Ion-Imprinted Polymer Structurally Preorganized Using a Phenanthroline-Divinylbenzoate Complex with the Cu(II) Ion as Template and Some Adsorption Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
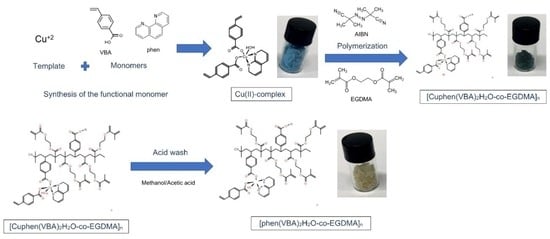
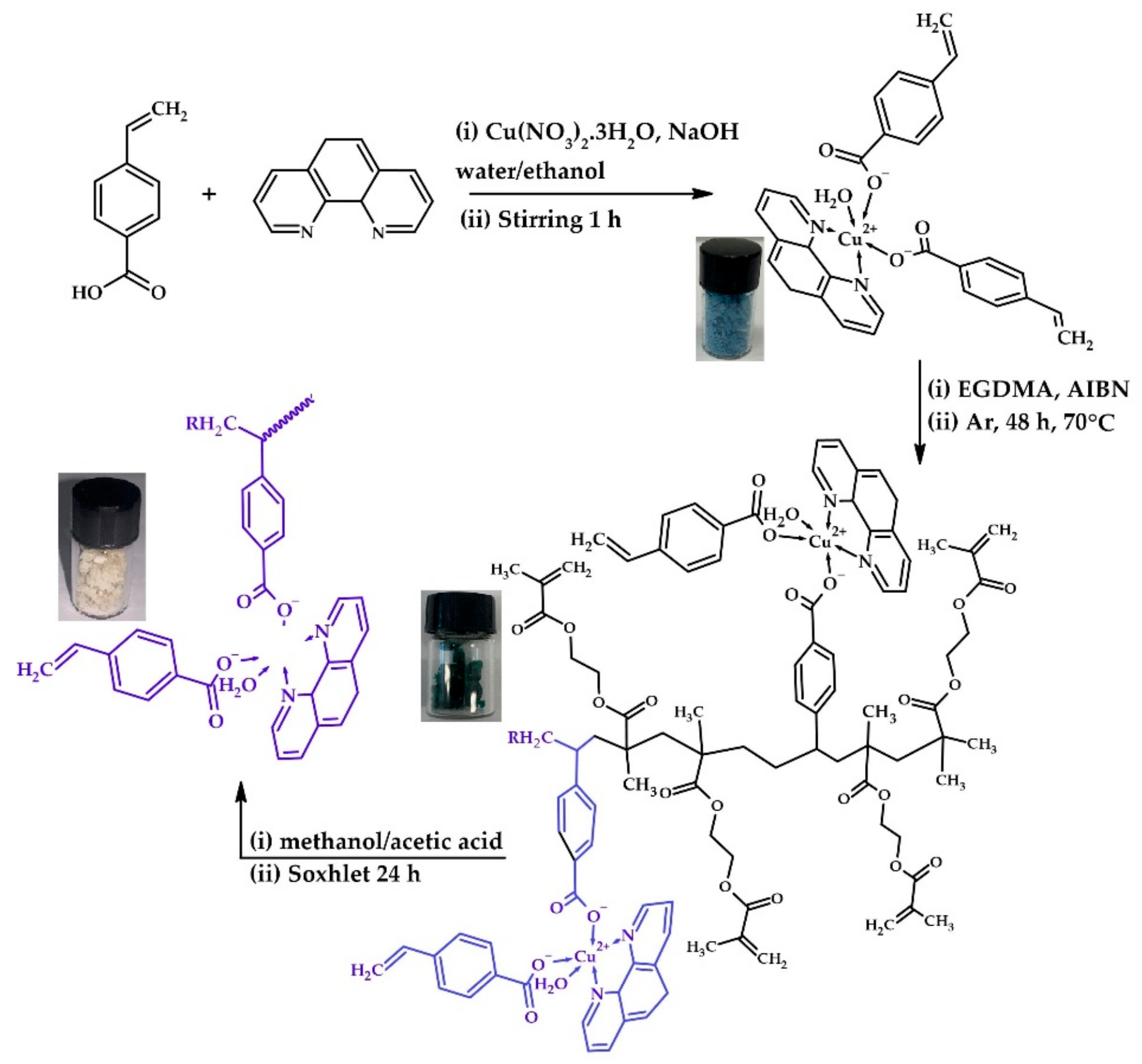
2.3. Synthesis of [Cuphen(VBA)2H2O] Functional Monomer
2.4. Preparation of [Cuphen(VBA)2H2O-co-EGDMA]n (MIP), the NIIP, and [Phen (VBA)2H2O-co-EGDMA]n (IIP)
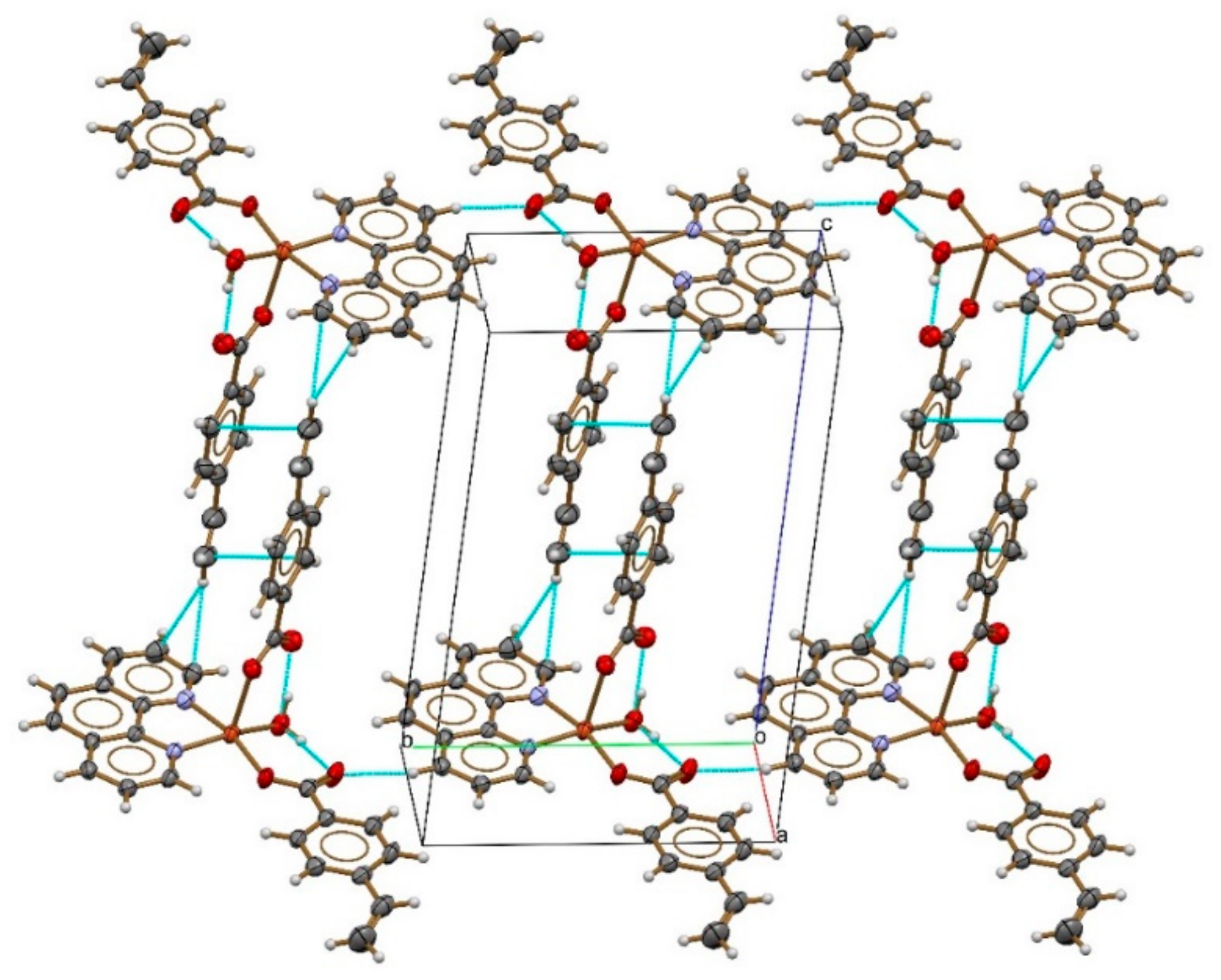
2.5. Crystallography of the Functional Monomer, [Cuphen(VBA)2H2O]
2.6. Surface Area
2.7. Adsorption Capacity and Adsorption Isotherm
2.8. Selective Recognition
3. Results and Discussion
3.1. Crystal Structure of the Functional Monomer
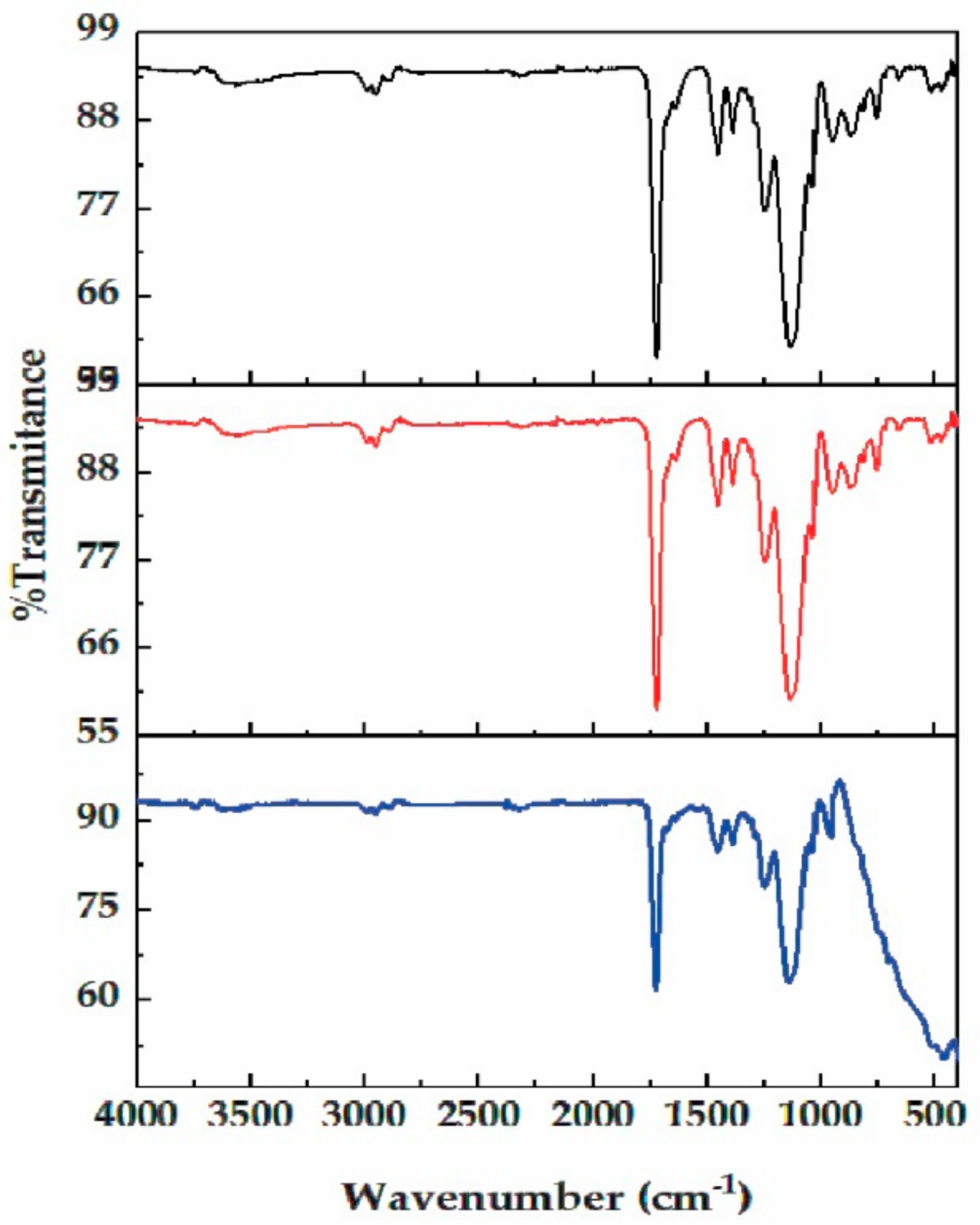
3.2. Synthesis and Characterization
3.3. Surface Area Properties
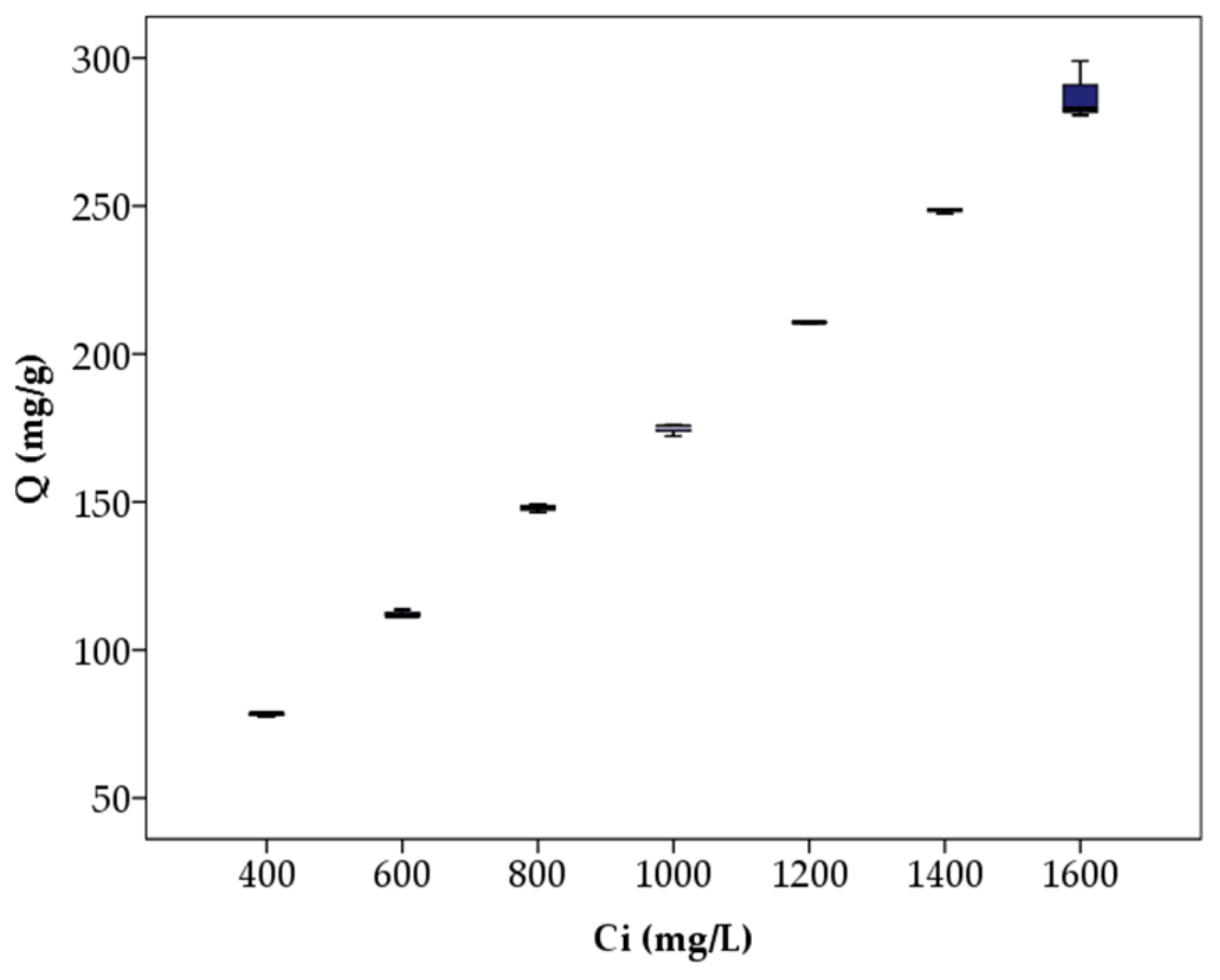
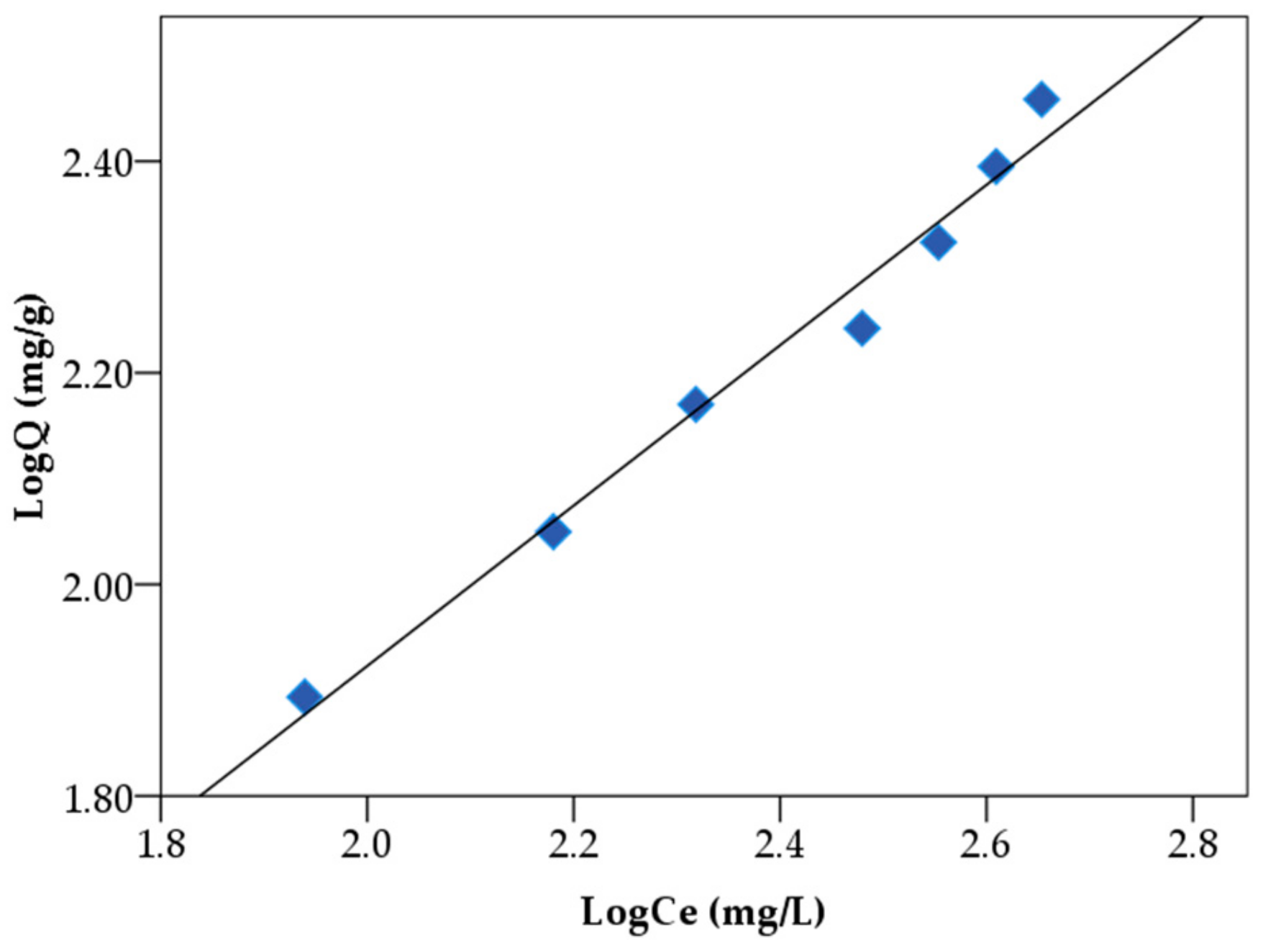
3.4. Static Adsorption Capacity and Adsorption Isotherm
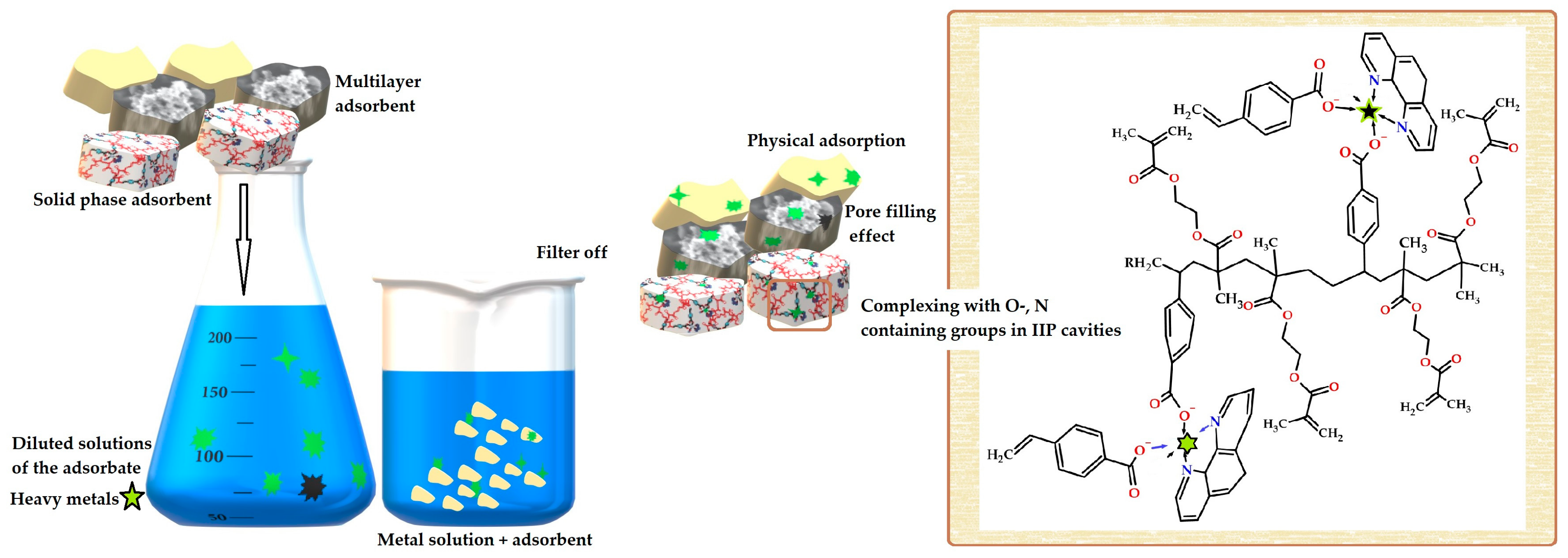
3.5. Selective Recognition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, D.A.E.A.; Slima, D.F.; Al-Yasi, H.M.; Hassan, L.M.; Galal, T.M. Risk assessment of trace metals in Solanum lycopersicum L. (tomato) grown under wastewater irrigation conditions. Environ. Sci. Pollut. Res. 2023, 1–12. [Google Scholar] [CrossRef]
- Cohuo, S.; Moreno-López, A.; Escamilla-Tut, N.Y.; Pérez-Tapia, A.M.; Santos-Itzá, I.; Macario-González, L.A.; Villegas-Sánchez, C.A.; Medina-Quej, A. Assessment of Water Quality and Heavy Metal Environmental Risk on the Peri-Urban Karst Tropical Lake La Sabana, Yucatán Peninsula. Water 2023, 15, 390. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Renu, K.; Gopalakrishnan, A.V.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; Dey, A.; Vellingiri, B.; George, A.; Madhyastha, H.; et al. Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection. Sustainability 2023, 15, 1195. [Google Scholar] [CrossRef]
- Amoo, T.E.; Amoo, K.O.; Adeeyo, O.A.; Ogidi, C.O. Kinetics and Equilibrium Studies of the Adsorption of Copper(II) ions from Indsutrial Wastwater using activated carbons derived from Sugarcane Bagasse. Int. J. Chem. Eng. 2022, 2022, 1–24. [Google Scholar] [CrossRef]
- Haque, J.U.; Siddique, M.A.B.; Islam, M.S.; Ali, M.M.; Tokatli, C.; Islam, A.; Pal, S.C.; Idris, A.M.; Malafaia, G.; Islam, A.R.M.T. Effects of COVID-19 era on a subtropical river basin in Bangladesh: Heavy metal(loid)s distribution, sources and probable human health risks. Sci. Total Environ. 2023, 857, 159383. [Google Scholar] [CrossRef]
- Maftouh, A.; El Fatni, O.; El Hajjaji, S.; Jawish, M.W.; Sillanpää, M. Comparative review of different adsorption techniques used in heavy metals removal in water. Biointerface Res. Appl. Chem. 2023, 13, 397. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.C.; Lai, S.H. Current development of geopolymer as alternative adsorbent for heavy metal removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy metals removal from water by efficient adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Özgecan, E.; Yeşeren, S.; Müge, A.; Adil, D. Molecularly Imprinted Polymers for Removal of Metal Ions: An Alternative Treatment Method. Biomimetics 2018, 3, 38. [Google Scholar] [CrossRef]
- Gaurav, S.; Balasubramanian, K. Molecularly Imprinted Polymers for Selective Recognition and Extraction of Heavy Metal Ions and Toxic Dyes. J. Chem. Eng. Data 2020, 65, 396–418. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Edu. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.36.32; Oxford Diffraction Ltd.: Abingdon, UK, 2013.
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Cryst. 1995, A51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Kuras, M.J.; Perz, K.; Kołodziejski, W.L. Synthesis, characterization and application of a novel zinc(II) ion-imprinted polymer. Polym. Bull. 2017, 74, 5029–5048. [Google Scholar] [CrossRef]
- Kaewprasit, C.; Hequet, E.; Abidi, N.; Gourlot, J.P. Application of methylene blue adsorption to cotton fiber specific surface area measurement. Part 1 methodology. J. Cotton Sci. 1998, 2, 164–173. [Google Scholar]
- Ahmadi, E.; Hajifatheali, H.; Valipoor, Z.; Marefat, M. Synthesis, characterization and analytical applications of Ni(II) ion-imprinted polymer prepared by N-(2-hydroxyphenyl)acrylamide. J. Polym. Res. 2021, 28, 181. [Google Scholar] [CrossRef]
- Ye, C.; Pan, Z.; Shen, Y. Facile Conversion of polystyrene waste into an efficient sorbent for water purification. Polymers 2022, 14, 4477. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, N.H.S.; Mehamod, F.S.; Yusof, N.F.; Jani, A.M.M.; Suah, F.B.M.; Ariffin, M.M.; Zulkefli, N.A. Preparation and adsorption studies of molecularly imprinted polymer for selective recognition of tryptophan. Malays. J. Anal. Sci. 2022, 26, 215–228. [Google Scholar]
- Bivián-Castro, E.Y.; López, M.G.; Pedraza-Reyes, M.; Bernes, S.; Mendoza-Díaz, G. Synthesis, Bioinorg. Chem. Appl. 2009, 2009, 1–8. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2_-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Nobuhiro, Y.; Hidehiro, U.; Yuji, O. 4-Vinylbenzoic acid and 9-vinylanthracene. Acta Cryst. 2000, C56, 1364–1366. [Google Scholar]
- Androvic, L.; Bartácek, J.; Sedlák, M. Recent advances in the synthesis and applications of azo initiators. Res. Chem. Intermed. 2016, 42, 5133–5145. [Google Scholar] [CrossRef]
- Meléndez-Marmolejo, J.; Díaz de León, L. Polímeros de Impresión Molecular; Universitarios Potosinos: San Luís Potosí, México, 2020; pp. 16–21. [Google Scholar]
- Benedetti, C.; Cazzolaro, A.; Carraro, M.; Graf, R.; Landfester, K.; Gross, S.; Muñoz-Espí, R. Dual Role of Zirconium Oxoclusters in Hybrid Nanoparticles: Cross-Linkers and Catalytic Sites. ACS Appl. Mater. Interfaces 2016, 8, 26275–26284. [Google Scholar] [CrossRef]
- Soledad-Rodríguez, B.E. Caracterización de un polímero de impronta molecular para la ampicilina por NIR. Comparación entre NIR y FT-IR. Rev. Tekhné. 2018, 21, 15–27. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; J. Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 232–233. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, I. Surface properties of activated carbon prepared from wastes. Surf. Interface Anal. 2008, 40, 612–615. [Google Scholar] [CrossRef]
- Sulyman, E.Z.; Sedeeq, S.H.; Saad-Aldeen, R.A.; Hammadi, E.Q. Study the specifications of activated carbon prepared from avocado seeds using carbonation and chemical activation. Int. J. Appl. Sci. Technol. 2022, 69–79. [Google Scholar] [CrossRef]
- Sing, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef] [PubMed]
- Sabela, M.I.; Kunene, K.; Kanchi, S.; Xhakaza, N.M.; Bathinapatla, A.; Mdluli, P.; Sharma, D.; Bisetty, K. Removal of copper (II) from wastewater using green vegetable waste derived activated carbon: An approach to equilibrium and kinetic study. Arab. J. Chem. 2019, 12, 4331–4339. [Google Scholar] [CrossRef]


| Empirical Formula | C30H24CuN2O5 |
|---|---|
| Formula weight | 556.05 |
| Temperature | 130(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Triclinic |
| Space group | P-1 |
| Unit cell dimensions | a = 8.0188(4) Å |
| b = 10.7541(10) Å | |
| c = 15.7861(14) Å | |
| α = 101.821(7)° | |
| β = 94.259(6)° | |
| γ = 109.056(7)° | |
| Volume | 1244.68(18) Å3 |
| Z | 2 |
| Density (calculated) | 1.484 g/cm3 |
| Absorption coefficient | 0.923 mm−1 |
| F(000) | 574 |
| Crystal size | 0.540 × 0.380 × 0.240 mm3 |
| Theta range for data collection | 3.751 to 26.055° |
| Index ranges | −9 <= h <= 9, −13 <= k <= 10, −19 <= l <= 19 |
| Reflections collected | 8643 |
| Independent reflections | 4908 [R(int) = 0.0293] |
| Completeness to theta = 25.242° | 99.7% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 4908/5/350 |
| Goodness-of-fit on F2 | 1.056 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0412, wR2 = 0.0950 |
| R indices (all data) | R1 = 0.0528, wR2 = 0.1029 |
| Largest diff. peak and hole | 0.483 and −0.556 e.Å−3 |
| Bond Lengths (Å) | Bond Angles (°) | ||
|---|---|---|---|
| C(1)—N(1) | 1.323(3) | O(3)—Cu(1)—O(1W) | 94.99(8) |
| C(1)—C(2) | 1.402(4) | O(3)—Cu(1)—N(2) | 89.33(8) |
| C(13)—O(1) | 1.258(3) | O(1W)—Cu(1)—N(2) | 165.47(8) |
| C(13)—O(2) | 1.266(3) | O(3)—Cu(1)—N(1) | 166.89(8) |
| C(29)—C(30) | 1.301(4) | O(1W)—Cu(1)—N(1) | 91.43(8) |
| Cu(1)—O(3) | 1.9493(18) | N(2)—Cu(1)—N(1) | 81.77(8) |
| Cu(1)—O(1W) | 1.9823(19) | O(3)—Cu(1)—O(1) | 99.29(7) |
| Cu(1)—N(2) | 2.007(2) | O(1W)—Cu(1)—O(1) | 91.17(7) |
| Cu(1)—N(1) | 2.026(2) | N(2)—Cu(1)—O(1) | 101.83(7) |
| Cu(1)—O(1) | 2.2622(17) | N(1)—Cu(1)—O(1) | 91.97(7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bivián-Castro, E.Y.; Zepeda-Navarro, A.; Guzmán-Mar, J.L.; Flores-Alamo, M.; Mata-Ortega, B. Ion-Imprinted Polymer Structurally Preorganized Using a Phenanthroline-Divinylbenzoate Complex with the Cu(II) Ion as Template and Some Adsorption Results. Polymers 2023, 15, 1186. https://doi.org/10.3390/polym15051186
Bivián-Castro EY, Zepeda-Navarro A, Guzmán-Mar JL, Flores-Alamo M, Mata-Ortega B. Ion-Imprinted Polymer Structurally Preorganized Using a Phenanthroline-Divinylbenzoate Complex with the Cu(II) Ion as Template and Some Adsorption Results. Polymers. 2023; 15(5):1186. https://doi.org/10.3390/polym15051186
Chicago/Turabian StyleBivián-Castro, Egla Yareth, Abraham Zepeda-Navarro, Jorge Luis Guzmán-Mar, Marcos Flores-Alamo, and Brenda Mata-Ortega. 2023. "Ion-Imprinted Polymer Structurally Preorganized Using a Phenanthroline-Divinylbenzoate Complex with the Cu(II) Ion as Template and Some Adsorption Results" Polymers 15, no. 5: 1186. https://doi.org/10.3390/polym15051186
APA StyleBivián-Castro, E. Y., Zepeda-Navarro, A., Guzmán-Mar, J. L., Flores-Alamo, M., & Mata-Ortega, B. (2023). Ion-Imprinted Polymer Structurally Preorganized Using a Phenanthroline-Divinylbenzoate Complex with the Cu(II) Ion as Template and Some Adsorption Results. Polymers, 15(5), 1186. https://doi.org/10.3390/polym15051186