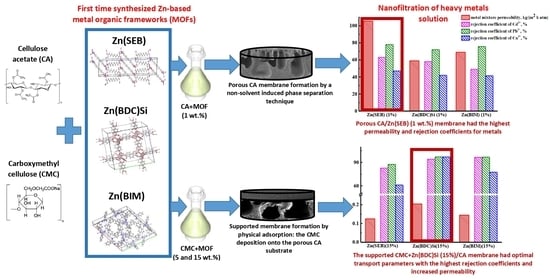
Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.2.1. Porous Membranes
2.2.2. Supported Membranes
2.3. Nanofiltration Experiment
2.4. Fourier-Transform Infrared Spectroscopy
2.5. Scanning Electron Microscopy
2.6. Atomic Force Microscopy
2.7. Contact Angle Measurement
2.8. Standard Porosimetry Method
2.9. Mechanical Properties Investigation
3. Results
3.1. Development and Investigation of Porous CA-Based Membranes
3.1.1. Transport Properties of the Porous CA-Based Membranes
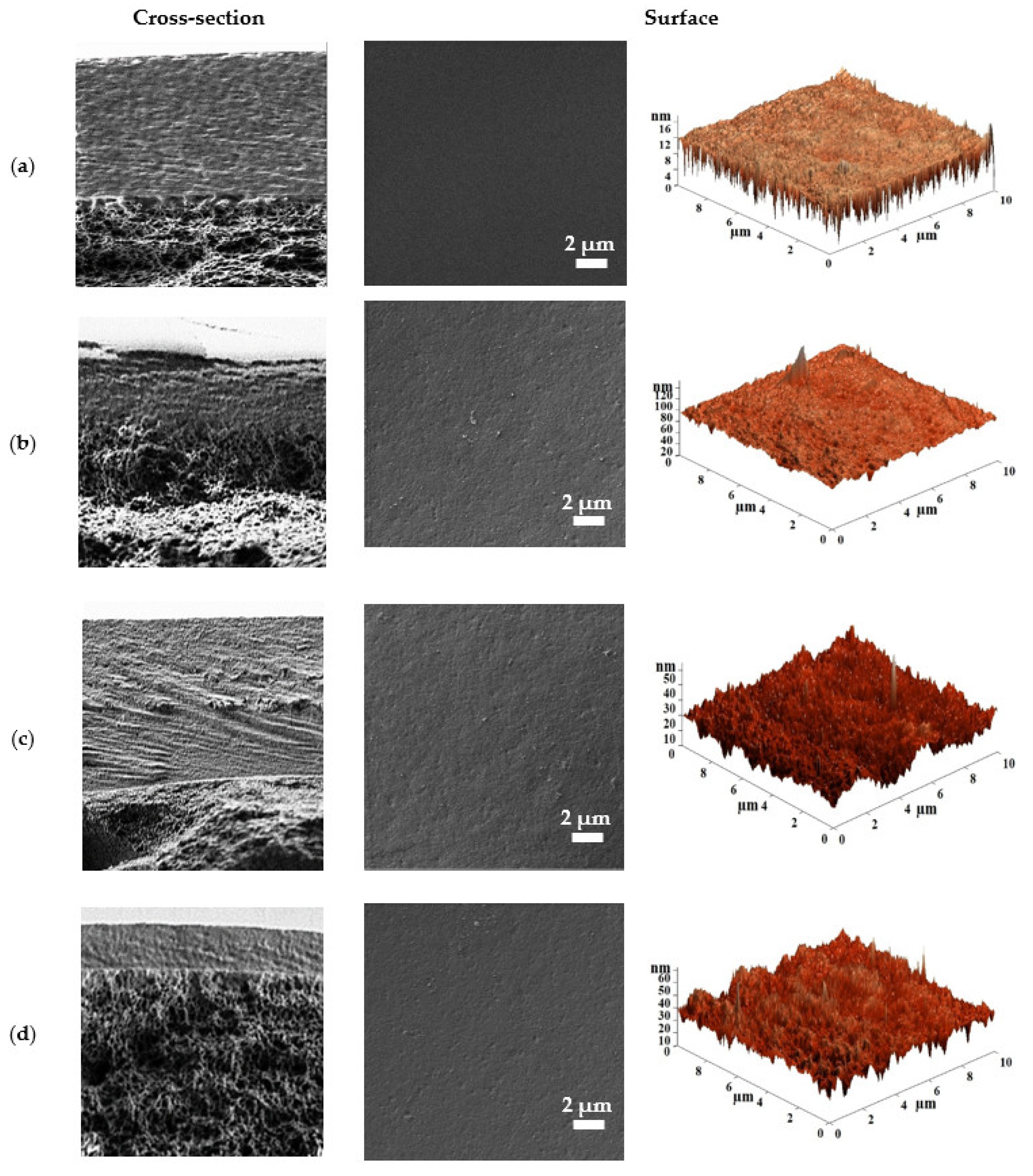
3.1.2. Characterization of the Porous CA-Based Membranes
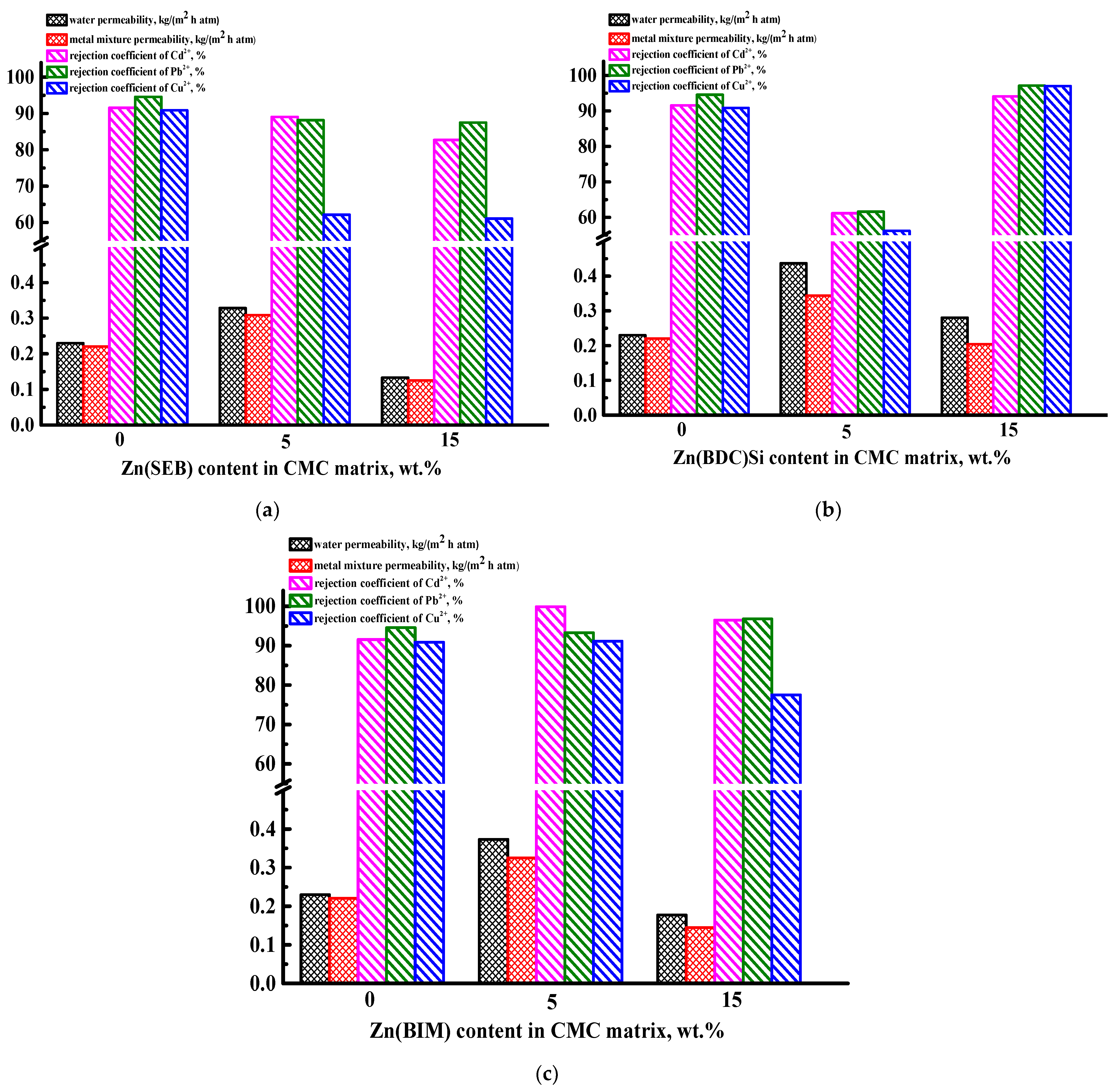
3.2. Development and Investigation of Supported CMC-Based Membranes
3.2.1. Transport Properties of the Supported CMC-Based Membranes
3.2.2. Characterization of the Supported CMC-Based Membranes
3.3. Investigation of Membrane Performance in Nanofiltration of Wastewater from Galvanic Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, Y.; Shu, L.; Liu, L.; Wu, Y.; Xie, L.-H.; Zhao, M.-J.; Li, J.-R. A high-flux mixed matrix nanofiltration membrane with highly water-dispersible MOF crystallites as filler. J. Membr. Sci. 2019, 591, 117360. [Google Scholar] [CrossRef]
- Gnanasekaran, G.; Balaguru, S.; Arthanareeswaran, G.; Das, D.B. Removal of hazardous material from wastewater by using metal organic framework (MOF) embedded polymeric membranes. Sep. Sci. Technol. 2019, 54, 434–446. [Google Scholar] [CrossRef]
- Luo, X.-P.; Fu, S.-Y.; Du, Y.-M.; Guo, J.-Z.; Li, B. Adsorption of methylene blue and malachite green from aqueous solution by sulfonic acid group modified MIL-101. Microporous Mesoporous Mater. 2017, 237, 268–274. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Ahmed, I.; Jhung, S.H. Remarkable adsorbent for phenol removal from fuel: Functionalized metal–organic framework. Fuel 2016, 174, 43–48. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2021, 12, 14. [Google Scholar] [CrossRef]
- Idress, H.; Zaidi, S.Z.J.; Sabir, A.; Shafiq, M.; Khan, R.U.; Harito, C.; Hassan, S.; Walsh, F.C. Cellulose acetate based Complexation-NF membranes for the removal of Pb(II) from waste water. Sci. Rep. 2021, 11, 1806. [Google Scholar] [CrossRef]
- Mondal, P.; Purkait, M.K. Green synthesized iron nanoparticles supported on pH responsive polymeric membrane for nitrobenzene reduction and fluoride rejection study: Optimization approach. J. Clean. Prod. 2018, 170, 1111–1123. [Google Scholar] [CrossRef]
- Otvagina, K.; Penkova, A.; Dmitrenko, M.; Kuzminova, A.; Sazanova, T.; Vorotyntsev, A.; Vorotyntsev, I. Novel Composite Membranes Based on Chitosan Copolymers with Polyacrylonitrile and Polystyrene: Physicochemical Properties and Application for Pervaporation Dehydration of Tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K. (Ed.) Smart Materials for Waste Water Applications; Wiley: Chichester, UK, 2016; ISBN 9781119041184. [Google Scholar]
- Su, J.; Yang, Q.; Teo, J.F.; Chung, T.-S. Cellulose acetate nanofiltration hollow fiber membranes for forward osmosis processes. J. Membr. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane. In Saline Water Conversion—II; ACS Publications: Washington, DC, USA, 1963; pp. 117–132. [Google Scholar]
- Alhalili, Z.; Romdhani, C.; Chemingui, H.; Smiri, M. Removal of dithioterethiol (DTT) from water by membranes of cellulose acetate (AC) and AC doped ZnO and TiO2 nanoparticles. J. Saudi Chem. Soc. 2021, 25, 101282. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. Preparation of full-bio-based nanofiltration membranes. J. Membr. Sci. 2021, 618, 118674. [Google Scholar] [CrossRef]
- Rasool, M.A.; Van Goethem, C.; Vankelecom, I.F.J. Green preparation process using methyl lactate for cellulose-acetate-based nanofiltration membranes. Sep. Purif. Technol. 2020, 232, 115903. [Google Scholar] [CrossRef]
- Ounifi, I.; Ursino, C.; Santoro, S.; Chekir, J.; Hafiane, A.; Figoli, A.; Ferjani, E. Cellulose acetate nanofiltration membranes for cadmium remediation. J. Membr. Sci. Res. 2020, 6, 226–234. [Google Scholar] [CrossRef]
- Shao, L.-L.; An, Q.-F.; Ji, Y.-L.; Zhao, Q.; Wang, X.-S.; Zhu, B.-K.; Gao, C.-J. Preparation and characterization of sulfated carboxymethyl cellulose nanofiltration membranes with improved water permeability. Desalination 2014, 338, 74–83. [Google Scholar] [CrossRef]
- Jabbarvand Behrouz, S.; Khataee, A.; Safarpour, M.; Arefi-Oskoui, S.; Woo Joo, S. Carboxymethyl cellulose/polyethersulfone thin-film composite membranes for low-pressure desalination. Sep. Purif. Technol. 2021, 269, 118720. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, R.; Bai, R. Poly (vinyl alcohol)/carboxymethyl cellulose sodium blend composite nanofiltration membranes developed via interfacial polymerization. J. Membr. Sci. 2015, 493, 654–663. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Lü, Z.; Yu, S. Modification of polysulfone ultrafiltration membrane by sequential deposition of cross-linked poly(vinyl alcohol) (PVA) and sodium carboxymethyl cellulose (CMCNa) for nanofiltration. Desalin. Water Treat. 2016, 57, 17658–17669. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, P.; Huang, W.; Yu, S.; Liu, M.; Gao, C. High-flux composite hollow fiber nanofiltration membranes fabricated through layer-by-layer deposition of oppositely charged crosslinked polyelectrolytes for dye removal. J. Membr. Sci. 2015, 492, 312–321. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, Y.; Zhou, Q.; Shuai, S.; Lü, Z.; Gao, C. Facile modification of polypropylene hollow fiber microfiltration membranes for nanofiltration. Desalination 2012, 298, 49–58. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Cheng, Q.; Lü, Z.; Liu, M.; Gao, C. Application of thin-film composite hollow fiber membrane to submerged nanofiltration of anionic dye aqueous solutions. Sep. Purif. Technol. 2012, 88, 121–129. [Google Scholar] [CrossRef]
- Zhao, Q.; Ji, Y.-L.; Wu, J.-K.; Shao, L.-L.; An, Q.-F.; Gao, C.-J. Polyelectrolyte complex nanofiltration membranes: Performance modulation via casting solution pH. RSC Adv. 2014, 4, 52808–52814. [Google Scholar] [CrossRef]
- Ji, Y.; An, Q.; Zhao, Q.; Chen, H.; Qian, J.; Gao, C. Fabrication and performance of a new type of charged nanofiltration membrane based on polyelectrolyte complex. J. Membr. Sci. 2010, 357, 80–89. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Penkova, A. Novel PDMS-b-PPO Membranes Modified with Graphene Oxide for Efficient Pervaporation Ethanol Dehydration. Membranes 2022, 12, 832. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Hliavitskaya, T.A.; Ulbricht, M. One-Step Preparation of Antifouling Polysulfone Ultrafiltration Membranes via Modification by a Cationic Polyelectrolyte Based on Polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers 2020, 12, 864. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Liamin, V.; Plisko, T.; Burts, K.; Bildyukevich, A.; Ermakov, S.; Penkova, A. Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance. Polymers 2021, 13, 2804. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Loginova, E.; Plisko, T.; Burts, K.; Bildyukevich, A.; Penkova, A. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO2 for enhanced antifouling performance in water treatment. Sep. Purif. Technol. 2022, 286, 120500. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Kuzminova, A.I.; Zolotarev, A.A.; Korniak, A.S.; Ermakov, S.S.; Su, R.; Penkova, A.V. Novel mixed matrix membranes based on polyelectrolyte complex modified with fullerene derivatives for enhanced pervaporation and nanofiltration. Sep. Purif. Technol. 2022, 298, 121649. [Google Scholar] [CrossRef]
- Gribanova, E.V.; Larionov, M.I. Application of contact angle dependence on ph for estimation of acid-base properties of oxide surfaces. Vestn. St. -Petersbg. Univ. Ser. 4. PHYSICS Chem. 2014, 1, 401–407. [Google Scholar]
- Plisko, T.V.; Liubimova, A.S.; Bildyukevich, A.V.; Penkova, A.V.; Dmitrenko, M.E.; Mikhailovskii, V.Y.; Melnikova, G.B.; Semenov, K.N.; Doroshkevich, N.V.; Kuzminova, A.I. Fabrication and characterization of polyamide-fullerenol thin film nanocomposite hollow fiber membranes with enhanced antifouling performance. J. Membr. Sci. 2018, 551, 20–36. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Atta, R.R.; Zolotarev, A.A.; Plisko, T.V.; Mazur, A.S.; Solovyev, N.D.; Ermakov, S.S. The development and study of novel membrane materials based on polyphenylene isophthalamide—Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ali, F.A.A.; Alhoshan, M. A highly permeable zinc-based MOF/polyphenylsulfone composite membrane with elevated antifouling properties. Chem. Commun. 2020, 56, 5231–5234. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.S.; Ali, F.A.A.; Mishra, U.; Hamid, A.A. Thin-Film Nanocomposite Membrane Incorporated with Porous Zn-Based Metal–Organic Frameworks: Toward Enhancement of Desalination Performance and Chlorine Resistance. ACS Appl. Mater. Interfaces 2021, 13, 28818–28831. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88. [Google Scholar] [CrossRef]
- Jun, B.-M.; Al-Hamadani, Y.A.J.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of metal-organic framework based membranes in water purification: A review. Sep. Purif. Technol. 2020, 247, 116947. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Venna, S.R.; Mohamed, M.H.; Gipple, M.J.; Hopkinson, D.P. Dual-Layer MOF Composite Membranes with Tuned Interface Interaction for Postcombustion Carbon Dioxide Separation. Cell Rep. Phys. Sci. 2020, 1, 100059. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Myznikov, D.; Selyutin, A.; Su, R.; Penkova, A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes 2022, 12, 908. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Mazur, A.; Ermakov, S.; Penkova, A. Novel Pervaporation Membranes Based on Biopolymer Sodium Alginate Modified by FeBTC for Isopropanol Dehydration. Sustainability 2021, 13, 6092. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, H.Y.; Zhao, D.; Wang, J. Metal–Organic Frameworks (MOFs)-boosted filtration membrane technology for water sustainability. APL Mater. 2020, 8, 040902. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Patapeejumruswong, M.; Weiss, J.; Supaphol, P.; Yoovidhya, T. Electrospinning of food-grade nanofibers from cellulose acetate and egg albumen blends. J. Food Eng. 2010, 98, 370–376. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Mazur, A.; Semenov, K.; Solovyev, N.; Penkova, A. Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol. Polymers 2022, 14, 691. [Google Scholar] [CrossRef]
- Sui, X.; Shao, C.; Liu, Y. Photoluminescence of polyethylene oxide–ZnO composite electrospun fibers. Polymer 2007, 48, 1459–1463. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, H.; Wang, Q.; Ma, W.; Yang, G.; Xu, S.; Li, S.; Su, G.; Qu, Y.; Zhang, M.; et al. Optimization of a MOF Blended with Modified Polyimide Membrane for High-Performance Gas Separation. Membranes 2021, 12, 34. [Google Scholar] [CrossRef]
- Yang, Z.; Ao, D.; Guo, X.; Nie, L.; Qiao, Z.; Zhong, C. Preparation and characterization of small-size amorphous MOF mixed matrix membrane. Sep. Purif. Technol. 2021, 272, 118860. [Google Scholar] [CrossRef]
- Taghipour, A.; Rahimpour, A.; Rastgar, M.; Sadrzadeh, M. Ultrasonically synthesized MOFs for modification of polymeric membranes: A critical review. Ultrason. Sonochem. 2022, 90, 106202. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quemener, D. MOF-mixed matrix membranes: Precise dispersion of MOF particles with better compatibility via a particle fusion approach for enhanced gas separation properties. J. Membr. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Qiu, C.; Panwisawas, C.; Ward, M.; Basoalto, H.C.; Brooks, J.W.; Attallah, M.M. On the role of melt flow into the surface structure and porosity development during selective laser melting. Acta Mater. 2015, 96, 72–79. [Google Scholar] [CrossRef]
- Burts, K.S.; Plisko, T.V.; Bildyukevich, A.V.; Penkova, A.V.; Pratsenko, S.A. Modification of polysulfone ultrafiltration membranes using block copolymer Pluronic F127. Polym. Bull. 2021, 78, 6549–6576. [Google Scholar] [CrossRef]
- Xu, T.-C.; Wang, C.-S.; Hu, Z.-Y.; Zheng, J.-J.; Jiang, S.-H.; He, S.-J.; Hou, H.-Q. High Strength and Stable Proton Exchange Membrane Based on Perfluorosulfonic Acid/Polybenzimidazole. Chin. J. Polym. Sci. 2022, 40, 764–771. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Ermakov, S.; Roizard, D.; Penkova, A. Enhanced pervaporation properties of PVA-based membranes modified with polyelectrolytes. application to IPA dehydration. Polymers 2020, 12, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Zhao, Q.; An, Q.; Qian, J.; Lee, K.; Lai, J. The chemical crosslinking of polyelectrolyte complex colloidal particles and the pervaporation performance of their membranes. J. Membr. Sci. 2011, 385–386, 132–140. [Google Scholar] [CrossRef]









| Membrane | Ra, nm | Contact Angle, ° | Total Porosity, % | Porosity Over Weight, cm3/g |
|---|---|---|---|---|
| CA-12 | 3.16 | 30 | 95.8 | 4.5 |
| CA-15 | 1.91 | 31 | 94.6 | 2.2 |
| CA-17 | 1.65 | 31 | 94.4 | 1.9 |
| CA-20 | 1.60 | 33 | 91.8 | 1.2 |
| CA-12/Zn(SEB) (1%) | 2.97 | 26 | 89.1 | 3.4 |
| CA-12/Zn(BDC)Si (1%) | 3.29 | 27 | 87.0 | 2.8 |
| CA-12/Zn(BIM) (1%) | 3.08 | 28 | 87.6 | 2.9 |
| Membrane | Ra, nm | Contact Angle of Water, ° |
|---|---|---|
| CMC/CA-15 | 1.1 | 54 |
| CMC + Zn(SEB) (15%)/CA-15 | 2.9 | 45 |
| CMC + Zn(BDC)Si (15%)/CA-15 | 3.4 | 50 |
| CMC + Zn(BIM) (15%)/CA-15 | 3.1 | 49 |
| Membrane | Permeability, kg/(m2 h atm) | Rejection Coefficient, % | ||||
|---|---|---|---|---|---|---|
| Cu2+ | Cd2+ | Cr3+ | Ni2+ | Zn2+ | ||
| CA-12 | 45 | 0 | 1 | 2 | 0 | 1 |
| CA-12/Zn(SEB) (1%) | 49 | 26 | 8 | 6 | 8 | 7 |
| CMC/CA-15 | 0.06 | 23 | 82 | 77 | 38 | 50 |
| CMC + Zn(BDC)Si (15%)/CA-15 | 0.10 | 80 | 93 | 79 | 91 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Selyutin, A.; Ermakov, S.; Penkova, A. Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions. Polymers 2023, 15, 1341. https://doi.org/10.3390/polym15061341
Dmitrenko M, Kuzminova A, Zolotarev A, Selyutin A, Ermakov S, Penkova A. Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions. Polymers. 2023; 15(6):1341. https://doi.org/10.3390/polym15061341
Chicago/Turabian StyleDmitrenko, Mariia, Anna Kuzminova, Andrey Zolotarev, Artem Selyutin, Sergey Ermakov, and Anastasia Penkova. 2023. "Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions" Polymers 15, no. 6: 1341. https://doi.org/10.3390/polym15061341
APA StyleDmitrenko, M., Kuzminova, A., Zolotarev, A., Selyutin, A., Ermakov, S., & Penkova, A. (2023). Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions. Polymers, 15(6), 1341. https://doi.org/10.3390/polym15061341