Copper II Complexes Based on Benzimidazole Ligands as a Novel Photoredox Catalysis for Free Radical Polymerization Embedded Gold and Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Irradiation Source
2.3. Free Radical Photopolymerization
2.4. Redox Potentials
2.5. FTIR Experiments
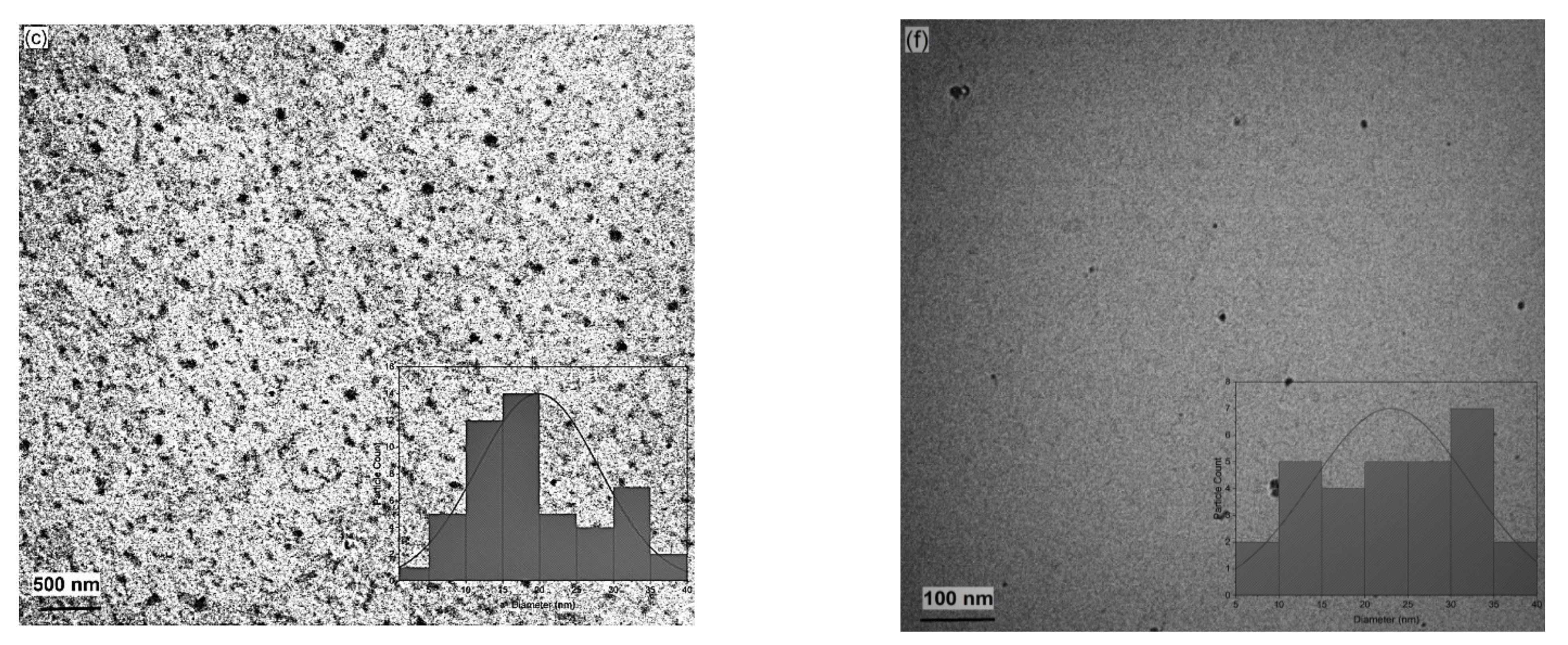
2.6. Transmission Electron Microscopy (TEM)
2.7. Fluorescence Experiments
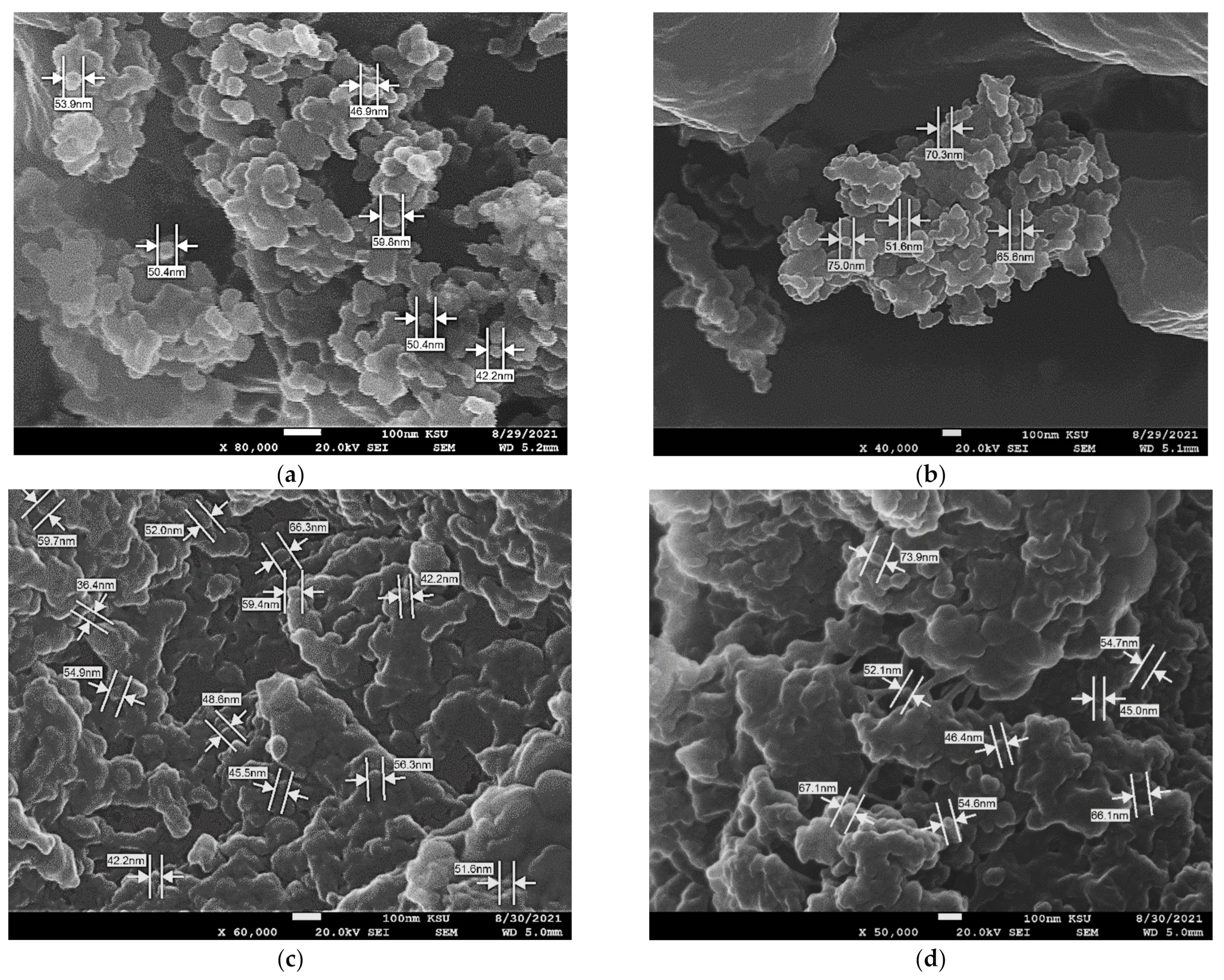
2.8. Scanning Image Microscope (SEM)
3. Results
3.1. Light Absorption of Cu-Complexes
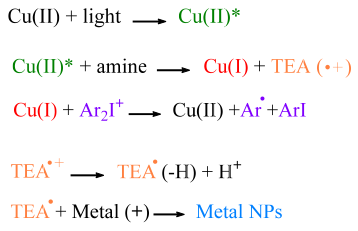
3.2. Copper II Complex Oxidation Process
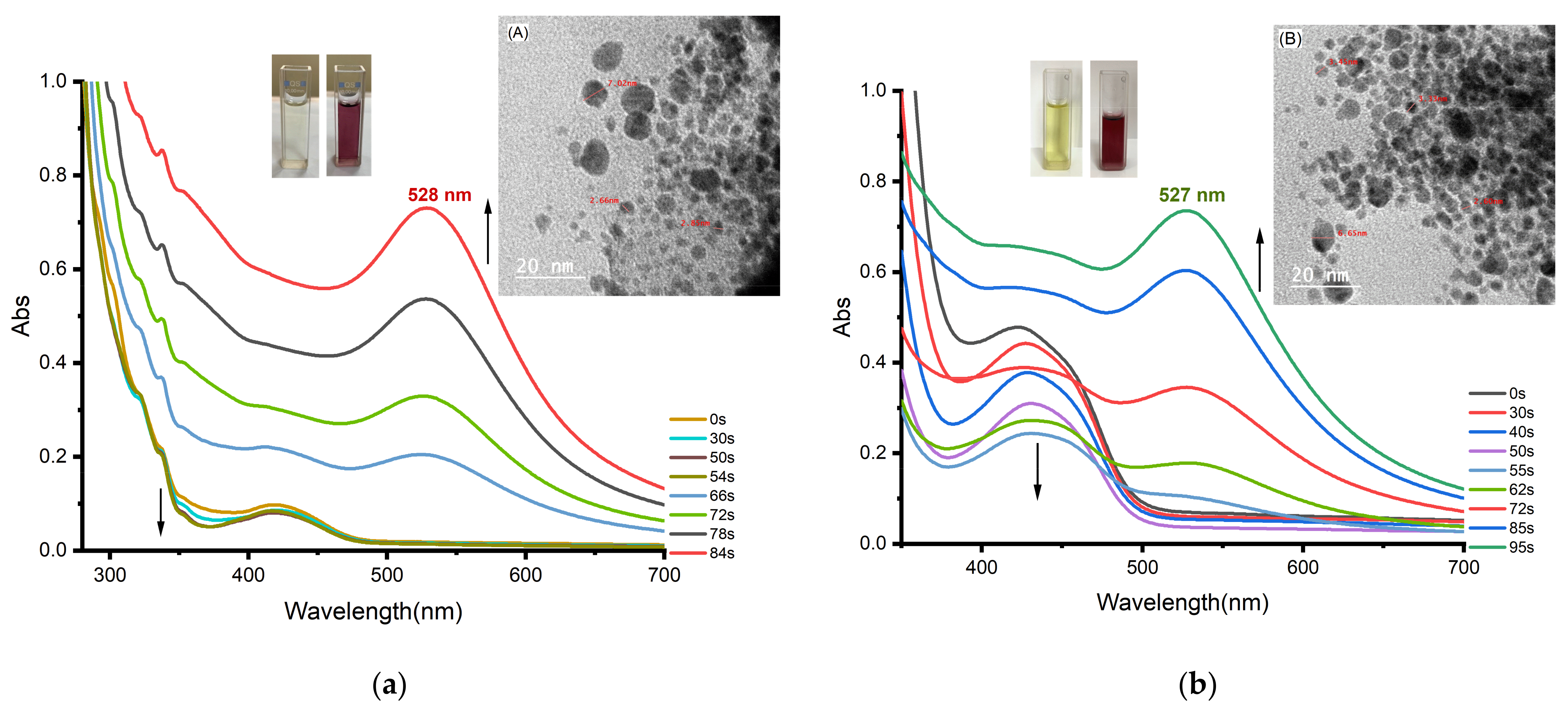
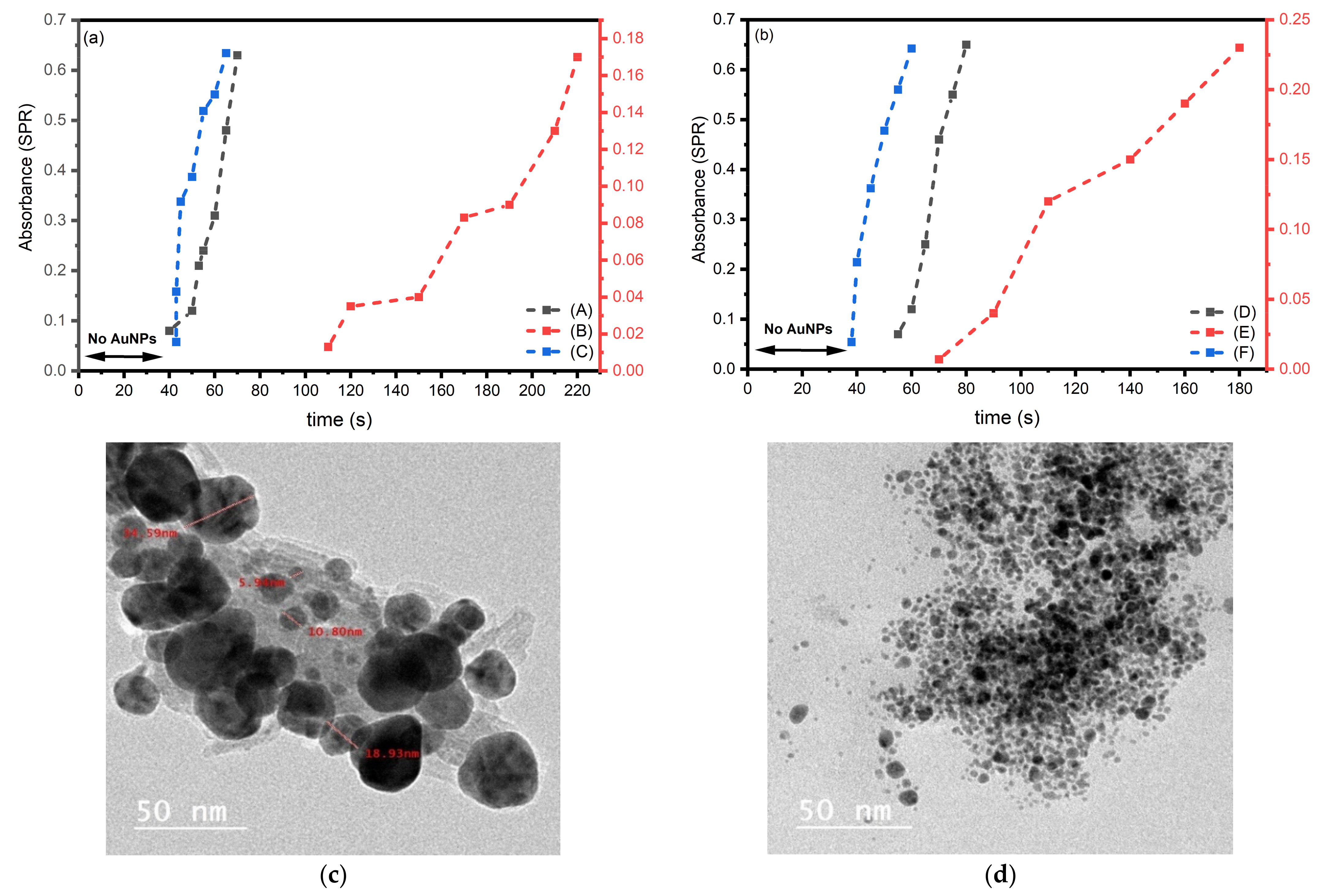
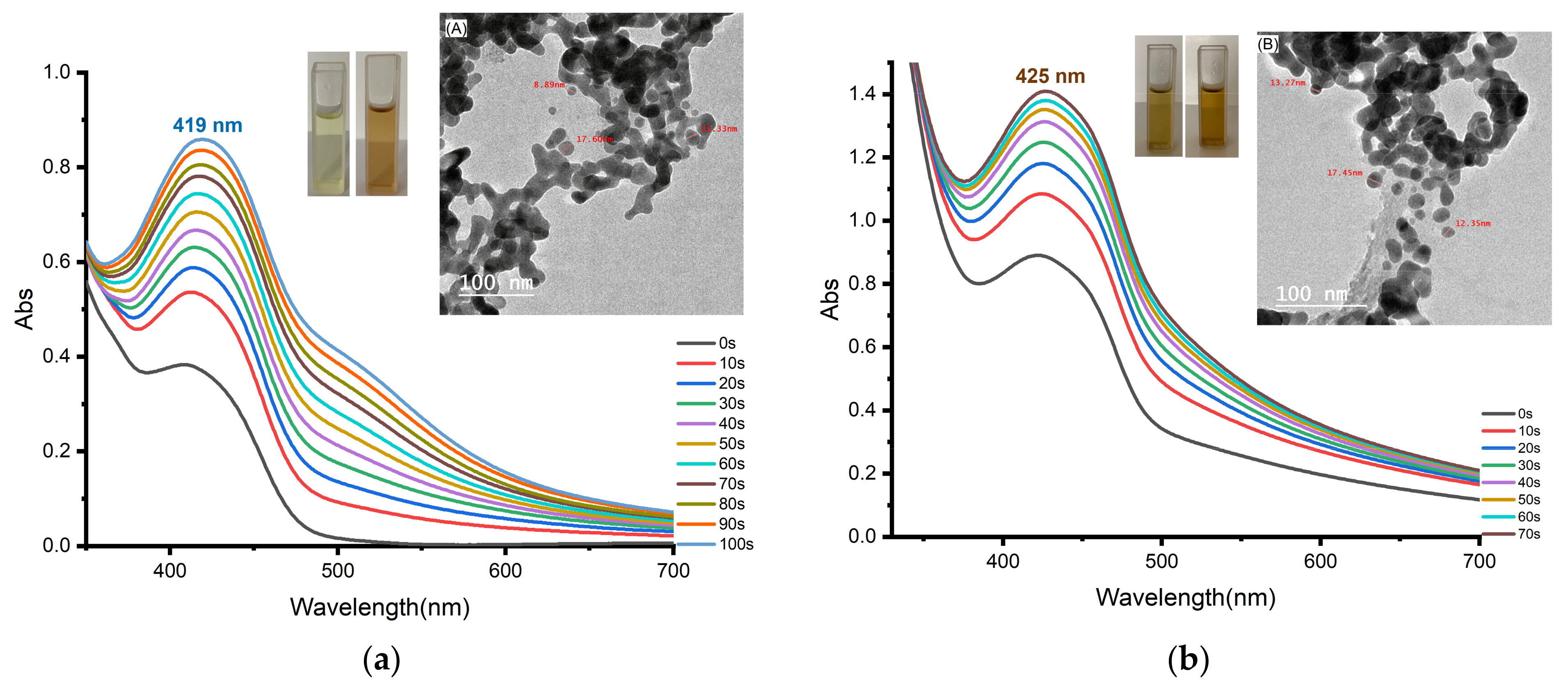
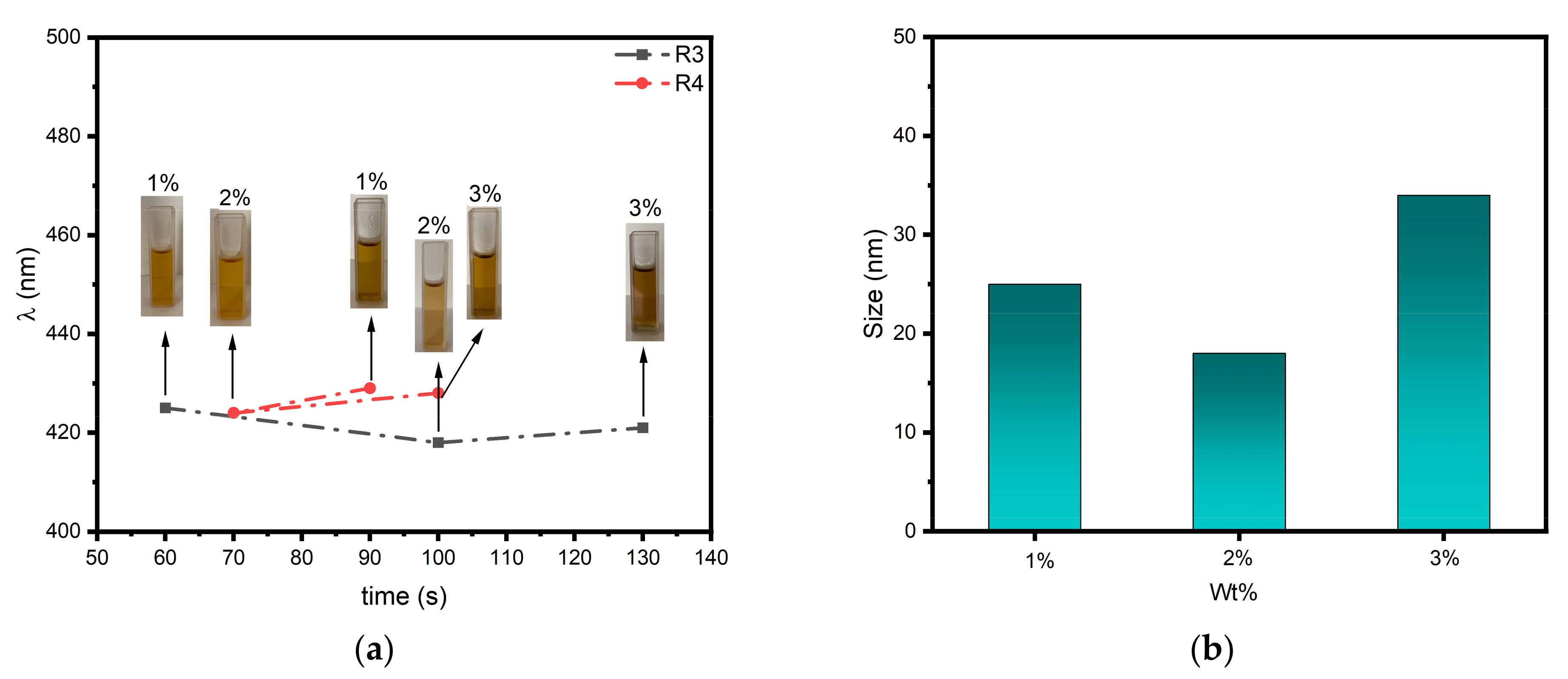
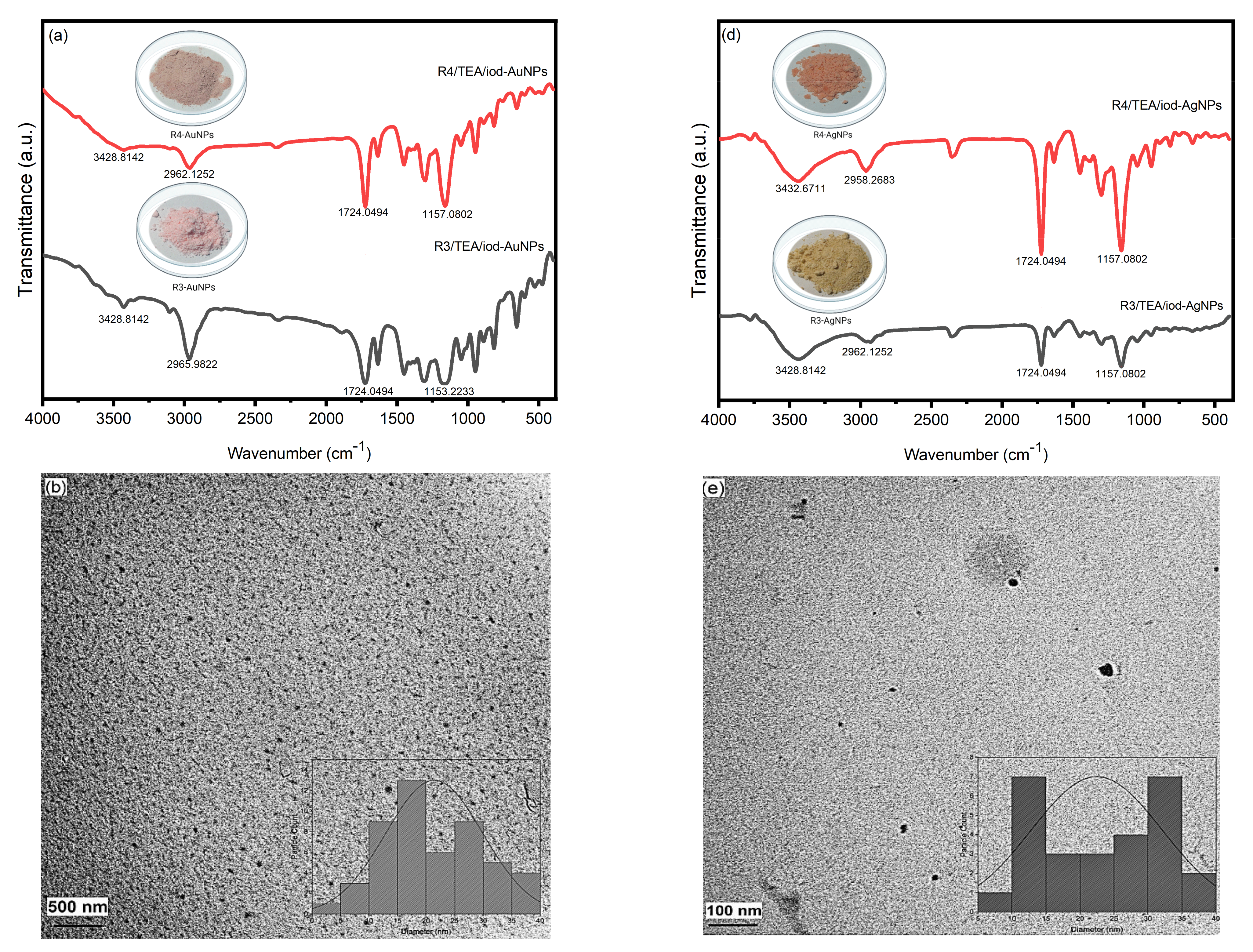
3.3. Photoinduced Synthesis of Au Nanoparticles:
3.4. Photoinduced Synthesis of Ag Nanoparticles
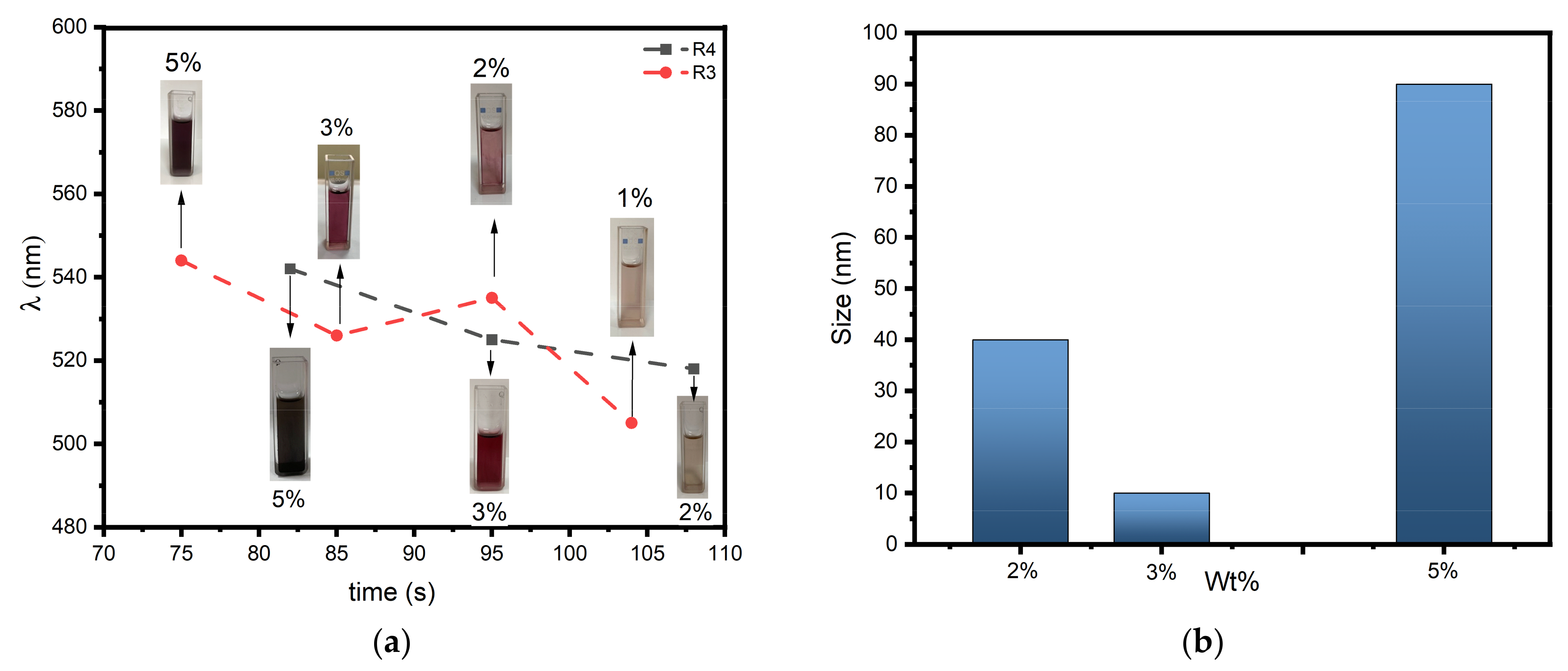
3.5. Fabrication of AuNPs and AgNPs Embedded Polymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, S.; Mishra, S.B. Sol–gel derived organic–inorganic hybrid materials: Synthesis, characterizations and applications. J. Sol-Gel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Zhang, J.Z. Ultrafast Studies of Electron Dynamics in Semiconductor and Metal Colloidal Nanoparticles: Effects of Size and Surface. Acc. Chem. Res. 1997, 30, 423–429. [Google Scholar] [CrossRef]
- Thomas, J.M. Colloidal metals: Past, present and future. Pure Appl. Chem. 1988, 60, 1517–1528. [Google Scholar] [CrossRef]
- Perenboom, J.; Wyder, P.; Meier, F. Electronic properties of small metallic particles. Phys. Rep. 1981, 78, 173–292. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Viswanathan, B.; Varadarajan, T.K. Photochemically reduced polyoxometalate assisted generation of silver and gold nanoparticles in composite films: A single step route. Nanoscale Res. Lett. 2007, 2, 175–183. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Z.; He, Y.; Ye, Y.; Zhou, J.; Zhang, J.; Ouyang, Q.; Tang, B.; Wang, X. Photoinduced synthesis of gold nanoparticle–bacterial cellulose nanocomposite and its application for in-situ detection of trace concentration of dyes in textile and paper. Cellulose 2018, 25, 3941–3953. [Google Scholar] [CrossRef]
- Batibay, G.S.; Gunkara, O.T.; Ocal, N.; Arsu, N. In-situ photoinduced formation of self–assembled Ag NPs using POSS-TX as nano-photoinitiator in PEGMEA/PEGDA polymer matrix and creating self-wrinkled pattern. J. Photochem. Photobiol. A Chem. 2018, 359, 73–79. [Google Scholar] [CrossRef]
- Balan, L.; Jin, M.; Malval, J.-P.; Chaumeil, H.; Defoin, A.; Vidal, L. Fabrication of Silver Nanoparticle-Embedded Polymer Promoted by Combined Photochemical Properties of a 2,7-Diaminofluorene Derivative Dye. Macromolecules 2008, 41, 9359–9365. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some Interesting Properties of Metals Confined in Time and Nanometer Space of Different Shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Balan, L.; Malval, J.-P.; Schneider, R.; Burget, D. Silver nanoparticles: New synthesis, characterization and photophysical properties. Mater. Chem. Phys. 2007, 104, 417–421. [Google Scholar] [CrossRef]
- Kong, H.; Jang, J. One-step fabrication of silver nanoparticle embedded polymer nanofibers by radical-mediated dispersion polymerization. Chem. Commun. 2006, 28, 3010–3012. [Google Scholar] [CrossRef] [PubMed]
- Scaiano, J.C.; Billone, P.; Gonzalez, C.M.; Marett, L.; Marin, M.L.; McGilvray, K.L.; Yuan, N. Photochemical routes to silver and gold nanoparticles. Pure Appl. Chem. 2009, 81, 635–647. [Google Scholar] [CrossRef]
- Korchev, A.S.; Bozack, M.J.; Slaten, B.L.; Mills, G. Polymer-Initiated Photogeneration of Silver Nanoparticles in SPEEK/PVA Films: Direct Metal Photopatterning. J. Am. Chem. Soc. 2003, 126, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Balan, L.; Melinte, V.; Buruiana, T.; Schneider, R.; Vidal, L. Controlling the morphology of gold nanoparticles synthesized photochemically in a polymer matrix through photonic parameters. Nanotechnology 2012, 23, 415705. [Google Scholar] [CrossRef]
- Sangermano, M.; Yagci, Y.; Rizza, G. In Situ Synthesis of Silver−Epoxy Nanocomposites by Photoinduced Electron Transfer and Cationic Polymerization Processes. Macromolecules 2007, 40, 8827–8829. [Google Scholar] [CrossRef]
- Sudeep, P.K.; Kamat, P.V. Photosensitized Growth of Silver Nanoparticles under Visible Light Irradiation: A Mechanistic Investigation. Chem. Mater. 2005, 17, 5404–5410. [Google Scholar] [CrossRef]
- Yagci, Y.; Sangermano, M.; Rizza, G. A visible light photochemical route to silver–epoxy nanocomposites by simultaneous polymerization–reduction approach. Polymer 2008, 49, 5195–5198. [Google Scholar] [CrossRef]
- Balan, L.; Malval, J.-P.; Schneider, R.; Le Nouen, D.; Lougnot, D.-J. In-situ fabrication of polyacrylate–silver nanocomposite through photoinduced tandem reactions involving eosin dye. Polymer 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Giuffrida, S.; Costanzo, L.L.; Ventimiglia, G.; Bongiorno, C. Photochemical synthesis of copper nanoparticles incorporated in poly(vinyl pyrrolidone). J. Nanoparticle Res. 2008, 10, 1183–1192. [Google Scholar] [CrossRef]
- Mitzscherling, S.; Cui, Q.; Koopman, W.; Bargheer, M. Dielectric function of two-phase colloid–polymer nanocomposite. Phys. Chem. Chem. Phys. 2015, 17, 29465–29474. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-Q.; Ren, B.; Wu, D.-Y. Surface-Enhanced Raman Scattering: From Noble to Transition Metals and from Rough Surfaces to Ordered Nanostructures. J. Phys. Chem. B 2002, 106, 9463–9483. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Pardoe, A.; McGilvray, K.L.; Chrétien, M.N.; Scaiano, J.C. Synthesis of copper nanoparticles mediated by photogenerated free radicals: Catalytic role of chloride anions. Photochem. Photobiol. Sci. 2010, 9, 766–774. [Google Scholar] [CrossRef]
- McGilvray, K.L.; Decan, M.R.; Wang, D.; Scaiano, J.C. Facile Photochemical Synthesis of Unprotected Aqueous Gold Nanoparticles. J. Am. Chem. Soc. 2006, 128, 15980–15981. [Google Scholar] [CrossRef] [PubMed]
- Maretti, L.; Billone, P.S.; Liu, Y.; Scaiano, J.C. Facile Photochemical Synthesis and Characterization of Highly Fluorescent Silver Nanoparticles. J. Am. Chem. Soc. 2009, 131, 13972–13980. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Stamplecoskie, K.G.; Hallett-Tapley, G.L. Photochemical Norrish type I reaction as a tool for metal nanoparticle synthesis: Importance of proton coupled electron transfer. Chem. Commun. 2012, 48, 4798–4808. [Google Scholar] [CrossRef]
- Marin, M.L.; McGilvray, K.L.; Scaiano, J.C. Photochemical Strategies for the Synthesis of Gold Nanoparticles from Au(III) and Au(I) Using Photoinduced Free Radical Generation. J. Am. Chem. Soc. 2008, 130, 16572–16584. [Google Scholar] [CrossRef]
- Çeper, T.; Arsu, N. Photochemically Prepared Gold/Polymer Nanocoatings: Formation of Gold Mirror. Macromol. Chem. Phys. 2017, 218, 1700030. [Google Scholar] [CrossRef]
- Tar, H.; Kashar, T.I.; Kouki, N.; Aldawas, R.; Graff, B.; Lalevée, J. Novel Copper Photoredox Catalysts for Polymerization: An In Situ Synthesis of Metal Nanoparticles. Polymers 2020, 12, 2293. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. A Novel Photopolymerization Initiating System Based on an Iridium Complex Photocatalyst. Macromol. Rapid Commun. 2011, 32, 917–920. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lepeltier, M.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural Effects in the Iridium Complex Series: Photoredox Catalysis and Photoinitiation of Polymerization Reactions under Visible Lights. Macromol. Chem. Phys. 2017, 218, 1700192. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Telitel, S.; Sun, J.; Zhao, J.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P. Iridium complexes incorporating coumarin moiety as catalyst photoinitiators: Towards household green LED bulb and halogen lamp irradiation. Polymer 2012, 53, 2803–2808. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Zinc-based metal complexes as new photocatalysts in polymerization initiating systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Copper Complexes in Radical Photoinitiating Systems: Applications to Free Radical and Cationic Polymerization upon Visible LEDs. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Kermagoret, A.; Versace, D.-L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper photoredox catalysts for polymerization upon near UV or visible light: Structure/reactivity/efficiency relationships and use in LED projector 3D printing resins. Polym. Chem. 2016, 8, 568–580. [Google Scholar] [CrossRef]
- Cuttell, D.G.; Kuang, S.-M.; Fanwick, P.E.; McMillin, D.R.; Walton, R.A. Simple Cu(I) Complexes with Unprecedented Excited-State Lifetimes. J. Am. Chem. Soc. 2001, 124, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, Q.; Cheng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly Efficient Electroluminescence from Green-Light-Emitting Electrochemical Cells Based on CuI Complexes. Adv. Funct. Mater. 2006, 16, 1203–1208. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Holler, M.; Moudam, O.; Nierengarten, J.-F.; Zhou, Z.; Wegh, R.T.; Welter, R. Highly Luminescent CuI Complexes for Light-Emitting Electrochemical Cells. Adv. Mater. 2006, 18, 1313–1316. [Google Scholar] [CrossRef]
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet Exciton Confinement in Green Organic Light-Emitting Diodes Containing Luminescent Charge-Transfer Cu(I) Complexes. Adv. Funct. Mater. 2012, 22, 2327–2336. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.C.; Vlassova, A.; Collins, S.K. Toward a Visible Light Mediated Photocyclization: Cu-Based Sensitizers for the Synthesis of [5]Helicene. Org. Lett. 2012, 14, 2988–2991. [Google Scholar] [CrossRef] [PubMed]
- Pirtsch, M.; Paria, S.; Matsuno, T.; Isobe, H.; Reiser, O. [Cu(dap)2Cl] As an Efficient Visible-Light-Driven Photoredox Catalyst in Carbon–Carbon Bond-Forming Reactions. Chem. A Eur. J. 2012, 18, 7336–7340. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Adzima, B.J.; Baker, N.H.; Bowman, C.N. Photopolymerization Reactions Using the Photoinitiated Copper (I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) Reaction. Adv. Mater. 2013, 25, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.A.; Erbse, A.H.; Bowman, C.N. Evaluation and development of novel photoinitiator complexes for photoinitiating the copper-catalyzed azide–alkyne cycloaddition reaction. Polym. Chem. 2013, 5, 1874–1882. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Zhao, J.; Lalevée, J.; Lu, H.; MacQueen, R.; Kable, S.H.; Schmidt, T.W.; Stenzel, M.H.; Xiao, P. A new role of curcumin: As a multicolor photoinitiator for polymer fabrication under household UV to red LED bulbs. Polym. Chem. 2015, 6, 5053–5061. [Google Scholar] [CrossRef]
- Deng, J.; Chen, W.; Deng, H. Synthesis of Dipyridyl Ketone Isonicotinoyl Hydrazone Copper(II) Complex: Structure, Anticancer Activity and Anticancer Mechanism. J. Fluoresc. 2016, 26, 1987–1996. [Google Scholar] [CrossRef]
- Chew, S.T.; Lo, K.M.; Lee, S.K.; Heng, M.P.; Teoh, W.Y.; Sim, K.S.; Tan, K.W. Copper complexes with phosphonium containing hydrazone ligand: Topoisomerase inhibition and cytotoxicity study. Eur. J. Med. Chem. 2014, 76, 397–407. [Google Scholar] [CrossRef]
- Vafazadeh, R.; Moghadas, Z.; Willis, A.C. Anion and solvent effects on the coordination behavior of N-(2-pyridinylmethylene)benzoylhydrazone with copper(II): Synthesis and structural characterization. J. Coord. Chem. 2015, 68, 4255–4271. [Google Scholar] [CrossRef]
- Wong, K.M.-C.; Hung, L.-L.; Lam, W.H.; Zhu, N.; Yam, V.W.-W. A Class of Luminescent Cyclometalated Alkynylgold (III) Complexes: Synthesis, Characterization, and Electrochemical, Photophysical, and Computational Studies of [Au (C∧ N∧ C)(C⋮CR)](C∧ N∧ C= κ3C, N, C Bis-cyclometalated 2, 6-Diphenylpyridyl). J. Am. Chem. Soc. 2007, 129, 4350–4365. [Google Scholar] [CrossRef]
- Rajam, B.M.; Ramasamy, P.; Mahalingam, U. Monodispersed gold nanoparticles as a probe for the detection of Hg2+ ions in water. Acta Chim. Slov. 2017, 64, 186–192. [Google Scholar] [CrossRef]
- Ghosh, D.; Chattopadhyay, N. Gold nanoparticles: Acceptors for efficient energy transfer from the photoexcited fluorophores. Opt. Photonics J. 2013, 3, 18–26. [Google Scholar] [CrossRef]
- Mutlu, S.; Metin, E.; Yuksel, S.A.; Bayrak, U.; Nuhoglu, C.; Arsu, N. In-situ photochemical synthesis and dielectric properties of nanocomposite thin films containing Au, Ag and MnO nanoparticles. Eur. Polym. J. 2020, 144, 110238. [Google Scholar] [CrossRef]
- Alsawafta, M.; Badilescu, S.; Paneri, A.; Truong, V.-V.; Packirisamy, M. Gold-Poly(methyl methacrylate) Nanocomposite Films for Plasmonic Biosensing Applications. Polymers 2011, 3, 1833–1848. [Google Scholar] [CrossRef]
- Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free Radical Photopolymerization and 3D Printing Using Newly Developed Dyes: Indane-1,3-Dione and 1H-Cyclopentanaphthalene-1,3-Dione Derivatives as Photoinitiators in Three-Component Systems. Catalysts 2020, 10, 463. [Google Scholar] [CrossRef]
- Balaji, D.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, Morphological Control, and Properties of Silver Nanoparticles in Potential Applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Malval, J.-P.; Jin, M.; Balan, L.; Schneider, R.; Versace, D.-L.; Chaumeil, H.; Defoin, A.; Soppera, O. Photoinduced Size-Controlled Generation of Silver Nanoparticles Coated with Carboxylate-Derivatized Thioxanthones. J. Phys. Chem. C 2010, 114, 10396–10402. [Google Scholar] [CrossRef]
- Zaier, M.; Vidal, L.; Hajjar-Garreau, S.; Balan, L. Generating highly reflective and conductive metal layers through a light-assisted synthesis and assembling of silver nanoparticles in a polymer matrix. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Tunable plasmonic glucose sensing based on the dissociation of Con A-aggregated dextran-coated gold colloids. Anal. Chim. Acta 2004, 517, 139–144. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, P.; Mukherjee, P.; Kapoor, S. Triethylamine induced synthesis of silver and bimetallic (Ag/Au) nanoparticles in glycerol and their antibacterial study. J. Nanostructure Chem. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Henglein, A. Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 1989, 89, 1861–1873. [Google Scholar] [CrossRef]
- Belloni, J. Contribution of radiation chemistry to the study of metal clusters. Radiat. Res. 1998, 150, S9. [Google Scholar] [CrossRef]
- Kapoor, S. Preparation, Characterization, and Surface Modification of Silver Particles. Langmuir 1998, 14, 1021–1025. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Manzoor, N.; Ahmed, J.; Asiri, A.M. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanasree, S.; Harini, D.; Rajaram, A.; Rajaram, R. Rapid synthesis of gold nanoparticles with Cissus quadrangularis extract using microwave irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 190–196. [Google Scholar] [CrossRef]
- Sundararajan, B.; Kumari, B.R. Novel synthesis of gold nanoparticles using Artemisia vulgaris L. leaf extract and their efficacy of larvicidal activity against dengue fever vector Aedes aegypti L. J. Trace Elem. Med. Biol. 2017, 43, 187–196. [Google Scholar] [CrossRef]
- Zhi, B.; Song, Q.; Mao, Y. Vapor deposition of polyionic nanocoatings for reduction of microglia adhesion. RSC Adv. 2018, 8, 4779–4785. [Google Scholar] [CrossRef]
- Baganizi, D.R.; Nyairo, E.; Duncan, S.A.; Singh, S.R.; Dennis, V.A. Interleukin-10 Conjugation to Carboxylated PVP-Coated Silver Nanoparticles for Improved Stability and Therapeutic Efficacy. Nanomaterials 2017, 7, 165. [Google Scholar] [CrossRef]
- Anyaogu, K.C.; Cai, X.; Neckers, D.C. Gold nanoparticle photosensitized radical photopolymerization. Photochem. Photobiol. Sci. 2008, 7, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Singh, J.; Raj, P.; Singh, N.; Singh, H.; Sharma, S.K.; Kim, D.Y.; Kaur, N. ZnO decorated with organic nanoparticles based sensor for the ratiometric selective determination of mercury ions. New J. Chem. 2016, 40, 1529–1534. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; ten Bokkel Huinink, W.W.; Swenerton, K.D.; Gianni, L.; Myles, J.; Van der Burg, M.E.; Vermoken, J.B.; Bruser KColombo, N. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: High-dose versus low-dose and long versus short infusion. J. Clin. Oncol. 1994, 12, 2654–2666. [Google Scholar] [CrossRef]
- Mohan, S.; Sundaraganesan, N.; Mink, J. FTIR and Raman studies on benzimidazole. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 1111–1115. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Ilakiamani, S.; Subramani, P.; Joshua, B.D. Comparison of experimental and ab initio HF and DFT vibrational spectra of benzimidazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 628–635. [Google Scholar] [CrossRef]
- Baranwal, B.P.; Talat, F.; Varma, A. Synthesis, spectral and thermal characterization of nano-sized, oxo-centered, trinuclear carboxylate-bridged chromium (III) complexes of hydroxycarboxylic acids. J. Mol. Struct. 2009, 920, 472–477. [Google Scholar] [CrossRef]
- Kolmas, J.; Jaklewicz, A.; Zima, A.; Buæko, M.; Paszkiewicz, Z.; Lis, J.; OElósarczyk, A.; Kolodziejski, W. Incorporation of carbonate and magnesium ions into synthetic hydroxyapatite: The effect on physicochemical properties. J. Mol. Struct. 2011, 987, 40–50. [Google Scholar] [CrossRef]
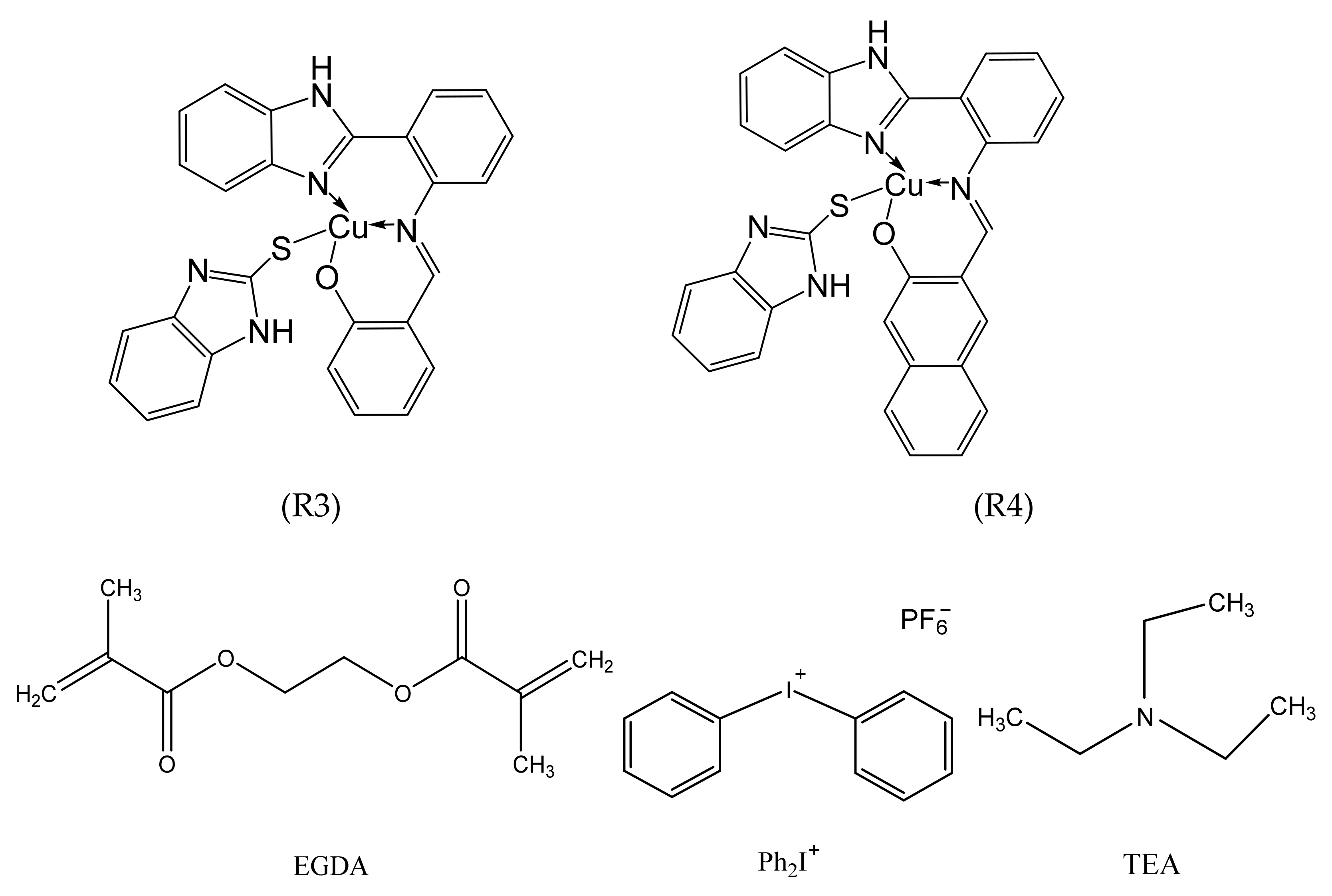


| Complexes | Solvent | λmax (nm) | ε (M−1 cm−1) |
|---|---|---|---|
| (R3) | DMF | 355 | 6220 |
| (R4) | DMF | 438 | 5820 |
| Eox vs. SCE* [V] (Cu Complexes) | Ered vs. SCE* [V] | E*[eV] | ∆Get (Cu Complexes/TEA) (eV) | ∆Get (Cu Complexes/Iod) (eV) |
|---|---|---|---|---|
| (R3) 1.01 | −0.59 | 2.95 | −1.28 | −1.74 |
| (R4) 1.05 | −0.47 | 3.13 | −1.58 | −1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhomaidan, L.M.; Tar, H.; Alnafisah, A.S.; Aroua, L.M.; KouKi, N.; Alminderej, F.M.; Lalevee, J. Copper II Complexes Based on Benzimidazole Ligands as a Novel Photoredox Catalysis for Free Radical Polymerization Embedded Gold and Silver Nanoparticles. Polymers 2023, 15, 1289. https://doi.org/10.3390/polym15051289
Alhomaidan LM, Tar H, Alnafisah AS, Aroua LM, KouKi N, Alminderej FM, Lalevee J. Copper II Complexes Based on Benzimidazole Ligands as a Novel Photoredox Catalysis for Free Radical Polymerization Embedded Gold and Silver Nanoparticles. Polymers. 2023; 15(5):1289. https://doi.org/10.3390/polym15051289
Chicago/Turabian StyleAlhomaidan, Lama M., Haja Tar, Abrar S. Alnafisah, Lotfi M. Aroua, Noura KouKi, Fahad M. Alminderej, and Jacques Lalevee. 2023. "Copper II Complexes Based on Benzimidazole Ligands as a Novel Photoredox Catalysis for Free Radical Polymerization Embedded Gold and Silver Nanoparticles" Polymers 15, no. 5: 1289. https://doi.org/10.3390/polym15051289
APA StyleAlhomaidan, L. M., Tar, H., Alnafisah, A. S., Aroua, L. M., KouKi, N., Alminderej, F. M., & Lalevee, J. (2023). Copper II Complexes Based on Benzimidazole Ligands as a Novel Photoredox Catalysis for Free Radical Polymerization Embedded Gold and Silver Nanoparticles. Polymers, 15(5), 1289. https://doi.org/10.3390/polym15051289








