Novel 1,2-Bismethacrylate-3-Eugenyl Propane for Resin Composites: Synthesis, Characterization, Rheological, and Degree of Conversion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of EgGAA
2.3. Purification of Synthesized Monomers
2.4. Synthesize of Silanized Silica
2.5. Preparation of Unfilled and Filled Resins
2.6. Monomer Characterization
2.7. Rheology Test
2.8. Degree of Conversion Test
2.9. Statistical Analysis
3. Results and Discussion
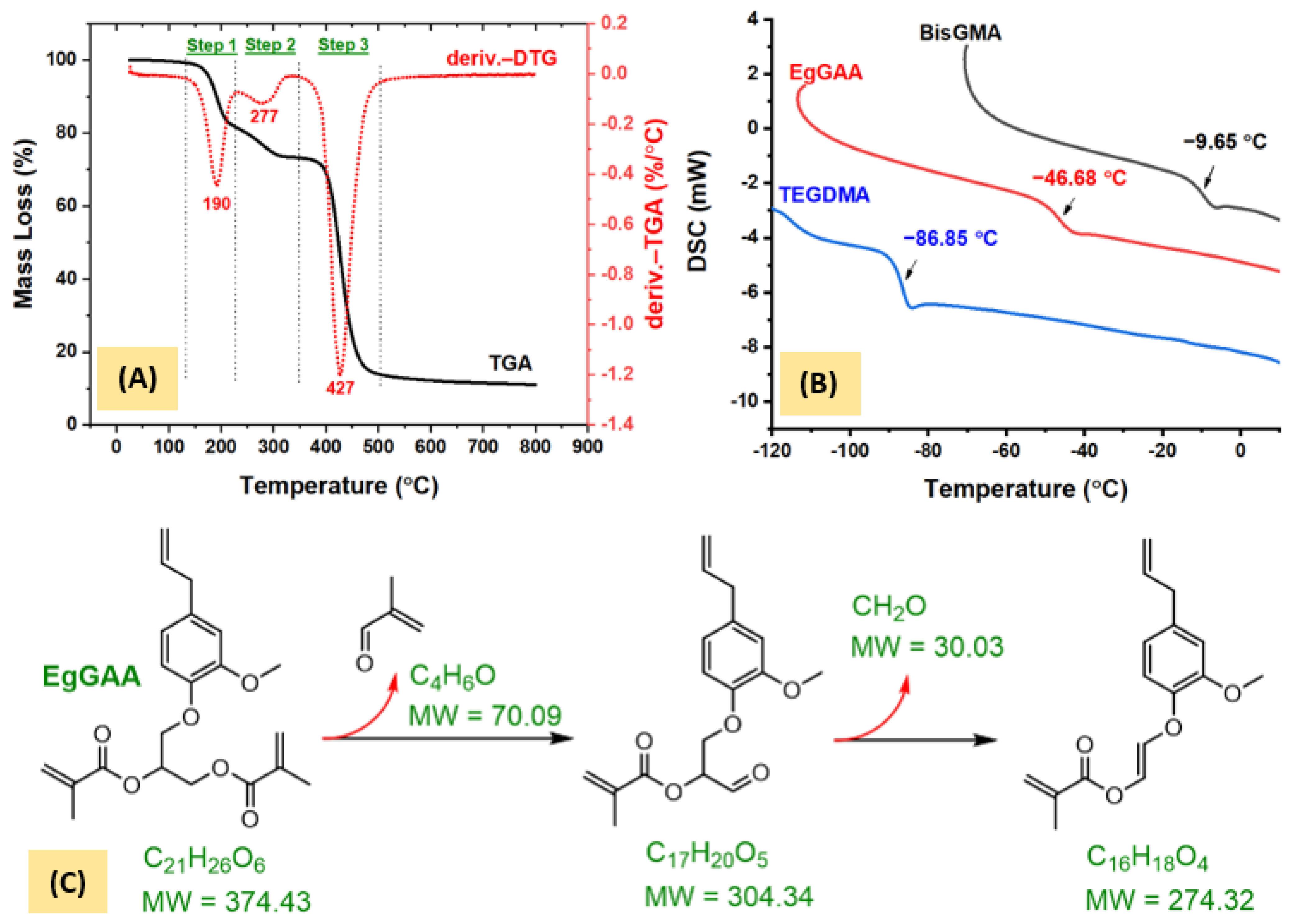
3.1. Synthesis of Monomers
3.2. Spectral Analysis
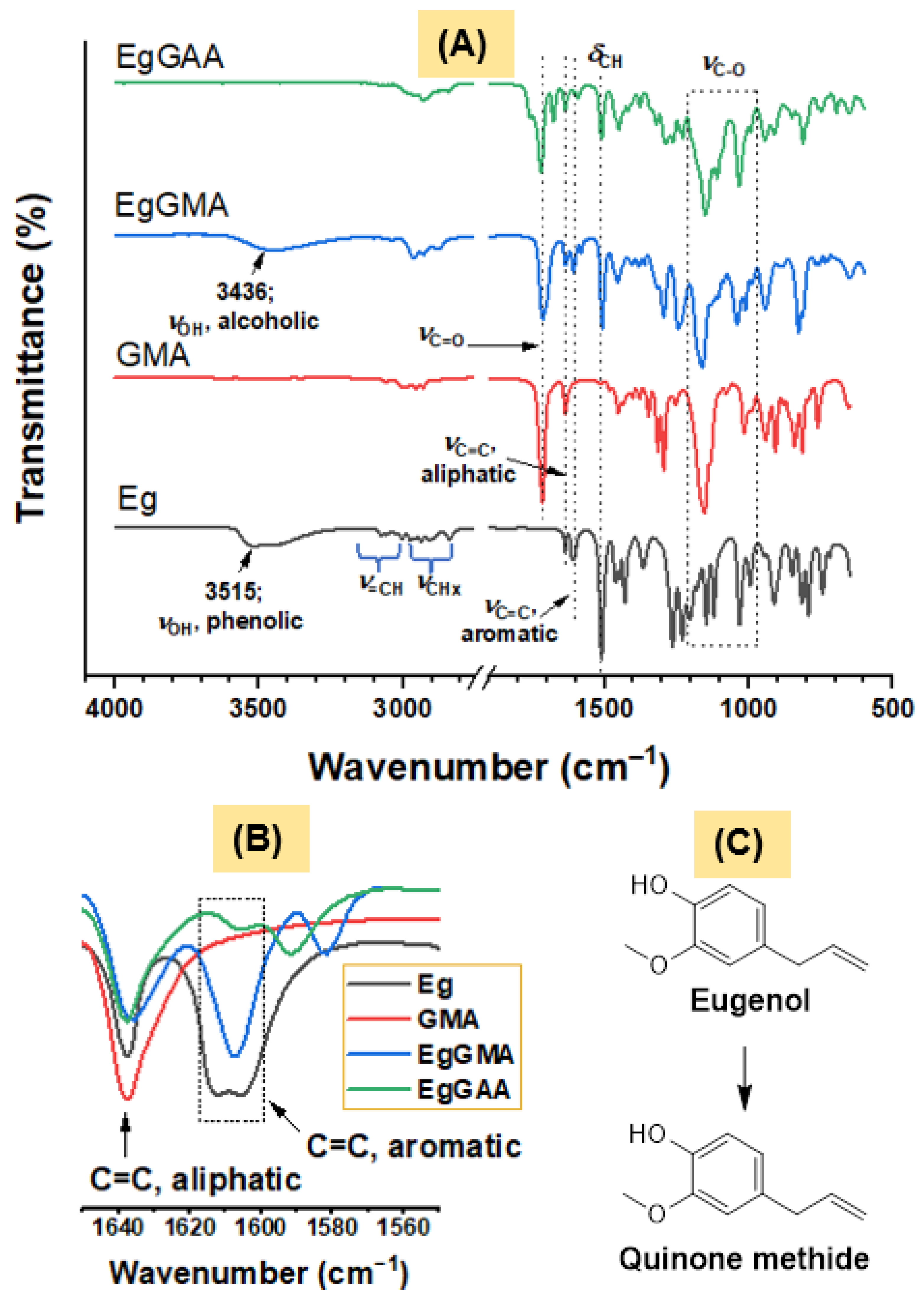
3.2.1. FTIR
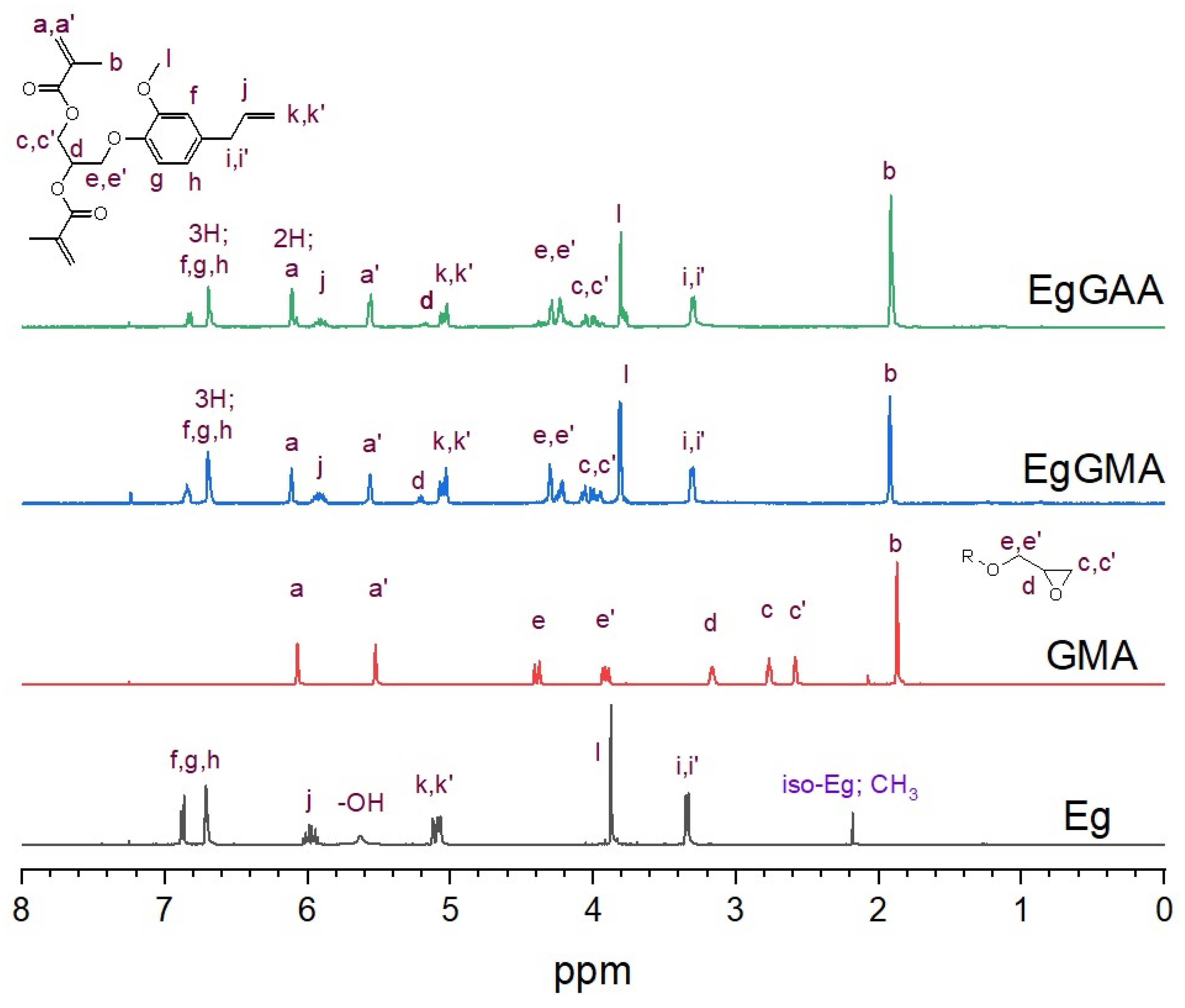
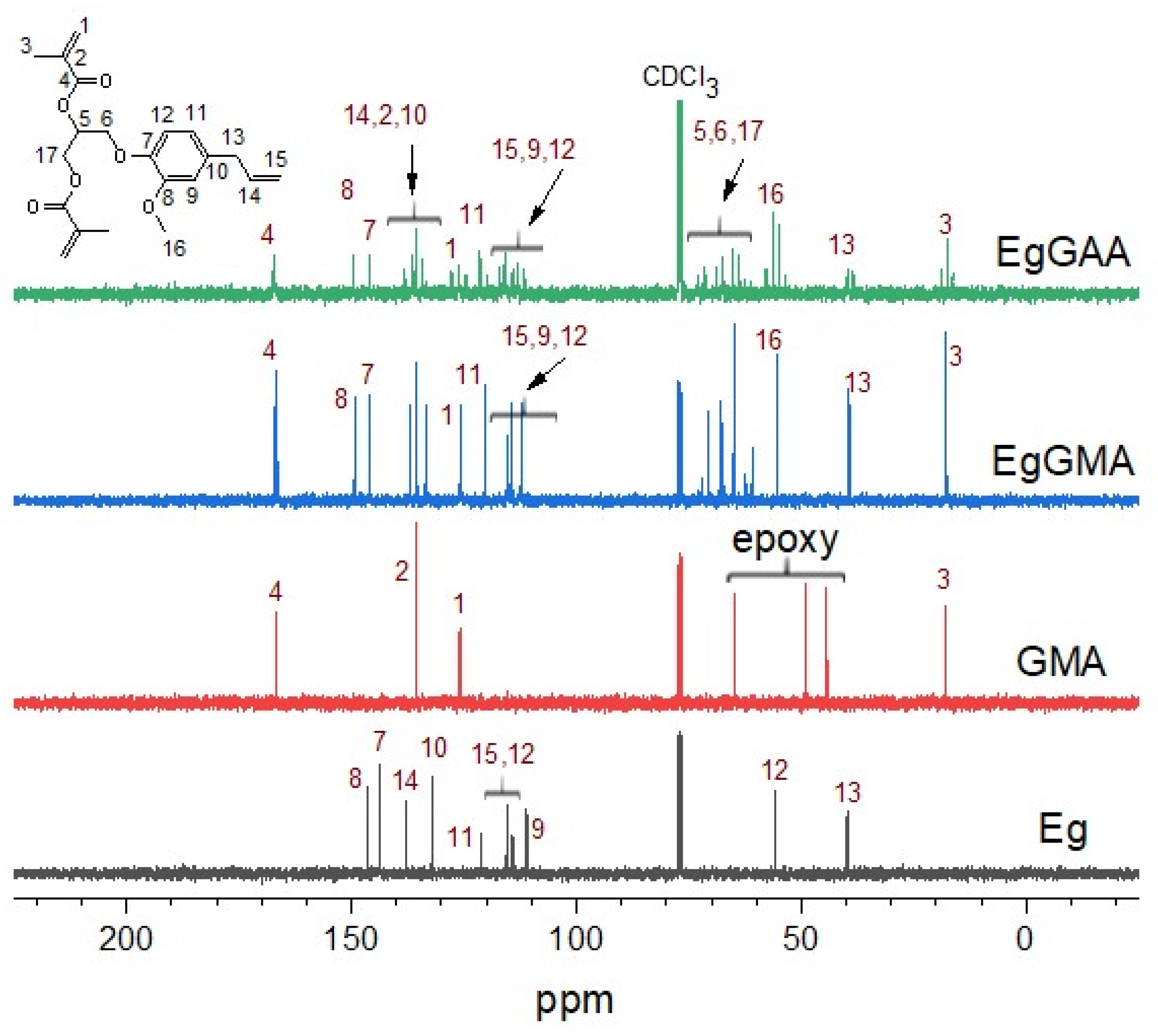
3.2.2. NMR Analysis
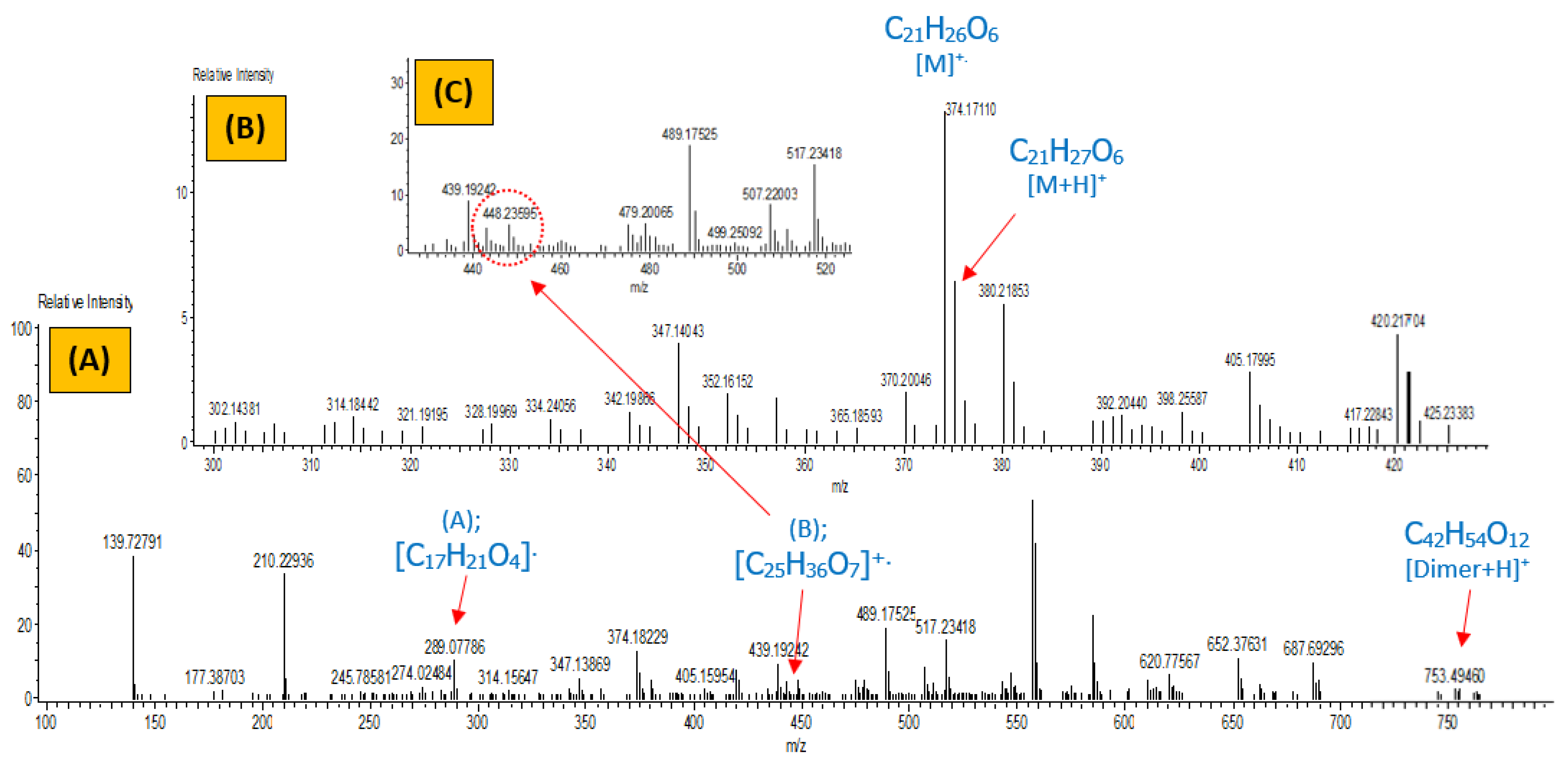
3.2.3. Mass Analysis
3.3. Thermal Analysis
3.3.1. TGA Analysis
3.3.2. DSC Analysis
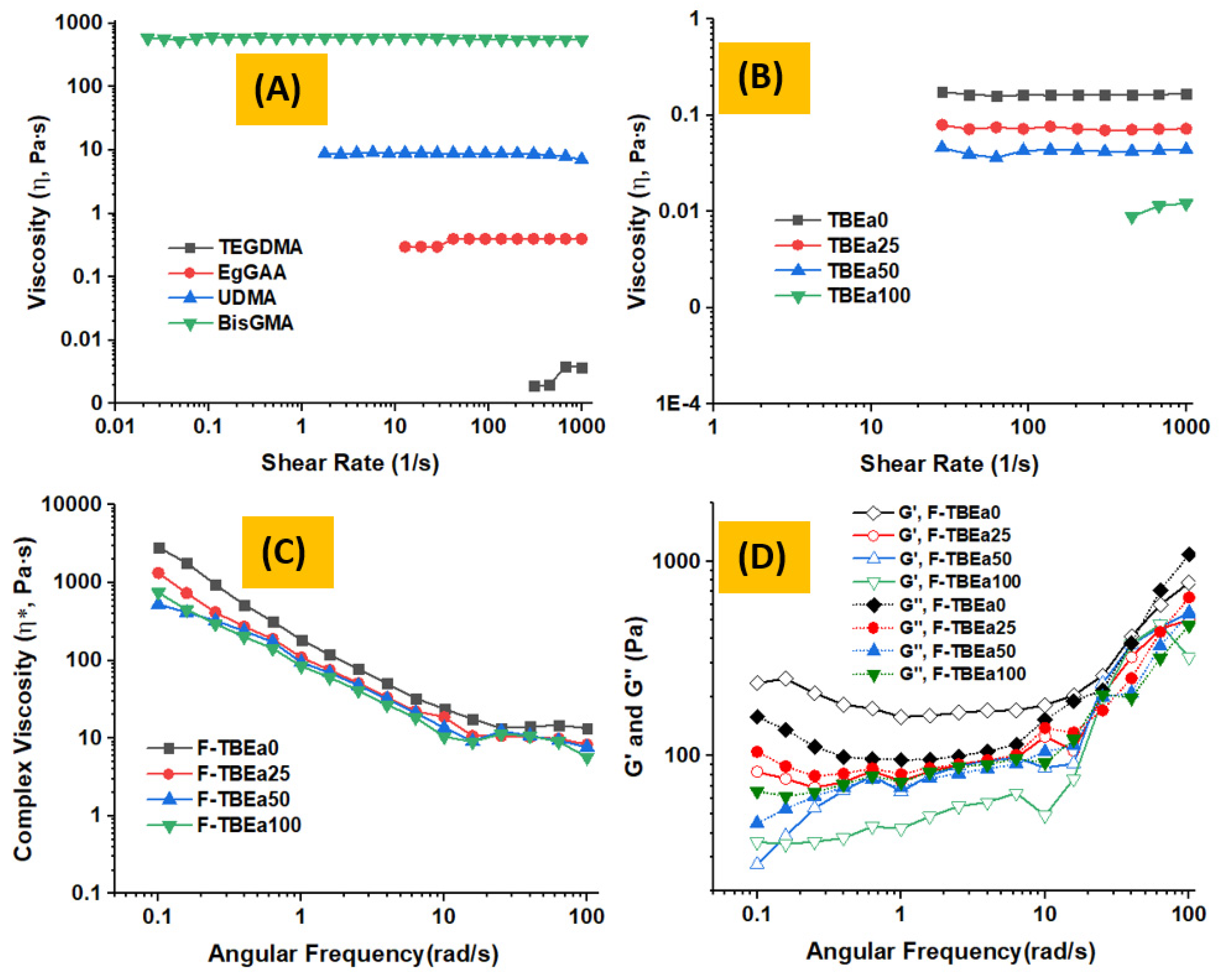
3.4. Rheology Analysis
3.5. Degree of Conversion Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina-Gutiérrez, S.; Manseri, A.; Ladmiral, V.; Bongiovanni, R.; Caillol, S.; Lacroix-Desmazes, P. Eugenol: A promising building block for synthesis of radically polymerizable monomers. Macromol. Chem. Phys. 2019, 220, 1900179. [Google Scholar] [CrossRef]
- Almaroof, A.; Niazi, S.; Rojo, L.; Mannocci, F.; Deb, S. Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations. Dent. Mater. 2017, 33, e239–e254. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, T.S. The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab. J. Chem. 2019, 12, 3225–3230. [Google Scholar] [CrossRef]
- Bazan, A.; Nowicki, P.; Półrolniczak, P.; Pietrzak, R. Thermal analysis of activated carbon obtained from residue after supercritical extraction of hops. J. Therm. Anal. Calorim. 2016, 125, 1199–1204. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Vargas Méndez, L.Y. Synthesis of eugenol-based monomers for sustainable epoxy thermoplastic polymers. J. Appl. Polym. Sci. 2022, 139, 52237. [Google Scholar] [CrossRef]
- Rojo, L.; Vazquez, B.; Parra, J.; López Bravo, A.; Deb, S.; San Roman, J. From natural products to polymeric derivatives of “eugenol”: A new approach for preparation of dental composites and orthopedic bone cements. Biomacromolecules 2006, 7, 2751–2761. [Google Scholar] [CrossRef]
- Alrahlah, A.; Al-Odayni, A.-B.; Saeed, W.S.; Al-Kahtani, A.; Alkhtani, F.M.; Al-Maflehi, N.S. Water Sorption, Water Solubility, and Rheological Properties of Resin-Based Dental Composites Incorporating Immobilizable Eugenol-Derivative Monomer. Polymers 2022, 14, 366. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Alotaibi, D.H.; Saeed, W.S.; Al-Kahtani, A.; Assiri, A.; Alkhtani, F.M.; Alrahlah, A. Eugenyl-2-Hydroxypropyl Methacrylate-Incorporated Experimental Dental Composite: Degree of Polymerization and In Vitro Cytotoxicity Evaluation. Polymers 2022, 14, 277. [Google Scholar] [CrossRef]
- Almaroof, A.; Niazi, S.; Rojo, L.; Mannocci, F.; Deb, S. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 929–939. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Wang, R.; Zhu, M. Synthesis and characterization of methacrylate-functionalized betulin derivatives as antibacterial comonomer for dental restorative resins. ACS Biomater. Sci. Eng. 2021, 7, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Mirah, M. Development of Experimental Dental Resin Composites with Potential Bioactivity. Ph.D. Thesis, University of Manchester, Manchester, UK, 2019. [Google Scholar]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.; Russell, R. Incorporation of bacterial inhibitor into resin composite. J. Dent. Res. 1994, 73, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Al-Odayni, A.-B.; Saeed, W.S.; Ahmed, A.Y.B.H.; Alrahlah, A.; Al-Kahtani, A.; Aouak, T. New monomer based on eugenol methacrylate, synthesis, polymerization and copolymerization with methyl methacrylate–characterization and thermal properties. Polymers 2020, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Patole, V.C.; Chaudhari, S.P. Eugenyl Methacrylate Microsponges Loaded with Eugenol Incorporated In Situ Gel for Treatment of Periodontitis. J. Pharm. Innov. 2021, 16, 408–418. [Google Scholar] [CrossRef]
- Habib, E.; Wang, R.; Zhu, X. Monodisperse silica-filled composite restoratives mechanical and light transmission properties. Dent. Mater. 2017, 33, 280–287. [Google Scholar] [CrossRef]
- Alrahlah, A.; Khan, R.; Al-Odayni, A.-B.; Saeed, W.S.; Bautista, L.S.; Vohra, F. Evaluation of synergic potential of rGO/SiO2 as hybrid filler for BisGMA/TEGDMA dental composites. Polymers 2020, 12, 3025. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Tauböck, T.T.; Attin, T.; Tarle, Z. Degree of conversion of experimental resin composites containing bioactive glass 45S5: The effect of post-cure heating. Sci. Rep. 2019, 9, 17245. [Google Scholar] [CrossRef]
- Saikia, B.J.; Dolui, S.K. Designing semiencapsulation based covalently self-healable poly (methyl methacrylate) composites by atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1842–1851. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Li, Y.-W.; Zheng, Y.-Q.; Luo, Z.; Gong, C.-R.; Xu, Y.; Wu, L.-X. Synthesis and characterization of a dual-curing resin for three-dimensional printing. J. Mater. Sci. 2019, 54, 5865–5876. [Google Scholar] [CrossRef]
- Chowdhry, B.Z.; Ryall, J.P.; Dines, T.J.; Mendham, A.P. Infrared and Raman spectroscopy of eugenol, isoeugenol, and methyl eugenol: Conformational analysis and vibrational assignments from density functional theory calculations of the anharmonic fundamentals. J. Phys. Chem. A 2015, 119, 11280–11292. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.S.; Rahayu, P.; Firdaus, M. Synthesis and Characterisation of Copoly-(Eugenol-N,N′-Methylene Bis (Acrylamide)). J. Phys. Sci. 2019, 30, 87–100. [Google Scholar] [CrossRef]
- Reis, A.V.; Fajardo, A.R.; Schuquel, I.T.; Guilherme, M.R.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): Is this reaction mechanism still unclear? J. Org. Chem. 2009, 74, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Bannach, G.; Cavalheiro, C.; Calixto, L.; Cavalheiro, E. Thermoanalytical study of monomers: BisGMA, BisEMA, TEGDMA, UDMA and their mixture. Braz. J. Therm. Anal 2015, 4, 28–34. [Google Scholar]
- Hutchinson, J. Determination of the glass transition temperature: Methods correlation and structural heterogeneity. J. Therm. Anal. Calorim. 2009, 98, 579–589. [Google Scholar] [CrossRef]
- Saen, P.; Atai, M.; Nodehi, A.; Solhi, L. Physical characterization of unfilled and nanofilled dental resins: Static versus dynamic mechanical properties. Dent. Mater. 2016, 32, e185–e197. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Li, M.; Yang, P.; Gu, Y. Effect of hydrogen bonds on structures and glass transition temperatures of maleimide–isobutene alternating copolymers: Molecular dynamics simulation study. Macromol. Theory Simul. 2013, 22, 107–114. [Google Scholar] [CrossRef]
- Lemon, M.T.; Jones, M.S.; Stansbury, J.W. Hydrogen bonding interactions in methacrylate monomers and polymers. J. Biomed. Mater. Res. Part A 2007, 83, 734–746. [Google Scholar] [CrossRef]

| Composite Code | Matrix, 34 wt% | Filler, 66 wt% | ||||||
|---|---|---|---|---|---|---|---|---|
| Resin Code | Diluent | Base | ||||||
| TEGDMA | BisGMA | EgGAA | ||||||
| wt% | mol% | wt% | mol% | wt% | mol% | |||
| F-TBEa0 | TBEa0 | 50.00 | 64.16 | 50.00 | 35.84 | 0.00 | 0.00 | Synthesized nanosilica (S-SiO2) |
| F-TBEa25 | TBEa25 | 50.00 | 62.11 | 37.50 | 26.02 | 12.50 | 11.87 | |
| F-TBEa50 | TBEa50 | 50.00 | 60.18 | 25.00 | 16.81 | 25.00 | 23.01 | |
| F-TBEa100 | TBEa100 | 50.00 | 41.84 | 0.00 | 0.00 | 50.00 | 58.16 | |
| Rheological Results | Degree of Conversion (DC; %), n = 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Viscosity (Pa·s), n = 3 | Complex Viscosity (Pa·s), n = 3 | |||||||||
| Monomer | Resin | Composite | ||||||||
| Monomer Code | Av. | SD | Resin Code | Av. | SD | Composite Code | At 1.0 (rad/s); Av. | At 1.0 (rad/s); SD | Av. | SD |
| BisGMA | 580.977 A | 12.648 | TBEa0 | 0.164 A | 0.034 | F–TBEa0 | 174.553 A | 20.917 | 61.22 A,C | 3.05 |
| UDMA | 9.106 B | 0.530 | TBEa25 | 0.074 B | 0.002 | F–TBEa25 | 102.726 B | 6.715 | 59.85 A | 2.44 |
| EgGAA | 0.379 B | 0.014 | TBEa50 | 0.042 C | 0.002 | F–TBEa50 | 92.543 B | 23.248 | 59.50 A | 3.07 |
| TEGDMA | 0.003 B | 0.000 | TBEa100 | 0.010 D | 0.002 | F–TBEa100 | 66.269 B | 5.990 | 52.54 B | 5.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kahtani, H.M.; Al-Odayni, A.-B.; Saeed, W.S.; Robaian, A.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Novel 1,2-Bismethacrylate-3-Eugenyl Propane for Resin Composites: Synthesis, Characterization, Rheological, and Degree of Conversion. Polymers 2023, 15, 1481. https://doi.org/10.3390/polym15061481
Al-Kahtani HM, Al-Odayni A-B, Saeed WS, Robaian A, Al-Kahtani A, Aouak T, Alrahlah A. Novel 1,2-Bismethacrylate-3-Eugenyl Propane for Resin Composites: Synthesis, Characterization, Rheological, and Degree of Conversion. Polymers. 2023; 15(6):1481. https://doi.org/10.3390/polym15061481
Chicago/Turabian StyleAl-Kahtani, Haifa Masfeer, Abdel-Basit Al-Odayni, Waseem Sharaf Saeed, Ali Robaian, Abdullah Al-Kahtani, Taieb Aouak, and Ali Alrahlah. 2023. "Novel 1,2-Bismethacrylate-3-Eugenyl Propane for Resin Composites: Synthesis, Characterization, Rheological, and Degree of Conversion" Polymers 15, no. 6: 1481. https://doi.org/10.3390/polym15061481
APA StyleAl-Kahtani, H. M., Al-Odayni, A.-B., Saeed, W. S., Robaian, A., Al-Kahtani, A., Aouak, T., & Alrahlah, A. (2023). Novel 1,2-Bismethacrylate-3-Eugenyl Propane for Resin Composites: Synthesis, Characterization, Rheological, and Degree of Conversion. Polymers, 15(6), 1481. https://doi.org/10.3390/polym15061481









