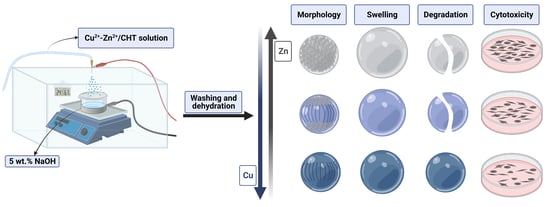
Preparation and Properties of Bimetallic Chitosan Spherical Microgels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bimetallic-Chitosan-Complex Solutions
2.3. Production of Bimetallic Chitosan Microgels
2.4. Characterization Methods
2.5. Enzymatic Degradation
2.6. Cell Cytotoxicity Assay
2.7. Statistical Analysis
3. Results and Discussion
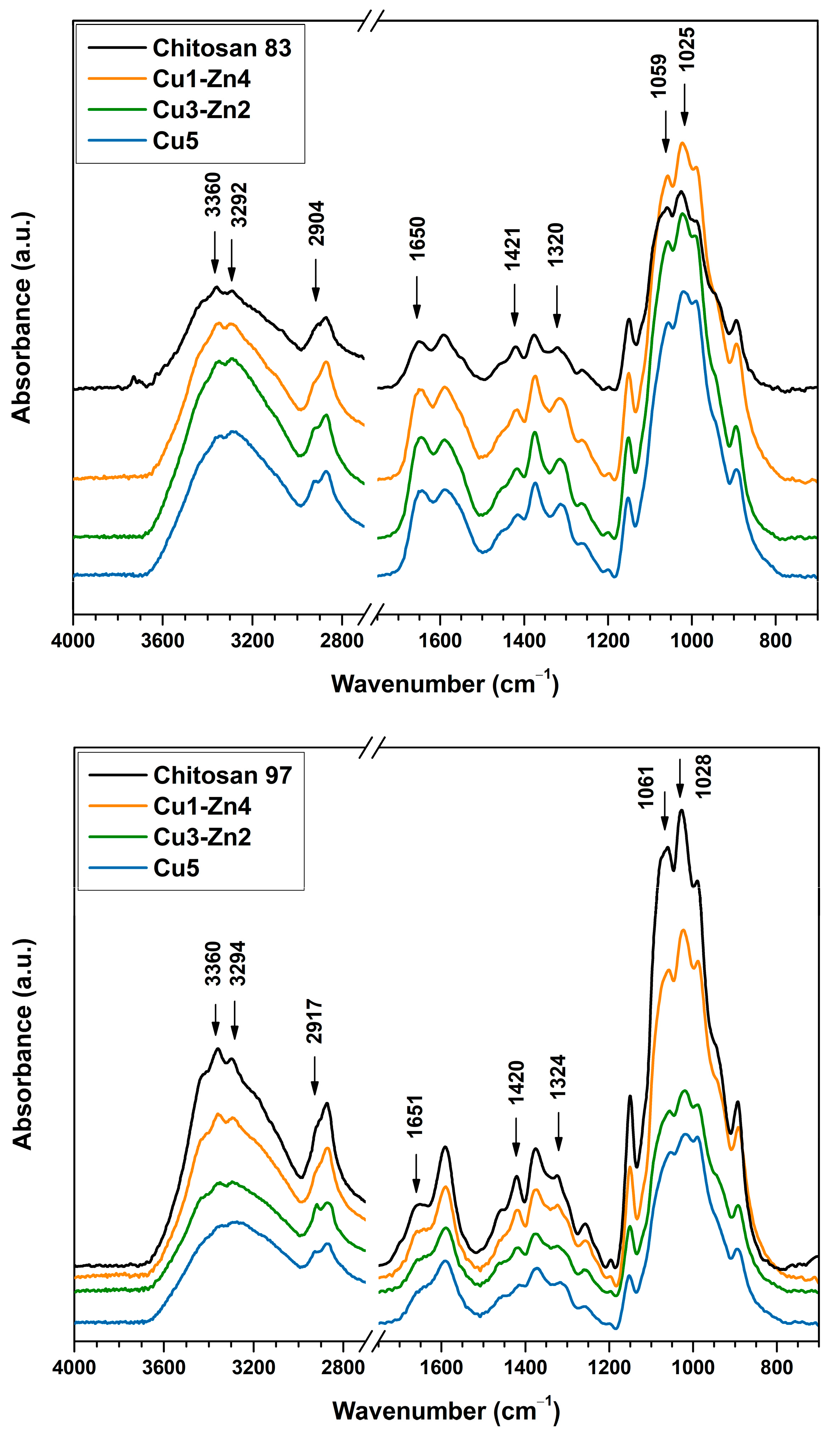
3.1. Structural Characterization
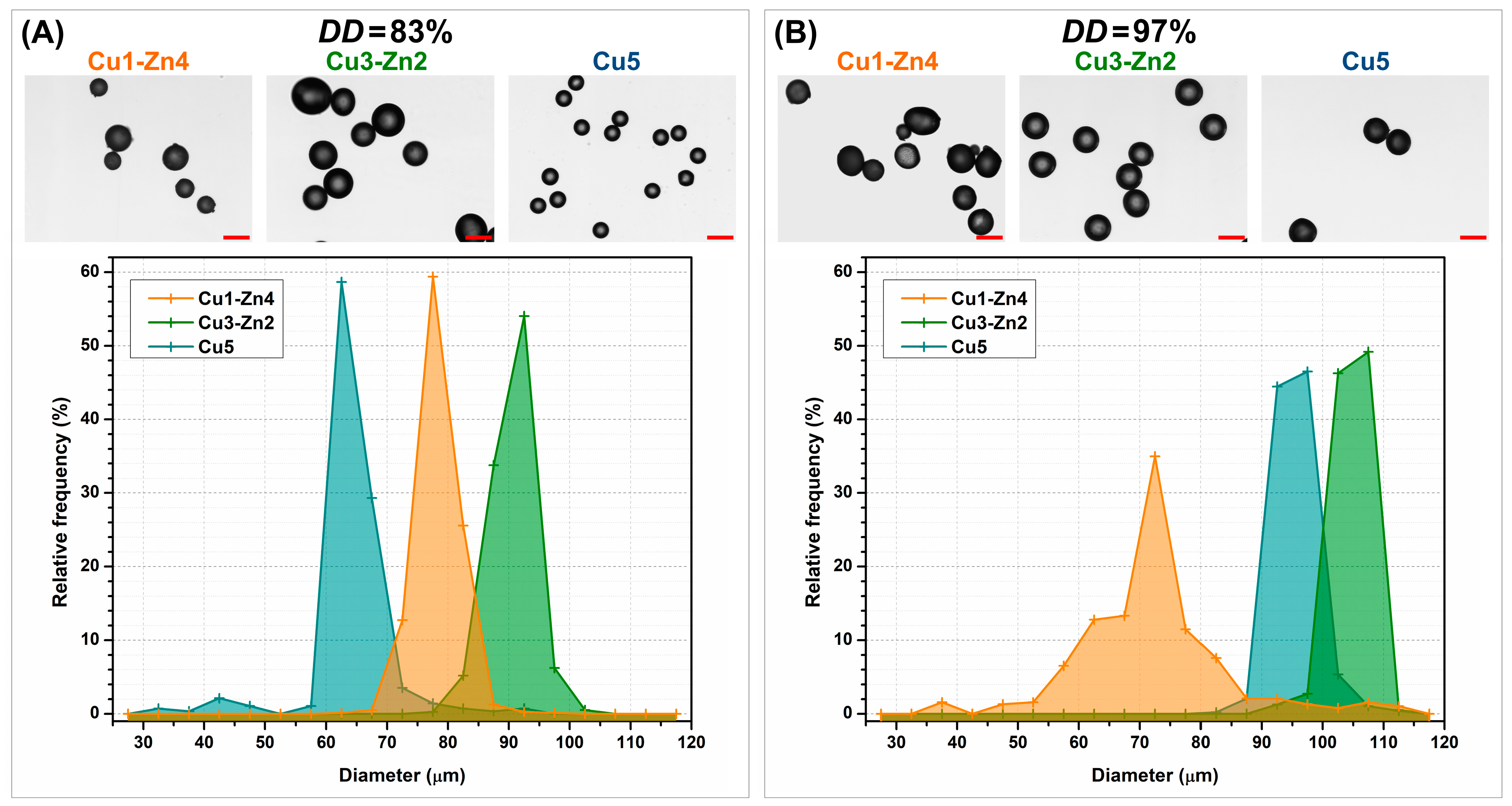
3.2. Size Distribution of Dry Microgels
3.3. Morphology of Dry Microgels
3.4. Swelling Property and Enzymatic Degradation
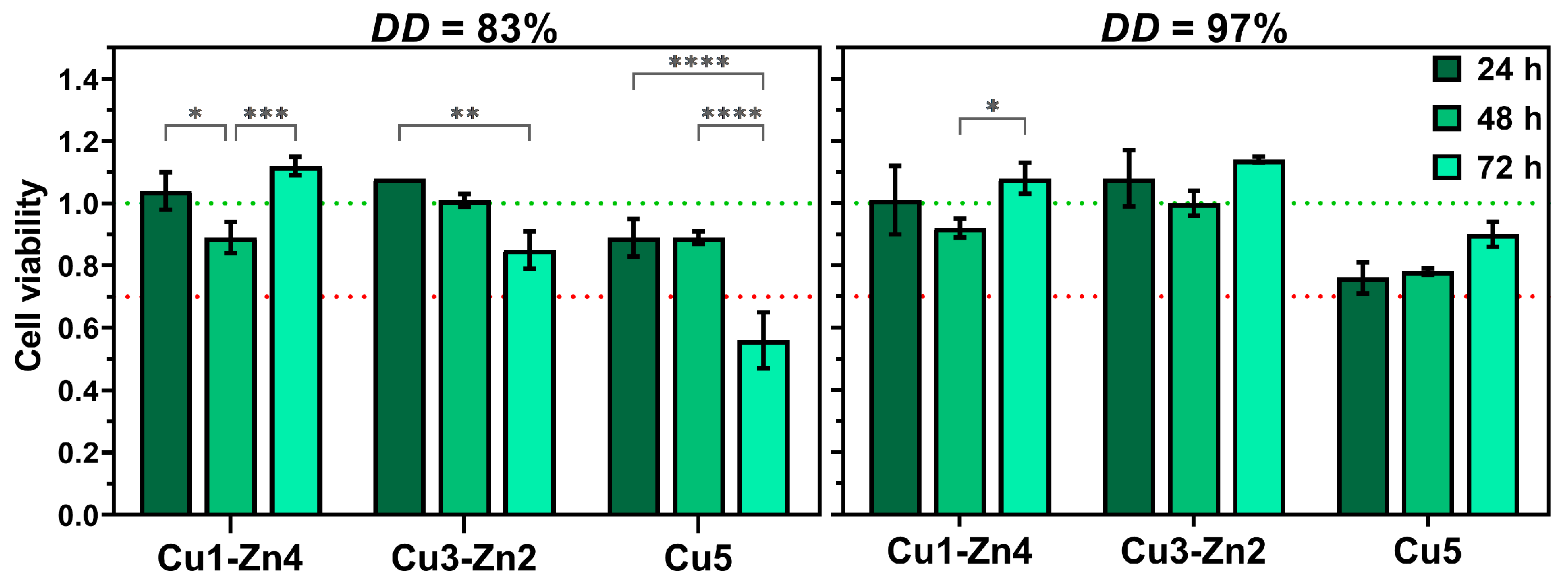
3.5. Cell Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, H.; Li, X.; Gao, W.; Fu, X.; Fang, R.H.; Zhang, L.; Zhang, K. Tissue repair and regeneration with endogenous stem cells. Nat. Rev. Mater. 2018, 3, 174–193. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef] [PubMed]
- Kurtuldu, F.; Kaňková, H.; Beltrán, A.M.; Liverani, L.; Galusek, D.; Boccaccini, A.R. Anti-inflammatory and antibacterial activities of cerium-containing mesoporous bioactive glass nanoparticles for drug-free biomedical applications. Mater. Today Bio. 2021, 12, 100150. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, U.; Kowol, C.R.; Keppler, B.K.; Hartinger, C.G.; Berger, W.; Heffeter, P.; Hager, S.; Pape, V.F.; Pósa, V.; Montsch, B.; et al. Anticancer Activity of Metal Complexes: Involvement of Redox Processes. Antioxid. Redox Signal. 2011, 15, 1085–1127. [Google Scholar] [CrossRef]
- Ciriza, J.; Rodríguez-Romano, A.; Nogueroles, I.; Gallego-Ferrer, G.; Cabezuelo, R.M.; Pedraz, J.L.; Rico, P. Borax-loaded injectable alginate hydrogels promote muscle regeneration in vivo after an injury. Mater. Sci. Eng. C 2021, 123, 112003. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.; Maswal, M.; Dar, A.A. Synergistic effect of various metal ions on the mechanical, thixotropic, self-healing, swelling and water retention properties of bimetallic hydrogels of alginate. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 627, 127223. [Google Scholar] [CrossRef]
- Yang, R.; Li, G.; Zhuang, C.; Yu, P.; Ye, T.; Zhang, Y.; Shang, P.; Huang, J.; Cai, M.; Wang, L.; et al. Gradient bimetallic ion–based hydrogels for tissue microstructure reconstruction of tendon-to-bone insertion. Sci. Adv. 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Rogina, A.; Lončarević, A.; Antunović, M.; Marijanović, I.; Ivanković, M.; Ivanković, H. Tuning physicochemical and biological properties of chitosan through complexation with transition metal ions. Int. J. Biol. Macromol. 2019, 129, 645–652. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef]
- Gu, G.; Erişen, D.E.; Yang, K.; Zhang, B.; Shen, M.; Zou, J.; Qi, X.; Chen, S.; Xu, X. Antibacterial and anti-inflammatory activities of chitosan/copper complex coating on medical catheters: In vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1899–1910. [Google Scholar] [CrossRef]
- Brunel, F.; El Gueddari, N.E.; Moerschbacher, B.M. Complexation of copper(II) with chitosan nanogels: Toward control of microbial growth. Carbohydr. Polym. 2013, 92, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Zhou, Y.-N.; Li, X.-Y.; Huang, J.; Wahid, F.; Zhong, C.; Chu, L.-Q. Continuous production of antibacterial carboxymethyl chitosan-zinc supramolecular hydrogel fiber using a double-syringe injection device. Int. J. Biol. Macromol. 2020, 156, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, N.; Liverani, L.; Kurtuldu, F.; Galusek, D.; Boccaccini, A.R. Zinc improves antibacterial, anti-inflammatory and cell motility activity of chitosan for wound healing applications. Int. J. Biol. Macromol. 2022, 213, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.F.; Patrício, S.G.; Silva, A.S.; Mano, J.F. Freestanding Magnetic Microtissues for Tissue Engineering Applications. Adv. Healthc. Mater. 2022, 11, 2101532. [Google Scholar] [CrossRef]
- Correia, C.R.; Bjørge, I.M.; Zeng, J.; Matsusaki, M.; Mano, J.F. Liquefied Microcapsules as Dual-Microcarriers for 3D+3D Bottom-Up Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1901221. [Google Scholar] [CrossRef]
- Rogina, A.; Vidović, D.; Antunović, M.; Ivanković, M.; Ivanković, H. Metal ion-assisted formation of porous chitosan-based microspheres for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 1027–1035. [Google Scholar] [CrossRef]
- Lončarević, A.; Ivanković, M.; Rogina, A. Electrosprayed Chitosan–Copper Complex Microspheres with Uniform Size. Materials 2021, 14, 5630. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Z.; Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep. 2016, 6, 36005. [Google Scholar] [CrossRef]
- Li, P.; Feng, Z.; Yu, Z.; Chen, Y.; Li, P.; Yang, Z.; Li, S.; Jin, S. Preparation of chitosan-Cu2+/NH3 physical hydrogel and its properties. Int. J. Biol. Macromol. 2019, 133, 67–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Bai, X.; Xu, J.; Zhang, J.; Zhang, G.; Huang, C.; Liu, W.; Huang, C.; Xiong, X. Microfluidic preparation of magnetic chitosan microsphere and its adsorption towards Congo red. J. Polym. Res. 2023, 30, 77. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, E.; Perkas, N.; Tzanov, T.; Beddow, J.; Joyce, E.; Mason, T.J.; Blanes, M.; Mollá, K.; Patlolla, A.; et al. Chitosan and chitosan–ZnO-based complex nanoparticles: Formation, characterization, and antibacterial activity. J. Mater. Chem. B 2013, 1, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan- metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Ogawa, K.; Oka, K.; Yui, T. X-ray study of chitosan-transition metal complexes. Chem. Mater. 1993, 5, 726–728. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Gomes, J.R.; Jorge, M.; Gomes, P. Interaction of chitosan and chitin with Ni, Cu and Zn ions: A computational study. J. Chem. Thermodyn. 2014, 73, 121–129. [Google Scholar] [CrossRef]
- Neto, M.; Oliveira, M.B.; Mano, J.F. Microparticles in Contact with Cells: From Carriers to Multifunctional Tissue Modulators. Trends Biotechnol. 2019, 37, 1011–1028. [Google Scholar] [CrossRef]
- Maciel, M.M.; Correia, T.R.; Henriques, M.; Mano, J.F. Microparticles orchestrating cell fate in bottom-up approaches. Curr. Opin. Biotechnol. 2022, 73, 276–281. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Virolainen, E.; Conley, K.; Vahala, R. Chitosan–Zinc(II) Complexes as a Bio-Sorbent for the Adsorptive Abatement of Phosphate: Mechanism of Complexation and Assessment of Adsorption Performance. Polymers 2017, 10, 25. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef]
- Sousa, M.P.; Arab-Tehrany, E.; Cleymand, F.; Mano, J.F. Surface Micro- and Nanoengineering: Applications of Layer-by-Layer Technology as a Versatile Tool to Control Cellular Behavior. Small 2019, 15, 1901228. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Jing, D.; Li, J.; Gong, Y.; Zhao, N.; Zhang, X. Effects of the Degree of Deacetylation on the Physicochemical Properties and Schwann Cell Affinity of Chitosan Films. J. Biomater. Appl. 2005, 20, 157–177. [Google Scholar] [CrossRef]
- Bagheri-Khoulenjani, S.; Taghizadeh, S.; Mirzadeh, H. An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr. Polym. 2009, 78, 773–778. [Google Scholar] [CrossRef]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper–zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Shi, S.; Kong, X.; Guo, G.; Huang, M.; Luo, F.; Wei, Y.; Zhao, X.; Qian, Z. Preparation and characterization of a novel chitosan scaffold. Carbohydr. Polym. 2010, 80, 860–865. [Google Scholar] [CrossRef]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef]
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable Chitosan Nanoparticle Coatings on Titanium for the Delivery of BMP-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef]
- Bosch-Rué, E.; Díez-Tercero, L.; Rodríguez-González, R.; Bosch-Canals, B.M.; Perez, R.A. Assessing the potential role of copper and cobalt in stimulating angiogenesis for tissue regeneration. PLoS ONE 2021, 16, e0259125. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]





| Sample | Cu1-Zn4 | Cu3-Zn2 | Cu5 | |
|---|---|---|---|---|
| DD83 | w(Cu2+), % | 0.62 | 1.84 | 3.03 |
| w(Zn2+), % | 2.51 | 1.27 | - | |
| DD97 | w(Cu2+), % | 0.64 | 1.90 | 3.12 |
| w(Zn2+), % | 2.58 | 1.31 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarević, A.; Ostojić, K.; Urlić, I.; Rogina, A. Preparation and Properties of Bimetallic Chitosan Spherical Microgels. Polymers 2023, 15, 1480. https://doi.org/10.3390/polym15061480
Lončarević A, Ostojić K, Urlić I, Rogina A. Preparation and Properties of Bimetallic Chitosan Spherical Microgels. Polymers. 2023; 15(6):1480. https://doi.org/10.3390/polym15061480
Chicago/Turabian StyleLončarević, Andrea, Karla Ostojić, Inga Urlić, and Anamarija Rogina. 2023. "Preparation and Properties of Bimetallic Chitosan Spherical Microgels" Polymers 15, no. 6: 1480. https://doi.org/10.3390/polym15061480
APA StyleLončarević, A., Ostojić, K., Urlić, I., & Rogina, A. (2023). Preparation and Properties of Bimetallic Chitosan Spherical Microgels. Polymers, 15(6), 1480. https://doi.org/10.3390/polym15061480