Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the Titanium Nanosystem Catalysts
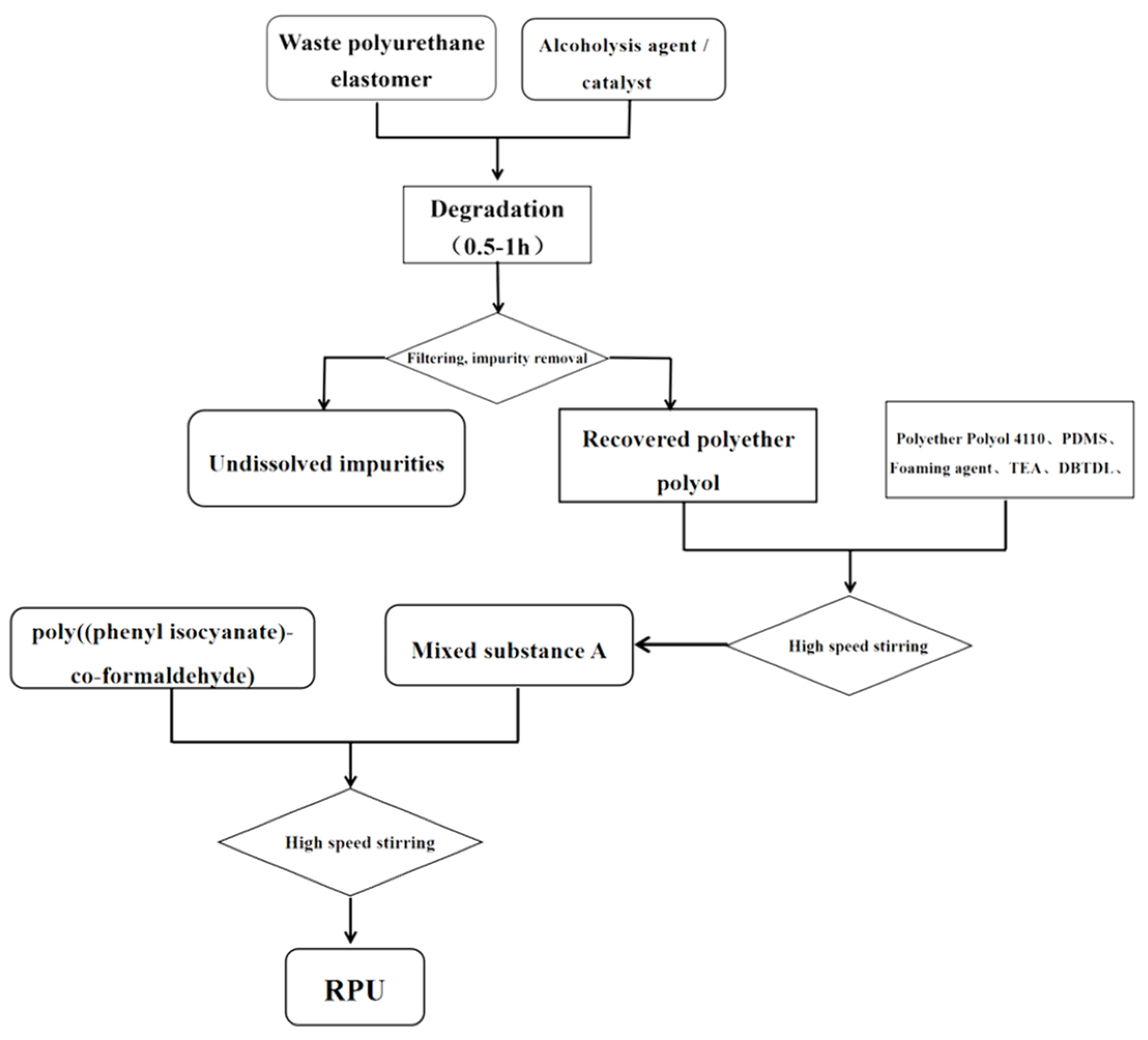
- The waste polyurethane elastomer was cut and processed to 5–10 mm small sections.
- We mixed 80 g small molecular alcohol and catalyst (KOH) and added them into a 1000 mL spherical reactor. We then placed the reactor in a heating jacket and stirred to 90 °C until the catalyst was completely dissolved.
- We added 80 g of chopped waste polyurethane elastomer into the reactor and heated it up to 180 °C. At that time, we accelerated the stirring speed and waited for the polyurethane elastomer to dissolve, gently stirring for 0.5–1 h.
- We stopped the reaction and cooled the mixture to room temperature before pouring it into a disposable plastic cup.
- The hydroxyl value and viscosity of the sample were measured, and the foaming experiment was carried out after the value had reached the standard.
- We took a certain amount of alcoholysis products and added the catalyst, foaming agent, and surfactant, stirring the mixture evenly. Then, we added polyphenyl polymethylene polyisocyanate (PAPI) at a ratio of 1:1 and immediately stirred with a high-speed mixer for 10 s until the mixture was uniform, and we waited for the foam to slowly form.
- The sample preparation and testing could then be carried out on the regenerated polyurethane hard foam, which was to be prepared after curing and cross-linking for 24 h.
2.3. Performance Test and Structural Characterization of the Polyurethane Rigid Foam
3. Results and Discussion
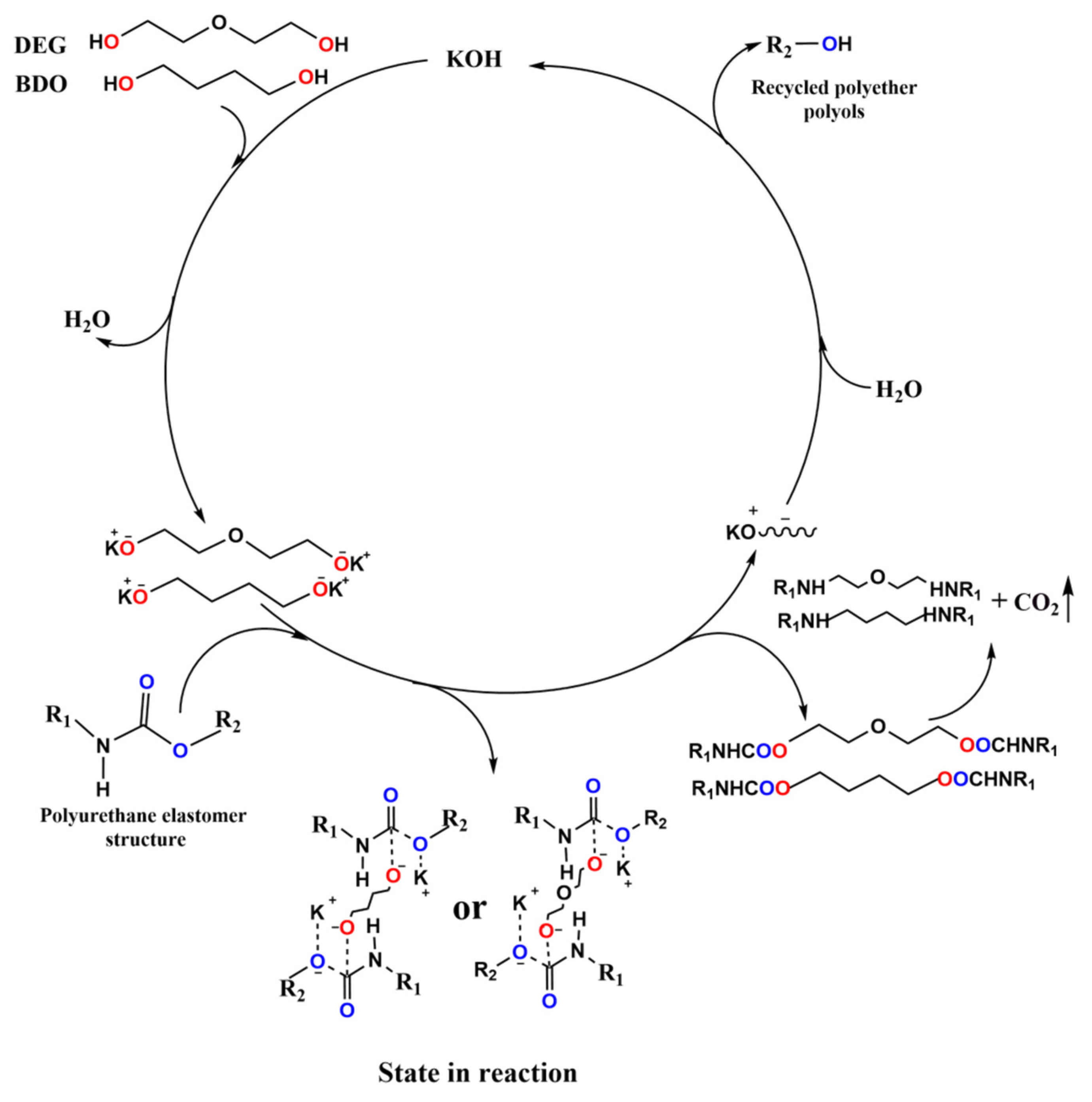
3.1. Degradation Mechanism of the Waste Polyurethane Elastomers
3.2. Small Molecular Alcohol Is Degraded and Foamed
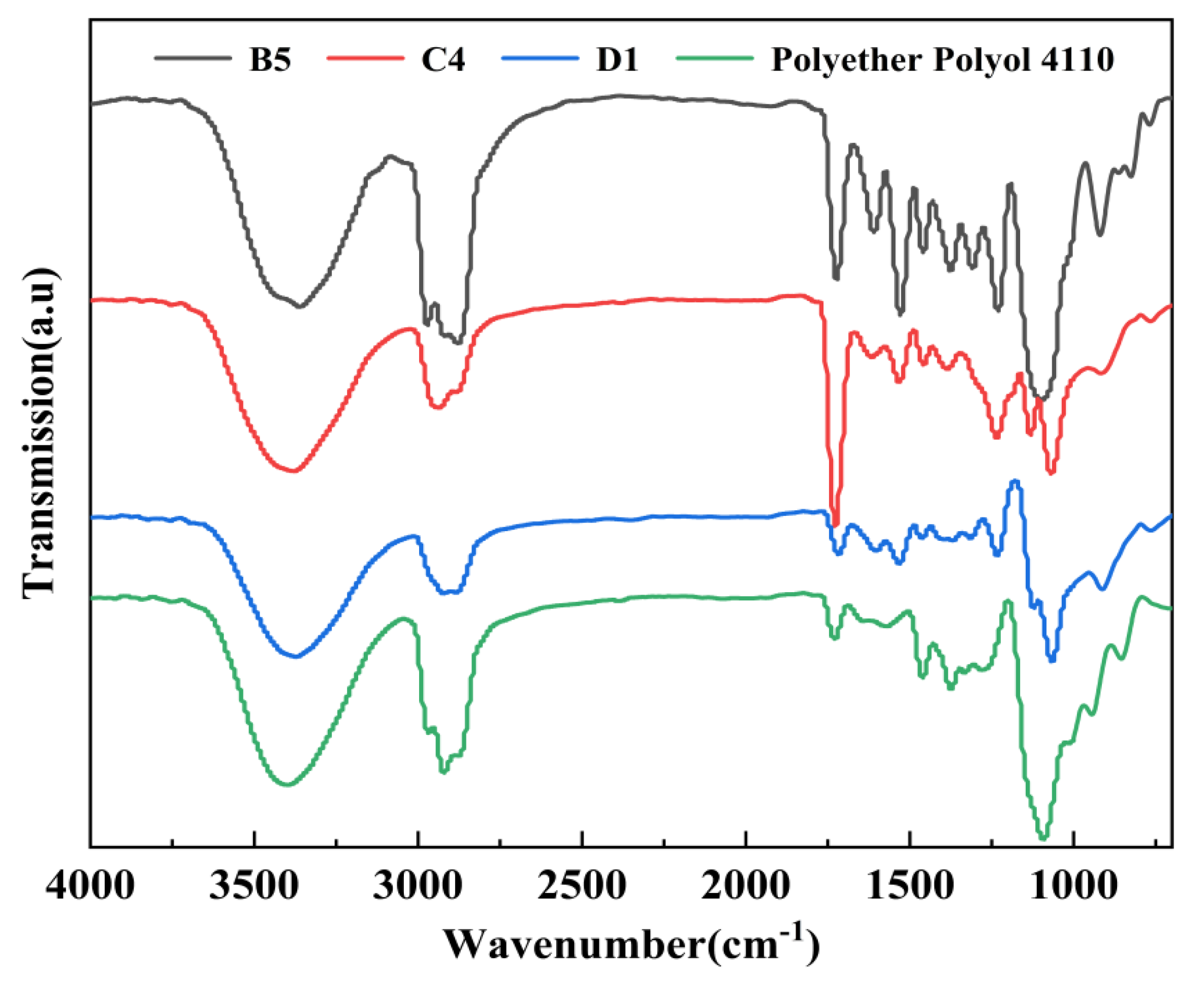
3.3. FT-IR Analysis of the Degradation Materials
3.4. Viscosity of Degradation Products
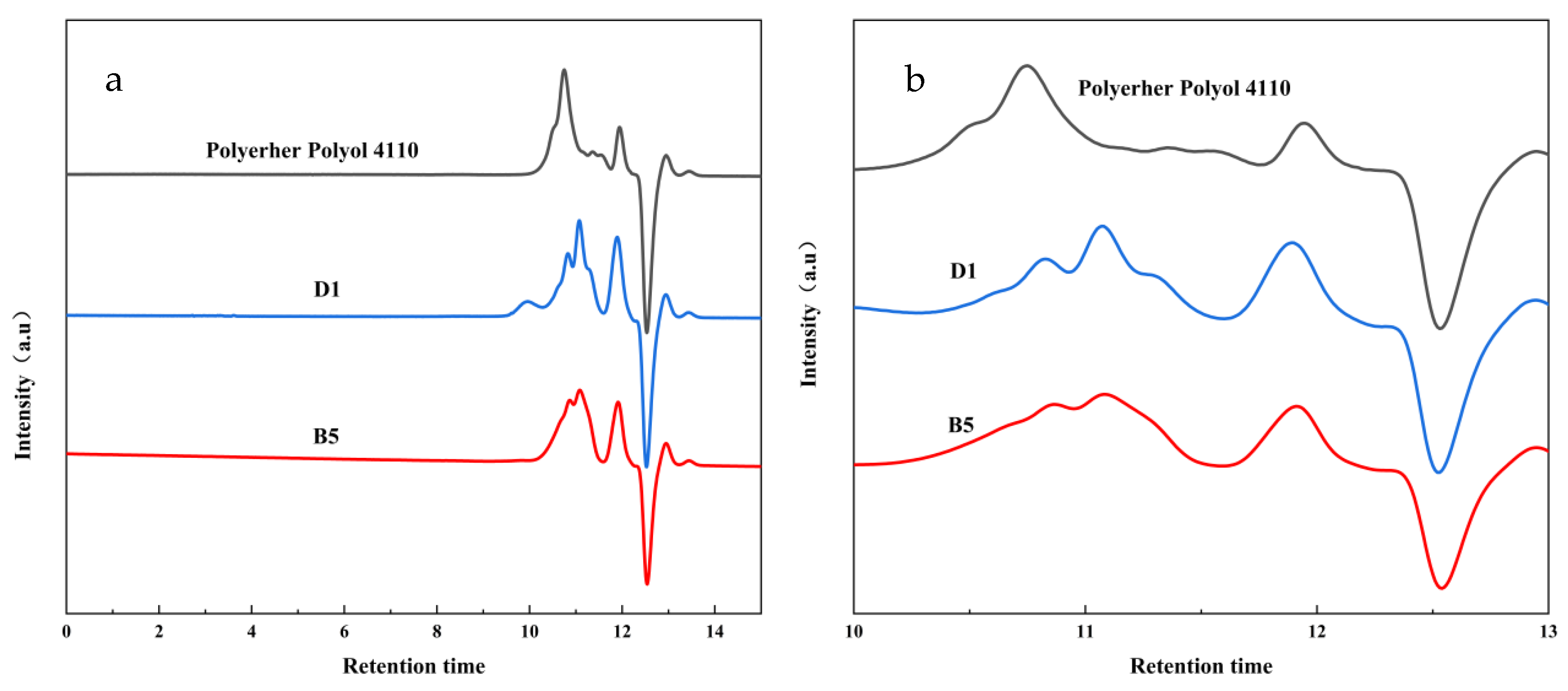
3.5. GPC Determination of the Degradation Products
3.6. Foaming Time of the Regenerated PU Rigid Foam
3.7. Water Absorption of the Regenerated PU Rigid Foam
3.8. Determination of Density, Strength, and Thermal Conductivity of the Recycled PU Hard Foam
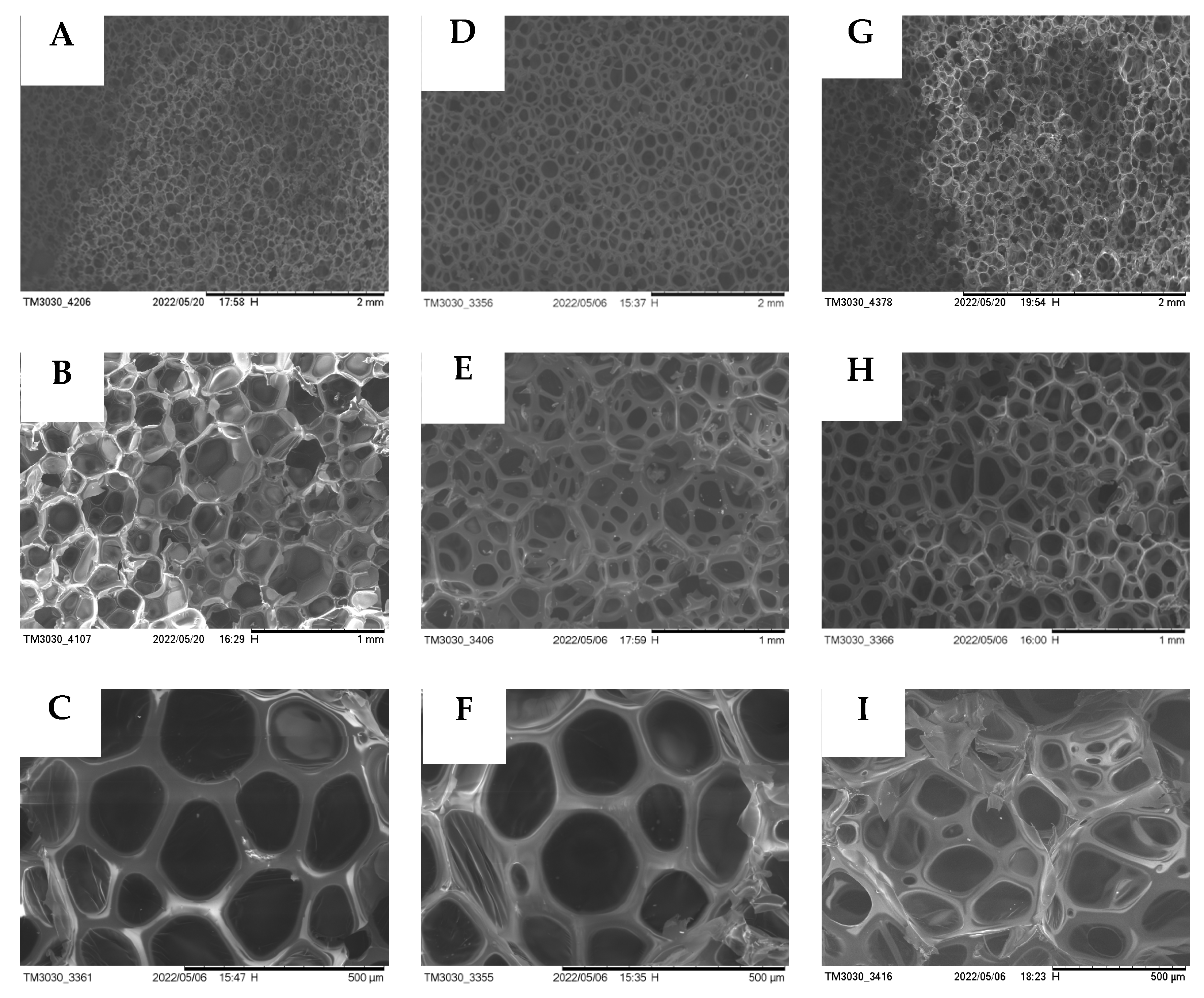
3.9. SEM Analysis of the Regenerated PU Rigid Foam
3.10. TG Analysis of the Regenerated PU Rigid Foam
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqba, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Advances in Waterborne Polyurethane and Polyurethane-Urea Dispersions and Their Eco-friendly Derivatives: A Review. Polymers 2021, 13, 409. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H.; Bian, Q.; Xiang, A.; Ge, X.; Liu, Q. Effect of Trolamine and Dibutyltindilaurate on the Structure and Properties of Polyurethaneimide Foams. Cell. Polym. 2015, 34, 119–136. [Google Scholar] [CrossRef]
- Hernandez, M.D.R.; Garcia, A.N.; Marcilla, A. Catalytic flash pyrolysis of HDPE in a fluidized bed reactor for recovery of fuel-like hydrocarbons. J. Anal. Appl. Pyrolysis 2007, 78, 272–281. [Google Scholar] [CrossRef]
- Gang, H.; Lee, D.; Choi, K.-Y.; Kim, H.-N.; Ryu, H.; Lee, D.-S.; Kim, B.-G. Development of High Performance Polyurethane Elastomers Using Vanillin-Based Green Polyol Chain Extender Originating from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 4582–4588. [Google Scholar] [CrossRef]
- Zhang, H.B.; Li, W.; Kong, X.; Yang, X.J. Effects of synthesis technology route on mechanical properties and phases/microstructures of polyurethane elastomers. Mater. Sci. Technol. 2007, 23, 1256–1259. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Huang, H. Thermoplastic polyurethane–urea elastomers with superior mechanical and thermal properties prepared from alicyclic diisocyanate and diamine. J. Appl. Polym. Sci. 2020, 137, 49575. [Google Scholar] [CrossRef]
- Jin, X.; Guo, N.; You, Z.; Tan, Y. Design and Performance of Polyurethane Elastomers Composed with Different Soft Segments. Materials 2020, 13, 4991. [Google Scholar] [CrossRef]
- Król, P.; Król, B. Structures, properties and applications of the polyurethane ionomers. J. Mater. Sci. 2020, 55, 73–87. [Google Scholar] [CrossRef]
- Elkhaoulani, A.; Arrakhiz, F.Z.; Benmoussa, K.; Bouhfid, R.; Qaiss, A. Mechanical and thermal properties of polymer composite based on natural fibers: Moroccan hemp fibers/polypropylene. Mater. Des. 2013, 49, 203–208. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste Rubber Recycling: A Review on the Evolution and Properties of Thermoplastic Elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, C. Polyurethane Elastomers; Applied Science Publisher, Ltd.: London, UK, 2020. [Google Scholar]
- Steube, M.; Johann, T.; Barent, R.D.; Müller, A.H.E.; Frey, H. Rational design of tapered multiblock copolymers for thermoplastic elastomers. Prog. Polym. Sci. 2022, 124, 101488. [Google Scholar] [CrossRef]
- Feghali, E.; Tauk, L.; Ortiz, P.; Vanbroekhoven, K.; Eevers, W. Catalytic chemical recycling of biodegradable polyesters. Polym. Degrad. Stab. 2020, 179, 109241. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Foundation, M.C. The New Plastics Economy: Rethinking the Future of Plastics; Ellen MacArthur Foundation: Cowes, UK, 2015. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Mizera, K.; Sałasińska, K.; Ryszkowska, J.; Kurańska, M.; Kozera, R. Effect of the Addition of Biobased Polyols on the Thermal Stability and Flame Retardancy of Polyurethane and Poly(urea)urethane Elastomers. Materials 2021, 14, 1805. [Google Scholar] [CrossRef]
- Zhang, G.; Yin, T.; Nian, G.; Suo, Z. Fatigue-resistant polyurethane elastomer composites. Extrem. Mech. Lett. 2021, 48, 101434. [Google Scholar] [CrossRef]
- Watando, H.; Saya, S.; Fukaya, T.; Fujieda, S.; Yamamoto, M. Improving chemical recycling rate by reclaiming polyurethane elastomer from polyurethane foam. Polym. Degrad. Stab. 2006, 91, 3354–3359. [Google Scholar] [CrossRef]
- Borda, J.; Pásztor, G.; Zsuga, M. Glycolysis of polyurethane foams and elastomers. Polym. Degrad. Stab. 2000, 68, 419–422. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, S.; Lu, Z.; Li, J.; Yang, X.; Qiao, Y.; Men, Y.; Sun, K.J. Healable, Recyclable, and Mechanically Tough Polyurethane Elastomers with Exceptional Damage Tolerance. Adv. Mater. 2020, 32, 2005759. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hou, X.; Chai, L.; Cui, X.; Wang, Y.; Deng, T. Efficient and green catalytic degradation of high crosslinked rigid PU foam and recovery value-added products via selective cleavage of C-O and C-N bonds. Polym. Degrad. Stab. 2020, 181, 109262. [Google Scholar] [CrossRef]
- Taourit, S.; Gac, P.; Fayolle, B. Relationship between network structure and ultimate properties in polyurethane during a chain scission process. Polym. Degrad. Stab. 2022, 201, 109971. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Li, J.-K. Glycolysis of rigid polyurethane from waste refrigerators. Polym. Degrad. Stab. 2002, 75, 413–421. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Cheng, C.-M.; Huang, H.-C. Glycolysis of waste flexible polyurethane foam. Polym. Degrad. Stab. 2003, 80, 103–111. [Google Scholar] [CrossRef]
- Zhao, L.; Semetey, V. Recycling Polyurethanes through Transcarbamoylation. ACS Omega 2021, 6, 4175–4183. [Google Scholar] [CrossRef]
- Rane, A.V.; Sabnis, A. Synthesis and Investigation of Aminolysates and obtained Poly Ester Amides. In Proceedings of the 3rd International Conference on Radiation Medicine (ICRM), Kottayam, India, 11–13 April 2014. [Google Scholar]
- Xu, W.; Lu, B.; Hu, Y.; Song, L.; Nie, S. Synthesis and characterization of novel fluorinated polyurethane elastomers based on 2,2-bis[4-(4-amino-2-trifluoromehyloxyphenyl) phenyl]propane. Polym. Adv. Technol. 2012, 23, 877–883. [Google Scholar] [CrossRef]
- Sang, S.; Li, Y.; Wang, K.; Tang, J. Application of blocked isocyanate in preparation of polyurethane(urea) elastomers. J. Appl. Polym. Sci. 2021, 138, 50582. [Google Scholar] [CrossRef]
- Salgado, C.; Arrieta, M.P.; Sessini, V.; Peponi, L.; López, D.; Fernández-García, M. Functional properties of photo-cross link able biodegradable polyurethane nanocomposites. Polym. Degrad. Stab. 2020, 178, 109204. [Google Scholar] [CrossRef]
- Vanbergen, T.; Verlent, I.; Geeter, J.D.; Haelterman, B.; Claes, L.; De Vos, D. Recycling of Flexible Polyurethane Foam by Split-Phase Alcoholysis: Identification of Additives and Alcoholyzing Agents to Reach Higher Efficiencies. ChemSusChem 2020, 13, 3835–3843. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Lyu, S.; Cheng, W.; Liu, S. Effect of different catalysts on recovery and reuse of waste polyurethane rigid foam. Mater. Res. Express 2021, 8, 035105. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; Lucas, A.D. Glycolysis of flexible polyurethane wastes containing polymeric polyols—ScienceDirect. Polym. Degrad. Stab. 2014, 109, 115–121. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Chen, C.; Li, H. Chemical degradation of thermoplastic polyurethane for recycling polyether polyol. Fibers Polym. 2011, 12, 857. [Google Scholar] [CrossRef]
- Blattmann, H.R.; Lauth, M.; Mülhaupt, R. Flexible and Bio-Based Nonisocyanate Polyurethane (NIPU) Foams. Macromol. Mater. Eng. 2016, 301, 944–952. [Google Scholar] [CrossRef]
- Strachota, A.; Strachotová, B.; Špírková, M. Comparison of Environmentally Friendly, Selective Polyurethane Catalysts. Mater. Manuf. Process. 2008, 23, 566–570. [Google Scholar] [CrossRef]
- Mondal, P.; Khakhar, D.V. Hydraulic resistance of rigid polyurethane foams. II. Effect of variation of surfactant, water, and nucleating agent concentrations on foam structure and properties. J. Appl. Polym. Sci. 2010, 93, 2830–2837. [Google Scholar] [CrossRef]
- Shabani, A.; Fathi, A.; Erlwein, S.; Altstädt, V. Thermoplastic polyurethane foams: From autoclave batch foaming to bead foam extrusion. J. Cell. Plast. 2020, 57, 391–411. [Google Scholar] [CrossRef]
- Molero, C.; Lucas, A.D.; Rodríguez, J.F. Recovery of polyols from flexible polyurethane foam by “split-phase” glycolysis: Glycol influence. Polym. Degrad. Stab. 2006, 91, 221–228. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Nelson, M.C. The Relationship of Cell Morphology, Density, and Mechanical Properties in a Rigid Polyurethane Foam; University of Nevada: Las Vegas, NV, USA, 2003. [Google Scholar]
- Yang, S.; Liu, X.; Tang, G.; Long, H.; Wang, B.; Zhang, H.; Ji, Y.; Yang, Y. Fire retarded polyurethane foam composites based on steel slag/ammonium polyphosphate system: A novel strategy for utilization of metallurgical solid waste. Polym. Adv. Technol. 2022, 33, 452–463. [Google Scholar] [CrossRef]






| Reagent Name | The Purity | Factory of Production |
|---|---|---|
| Waste polyurethane elastomer | Industrial waste | Shanghai Hecheng Polymer Material Co., Ltd. |
| Ethylene glycol (EG) | AR | Tianjin Chemical Reagent Factory 1 |
| 1,2-Propanediol (PG) | AR | Tianjin Chemical Reagent Factory 1 |
| Butane-1,4-diol (BDO) | AR | Tianjin Kaitong Chemical Reagent Co., Ltd. |
| Diethylene glycol (DEG) | AR | China Pharmaceutical Guangzhou Chemical Reagent Company |
| Potassium hydroxide (KOH) | AR | Tianjin Tianli Chemical Reagent Co., Ltd. |
| Silicone oil stabilizer | CP | Guangzhou Feirui Chemical Co., LRD |
| TEA | AR | Shanghai Demao Chemical Co., Ltd. |
| Dibutyltin dilaurate | AR | Shanghai Jieer Technology Co., Ltd. |
| Foaming agent | CP | Shenzhen Huachang Chemical Co., Ltd. |
| Polyether 4110 | CP | Shandong Lianhaoyao New Material Co., Ltd. |
| Polyaryl polymethylene isocyanate (PAPI) | CP | Wuhan Fude Chemical Co., Ltd. |
| Name of Instrument | Model | Factory of Production |
|---|---|---|
| Electronic analytical balance | JA3003C | Sartorius Scientific Instruments (Beijing) Co., Ltd. |
| Cantilever constant speed power electric mixer | TJ-1200W | Changzhou Huaao Instrument Manufacturing Co., Ltd. |
| Spherical reactor (1 L) | ZNHW-200 | Shanghai Leighton Industrial Co., Ltd |
| Digital blast drying oven | WX881 | Wujiang Weixin Electric Heating Equipment Co., Ltd. |
| Digital viscometer | NDJ-5 | Shanghai Pingxuan Scientific Instrument Co., Ltd. |
| Disposable plastic cup | 350 mL | Topu Daily Chemicals (China) Co., Ltd. |
| Constant temperature heating sleeve | FDSG-420 | Wuxi Huachang Chemical Co., Ltd. |
| Number | Proportion | Viscosity (mPa·s) | Proportion of Catalyst | Wire Drawing Time (s) | Debonding Time (s) |
|---|---|---|---|---|---|
| A5 | 50:30 | 605.1 | - | 60 | 25 |
| B1 | 40:40 | 992.4 | 1:2 | 37 | 20 |
| B4 | 20:60 | 692.5 | 2:2 | 60 | 70 |
| B5 | 60:20 | 660.2 | 1:1 | 45 | 75 |
| 1:2 | 34 | 64 | |||
| C1 | 40:40 | 750.6 | - | 67 | 45 |
| 1:1 | 50 | 46 | |||
| 2:1 | 46 | 50 | |||
| C4 | 60:20 | 555.0 | - | 67 | 29 |
| 1:1 | 48 | 19 | |||
| D1 | 40:40 | 1208.4 | 1:1 | 56 | 32 |
| 2:2 | 21 | 22 | |||
| E3 | 60:20 | 484.2 | 2:2 | 74 | 66 |
| Number | Proportion | Density (Kg·m−3) | Water Absorption Rate (%) | Strength of Compression (MPa) | Coefficient of Thermal Conductivity (W·(m·K)−1) |
|---|---|---|---|---|---|
| A5 | 50:30 | 0.0399 | 1.9923 | 0.131 | 0.0180 |
| B1 | 40:40 | 0.0335 | 1.6748 | 0.137 | 0.0173 |
| B4 | 20:60 | 0.0312 | 1.5339 | 0.132 | 0.0202 |
| B5 | 60:20 | 0.0328 | 0.8853 | 0.146 | 0.0191 |
| 0.0397 | 1.0561 | 0.138 | 0.0182 | ||
| C1 | 40:40 | 0.0347 | 1.1618 | 0.133 | 0.0180 |
| 0.0303 | 1.2980 | 0.139 | 0.0182 | ||
| 0.0366 | 1.2802 | 0.143 | 0.0173 | ||
| C4 | 60:20 | 0.0340 | 0.8976 | 0.143 | 0.0174 |
| 0.0359 | 0.7265 | 0.162 | 0.0166 | ||
| D1 | 40:40 | 0.0362 | 0.9368 | 0.136 | 0.0164 |
| 0.0403 | 0.7630 | 0.176 | 0.0151 | ||
| E3 | 60:20 | 0.0325 | 1.0157 | 0.141 | 0.0155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Wang, X.; Guo, X.; Liu, S.; Li, Q.; Liu, Y. Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane. Polymers 2023, 15, 1445. https://doi.org/10.3390/polym15061445
Gu X, Wang X, Guo X, Liu S, Li Q, Liu Y. Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane. Polymers. 2023; 15(6):1445. https://doi.org/10.3390/polym15061445
Chicago/Turabian StyleGu, Xiaohua, Xiaoyao Wang, Xinyu Guo, Siwen Liu, Qi Li, and Yan Liu. 2023. "Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane" Polymers 15, no. 6: 1445. https://doi.org/10.3390/polym15061445
APA StyleGu, X., Wang, X., Guo, X., Liu, S., Li, Q., & Liu, Y. (2023). Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane. Polymers, 15(6), 1445. https://doi.org/10.3390/polym15061445