. |
| 3 | Soybean peels | 2 h | 200 °C | n.r. |
| 4 | Diaper cellulose | 2 h | 200 °C | n.r. |
| Entry | Matrix | Catalyst | Product | Yield | E-Factor | Ref. |
|---|---|---|---|---|---|---|
| 1 | Cigarette butts | Sc(OTf)3 | HMF | 23.3% (w/w) | 1.25 | This work a |
| 2 | Soybean peels | CH3COOH | furfural | 21.6%(w/w) | 12.76 | This work c |
| 3 | Cigarette butts | CH3COOH | AMF | 38.3% (w/w) | 19.90 | This work b |
| 4 | Cellulose | AlCl3 | HMF | 31% molar yield | 2.65 | [15] |
| 5 | Fructose | Sc(OTf)3/H2O | HMF | 38.5% molar yield | 3.12 | [17] d |
| 6 | Fructose | Sc(OTf)3/DMSO | HMF | 83.3% molar yield | 86.65 | [17] e |
| 7 | Xylose | CH3COOH | furfural | 80% molar yield | 40.04 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losito, O.; Casiello, M.; Fusco, C.; Mateos Cuadrado, H.; Monopoli, A.; Nacci, A.; D’Accolti, L. Eco-Friendly Catalytic Synthesis of Top Value Chemicals from Valorization of Cellulose Waste. Polymers 2023, 15, 1501. https://doi.org/10.3390/polym15061501
Losito O, Casiello M, Fusco C, Mateos Cuadrado H, Monopoli A, Nacci A, D’Accolti L. Eco-Friendly Catalytic Synthesis of Top Value Chemicals from Valorization of Cellulose Waste. Polymers. 2023; 15(6):1501. https://doi.org/10.3390/polym15061501
Chicago/Turabian StyleLosito, Onofrio, Michele Casiello, Caterina Fusco, Helena Mateos Cuadrado, Antonio Monopoli, Angelo Nacci, and Lucia D’Accolti. 2023. "Eco-Friendly Catalytic Synthesis of Top Value Chemicals from Valorization of Cellulose Waste" Polymers 15, no. 6: 1501. https://doi.org/10.3390/polym15061501
APA StyleLosito, O., Casiello, M., Fusco, C., Mateos Cuadrado, H., Monopoli, A., Nacci, A., & D’Accolti, L. (2023). Eco-Friendly Catalytic Synthesis of Top Value Chemicals from Valorization of Cellulose Waste. Polymers, 15(6), 1501. https://doi.org/10.3390/polym15061501














