Physicochemical Characterization and Antioxidant Properties of Chitosan and Sodium Alginate Based Films Incorporated with Ficus Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Procurement
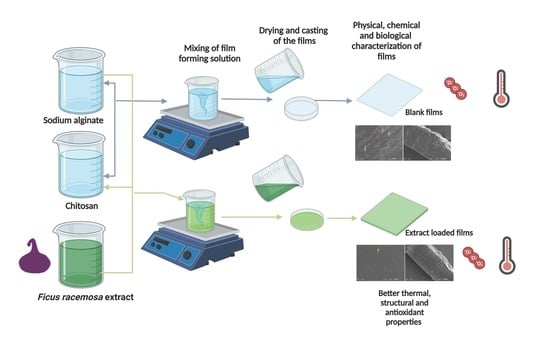
2.2. Film Preparation
2.3. Thickness
2.4. Mechanical Properties of Edible Films
2.5. Assessment of Water Solubility
2.6. Moisture Content
2.7. Determination of the Water Vapor Permeability
2.8. Transparency
2.9. Colour
2.10. Thermogravimetric Analysis
2.11. X-ray Diffraction
2.12. FTIR Analysis
2.13. SEM Analysis
2.14. Antioxidant Activity
2.15. Statistical Analysis
3. Results and Discussion
3.1. Thickness
3.2. Mechanical Properties
3.3. Moisture Content and Water Solubility
3.4. Water Vapor Permeability
3.5. Transparency
3.6. Colour Analysis
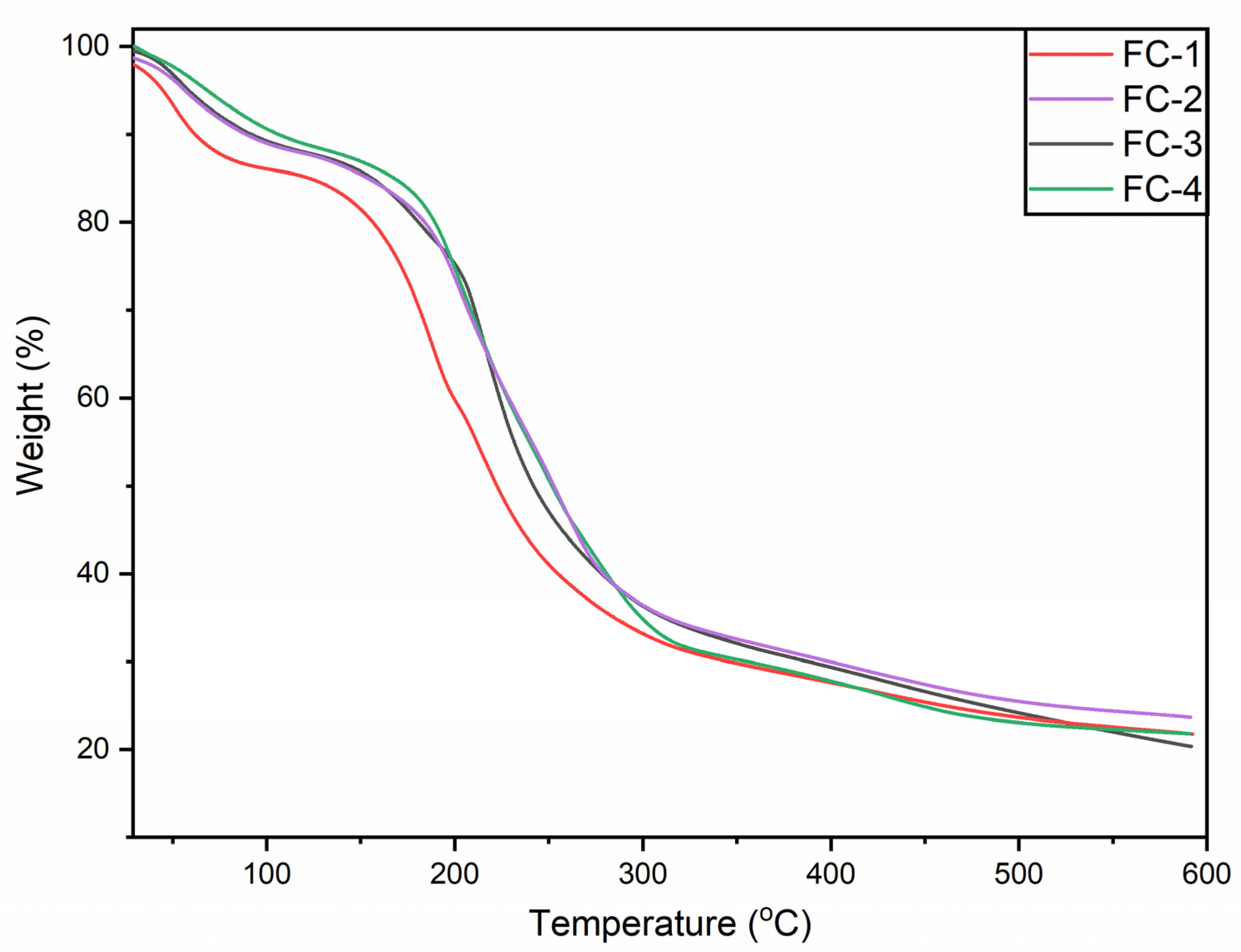
3.7. TGA
3.8. XRD
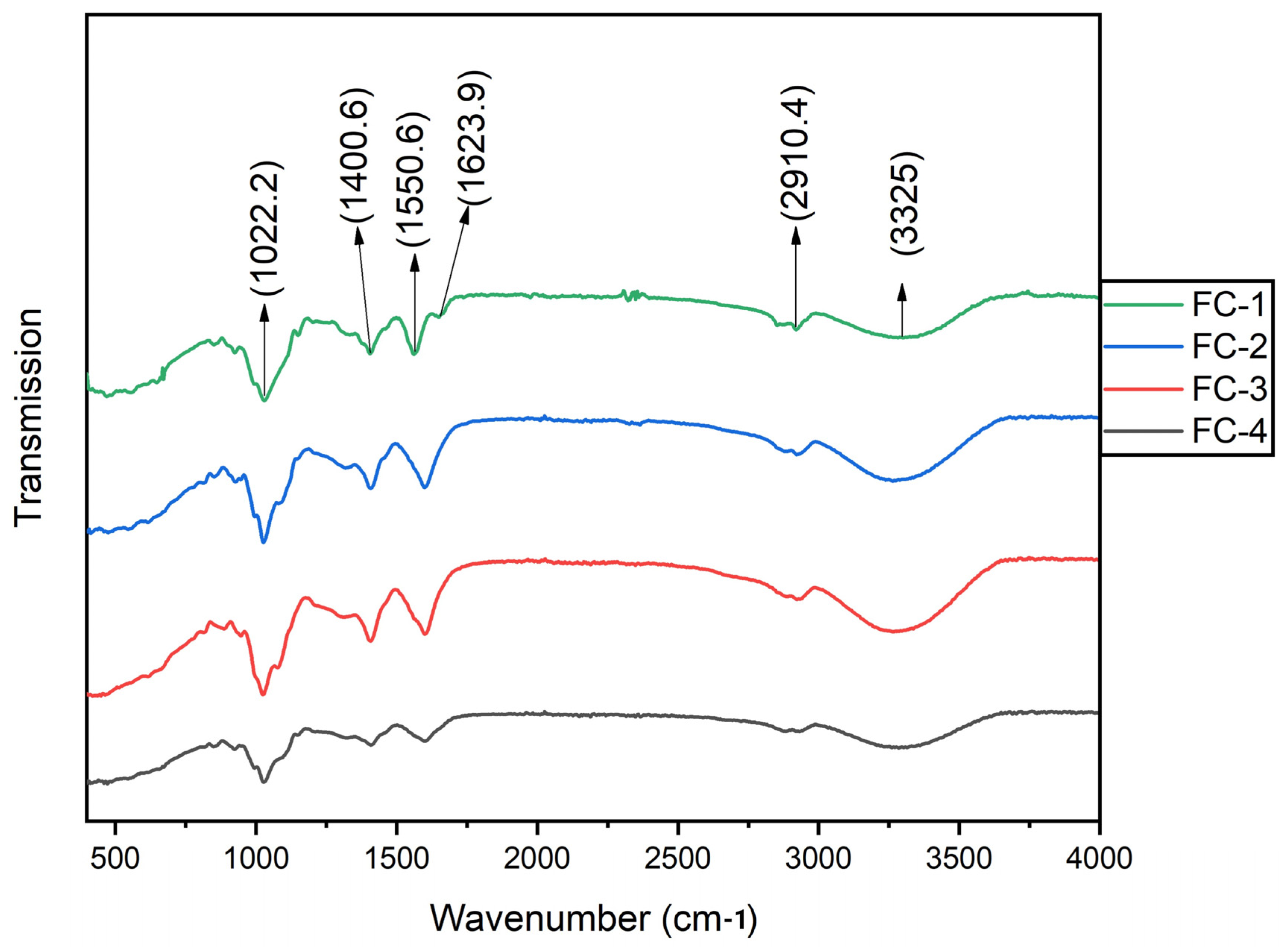
3.9. FTIR Analysis
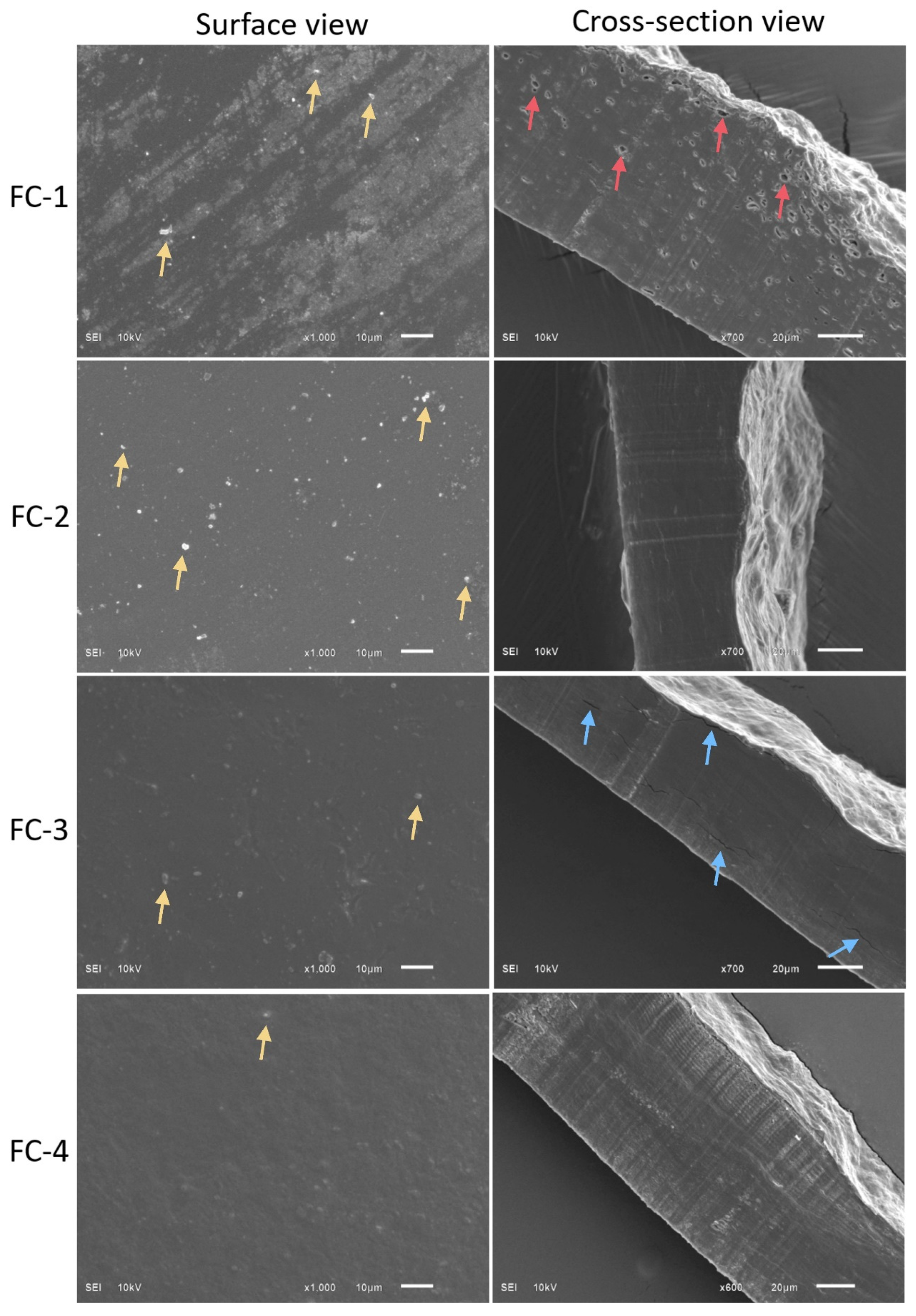
3.10. Scanning Electron Microscopy
3.11. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physico-Chemical, Mechanical Properties and Its Application in Food Preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Al-Harrasi, A.; Bhtaia, S.; Al-Azri, M.S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Mohan, S.; Sharma, A.; Behl, T. Development and characterization of chitosan and porphyran based composite edible films containing ginger essential oil. Polymers 2022, 14, 1782. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chu, Y.; Feng, X.; Gao, C.; Wu, D.; Cheng, W.; Meng, L.; Zhang, Y.; Tang, X. Effects of zein stabilized clove essential oil Pickering emulsion on the structure and properties of chitosan-based edible films. Int. J. Biol. Macromol. 2020, 156, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Khwaldia, K.; Basta, A.H.; Aloui, H.; El-Saied, H. Chitosan–caseinate bilayer coatings for paper packaging materials. Carbohydr. Polym. 2014, 99, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Khouri, J.; Penlidis, A.; Moresoli, C. Viscoelastic properties of crosslinked chitosan films. Processes 2019, 7, 157. [Google Scholar] [CrossRef]
- Aguila-Almanza, E.; Salgado-Delgado, R.; Vargas-Galarza, Z.; García-Hernández, E.; Hernández-Cocoletzi, H. Enzymatic depolimerization of chitosan for the preparation of functional membranes. J. Chem. 2019, 2019, 5416297. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Gheorghita, R.; Gutt, G.; Amariei, S. The use of edible films based on sodium alginate in meat product packaging: An eco-friendly alternative to conventional plastic materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Sun, R.; Zhu, J.; Wu, H.; Wang, S.; Li, W.; Sun, Q. Modulating layer-by-layer assembled sodium alginate-chitosan film properties through incorporation of cellulose nanocrystals with different surface charge densities. Int. J. Biol. Macromol. 2021, 180, 510–522. [Google Scholar] [CrossRef]
- Joseph, B.; Raj, S.J. Phytopharmacological properties of Ficus racemosa Linn-An overview. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 134–138. [Google Scholar]
- Jahan, I.A.; Nahar, N.; Mosihuzzaman, M.; Rokeya, B.; Ali, L.; Khan, A.A.; Makhmur, T.; Choudhary, M.I. Hypoglycaemic and antioxidant activities of Ficus racemose Linn. fruits. Nat. Prod. Res. 2009, 23, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Khan, N.U.; Singhal, K.C. Potential antifilarial activity of fruit extracts of Ficus racemosa Linn. against Setaria cervi in vitro. Experiment 2005, 43, 346–350. [Google Scholar]
- Rao, C.V.; Verma, A.R.; Vijayakumar, M.; Rastogi, S. Gastroprotective effect of standardized extract of Ficus glomerata fruit on experimental gastric ulcers in rats. J. Ethnopharmacol. 2008, 115, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, T.A.; Yadav, B.V.; Badole, S.L.; Bodhankar, S.L.; Dhaneshwar, S.R. Antihyperglycaemic activity of petroleum ether extract of Ficus racemosa fruits in alloxan induced diabetic mice. Pharmacologyonline 2007, 2, 504–515. [Google Scholar]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.B. Antimicrobial activity of buckwheat starch films containing zinc oxide nanoparticles against Listeria monocytogenes on mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Erdem, B.G.; Dıblan, S.; Kaya, S. Development and structural assessment of whey protein isolate/sunflower seed oil biocomposite film. Food Bioprod. Process. 2019, 118, 270–280. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Al-Azri, M.S.; Ullah, S.; Bekhit, A.E.-D.A.; Pratap-Singh, A.; Chatli, M.K.; Anwer, M.K.; Aldawsari, M.F. Preparation and physiochemical characterization of bitter Orange oil loaded sodium alginate and casein based edible films. Polymers 2022, 14, 3855. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Trajkovska Petkoska, A.; Khojah, E.; Sami, R.; Al-Mushhin, A.A. Chitosan edible films enhanced with pomegranate peel extract: Study on physical, biological, thermal, and barrier properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef] [PubMed]
- Nemazifard, M.; Kavoosi, G.; Marzban, Z.; Ezedi, N. Physical, mechanical, water binding, and antioxidant properties of cellulose dispersions and cellulose film incorporated with pomegranate seed extract. Int. J. Food Prop. 2017, 20, 1501–1514. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Kola, V. Plant Extracts as Additives in Biodegradable Films and Coatings in Active Food Packaging: Effects and Applications; Universidade do Algarve: Faro, Portugal, 2020. [Google Scholar]
- Augusto, A.; Dias, J.R.; Campos, M.J.; Alves, N.M.; Pedrosa, R.; Silva, S.F. Influence of Codium tomentosum extract in the properties of alginate and chitosan edible films. Foods 2018, 7, 53. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Zhang, Z.-H.; Li, L.; Yuan, M.-L.; Fan, J.; Zhao, T.-R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef]
- Dou, B.; Dupont, V.; Williams, P.T.; Chen, H.; Ding, Y. Thermogravimetric kinetics of crude glycerol. Bioresour. Technol. 2009, 100, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.Z.; Li, S.D.; Ou, C.Y.; Li, C.P.; Yang, L.; Zhang, C.H. Thermogravimetric analysis of chitosan. J. Appl. Polym. Sci. 2007, 105, 547–551. [Google Scholar] [CrossRef]
- Caykara, T.; Demirci, S. Preparation and characterization of blend films of poly (vinyl alcohol) and sodium alginate. J. Macromol. Sci. Part A 2006, 43, 1113–1121. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Chen, H. Characterization and functional properties of a pectin/tara gum based edible film with ellagitannins from the unripe fruits of Rubus chingii Hu. Food Chem. 2020, 325, 126964. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Rocha, C.M.; Avides, M.C.; Quintas, M.A.; Vicente, A.A. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J. Food Eng. 2011, 106, 111–118. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Z.; Duan, L.; Gao, G. A tough, stretchable, and extensively sticky hydrogel driven by milk protein. Polym. Chem. 2018, 9, 2617–2624. [Google Scholar] [CrossRef]
- Devi, N.; Paul, M. Gc-Ms And Ft-Ir Analysis Of Methanol Fruit Extract of Ficus Racemosa And Ficus Auriculata. Int. J. Pharm. Sci. Res. 2020, 40, 1679–1684. [Google Scholar]
- De Moura, M.R.; Aouada, F.A.; Avena-Bustillos, R.J.; McHugh, T.H.; Krochta, J.M.; Mattoso, L.H. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J. Food Eng. 2009, 92, 448–453. [Google Scholar] [CrossRef]
- Rai, S.K.; Chaturvedi, K.; Yadav, S.K. Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019, 91, 127–135. [Google Scholar]
- Yadav, R.K.; Nandy, B.C.; Maity, S.; Sarkar, S.; Saha, S. Phytochemistry, pharmacology, toxicology, and clinical trial of Ficus racemosa. Pharmacogn. Rev. 2015, 9, 73. [Google Scholar] [PubMed]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef] [PubMed]
- Zulfiker, A.H.M.; Saha, M.R.; Sarwar, S.; Nahar, L.; Hamid, K.; Rana, M.S. Hypoglycemic and in vitro antioxidant activity of ethanolic extracts of Ficus racemosa Linn. fruits. Am. J. Sci. Ind. Res. 2011, 2, 391–400. [Google Scholar] [CrossRef]
- Hasan, N.; Mamun, F.; Mamun, M.A.; Belal, H.; Rokon Ul, K.M.; Nishu, A.; Islam, K.; Tasnin, N.; Rahman, M.S.; Amin, Z.; et al. Phytochemical Investigation and Evaluation of In Vitro Antioxidant and Anti-Inflammatory Activity of Ficus Recemosa Fruit Extracts Using Different Solvents. Br. J. Med. Health Res. 2016, 3, 70–85. [Google Scholar]


| Sample Codes | The Composition of the Film-Forming Components |
|---|---|
| FC-1 | CS (1%) + SA (3%) + Gly (5%) |
| FC-2 | CS (1%) + SA (3%) + Gly (5%) + FC (0.5%) |
| FC-3 | CS (1%) + SA (3%) + Gly (5%) + FC (1%) |
| FC-4 | CS (1%) + SA (3%) + Gly (5%) + FC (1.5%) |
| Sample Codes | TS (Mpa) | EAB (%) | Film Thickness (mm) | Moisture Content (%) | WVP (×10−12 g·cm/cm2·s·Pa) |
|---|---|---|---|---|---|
| FC-1 | 2.136 ± 0.150 a | 16.212 ± 0.373 a | 0.040 ± 0.007 a | 24.16 ± 1.07 ab | 0.356 ± 0.006 a |
| FC-2 | 1.388 ± 0.109 b | 17.541 ± 0.104 b | 0.040 ± 0.010 ab | 26.66 ± 0.75 b | 0.348 ± 0.001 b |
| FC-3 | 1.170 ± 0.046 c | 19.176 ± 0.581 c | 0.054 ± 0.009 b | 30.87 ± 0.46 c | 0.337 ± 0.009 c |
| FC-4 | 1.046 ± 0.019 d | 21.475 ± 0.659 d | 0.053 ± 0.005 b | 34.82 ± 0.97 d | 0.333 ± 0.005 d |
| Sample | L | a* | b* | ΔE | Transparency % |
|---|---|---|---|---|---|
| FC-1 | 96.43 ± 0.15 a | −0.15 ± 0.02 a | 1.74 ± 0.07 a | 0.94 ± 0.010 a | 80.45 ± 0.65 a |
| FC-2 | 95.63 ± 0.22 a | −0.15 ± 0.02 a | 2.58 ± 0.22 b | 1.74 ± 0.17 b | 75.25 ± 0.12 b |
| FC-3 | 95.10 ± 0.11 a | −0.09 ± 0.00 b | 2.77 ± 0.25 b | 2.09 ± 0.27 c | 67.69 ± 0.33 c |
| FC-4 | 94.94 ± 0.09 a | −0.04 ± 0.01 c | 3.06 ± 0.07 c | 2.42 ± 0.09 d | 46.42 ± 2.04 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. Physicochemical Characterization and Antioxidant Properties of Chitosan and Sodium Alginate Based Films Incorporated with Ficus Extract. Polymers 2023, 15, 1215. https://doi.org/10.3390/polym15051215
Bhatia S, Al-Harrasi A, Shah YA, Jawad M, Al-Azri MS, Ullah S, Anwer MK, Aldawsari MF, Koca E, Aydemir LY. Physicochemical Characterization and Antioxidant Properties of Chitosan and Sodium Alginate Based Films Incorporated with Ficus Extract. Polymers. 2023; 15(5):1215. https://doi.org/10.3390/polym15051215
Chicago/Turabian StyleBhatia, Saurabh, Ahmed Al-Harrasi, Yasir Abbas Shah, Muhammad Jawad, Mohammed Said Al-Azri, Sana Ullah, Md Khalid Anwer, Mohammed F. Aldawsari, Esra Koca, and Levent Yurdaer Aydemir. 2023. "Physicochemical Characterization and Antioxidant Properties of Chitosan and Sodium Alginate Based Films Incorporated with Ficus Extract" Polymers 15, no. 5: 1215. https://doi.org/10.3390/polym15051215
APA StyleBhatia, S., Al-Harrasi, A., Shah, Y. A., Jawad, M., Al-Azri, M. S., Ullah, S., Anwer, M. K., Aldawsari, M. F., Koca, E., & Aydemir, L. Y. (2023). Physicochemical Characterization and Antioxidant Properties of Chitosan and Sodium Alginate Based Films Incorporated with Ficus Extract. Polymers, 15(5), 1215. https://doi.org/10.3390/polym15051215