Research on Temperature-Switched Dopamine Electrochemical Sensor Based on Thermosensitive Polymers and MWCNTs
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of PNIPAM
2.3. Preparation of Modified Electrode
2.4. Main Test Methods
2.5. Analysis of Actual Samples
3. Results
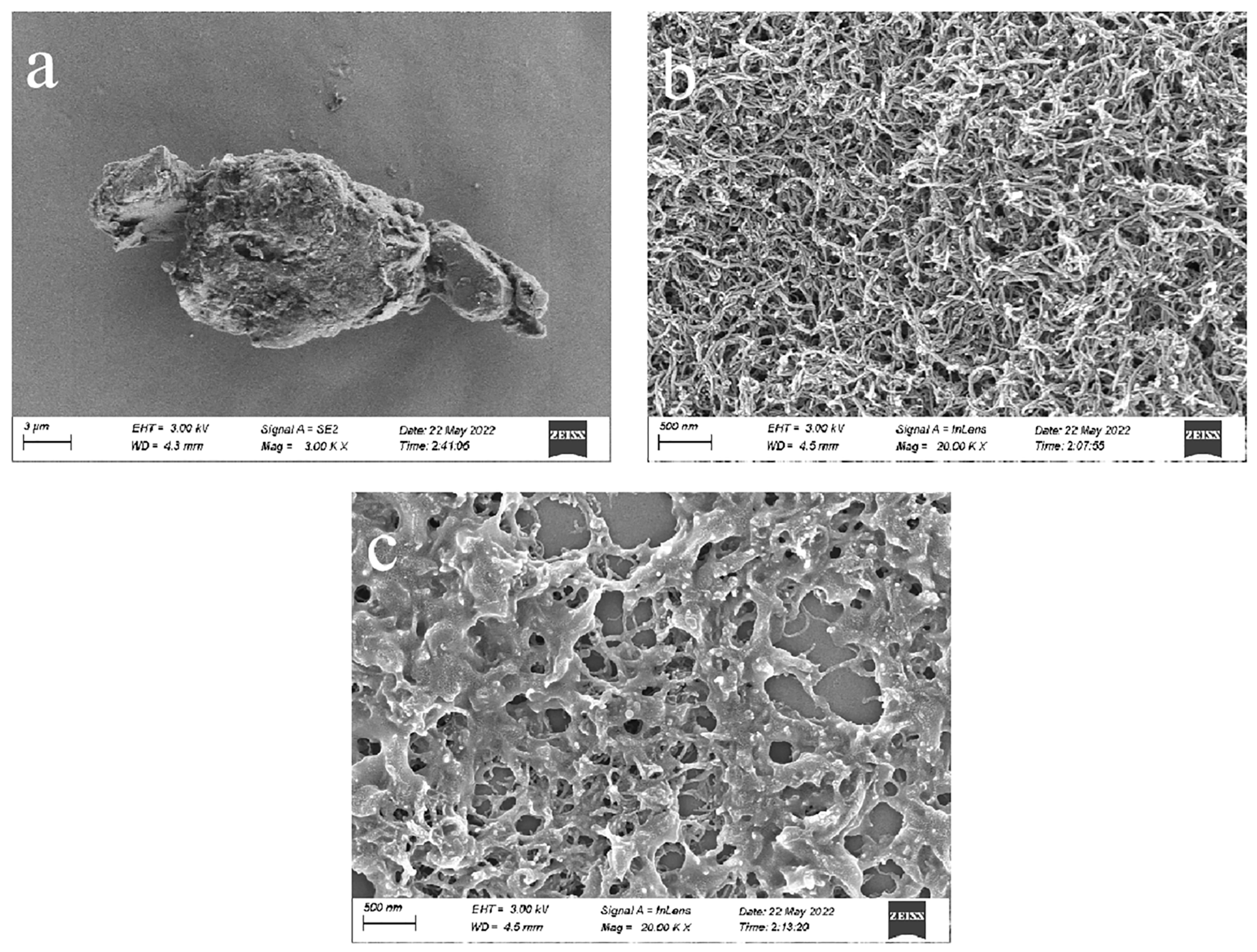
3.1. SEM Characterization
3.2. EIS Analysis
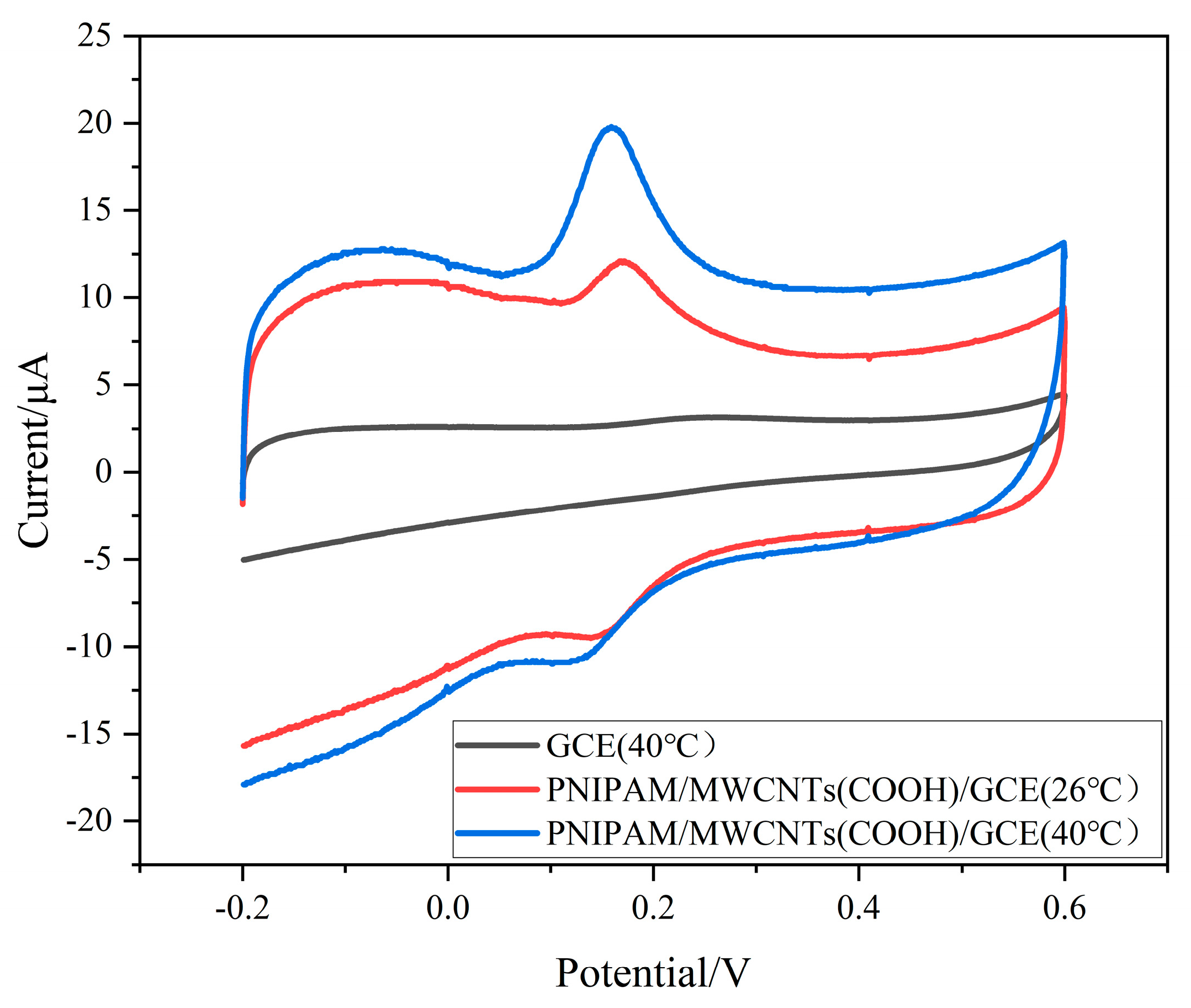
3.3. Dopamine-Detection Behavior of Different Materials
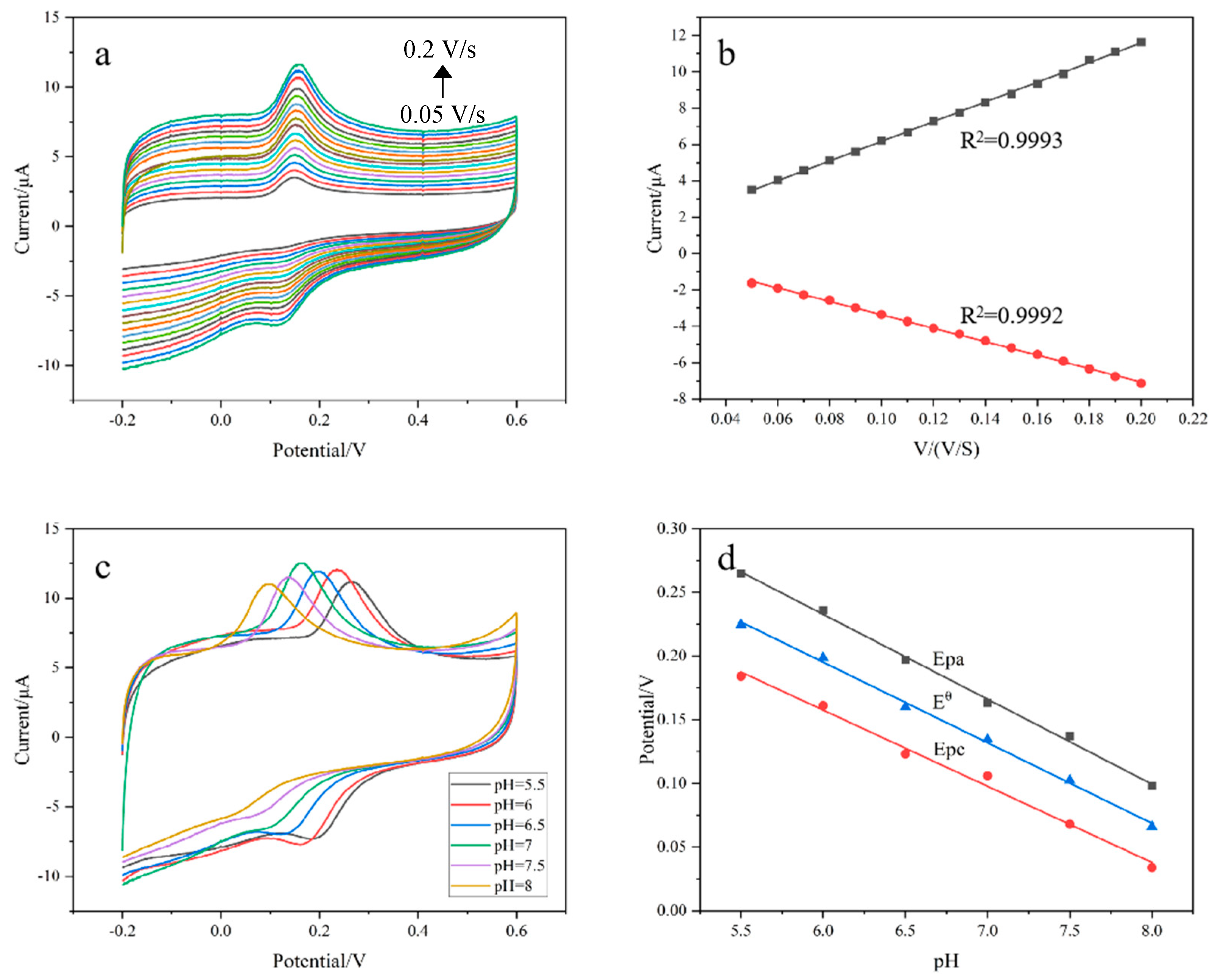
3.4. Effect of Scan Rate and pH
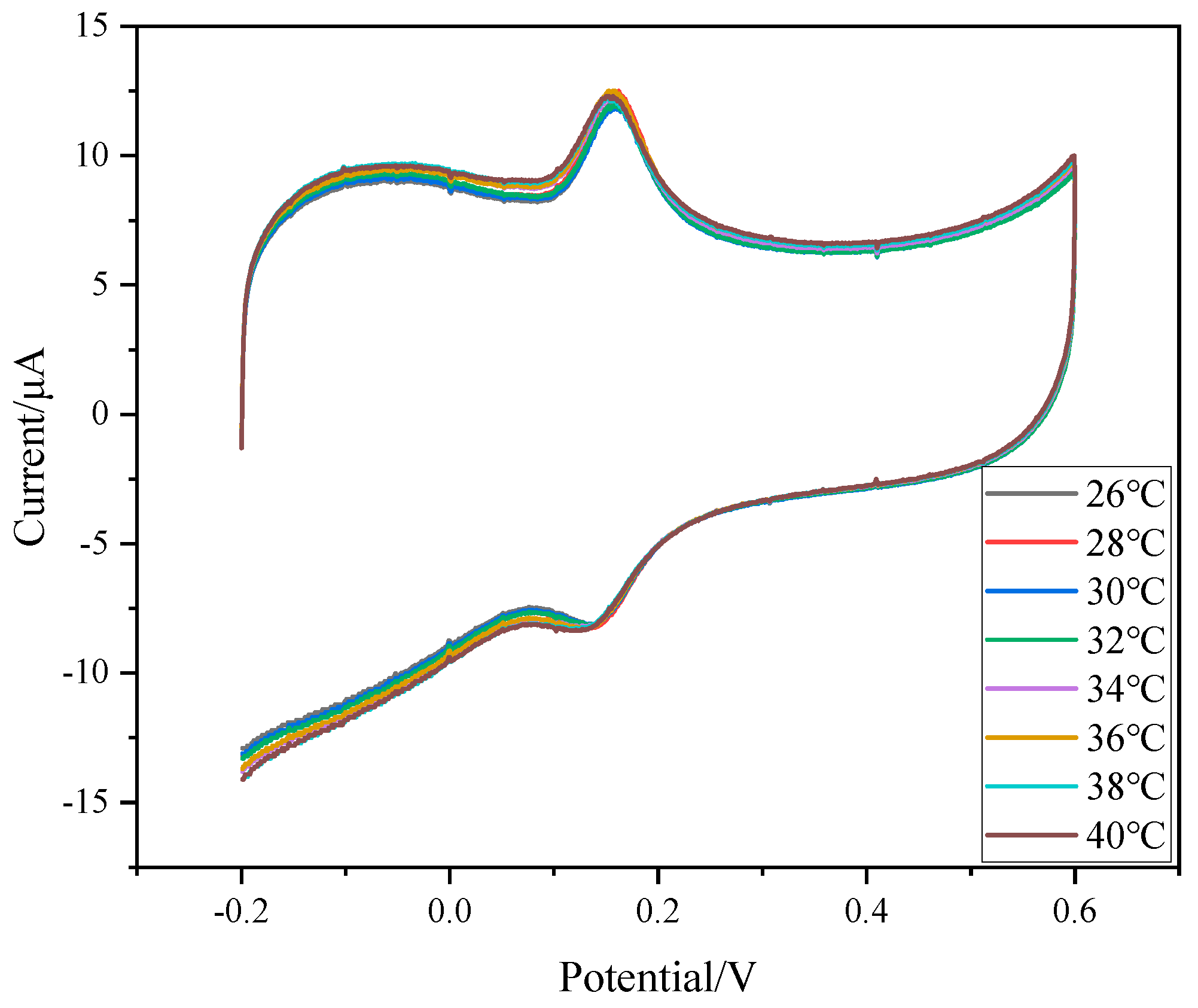
3.5. Temperature Response of DA on Composite Modified Electrode
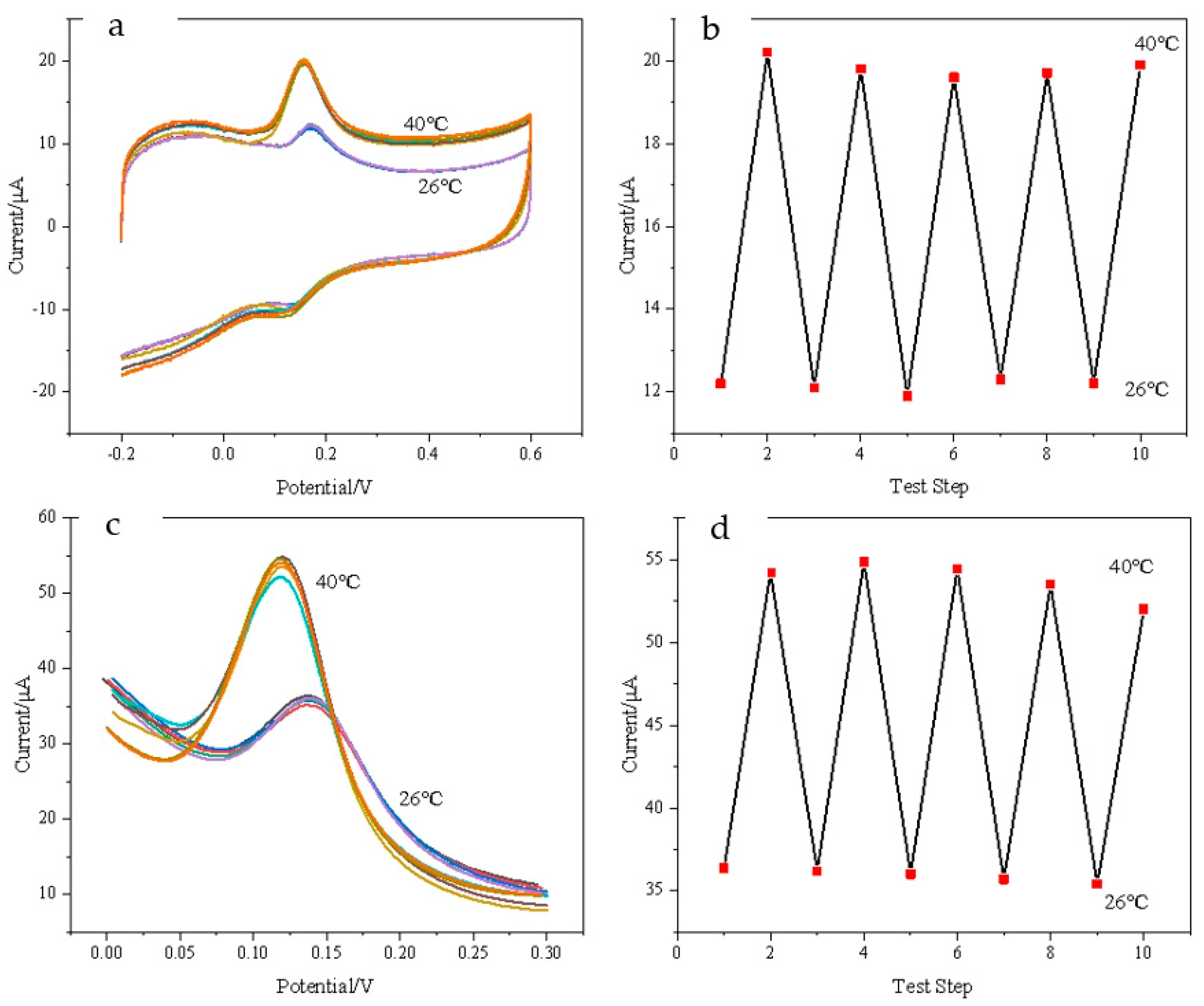
3.6. Temperature-Reversible Switch Response of DA on Composite Modified Electrode
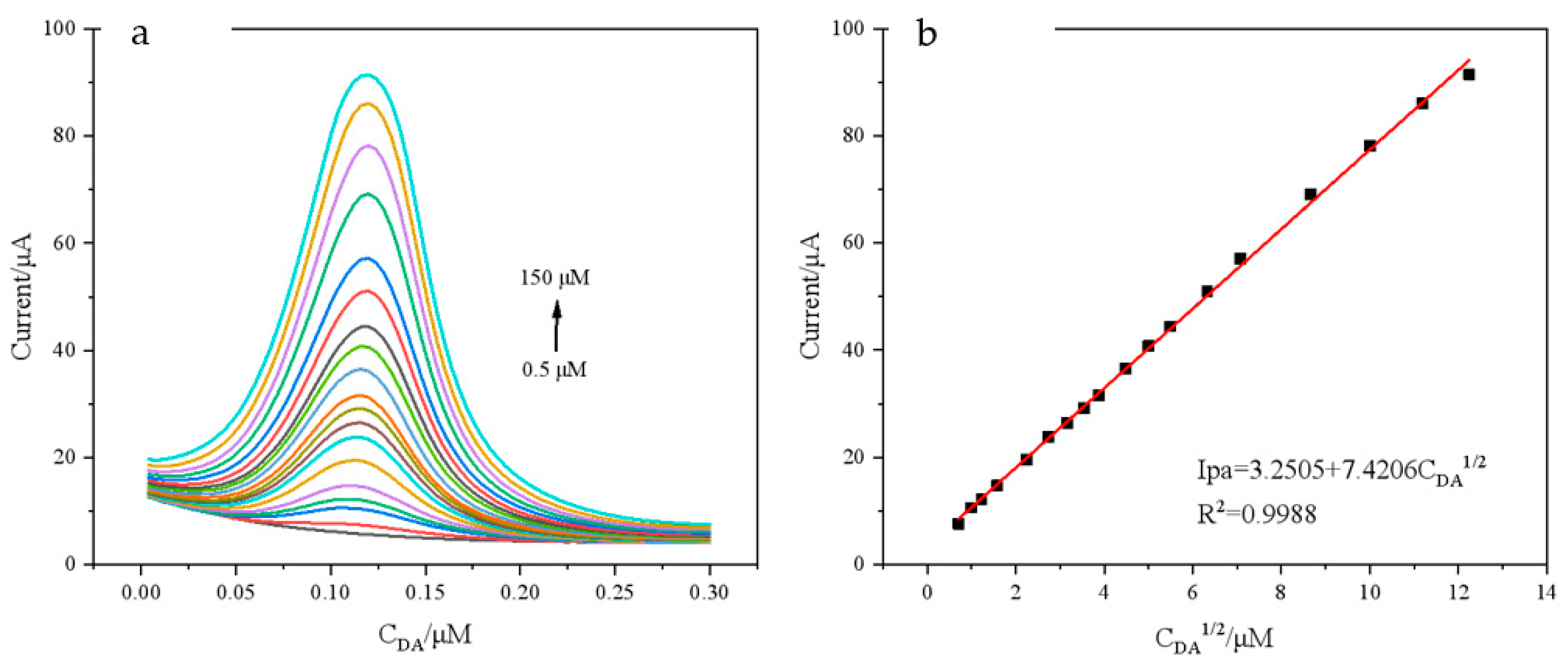
3.7. Determination of DA on PNIPAM/MWCNTs(COOH)/GCE
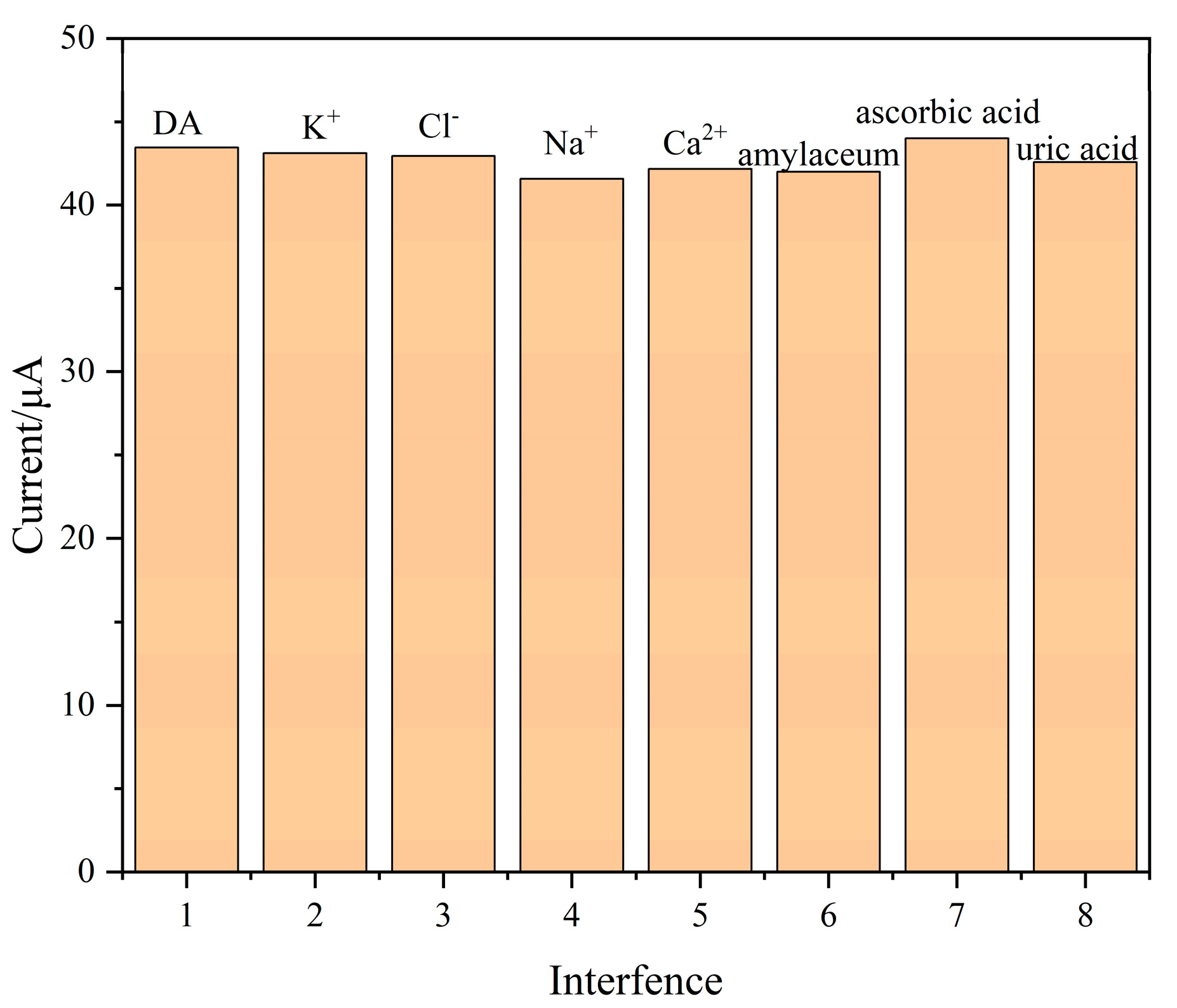
3.8. Stability, Reproducibility and Anti-Interference Test
3.9. Actual Sample Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical biosensing of dopamine neurotransmitter: A review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Shaheen Shah, S.; Naher, S.; Ali Ehsan, M.; Aziz, M.A.; Ahammad, A.S. Graphene and Carbon Nanotube-based Electrochemical Sensing Platforms for Dopamine. Chem.-Asian J. 2021, 16, 3516–3543. [Google Scholar] [CrossRef]
- Bucher, M.L.; Barrett, C.W.; Moon, C.J.; Mortimer, A.D.; Burton, E.A.; Greenamyre, J.T.; Hastings, T.G. Acquired dysregulation of dopamine homeostasis reproduces features of Parkinson’s disease. NPJ Parkinson’s Dis. 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Delva, N.C.; Stanwood, G.D. Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef]
- Rinne, J.O.; Säkö, E.; Paljärvi, L.; Mölsä, P.K.; Rinne, U.K. Brain dopamine D-1 receptors in senile dementia. J. Neurol. Sci. 1986, 73, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Samaha, A.N.; Khoo, S.Y.S.; Ferrario, C.R.; Robinson, T.E. Dopamine ‘ups and downs’ in addiction revisited. Trends Neurosci. 2021, 44, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yuan, C.; Shi, R.; Wang, S.; Wang, Y. Colorimetric detection of dopamine based on iodine-mediated etching of gold nanorods. Chin. J. Anal. Chem. 2021, 49, 60–67. [Google Scholar] [CrossRef]
- Khajehsharifi, H.; Pourbasheer, E.; Tavallali, H.; Sarvi, S.; Sadeghi, M. The comparison of partial least squares and principal component regression in simultaneous spectrophotometric determination of ascorbic acid, dopamine and uric acid in real samples. Arab. J. Chem. 2017, 10, S3451–S3458. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Francis, K.A.; Patel, J.; Jha, S.K.; Basu, S. A decoupler-free simple paper microchip capillary electrophoresis device for simultaneous detection of dopamine, epinephrine and serotonin. RSC Adv. 2020, 10, 25487–25495. [Google Scholar] [CrossRef]
- Pan, Q.; Xu, Z.; Deng, S.; Zhang, F.; Li, H.; Cheng, Y.; Zhou, B. A mechanochemically synthesized porous organic polymer derived CQD/chitosan-graphene composite film electrode for electrochemiluminescence determination of dopamine. RSC Adv. 2019, 9, 39332–39337. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Xie, Q.; Huang, S.; Liu, J.; Li, Z.; Yao, S. Simultaneous analysis of dopamine and homovanillic acid by high-performance liquid chromatography with wall-jet/thin-layer electrochemical detection. Analyst 2013, 138, 7246–7253. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, Z.; Wang, Z. A simple dopamine detection method based on fluorescence analysis and dopamine polymerization. Microchem. J. 2019, 145, 55–58. [Google Scholar] [CrossRef]
- Kannan, P.K.; Moshkalev, S.A.; Rout, C.S. Highly sensitive and selective electrochemical dopamine sensing properties of multilayer graphene nanobelts. Nanotechnology 2016, 27, 075504. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fen, Y.W. Recent advances in electrochemical and optical sensing of dopamine. Sensors 2020, 20, 1039–1086. [Google Scholar] [CrossRef] [PubMed]
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.U.; Boydston, A.J.; Nelson, A. Stimuli-responsive materials in additive manufacturing. Prog. Polym. Sci. 2019, 93, 36–67. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Cinay, G.E.; Erkoc, P.; Alipour, M.; Hashimoto, Y.; Sasaki, Y.; Akiyoshi, K.; Kizilel, S. Nanogel-integrated pH-responsive composite hydrogels for controlled drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 370–380. [Google Scholar] [CrossRef]
- Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Light-Responsive Polymer Membranes. Adv. Opt. Mater. 2019, 7, 1900252. [Google Scholar] [CrossRef]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.W.; Xia, Y. Stimuli-responsive materials for controlled release of theranostic agents. Adv. Funct. Mater. 2014, 24, 4206–4220. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, Q.; Wang, Y.; Du, X. Bioinspired actuators based on stimuli-responsive polymers. Chem.-Asian J. 2019, 14, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Moini Jazani, A.; Howell, E.P.; Oh, J.K.; Moffitt, M.G. Controlled Microfluidic Synthesis of Biological Stimuli-Responsive Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2019, 12, 177–190. [Google Scholar] [CrossRef]
- He, W.; Ma, Y.; Gao, X.; Wang, X.; Dai, X.; Song, J. Application of Poly (N-isopropylacrylamide) as Thermosensitive Smart Materials. J. Phys. Conf. Ser. 2020, 1676, 012063. [Google Scholar] [CrossRef]
- Dan, X.; Ruiyi, L.; Qinsheng, W.; Yongqiang, Y.; Guangli, W.; Zaijun, L. Thermal-switchable sensor based on palladium-graphene composite and poly (N-isopropylacrylamide) for electrochemical detection of 4-nitrophenol. Microchem. J. 2022, 172, 106970. [Google Scholar] [CrossRef]
- Mutharani, B.; Ranganathan, P.; Chen, S.M.; Chen, T.W.; Ali, M.A.; Mahmoud, A.H. Sonochemical synthesis of novel thermo-responsive polymer and tungsten dioxide composite for the temperature-controlled reversible “on-off” electrochemical detection of β-Blocker metoprolol. Ultrason. Sonochem. 2020, 64, 105008. [Google Scholar] [CrossRef] [PubMed]
- Feminus, J.J.; Manikandan, R.; Narayanan, S.S.; Deepa, P.N. Determination of gallic acid using poly (glutamic acid): Graphene modified electrode. J. Chem. Sci. 2019, 131, 11. [Google Scholar] [CrossRef]
- Huang, Z.N.; Zou, J.; Teng, J.; Liu, Q.; Yuan, M.M.; Jiao, F.P.; Jiang, X.Y.; Yu, J.G. A novel electrochemical sensor based on self-assembled platinum nanochains-multi-walled carbon nanotubes-graphene nanoparticles composite for simultaneous determination of dopamine and ascorbic acid. Ecotoxicol. Environ. Saf. 2019, 172, 167–175. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Shi, W.; Jiang, M.; Tian, L.; Su, M.; Gu, H. Pt nanoparticle decorated carbon nanotubes nanocomposite based sensing platform for the monitoring of cell-secreted dopamine. Sens. Actuators B 2021, 330, 129311. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Z.; Fan, W.; Wang, Y.; Li, G.; Cong, H.; Yuan, H. Carbon nanotube/carbon fiber electrodes via chemical vapor deposition for simultaneous determination of ascorbic acid, dopamine and uric acid. Arab. J. Chem. 2021, 13, 3266–3275. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Zhang, L.; Pei, T.; Li, E.; Wang, H.; Zhang, Q.; Xia, L. Temperature-Responsive Electrocatalysis Based on Poly (N-Isopropylacrylamide)-Modified Graphene Oxide (PNIPAm-GO). Chem.-Eur. J. 2019, 25, 1535–1542. [Google Scholar] [CrossRef]

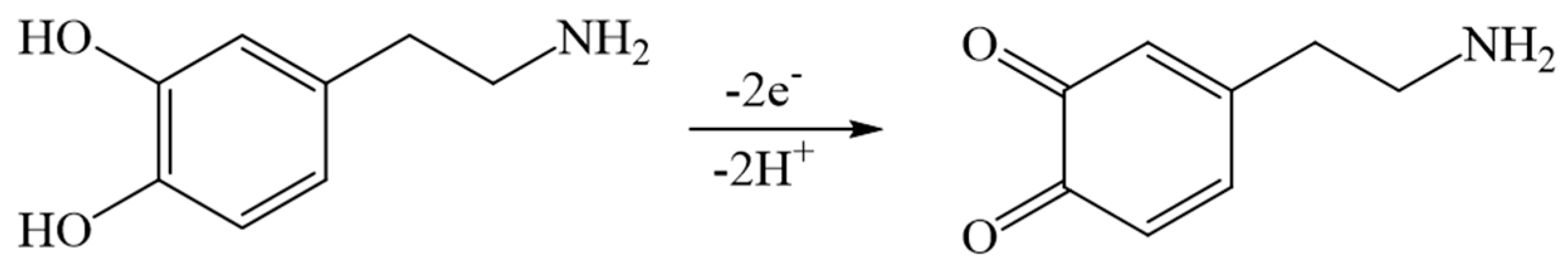


| Electrode | Technique Linear Range of DA (μM) | Linear Range of DA (μM) | LOD of HQ (μM) | Ref |
|---|---|---|---|---|
| PtNCs-MWCNTs-GNPs/GCE | DPV | 2–50 | 0.5 | [26] |
| Pt-MWCNTs/SPE | DPV | 0.005–1 | 0.002 | [27] |
| CNTs/CFE | DPV | 5–120.6 | 0.03 | [28] |
| PNIPAm-GO/GC | DPV | 3.9–174 | 1.3 | [29] |
| PNIPAM/MWCNTs(COOH)/GCE | DPV | 0.5–150 | 0.119 | This work |
| Sample | Added/μM | Found/μM | Recovery/% |
|---|---|---|---|
| 1 | 25 | 24.7 | 98.8 |
| 2 | 50 | 49.35 | 98.7 |
| 3 | 60 | 62.64 | 104.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Feng, Z.; Lin, F.; Zhao, Y.; Hu, Y.; Yang, Q.; Zou, Y.; Zhao, Y.; Yang, R. Research on Temperature-Switched Dopamine Electrochemical Sensor Based on Thermosensitive Polymers and MWCNTs. Polymers 2023, 15, 1465. https://doi.org/10.3390/polym15061465
Wang H, Feng Z, Lin F, Zhao Y, Hu Y, Yang Q, Zou Y, Zhao Y, Yang R. Research on Temperature-Switched Dopamine Electrochemical Sensor Based on Thermosensitive Polymers and MWCNTs. Polymers. 2023; 15(6):1465. https://doi.org/10.3390/polym15061465
Chicago/Turabian StyleWang, Haixiu, Zufei Feng, Fupeng Lin, Yan Zhao, Yangfan Hu, Qian Yang, Yiming Zou, Yingjuan Zhao, and Rong Yang. 2023. "Research on Temperature-Switched Dopamine Electrochemical Sensor Based on Thermosensitive Polymers and MWCNTs" Polymers 15, no. 6: 1465. https://doi.org/10.3390/polym15061465
APA StyleWang, H., Feng, Z., Lin, F., Zhao, Y., Hu, Y., Yang, Q., Zou, Y., Zhao, Y., & Yang, R. (2023). Research on Temperature-Switched Dopamine Electrochemical Sensor Based on Thermosensitive Polymers and MWCNTs. Polymers, 15(6), 1465. https://doi.org/10.3390/polym15061465






