Epoxidized and Maleinized Hemp Oil to Develop Fully Bio-Based Epoxy Resin Based on Anhydride Hardeners
Abstract
1. Introduction
2. Materials and Methods
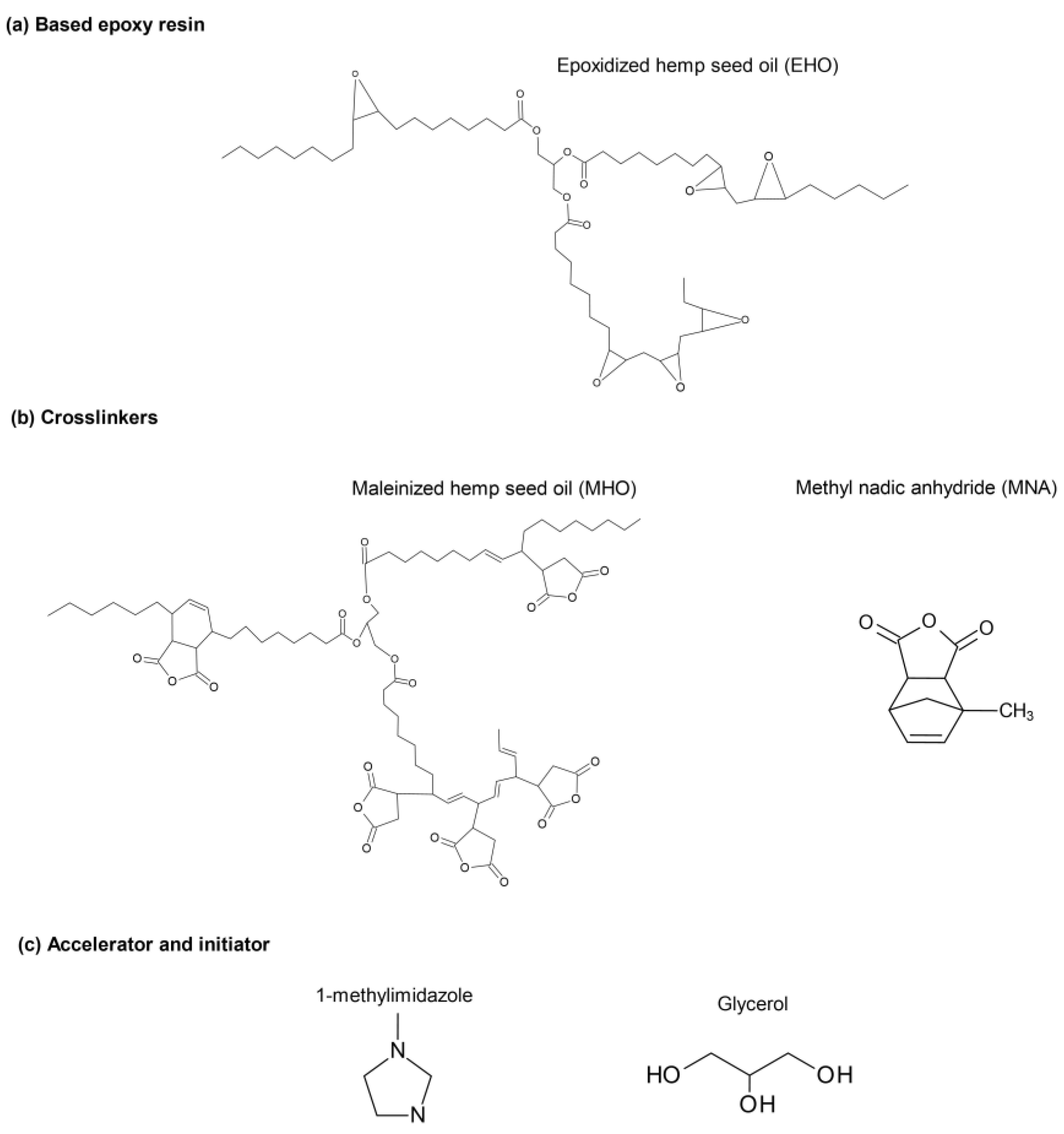
2.1. Materials
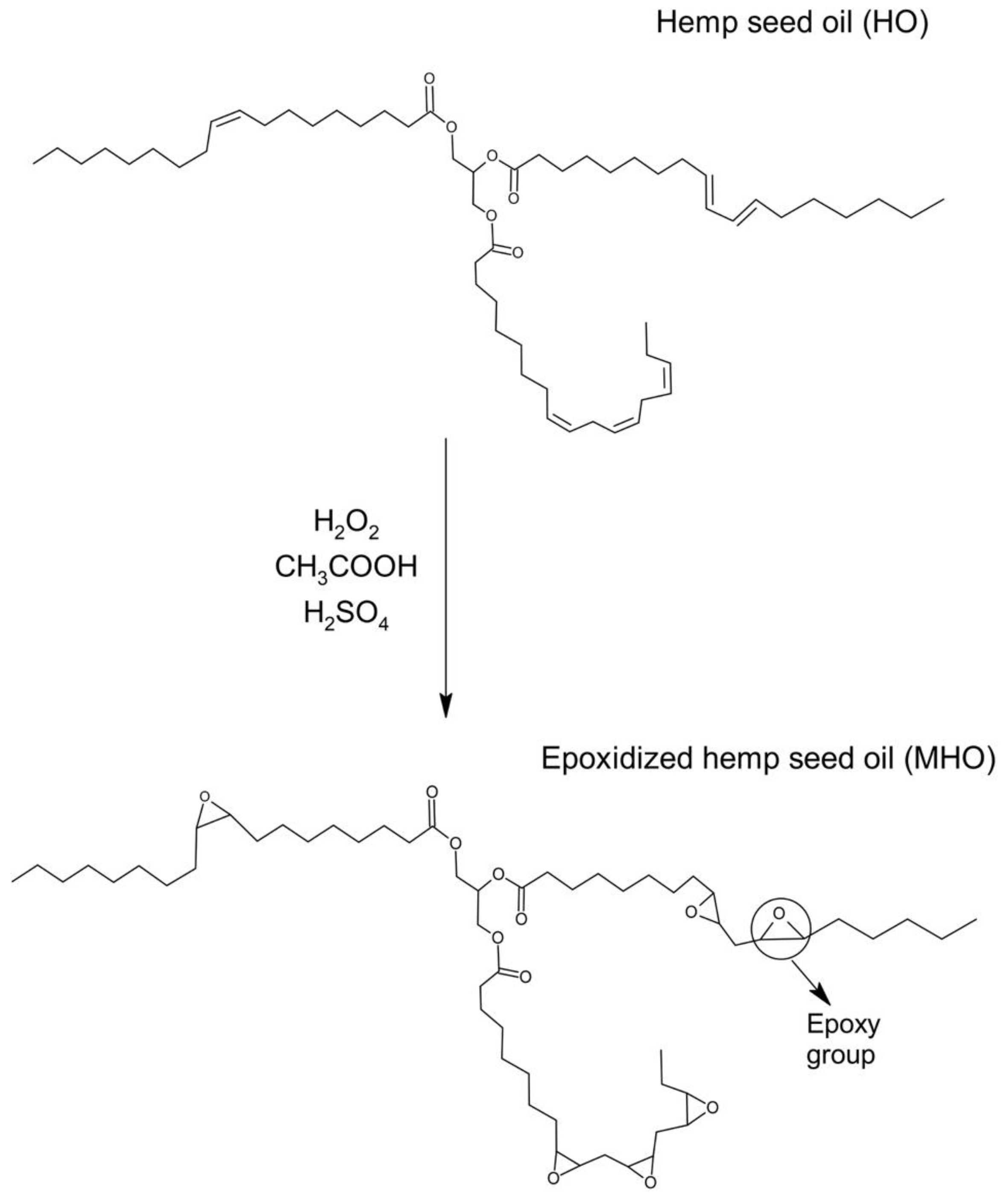
2.2. Epoxidation Process
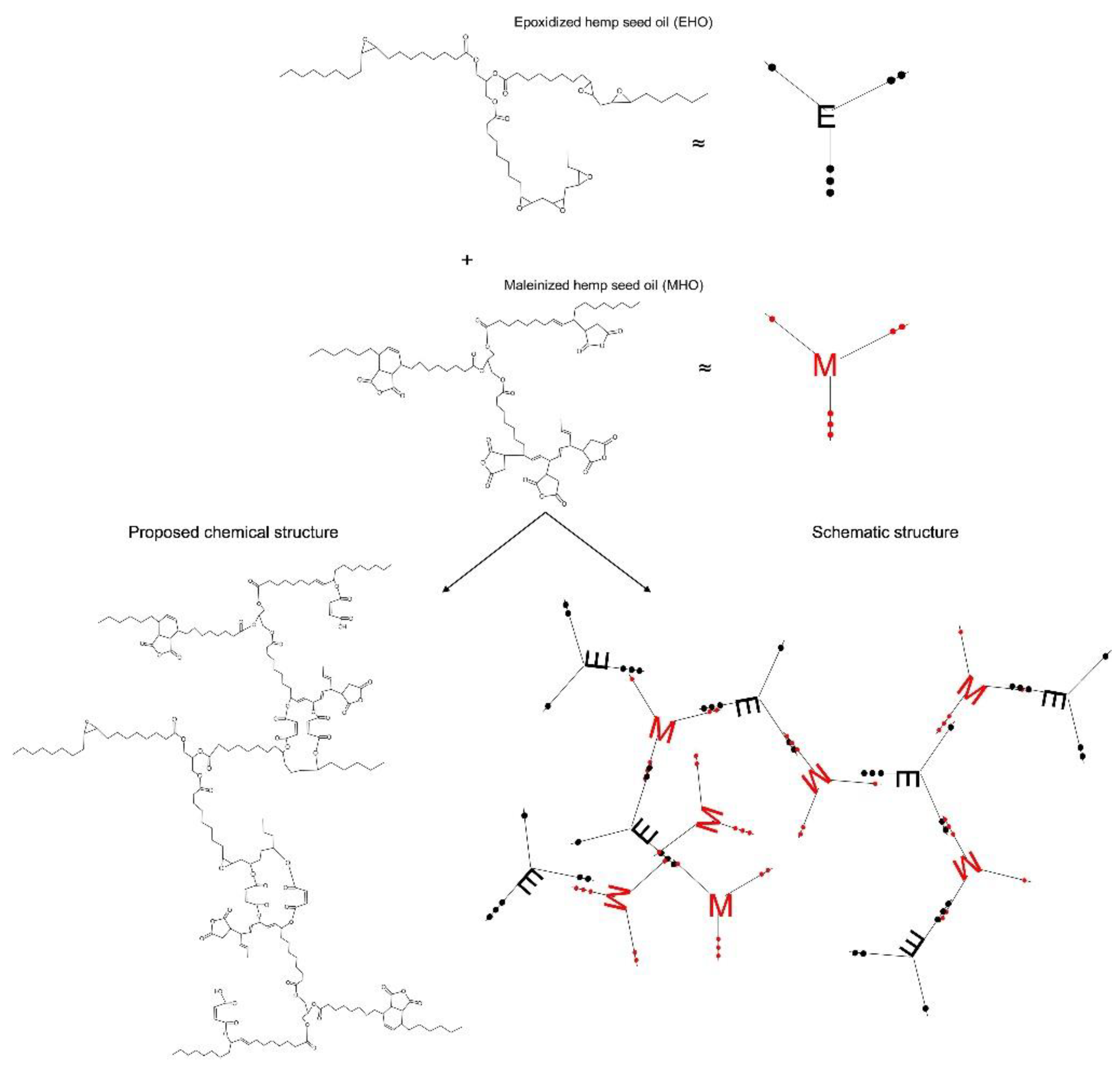
2.3. Maleinization Process
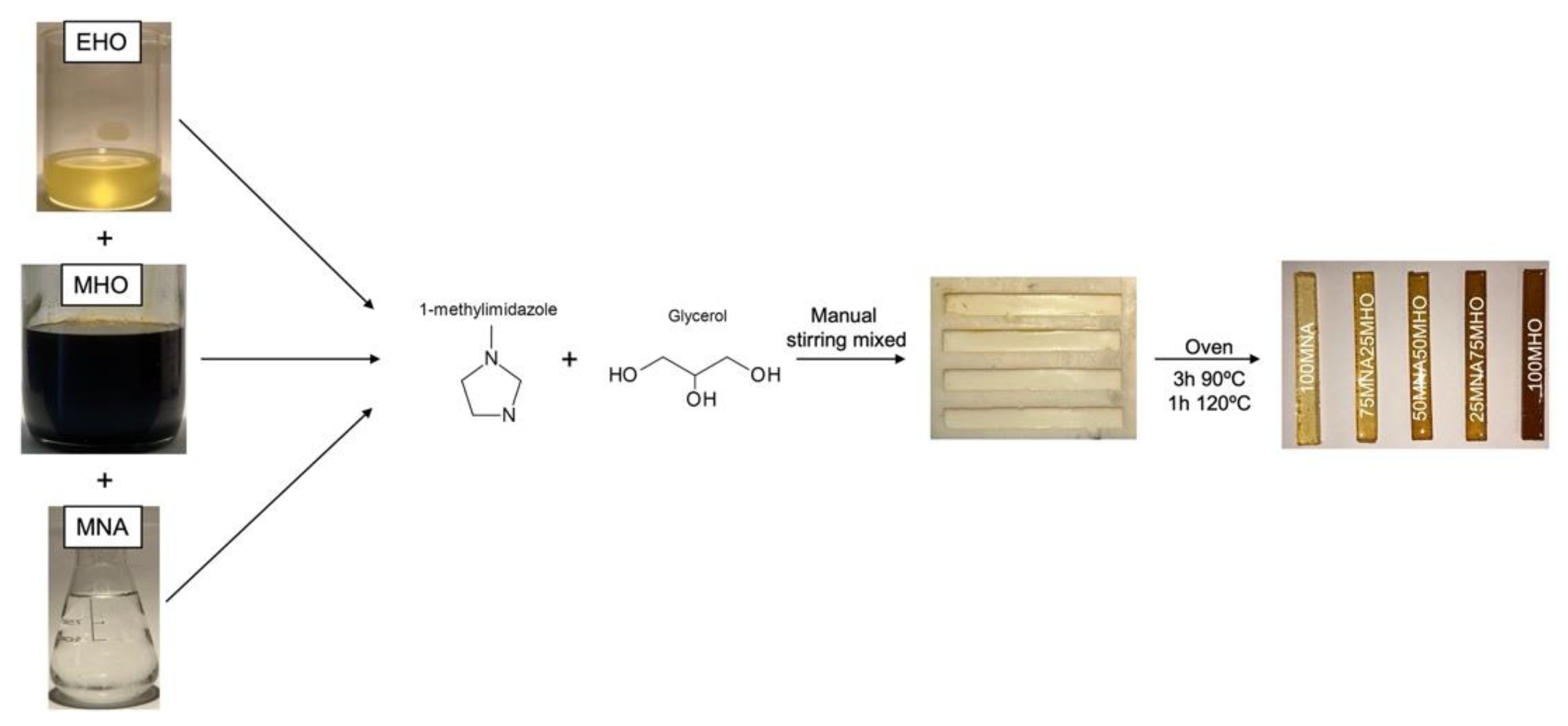
2.4. Sample Preparation
2.5. Oxirane Oxygen Content (Oo) and Acid Value
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Mechanical Characterization
2.8. Thermal Characterization
2.9. Thermomechanical Characterization
2.10. Morphological Characterization
3. Results and Discussion
3.1. Chemical Properties
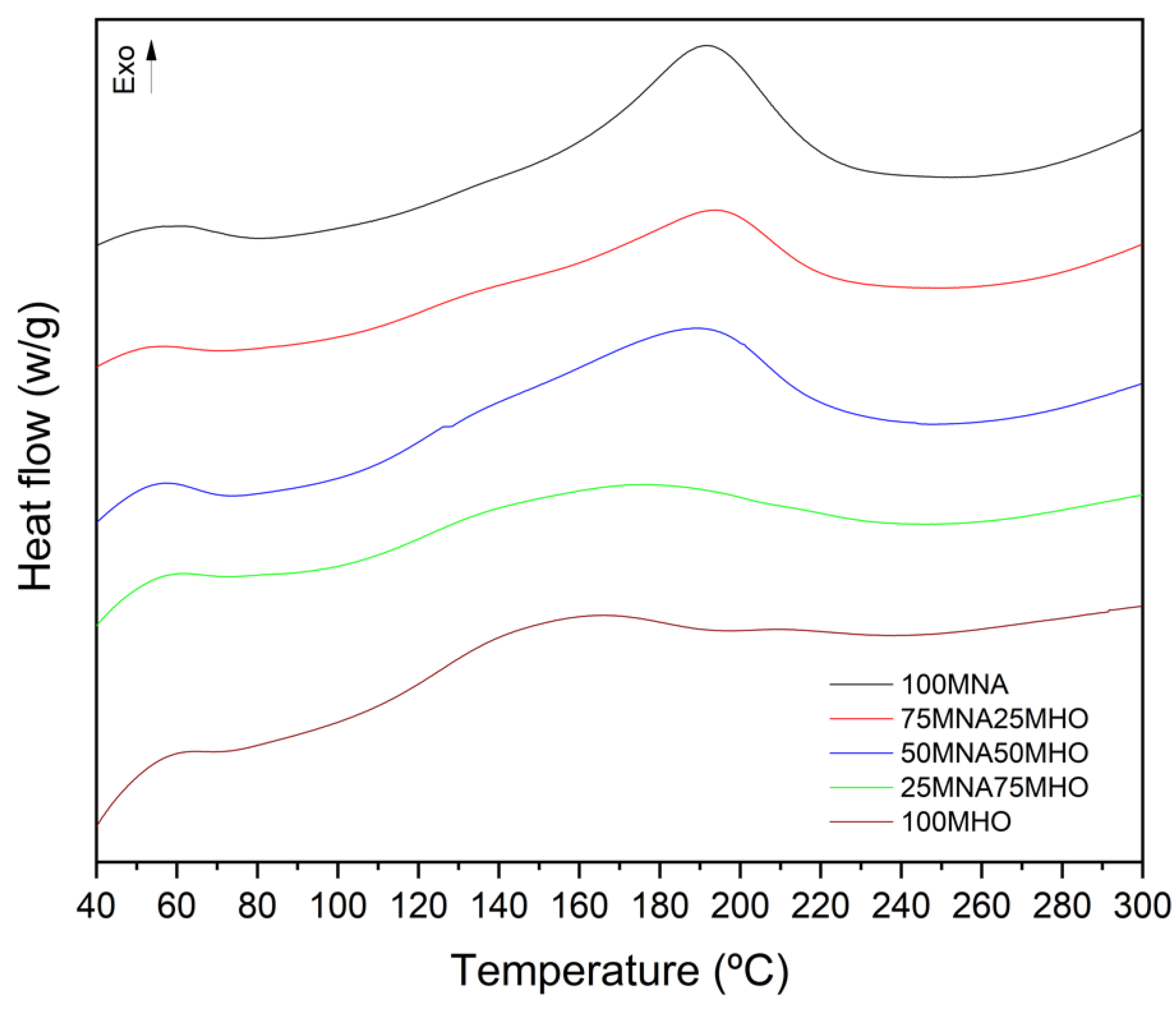
3.2. Thermal Properties
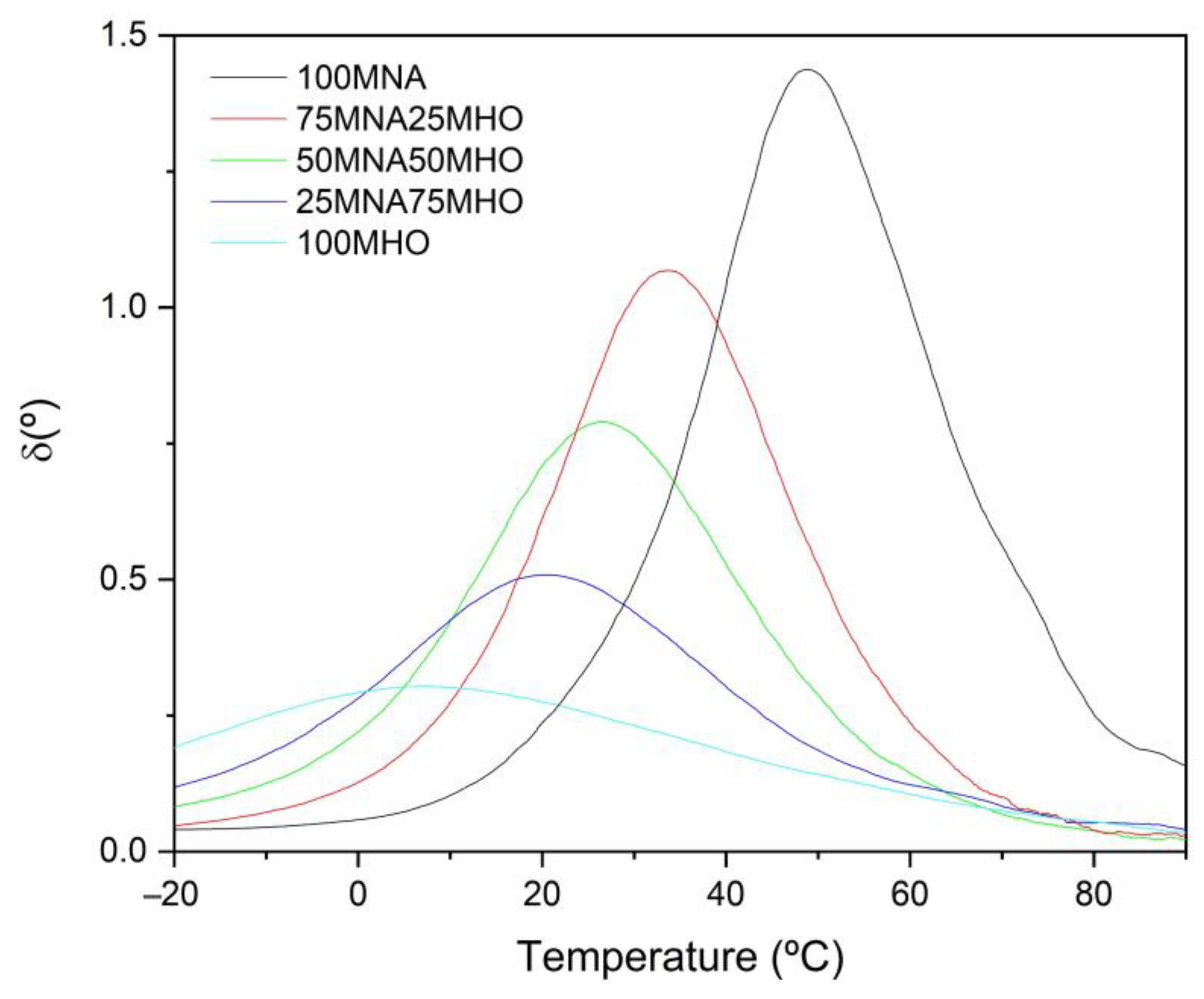
3.3. Thermomechanical Properties
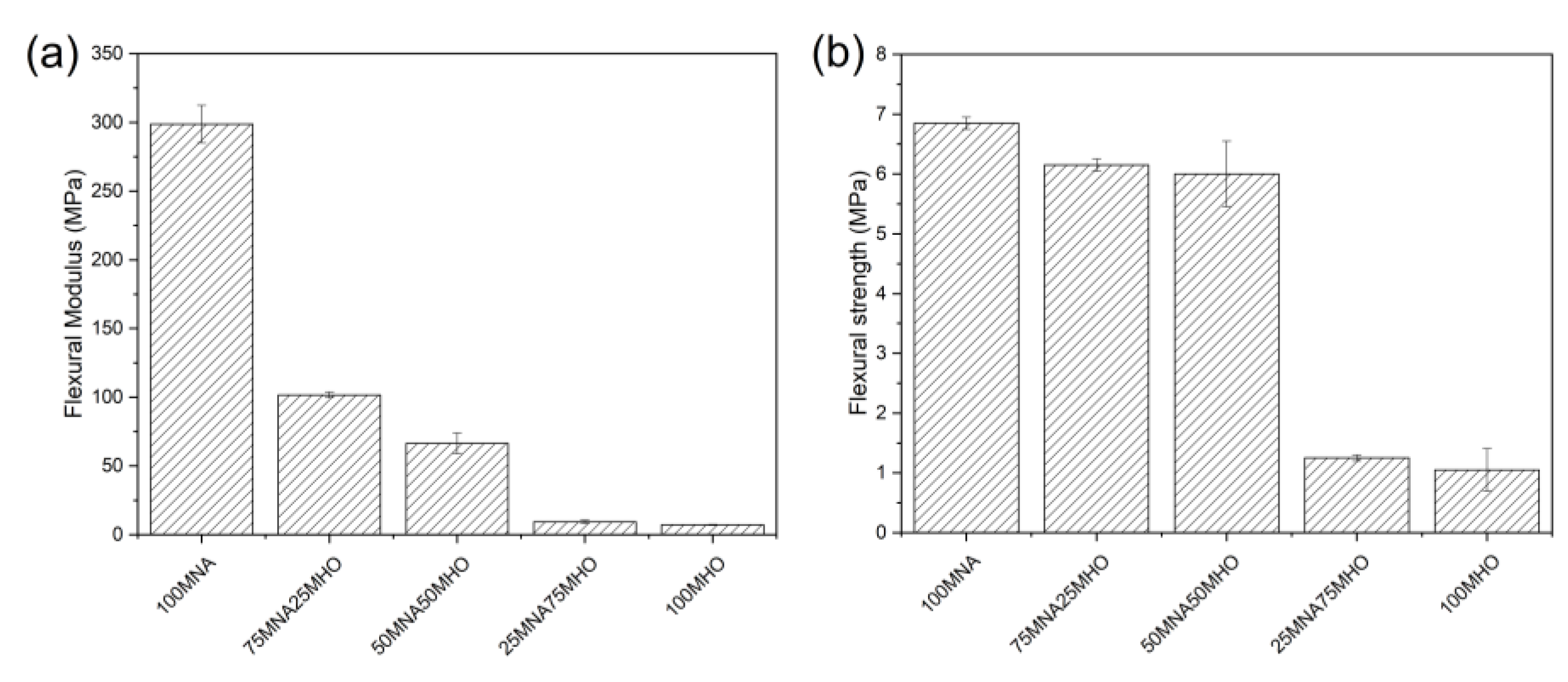
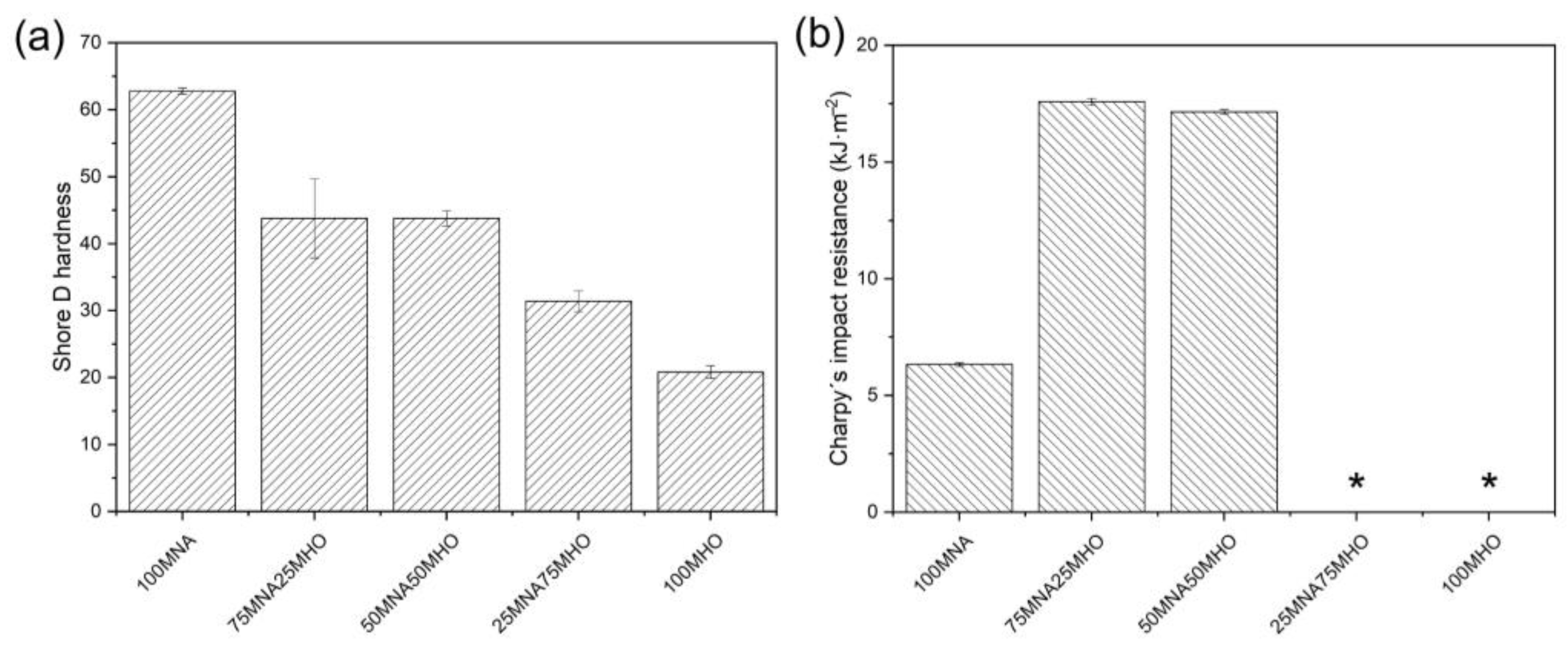
3.4. Mechanical Properties
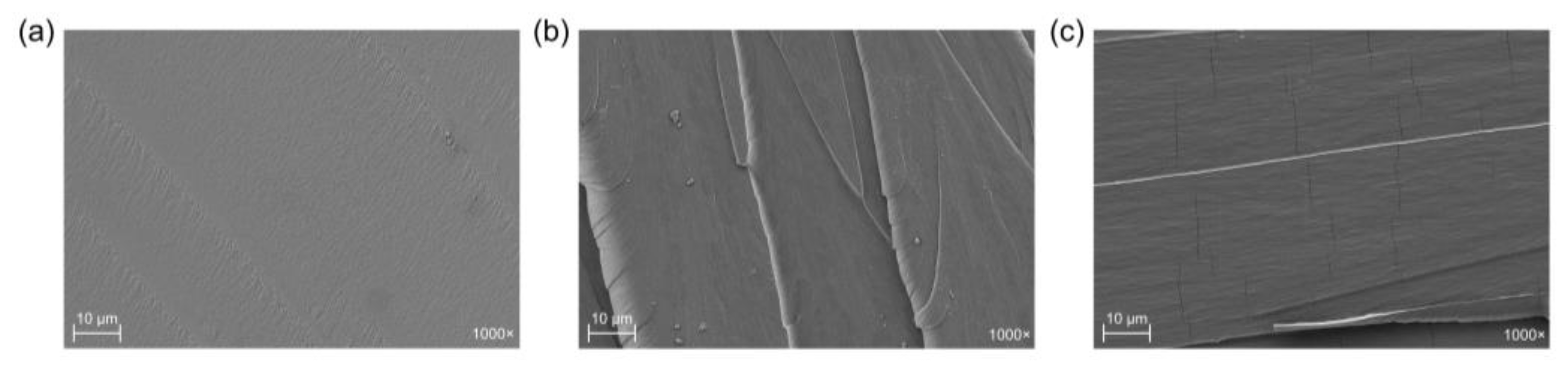
3.5. Morphological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, L.; Worrell, E.; Patel, M. Present and future development in plastics from biomass. Biofuels Bioprod. Biorefin. 2010, 4, 25–40. [Google Scholar] [CrossRef]
- Mohan, P. A Critical Review: The Modification, Properties, and Applications of Epoxy Resins. Polym.-Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Njuguna, J.; Wambua, P.; Pielichowski, K.; Kayvantash, K. Natural Fibre-Reinforced Polymer Composites and Nanocomposites for Automotive Applications; Springer: Berlin, Germany, 2011; pp. 661–700. [Google Scholar] [CrossRef]
- Ray, D.; Ghorui, S.; Bandyopadhyay, N.R.; Sengupta, S.; Kar, T. New Materials from Maleated Castor Oil/Epoxy Resin Blend Reinforced with Fly Ash. Ind. Eng. Chem. Res. 2012, 51, 2603–2608. [Google Scholar] [CrossRef]
- Hamerton, I.; Mooring, L. The Use of Thermosets in Aerospace Applications; Woodhead Publ Ltd.: Cambridge, UK, 2012; pp. 189–227. [Google Scholar]
- Anusic, A.; Resch-Fauster, K.; Mahendran, A.R.; Wuzella, G. Anhydride Cured Bio-Based Epoxy Resin: Effect of Moisture on Thermal and Mechanical Properties. Macromol. Mater. Eng. 2019, 304, 1900031. [Google Scholar] [CrossRef]
- Alam, M.; Akra, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Prabhu, L.; Krishnaraj, V.; Sathish, S.; Gokulkumar, S.; Karthi, N.; Rajeshkumar, L.; Balaji, D.; Vigneshkumar, N.; Elango, K.S. A review on natural fiber reinforced hybrid composites: Chemical treatments, manufacturing methods and potential applications. In Proceedings of the 2nd International Conference on Materials, Manufacturing, and Machining for Industry 4.0, Electr Network, Virtual, 9–10 October 2020; pp. 8080–8085. [Google Scholar]
- Ramesh, M. Flax (Linum usitatissimum L.) fibre reinforced polymer composite materials: A review on preparation, properties and prospects. Prog. Mater. Sci. 2019, 102, 109–166. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef]
- Boquillon, N.; Fringant, C. Polymer networks derived from curing of epoxidised linseed oil: Influence of different catalysts and anhydride hardeners. Polymer 2000, 41, 8603–8613. [Google Scholar] [CrossRef]
- Menager, C.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. Polymerization kinetic pathways of epoxidized linseed oil with aliphatic bio-based dicarboxylic acids. J. Polym. Sci. 2020, 58, 1717–1727. [Google Scholar] [CrossRef]
- Yue, H.B.; Cui, Y.D.; Shuttleworth, P.S.; Clark, J.H. Preparation and characterisation of bioplastics made from cottonseed protein. Green Chem. 2012, 14, 2009–2016. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Ferri, J.M.; Cardona, S.C.; Lora, J.; Fombuena, V. Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid). Polymers 2021, 13, 1283. [Google Scholar] [CrossRef] [PubMed]
- Fombuena, V.; Petrucci, R.; Dominici, F.; Jorda-Vilaplana, A.; Montanes, N.; Torre, L. Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers 2019, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Lerma-Canto, A.; Gomez-Caturla, J.; Herrero-Herrero, M.; Garcia-Garcia, D.; Fombuena, V. Development of Polylactic Acid Thermoplastic Starch Formulations Using Maleinized Hemp Oil as Biobased Plasticizer. Polymers 2021, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Samper, M.D.; Fombuena, V.; Boronat, T.; Garcia-Sanoguera, D.; Balart, R. Thermal and Mechanical Characterization of Epoxy Resins (ELO and ESO) Cured with Anhydrides. J. Am. Oil Chem. Soc. 2012, 89, 1521–1528. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131, 9016–9028. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of hempseed oil extraction by n-hexane. Ind. Crops Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- ITC. Trade Map. Available online: https://www.trademap.org/Index.aspx (accessed on 22 December 2022).
- Shuttleworth, P.S.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Flexible bionanocomposites from epoxidized hemp seed oil thermosetting resin reinforced with halloysite nanotubes. J. Phys. Chem. B 2017, 121, 2454–2467. [Google Scholar] [CrossRef]
- Manthey, N.W.; Cardona, F.; Francucci, G.; Aravinthan, T. Thermo-mechanical properties of epoxidized hemp oil-based bioresins and biocomposites. J. Reinf. Plast. Compos. 2013, 32, 1444–1456. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Lerma-Canto, A.; Cardona, S.C.; Lora, J.; Fombuena, V. Physicochemical Characterization of Novel Epoxidized Vegetable Oil from Chia Seed Oil. Materials 2022, 15, 3250. [Google Scholar] [CrossRef] [PubMed]
- Otabor, G.O.; Ifijen, I.H.; Mohammed, F.U.; Aigbodion, A.I.; Ikhuoria, E.U. Alkyd resin from rubber seed oil/linseed oil blend: A comparative study of the physiochemical properties. Heliyon 2019, 5, e01621. [Google Scholar] [CrossRef]
- Benaniba, M.T.; Belhaneche-Bensemra, N.; Gelbard, G. Epoxidation of sunflower oil with peroxoacetic acid in presence of ion exchange resin by various processes. Energy Educ. Sci. Technol. 2008, 21, 71–82. [Google Scholar]
- Huang, Y.B.; Yao, M.Y.; Xin, P.P.; Zhou, M.C.; Yang, T.; Pan, H. Influence of alkenyl structures on the epoxidation of unsaturated fatty acid methyl esters and vegetable oils. RSC Adv. 2015, 5, 74783–74789. [Google Scholar] [CrossRef]
- de Quadros, J.V.; Giudici, R. Epoxidation of soybean oil at maximum heat removal and single addition of all reactants. Chem. Eng. Process.-Process Intensif. 2016, 100, 87–93. [Google Scholar] [CrossRef]
- Sun, S.D.; Yang, G.L.; Bi, Y.L.; Liang, H. Enzymatic Epoxidation of Corn Oil by Perstearic Acid. J. Am. Oil Chem. Soc. 2011, 88, 1567–1571. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Development of completely bio-based epoxy networks derived from epoxidized linseed and castor oil cured with citric acid. Polym. Adv. Technol. 2018, 29, 2080–2090. [Google Scholar] [CrossRef]
- Derawi, D.; Salimon, J. Effect of Performic Acid Concentration on Epoxidation of Palm Olein. Sains Malays. 2011, 40, 1023–1027. [Google Scholar]
- Corcuera, M.A.; De la Caba, K.; Gabilondo, N.; Marieta, C.; Kortaberria, G.; Eceiza, A. Rheokinetic and dynamic mechanical analysis of tetrafunctional epoxy/anhydride mixtures. Influence of stoichiometry and cure conditions. High Perform. Polym. 2006, 18, 17–30. [Google Scholar] [CrossRef]
- Fombuena, V. Study of the properties of thermoset materials derived from epoxidized soybean oil and protein fillers. J. Am. Oil Chem. Soc. 2013, 90, 449–457. [Google Scholar] [CrossRef]
- De, B.; Gupta, K.; Mandal, M.; Karak, N. Biodegradable hyperbranched epoxy from castor oil-based hyperbranched polyester polyol. ACS Sustain. Chem. Eng. 2014, 2, 445–453. [Google Scholar] [CrossRef]
- Samper Madrigal, M.D. Desarrollo y Optimización De’green Composites’ Basados en Matrices Derivadas de Aceites Vegetales Modificados y Refuerzos de Fibras Minerales. Doctoral Dissertation, Universitat Politècnica de València, Valencia, Spain, 2015. [Google Scholar]
- López Téllez, G.; Vigueras-Santiago, E.; Hernández-López, S. Characterization of linseed oil epoxidized at different percentages. Superf. Vacío 2009, 22, 5–10. [Google Scholar]
- Boga, K.; Gaddam, S.K.; Chepuri, R.R.; Palanisamy, A. Development of biobased polyurethane-imides from maleinized cottonseed oil and castor oil. Polym. Adv. Technol. 2019, 30, 2742–2749. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Perez-Nakai, A.; Torres-Roca, E.; Lora-Garcia, J.; Fombuena, V. Development of a novel epoxy resin based on epoxidized chia oil as matrix and maleinized chia oil as bio-renewable crosslinker. J. Appl. Polym. Sci. 2022, 140, e53574. [Google Scholar] [CrossRef]
- Omonov, T.S.; Kharraz, E.; Curtis, J.M. The epoxidation of canola oil and its derivatives. RSC Adv. 2016, 6, 92874–92886. [Google Scholar] [CrossRef]
- Rosas-Mendoza, M.E.; Coria-Hernández, J.; Meléndez-Pérez, R.; Arjona-Román, J.L. Characteristics of chia (Salvia hispanica L.) seed oil extracted by ultrasound assistance. J. Mex. Chem. Soc. 2017, 61, 326–335. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Bernardi, L.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Development of environmentally friendly composite matrices from epoxidized cottonseed oil. Eur. Polym. J. 2015, 63, 1–10. [Google Scholar] [CrossRef]
- Samper, M.D.; Ferri, J.M.; Carbonell-Verdu, A.; Balart, R.; Fenollar, O. Properties of biobased epoxy resins from epoxidized linseed oil (ELO) crosslinked with a mixture of cyclic anhydride and maleinized linseed oil. Express Polym. Lett. 2019, 13, 407–418. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, P.; Huang, K.; Zhang, J. Study of green epoxy resins derived from renewable cinnamic acid and dipentene: Synthesis, curing and properties. RSC Adv. 2014, 4, 8525–8532. [Google Scholar] [CrossRef]
- Fombuena, V.; Bernardi, L.; Fenollar, O.; Boronat, T.; Balart, R. Characterization of green composites from biobased epoxy matrices and bio-fillers derived from seashell wastes. Mater. Des. 2014, 57, 168–174. [Google Scholar] [CrossRef]
- Rösch, J.; Mülhaupt, R. Polymers from renewable resoureces: Polyester resins and blends based upon anhydride-cured epoxidized soybean oil. Polym. Bull. 1993, 31, 679–685. [Google Scholar] [CrossRef]
- España, J.; Sánchez-Nacher, L.; Boronat, T.; Fombuena, V.; Balart, R. Properties of biobased epoxy resins from epoxidized soybean oil (ESBO) cured with maleic anhydride (MA). J. Am. Oil Chem. Soc. 2012, 89, 2067–2075. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Development of toughened bio-based epoxy with epoxidized linseed oil as reactive diluent and cured with bio-renewable crosslinker. Polym. Adv. Technol. 2018, 29, 565–574. [Google Scholar] [CrossRef]


| Vegetable Oils | Theoretical Oxirane Oxygen Content | References |
|---|---|---|
| Chia seed | 11.05 | [23] |
| Linseed | 10.60 | [24] |
| Hemp | 10.53 | Present study |
| Sunflower | 7.57 | [25] |
| Olive | 7.41 | [26] |
| Soybean | 7.36 | [27] |
| Corn | 6.76 | [28] |
| Castor | 5.03 | [29] |
| Palm | 3.76 | [30] |
| Code | MNA (%) | MHO (%) | Glycerol (wt.%) | 1-Methylimidazole (wt.%) |
|---|---|---|---|---|
| 100MNA | 100 | 0 | 0.8 | 2 |
| 75MNA25MHO | 75 | 25 | 0.8 | 2 |
| 50MNA50MHO | 50 | 50 | 0.8 | 2 |
| 25MNA75MHO | 25 | 75 | 0.8 | 2 |
| 100MHO | 0 | 100 | 0.8 | 2 |
| Code | Onset (°C) | Peak Temperature (°C) | Endset (°C) | Enthalpy (J·g−1) | % Cure * |
|---|---|---|---|---|---|
| 100MNA | 145 | 191 | 225 | 189.4 | 93 |
| 75MNA25MHO | 138 | 192 | 225 | 141.3 | 93 |
| 50MNA50MHO | 122 | 188 | 216 | 135.4 | 90 |
| 25MNA75MHO | 115 | 170 | 215 | 115.8 | 90 |
| 100MHO | 111 | 155 | 210 | 83.3 | 85 |
| Code | Crosslinking Onset (δ ≈ 90°) (s) | Gel Time (δ = 45°) (s) | Crosslinking Endset (δ ≈ 0°) (s) |
|---|---|---|---|
| 100MNA | 10,200 | 11,500 | 15,270 |
| 75MNA25MHO | 4690 | 5433 | 7900 |
| 50MNA50MHO | 2000 | 2649 | 3690 |
| 25MNA75MHO | 920 | 1350 | 2900 |
| 100MHO | 555 | 1006 | 1920 |
| Code | Tg (°C) |
|---|---|
| 100MNA | 48.7 |
| 75MNA25MHO | 33.7 |
| 50MNA50MHO | 26.7 |
| 25MNA75MHO | 20.1 |
| 100MHO | 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerma-Canto, A.; Samper, M.D.; Dominguez-Candela, I.; Garcia-Garcia, D.; Fombuena, V. Epoxidized and Maleinized Hemp Oil to Develop Fully Bio-Based Epoxy Resin Based on Anhydride Hardeners. Polymers 2023, 15, 1404. https://doi.org/10.3390/polym15061404
Lerma-Canto A, Samper MD, Dominguez-Candela I, Garcia-Garcia D, Fombuena V. Epoxidized and Maleinized Hemp Oil to Develop Fully Bio-Based Epoxy Resin Based on Anhydride Hardeners. Polymers. 2023; 15(6):1404. https://doi.org/10.3390/polym15061404
Chicago/Turabian StyleLerma-Canto, Alejandro, Maria D. Samper, Ivan Dominguez-Candela, Daniel Garcia-Garcia, and Vicent Fombuena. 2023. "Epoxidized and Maleinized Hemp Oil to Develop Fully Bio-Based Epoxy Resin Based on Anhydride Hardeners" Polymers 15, no. 6: 1404. https://doi.org/10.3390/polym15061404
APA StyleLerma-Canto, A., Samper, M. D., Dominguez-Candela, I., Garcia-Garcia, D., & Fombuena, V. (2023). Epoxidized and Maleinized Hemp Oil to Develop Fully Bio-Based Epoxy Resin Based on Anhydride Hardeners. Polymers, 15(6), 1404. https://doi.org/10.3390/polym15061404









