3,4-Enhanced Polymerization of Isoprene Catalyzed by Side-Arm Tridentate Iminopyridine Iron Complex with High Activity: Optimization via Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization of Isoprene
2.3. Characterization
2.4. Synthesis of Iron Complexes
3. Results and Discussion
3.1. Analytical Data for Ligands and Iminopyridine Iron Complexes
3.1.1. Characterization of [N, N, O] (L 1) and [N, N, O]FeCl2 (Fe 1)


3.1.2. Characterization of [N, N, O] (L 2) and [N, N, O]FeCl2 (Fe 2)


3.1.3. Characterization of [N, N, S] (L 3) and [N, N, S]FeCl2 (Fe 3)
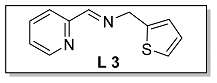

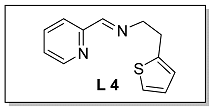
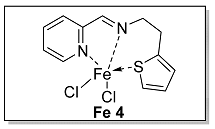
3.1.4. Characterization of [N, N, S] (L 4) and [N, N, S]FeCl2 (Fe 4)
3.2. Optimization of Single Factors
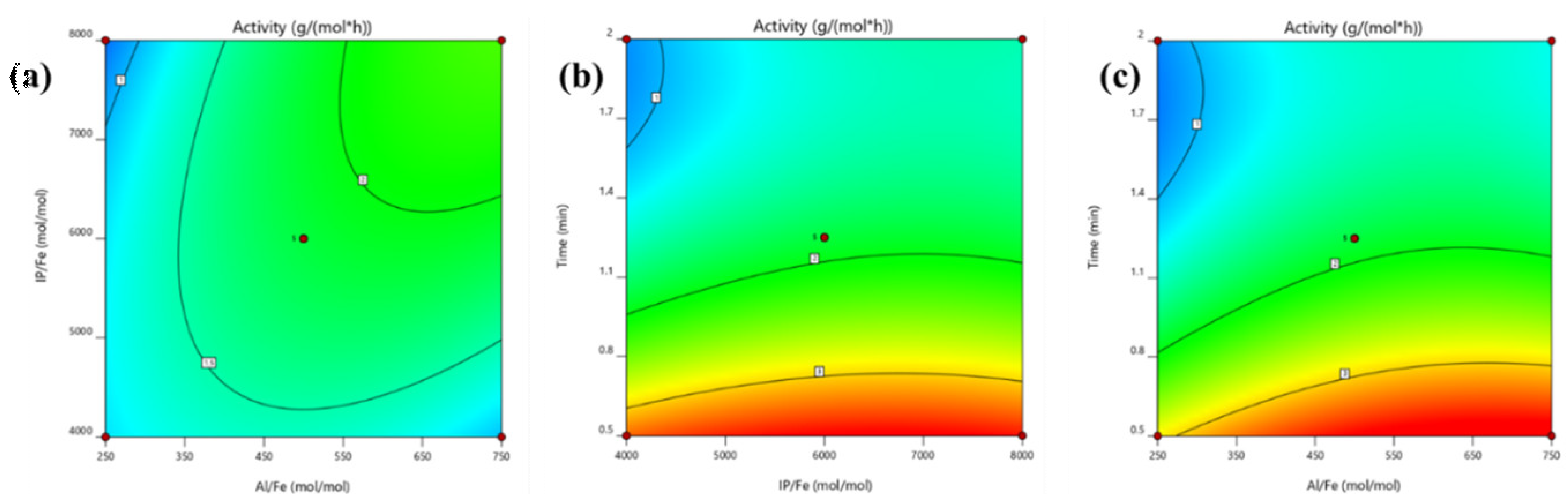
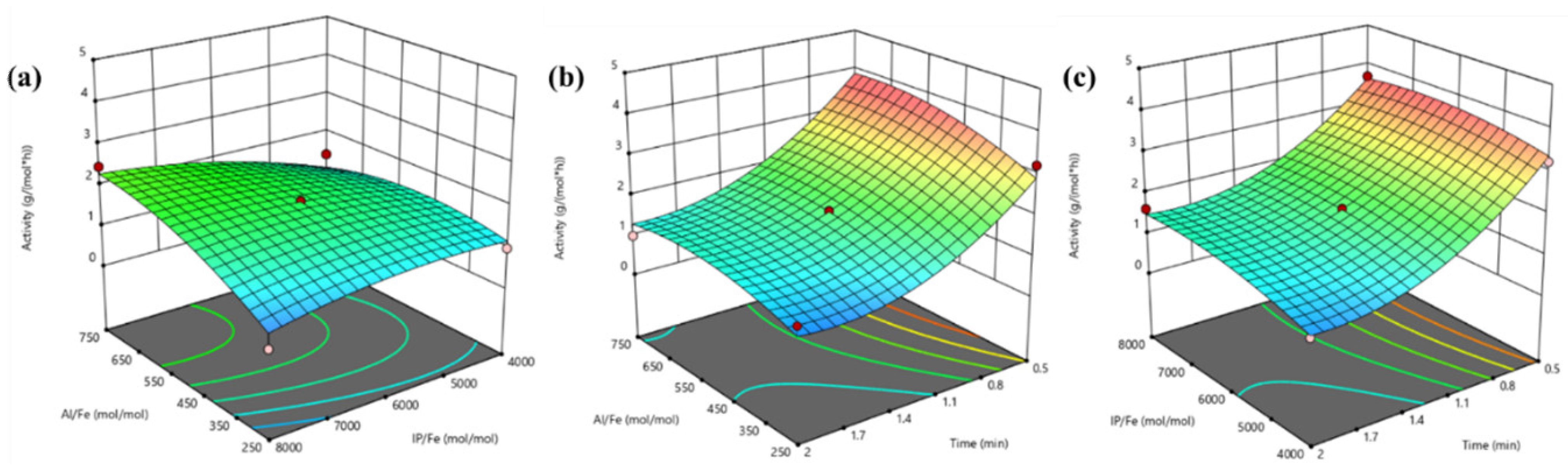
3.3. Response Surface Methodology (RSM) Design and Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouardad, S.; Bakleh, M.E.; Kostjuk, S.V.; Ganachaud, F.; Puskas, J.E.; Deffieux, A.; Peruch, F. Bio-inspired cationic polymerization of isoprene and analogues: State-of-the-art. Polym. Int. 2012, 61, 149–156. [Google Scholar] [CrossRef]
- Cherian, S.; Ryu, S.B.; Cornish, K. Natural rubber biosynthesis in plants, the rubber transferase complex, and metabolic engineering progress and prospects. Plant. Biotechnol. J. 2019, 17, 2041–2061. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Takahash, S. Molecular mechanisms of natural rubber biosynthesis. Annu. Rev. Biochem. 2020, 89, 821–851. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kang, M.Y.; Han, K.H. Identification of natural rubber and characterization of rubber biosynthetic activity in fig tree. Plant Physiol. 2000, 123, 1133–1142. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, D.; Wang, B.; Liu, B.; Yang, Y. Polymerization of 1,3-conjugated dienes with rare-earth metal precursors. Struct. Bond. 2010, 137, 49–108. [Google Scholar] [CrossRef]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes polymerization: Where we are and what lies ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L.; Pirozzi, B.; Napolitano, R. Synthesis and characterization of syndiotactic 3,4-polyisoprene prepared with diethylbis(2,2′-bipyridine)iron–MAO. Polymer 2004, 45, 2871–2875. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Hou, Z. Unprecedented isospecific 3,4-polymerization of isoprene by cationic rare earth metal alkyl species resulting from a binuclear precursor. J. Am. Chem. Soc. 2005, 127, 14562–14563. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Sun, G.; Liu, J.; Wang, M.; Li, S.; Cui, D. 3,4-Polymerization of isoprene by using NSN- and NPN-ligated rare earth metal precursors: Switching of stereo selectivity and mechanism. Macromolecules 2014, 47, 4971–4978. [Google Scholar] [CrossRef]
- Geng, J.; Sun, Y.; Hua, J. 1,2- and 3,4-Rich Polyisoprene Synthesized by Mo(VI)-based catalyst with phosphorus ligand. Polym. Sci. Ser. B 2016, 58, 495–502. [Google Scholar] [CrossRef]
- Gurina, G.A.; Kissel, A.A.; Lyubov, D.M.; Luconi, L.; Rossin, A.; Tuci, G.; Cherkasov, A.V.; Lyssenko, K.A.; Shavyrin, A.S.; Ob’edkov, A.M.; et al. Bis(alkyl) scandium and yttrium complexes coordinated by an amidopyridinate ligand: Synthesis, characterization and catalytic performance in isoprene polymerization, hydroelementation and carbon dioxide hydrosilylation. Dalton Trans. 2020, 49, 638–650. [Google Scholar] [CrossRef]
- Ren, W.; Liu, H.; You, F.; Mao, P.; So, Y.M.; Kang, X.; Shi, X. Unsymmetrical diarylamido-based rare-earth alkyl complexes: Their synthesis and catalytic performance in isoprene polymerization. Dalton Trans. 2021, 50, 1334–1343. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, H.; Li, W.; Jia, X.; Zhang, X.; Gong, D. Polymerization of isoprene promoted by aminophosphine(ory)-fused bipyridine cobalt complexes: Precise control of molecular weight and cis-1,4-alt-3,4 sequence. Inorg. Chem. 2018, 57, 4088–4097. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Gan, Q.; Ying, W.; Hu, Z.; Tang, F.; Luo, W.; Luo, Y.; Jian, Z.; Gong, D. Synthesis and properties investigation of hydroxyl functionalized polyisoprene prepared by cobalt catalyzed co-polymerization of isoprene and hydroxylmyrcene. Polym. Chem. 2020, 11, 2034–2043. [Google Scholar] [CrossRef]
- Buonerba, A.; Fienga, M.; Milione, S.; Cuomo, C.; Grassi, A.; Proto, A.; Capacchione, C. Binary copolymerization of p-methylstyrene with butadiene and isoprene catalyzed by titanium compounds showing different stereoselectivity. Macromolecules 2013, 46, 8449–8457. [Google Scholar] [CrossRef]
- Peng, W.; Xie, J.M.; Zhang, J.Y.; Yang, X.; He, A.H. Isoprene polymerizations catalyzed by TiCl4/MgCl2 type Ziegler-Natta catalysts with different titanium contents. Mol. Catal. 2020, 494, 111110. [Google Scholar] [CrossRef]
- Nakayama, Y.; Baba, Y.; Yasuda, H.; Kawakita, K.; Ueyama, N. Stereospecific polymerization of conjugated dienes by single site iron complexes having chelating N,N,N-donor ligands. Macromolecules 2003, 36, 7953–7958. [Google Scholar] [CrossRef]
- Guo, L.; Jing, X.; Xiong, S.; Liu, W.; Liu, Y.; Liu, Z.; Chen, C. Influences of alkyl and aryl substituents on iminopyridine Fe(II)- and Co(II)-catalyzed isoprene polymerization. Polymers 2016, 8, 389. [Google Scholar] [CrossRef]
- Ricci, G.; Morganti, D.; Sommazzi, A.; Santi, R.; Masi, F. Polymerization of 1,3-dienes with iron complexes based catalysts: Influence of the ligand on catalyst activity and stereospecificity. J. Mol. Catal. A Chem. 2003, 204–205, 287–293. [Google Scholar] [CrossRef]
- Gong, D.; Jia, X.; Wang, B.; Wang, F.; Zhang, C.; Zhang, X.; Jiang, L.; Dong, W. Highly trans-1,4 selective polymerization of 1,3-butadiene initiated by iron(III) bis(imino)pyridyl complexes. Inorg. Chim. Acta 2011, 373, 47–53. [Google Scholar] [CrossRef]
- Gong, D.; Jia, X.; Wang, B.; Zhang, X.; Jiang, L. Synthesis, characterization, and butadiene polymerization of iron(III), iron(II) and cobalt(II) chlorides bearing 2,6-bis(2-benzimidazolyl)pyridyl or 2,6-bis(pyrazol)pyridine ligand. J. Organomet. Chem. 2012, 702, 10–18. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Ma, J.; Feng, C.; Wang, S.; Zhao, K.Q.; Sun, W.H.; Redshaw, C.; Solan, G.A. Bi- and tri-dentate imino-based iron and cobalt pre-catalysts for ethylene oligo-/polymerization. Inorg. Chem. Front. 2014, 1, 14–34. [Google Scholar] [CrossRef]
- Raynaud, J.; Wu, J.Y.; Ritter, T. Iron-catalyzed polymerization of isoprene and other 1,3-dienes. Angew. Chem. Int. Ed. 2012, 51, 11805–11808. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, O.H.; Champouret, Y.; Visseaux, M. Highly active iminopyridyl iron-based catalysts for the polymerization of isoprene. Molecules 2019, 24, 3024. [Google Scholar] [CrossRef]
- Zhu, J.B.; Chen, H.; Liao, S.; Li, Y.X.; Tang, Y. A side arm-assisted phosphine for catalytic ylide intramolecular cyclopropanation. Org. Chem. Front. 2014, 1, 1035–1039. [Google Scholar] [CrossRef]
- Liao, S.; Sun, X.L.; Tang, Y. Side arm strategy for catalyst design: Modifying bisoxazolines for remote control of enantioselection and related. Acc. Chem. Res 2014, 47, 2260–2272. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sun, X.L.; Wang, F.; Tang, Y. Sa-BOX/copper catalysts for highly syndio-specific atom transfer radical polymerization of methyl methacrylate. ACS Catal. 2017, 7, 4692–4696. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Zhao, M.; Wang, L.; Jing, C.; Wang, P.; Wang, X.; Wang, Q. Influences of fluorine substituents on iminopyridine Fe(II)- and Co(II)-catalyzed isoprene polymerization. Polymers 2018, 10, 934. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Mahmood, Q.; Jing, C.; Zhu, G.; Zhang, X.; Wang, X.; Wang, Q. Controlled isoprene polymerization mediated by iminopyridine-iron (II) acetylacetonate pre-catalysts. Appl. Organomet. Chem. 2019, 33, e4836. [Google Scholar] [CrossRef]
- Jing, C.; Wang, L.; Mahmood, Q.; Zhao, M.; Zhu, G.; Zhang, X.; Wang, X.; Wang, Q. Synthesis and characterization of aminopyridine iron(II) chloride catalysts for isoprene polymerization: Sterically controlled monomer enchainment. Dalton Trans. 2019, 48, 7862–7874. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Hou, H.; Zhu, G.; Han, Z.; Yang, W.; Chen, X.; Wang, Q. An unsymmetrical binuclear iminopyridine-iron complex and its catalytic isoprene polymerization. Chem. Commun. 2020, 56, 8846–8849. [Google Scholar] [CrossRef]
- Jing, C.; Wang, L.; Zhu, G.; Hou, H.; Zhou, L.; Wang, Q. Enhancing thermal stability in aminopyridine iron(II)-catalyzed polymerization of conjugated dienes. Organometallics 2020, 39, 4019–4026. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, L.; Mahmood, Q.; Zhou, L.; Wang, Q. Ligand-regulated polymerization of conjugated dienes catalyzed by confined iminopyridine iron complexes with high activity and thermal stability. Polym. Test. 2021, 102, 107317. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, G.; Mahmood, Q.; Zhao, M.; Wang, L.; Jing, C.; Wang, X.; Wang, Q. Iminoimidazole-based Co(II) and Fe(II) complexes: Syntheses, characterization, and catalytic behaviors for isoprene polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 767–775. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, Y.; Zhang, X.; Wang, L.; Zhu, G.; Wang, Q. Synthesis, characterization and catalytic property studies for isoprene polymerization of iron complexes bearing unionized pyridine-oxime ligands. Polymers 2022, 14, 3612. [Google Scholar] [CrossRef]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, A.; Pettersen, J.; Bergman, R. Experimental design and optimization. Chemom. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–838. [Google Scholar] [CrossRef]
- Srewaradachpisal, S.; Dechwayukul, C.; Chatpun, S.; Spontak, R.J.; Thongruang, W. Optimization of the rubber formulation for footwear applications from the response surface method. Polymers 2020, 12, 2032. [Google Scholar] [CrossRef]
- Shokri, A.A.; Talebi, S.; Salami-Kalajahi, M. Polymerization of 1,3-butadiene using neodymium versatate: Optimization of NdV3/TEAL/EASC molar ratios via response surface methodology (RSM). Polym. Bull. 2020, 77, 5245–5260. [Google Scholar] [CrossRef]
- Shokri, A.A.; Talebi, S.; Salami-Kalajahi, M. Optimization of 1,3-butadiene monomer coordination polymerization using response surface methodology (RSM). Polyolefins J. 2021, 8, 63–72. [Google Scholar] [CrossRef]
- Le, Y.; Guan, Y.; Ma, X.; Zhang, W. Preparation and boron removal performance of glycidol modified PANI nanorods: An optimization study based on response surface methodology. Polymers 2023, 15, 459. [Google Scholar] [CrossRef] [PubMed]

| Entry a | Cat. | Activity b | Yield (%) | Microstructure % c | Mn d | Mw/Mn d | |
|---|---|---|---|---|---|---|---|
| 1,4 | 3,4 | ||||||
| 1 | Fe 1 | 8.0 | 97 | 40 | 60 | 38.0 | 2.9 |
| 2 | Fe 2 | 8.2 | >99 | 43 | 57 | 37.4 | 2.1 |
| 3 | Fe 3 | 7.9 | 96 | 38 | 62 | 42.7 | 2.6 |
| 4 | Fe 4 | 8.1 | 99 | 43 | 57 | 33.2 | 3.1 |
| Entry a | Time (min) | Al:Fe:Ip | Activity b | Yield (%) | Microstructure % c | Mn d | Mw/Mn d | |
|---|---|---|---|---|---|---|---|---|
| 1,4 | 3,4 | |||||||
| 1 | 10 | 500:1:2000 | 0.8 | >99 | 43 | 57 | 37.4 | 2.1 |
| 2 | 6 | 500:1:2000 | 1.4 | >99 | 44 | 56 | 42.9 | 2.1 |
| 3 | 1 | 500:1:2000 | 8.2 | >99 | 44 | 56 | 44.9 | 2.4 |
| 4 | 1 | 500:1:4000 | 16.4 | >99 | 42 | 58 | 48.3 | 2.0 |
| 5 | 1 | 500:1:6000 | 23.1 | 94 | 44 | 56 | 40.5 | 2.1 |
| 6 | 1 | 500:1:8000 | 22.0 | 67 | 42 | 58 | 23.4 | 2.0 |
| 7 | 1 | 1000:1:6000 | 24.6 | >99 | 43 | 57 | 35.5 | 2.1 |
| 8 | 1 | 750:1:6000 | 23.6 | 96 | 43 | 57 | 25.2 | 2.0 |
| 9 | 1 | 250:1:6000 | 20.9 | 85 | 43 | 57 | 33.4 | 2.1 |
| Independent Variables | Symbols | Levels | ||
|---|---|---|---|---|
| Low Level (−1) | Mean Level (0) | High Level (1) | ||
| Al/Fe | X1 | 250 | 500 | 750 |
| Ip/Fe | X2 | 4000 | 6000 | 8000 |
| t (min) | X3 | 0.5 | 1.25 | 2 |
| Run a | X1 | X2 | X3 | Y |
|---|---|---|---|---|
| 1 | 0 (500) | 0 (6000) | 0 (1.25) | 1.855 |
| 2 | 1 (750) | 0 (6000) | −1 (0.50) | 3.642 |
| 3 | −1 (250) | 0 (6000) | 0 (1.25) | 1.848 |
| 4 | 0 (500) | 0 (6000) | 0 (1.25) | 1.799 |
| 5 | 0 (500) | 0 (6000) | 0 (1.25) | 1.863 |
| 6 | −1 (250) | −1 (4000) | 0 (1.25) | 0.974 |
| 7 | 0 (500) | 1 (8000) | −1 (0.50) | 3.682 |
| 8 | −1 (250) | 0 (6000) | −1 (0.50) | 3.204 |
| 9 | 1 (750) | 1 (8000) | 0 (1.25) | 2.458 |
| 10 | 1 (750) | 0 (6000) | 1 (2.00) | 0.983 |
| 11 | −1 (250) | 1 (8000) | 0 (1.25) | 0.413 |
| 12 | 0 (500) | 1 (8000) | 1 (2.00) | 1.615 |
| 13 | −1 (250) | 0 (6000) | 1 (2.00) | 1.093 |
| 14 | 1 (750) | −1 (4000) | 0 (1.25) | 1.393 |
| 15 | 0 (500) | −1 (4000) | 1 (2.00) | 0.828 |
| 16 | 0 (500) | −1 (4000) | −1 (0.50) | 3.224 |
| 17 | 0 (500) | 0 (6000) | 0 (1.25) | 1.873 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 15.38 | 9 | 1.71 | 18.29 | 0.0005 |
| X1-Al/Fe | 0.975 | 1 | 0.975 | 10.44 | 0.0144 |
| X2-IP/Fe | 0.3815 | 1 | 0.3815 | 4.08 | 0.083 |
| X3-Time | 10.66 | 1 | 10.66 | 114.09 | <0.0001 |
| X1 X2 | 0.6604 | 1 | 0.6604 | 7.07 | 0.0325 |
| X1 X3 | 0.0749 | 1 | 0.0749 | 0.8022 | 0.4002 |
| X2 X3 | 0.027 | 1 | 0.027 | 0.2891 | 0.6074 |
| X12 | 0.4382 | 1 | 0.4382 | 4.69 | 0.067 |
| X22 | 0.1959 | 1 | 0.1959 | 2.1 | 0.1909 |
| X32 | 2.09 | 1 | 2.09 | 22.42 | 0.0021 |
| Residual | 0.6539 | 7 | 0.0934 | ||
| Lack of Fit | 0.6506 | 3 | 0.2169 | 260.35 | <0.0001 |
| Pure Error | 0.0033 | 4 | 0.0008 | ||
| Cor Total | 16.03 | 16 | |||
| R2 | 0.9592 | ||||
| Adjusted R2 | 0.9068 | ||||
| Adeq Precision | 13.2168 |
| Entry a | Cat. | Activity b |
|---|---|---|
| 1 | Fe 2 | 4.0929 |
| 2 | Fe 2 | 3.9703 |
| 3 | Fe 2 | 4.2034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Zhang, Y.; Wang, L.; Zhu, G.; Kuang, J.; Zhu, G.; Xu, G.; Wang, Q. 3,4-Enhanced Polymerization of Isoprene Catalyzed by Side-Arm Tridentate Iminopyridine Iron Complex with High Activity: Optimization via Response Surface Methodology. Polymers 2023, 15, 1231. https://doi.org/10.3390/polym15051231
Han Z, Zhang Y, Wang L, Zhu G, Kuang J, Zhu G, Xu G, Wang Q. 3,4-Enhanced Polymerization of Isoprene Catalyzed by Side-Arm Tridentate Iminopyridine Iron Complex with High Activity: Optimization via Response Surface Methodology. Polymers. 2023; 15(5):1231. https://doi.org/10.3390/polym15051231
Chicago/Turabian StyleHan, Zhenyu, Yongqiang Zhang, Liang Wang, Guangqian Zhu, Jia Kuang, Guangyu Zhu, Guangqiang Xu, and Qinggang Wang. 2023. "3,4-Enhanced Polymerization of Isoprene Catalyzed by Side-Arm Tridentate Iminopyridine Iron Complex with High Activity: Optimization via Response Surface Methodology" Polymers 15, no. 5: 1231. https://doi.org/10.3390/polym15051231
APA StyleHan, Z., Zhang, Y., Wang, L., Zhu, G., Kuang, J., Zhu, G., Xu, G., & Wang, Q. (2023). 3,4-Enhanced Polymerization of Isoprene Catalyzed by Side-Arm Tridentate Iminopyridine Iron Complex with High Activity: Optimization via Response Surface Methodology. Polymers, 15(5), 1231. https://doi.org/10.3390/polym15051231






