Development of an Fe3O4 Surface-Grafted Carboxymethyl Chitosan Molecularly Imprinted Polymer for Specific Recognition and Sustained Release of Salidroside
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Instruments and Methods
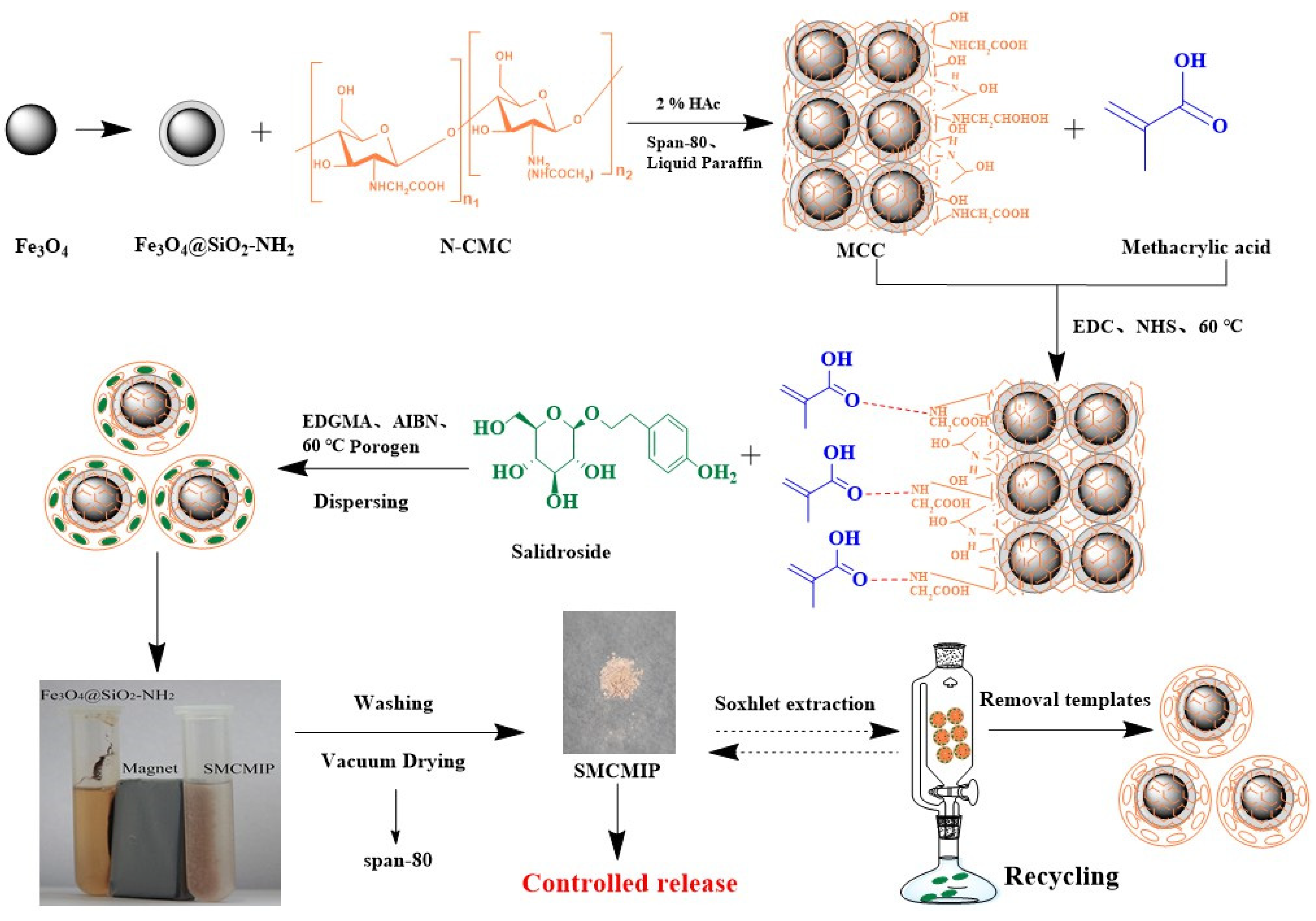
2.3. Preparation of Fe3O4@SiO2-NH2
2.4. Preparation of Fe3O4@SiO2-NH2-CC (MCC)
2.5. Preparation of SMCMIP and SMCNIP
2.6. Adsorption of SMCMIP
2.7. Solubility of SMCMIP
2.8. In Vitro Drug Release
2.9. In Vitro Release Kinetics
2.10. Proliferation Test on Intestinal Epithelial Cells (IPEC-J2)
3. Results and Discussion
3.1. Synthesis of SMCMIP
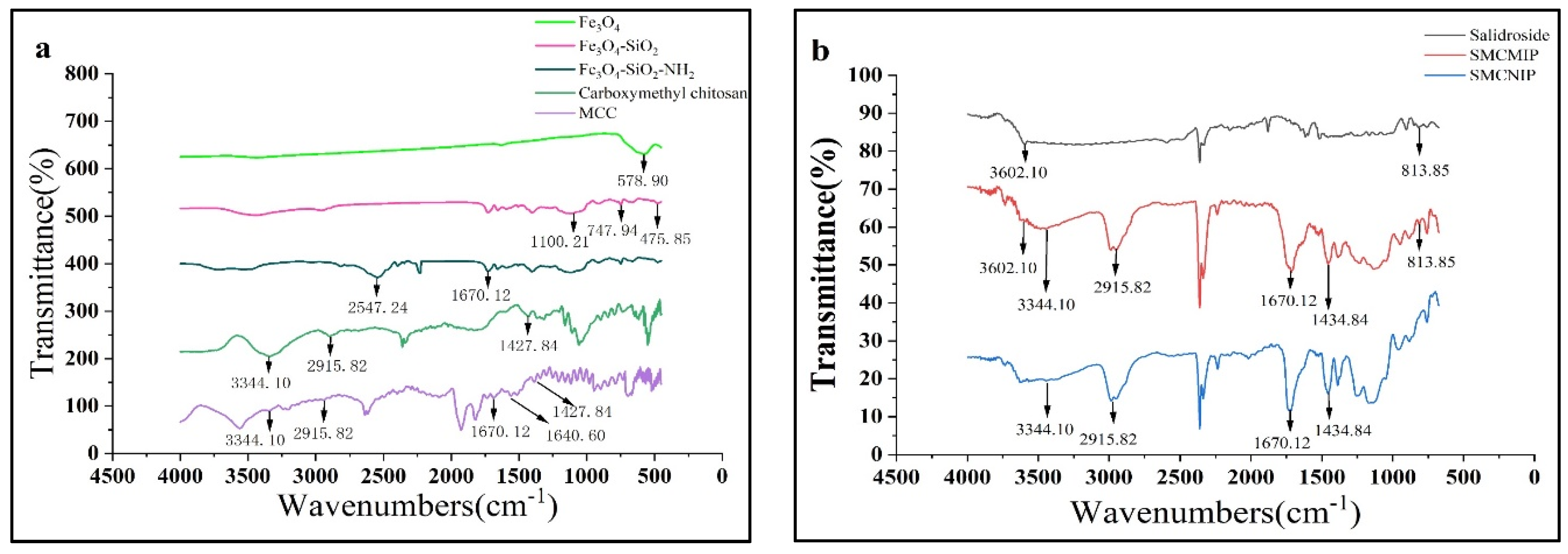
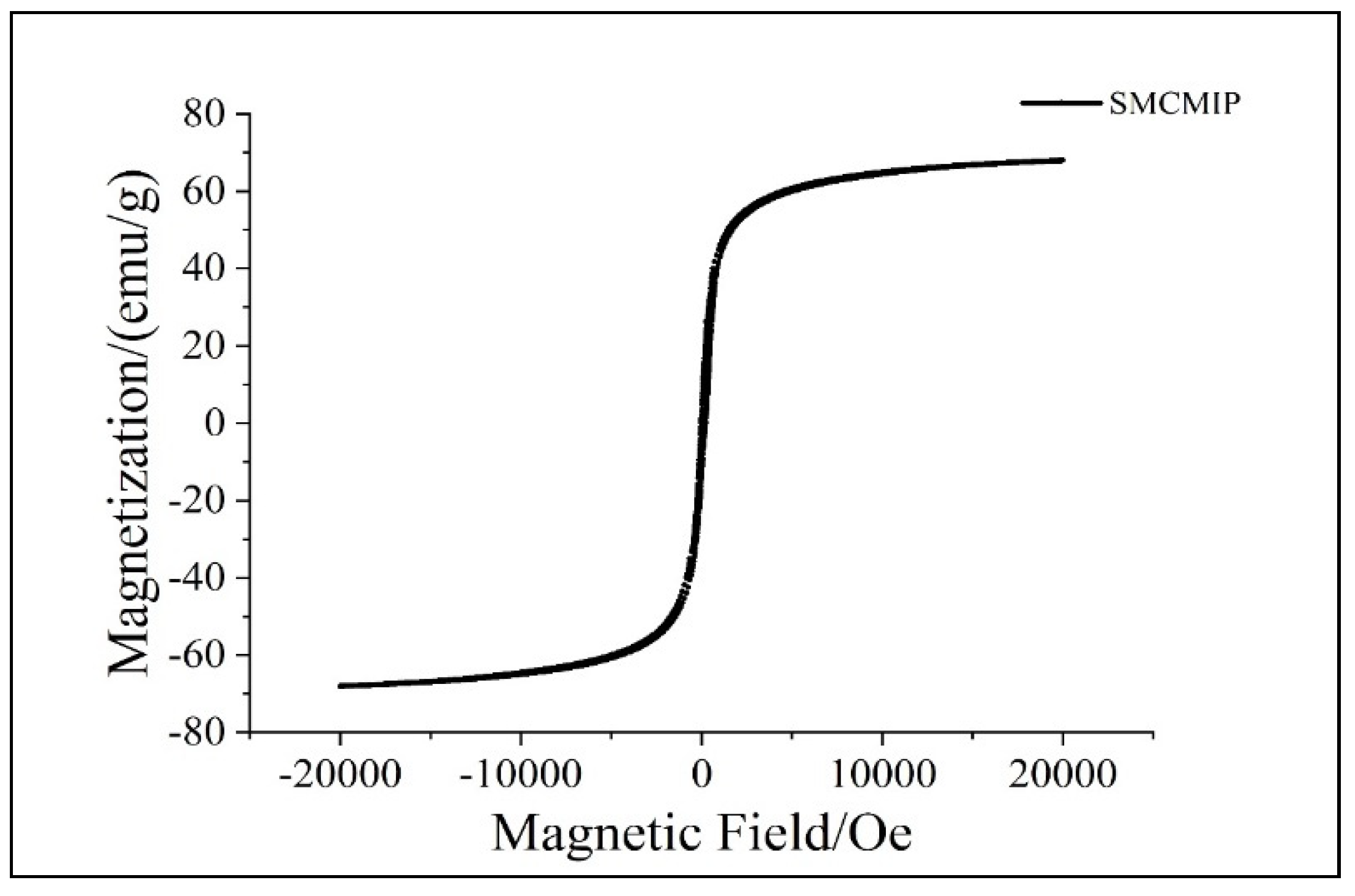
3.2. Characterization of SMCMIP
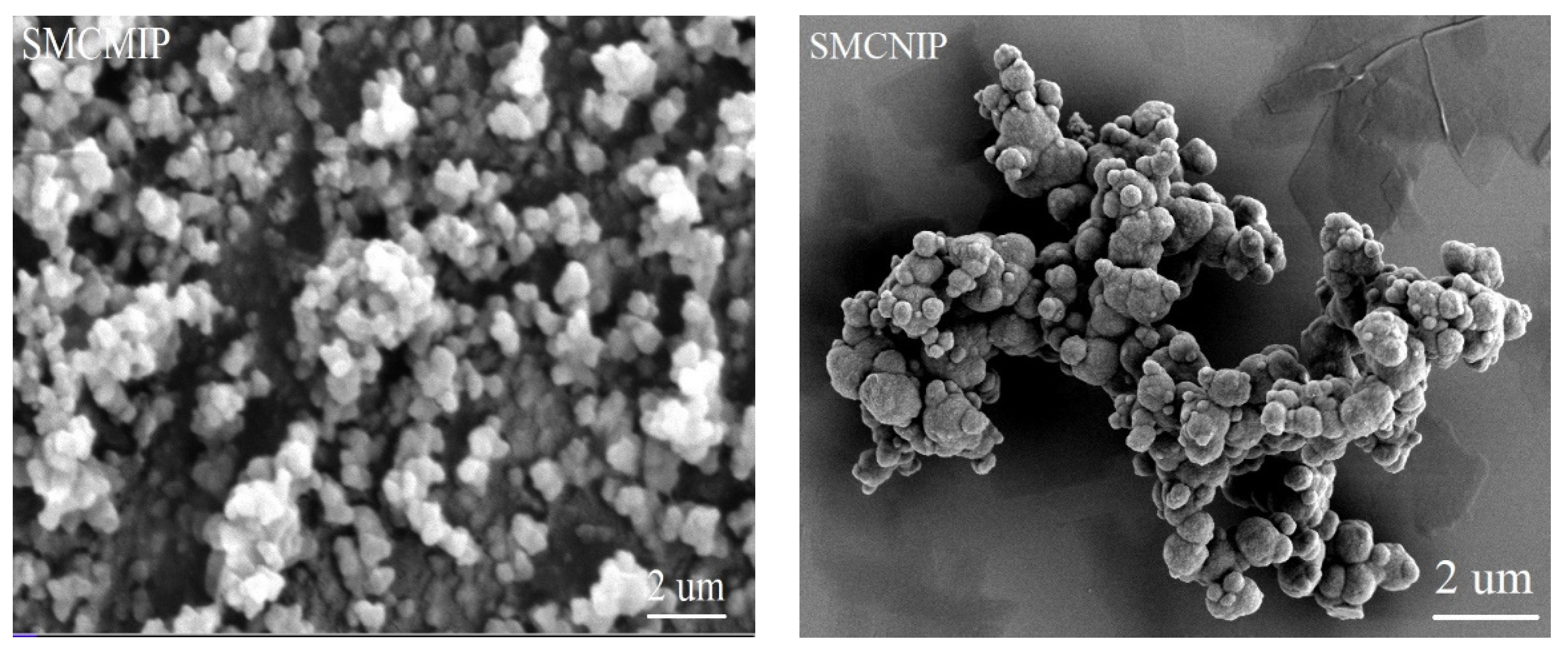
3.3. Morphology of SMCMIP by SEM
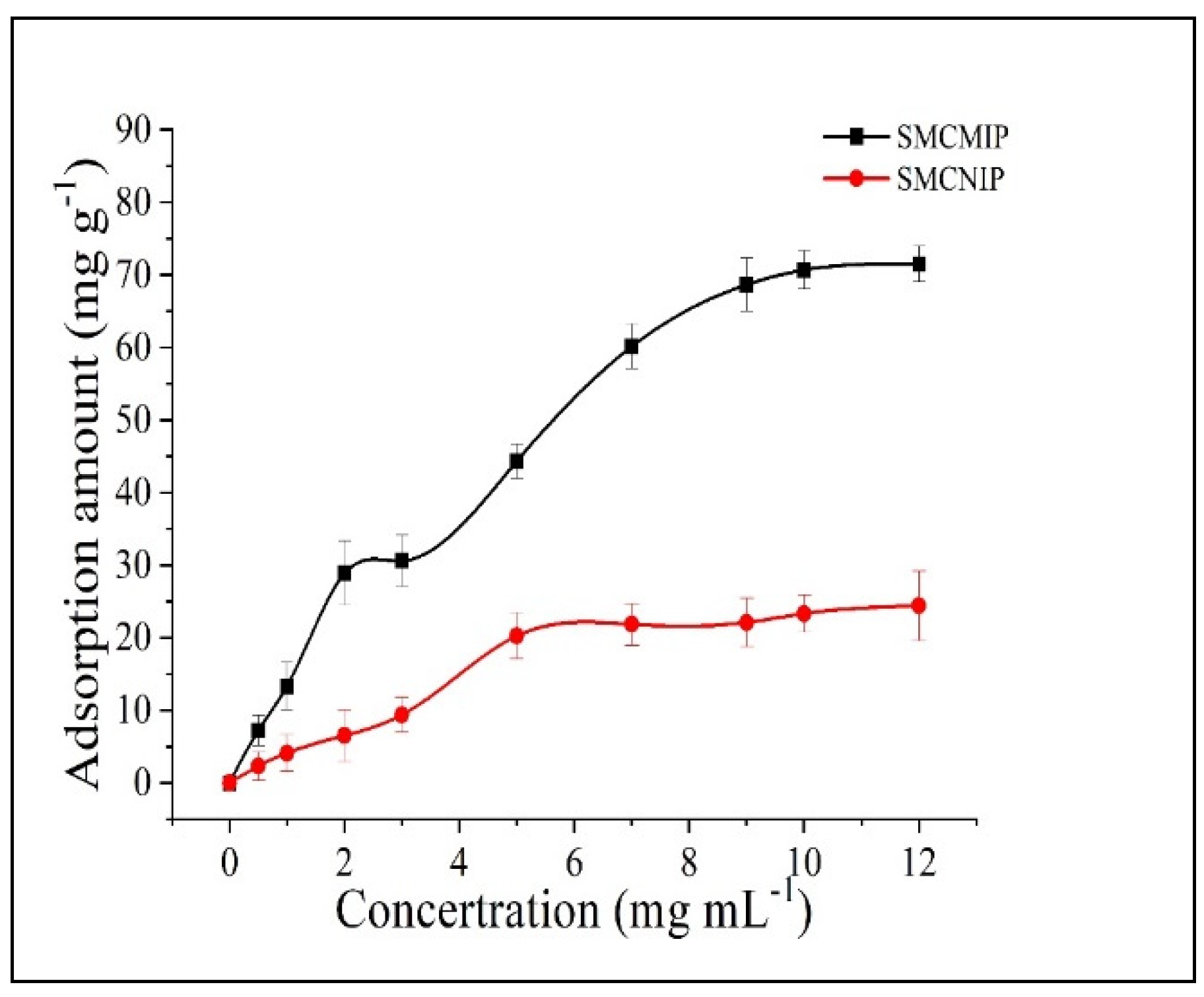
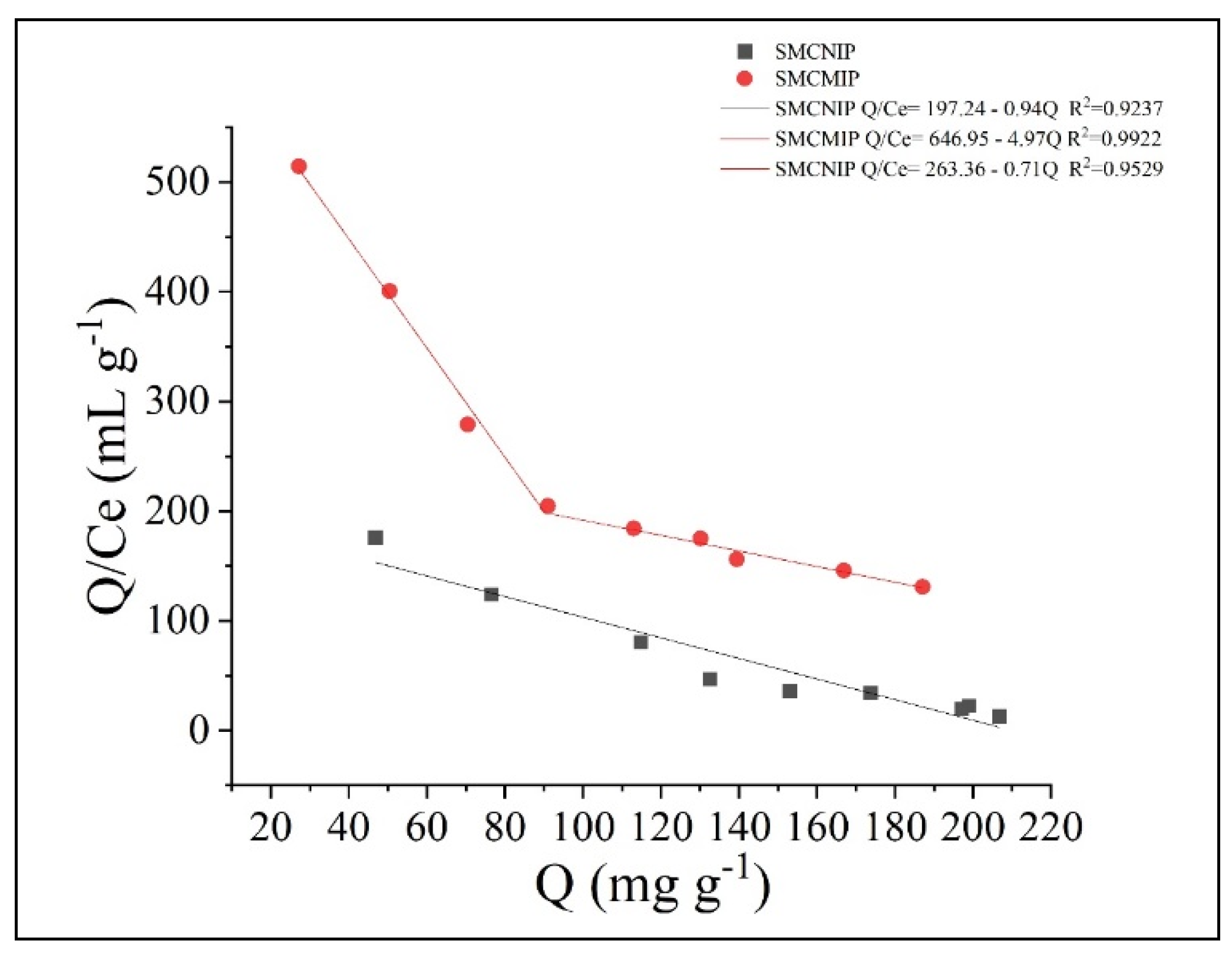
3.4. Adsorption Isotherms of SMCMIP
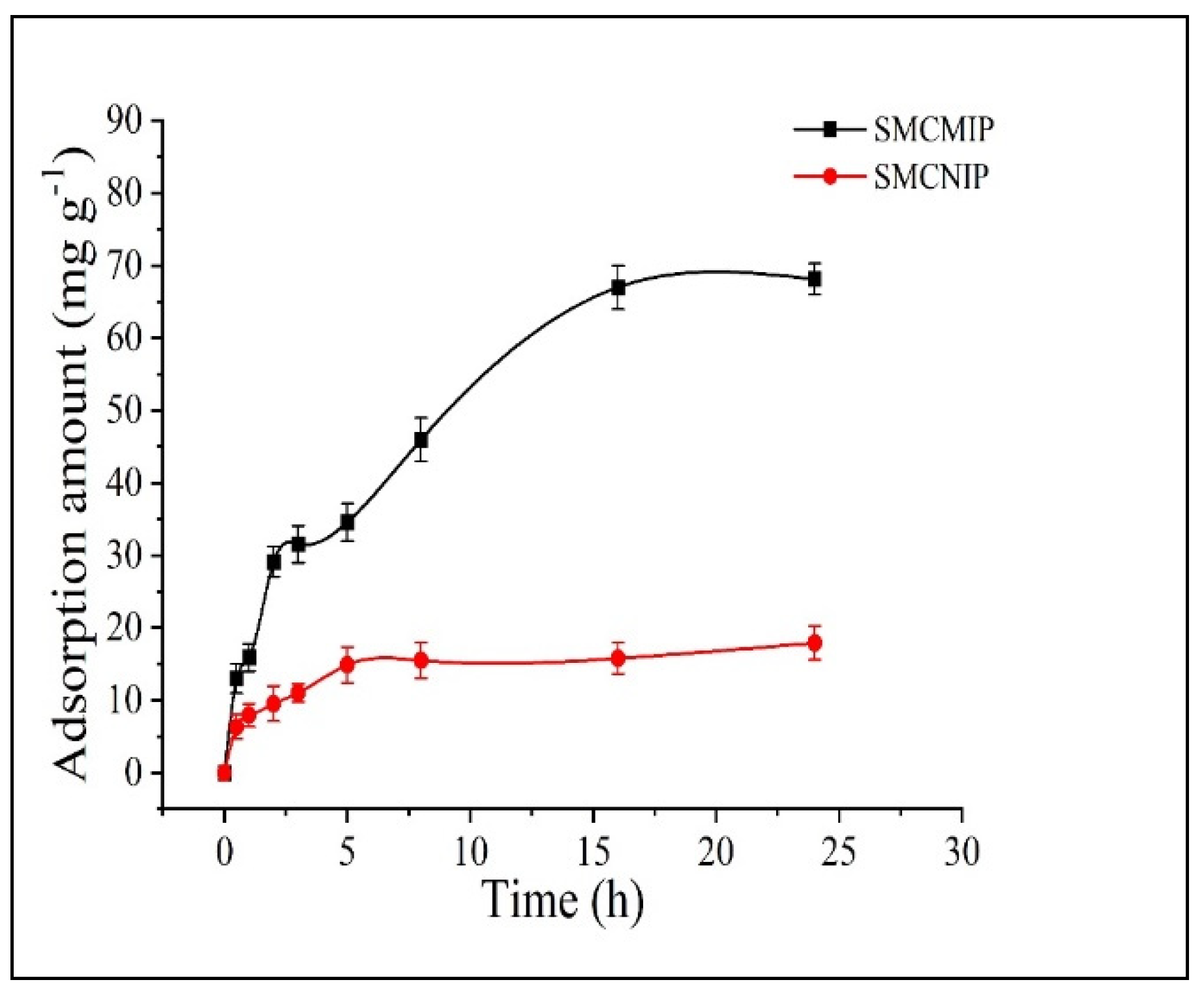
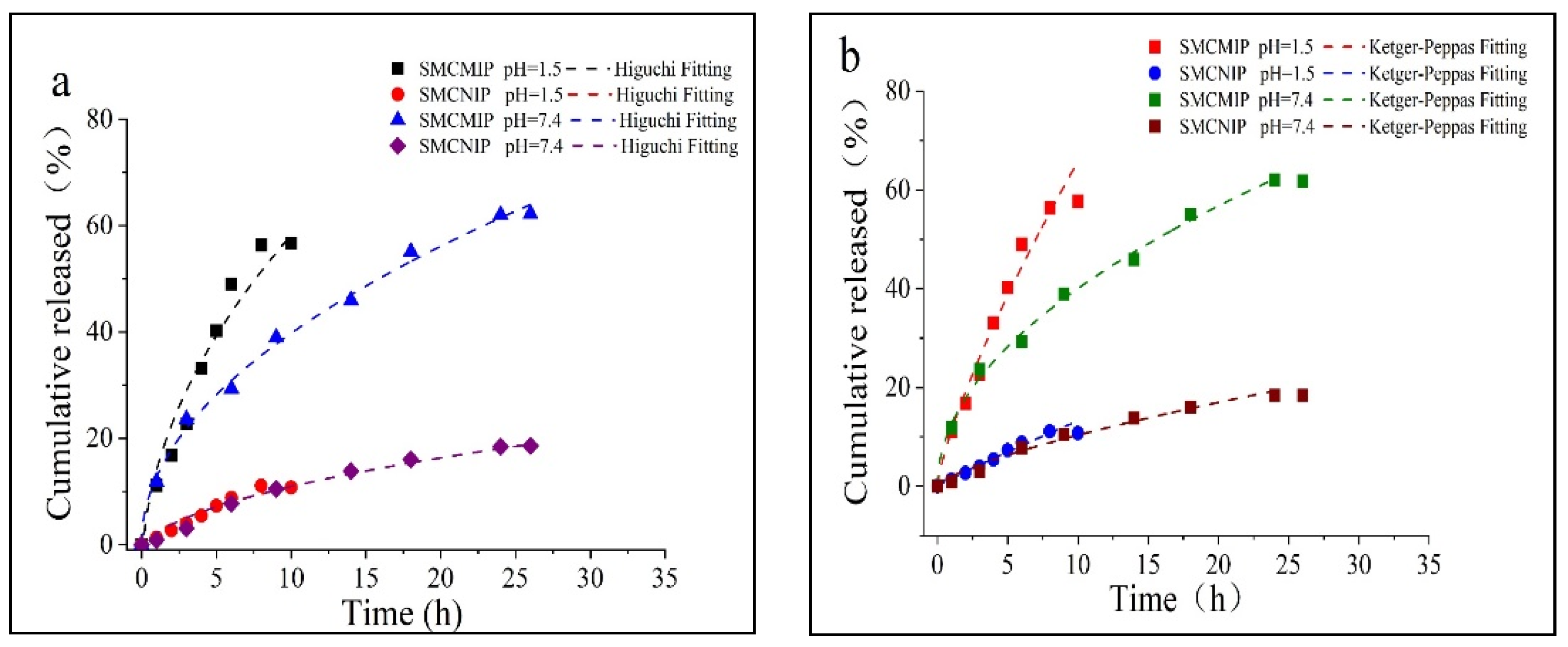
3.5. In Vitro Sustained Release of SMCMIP
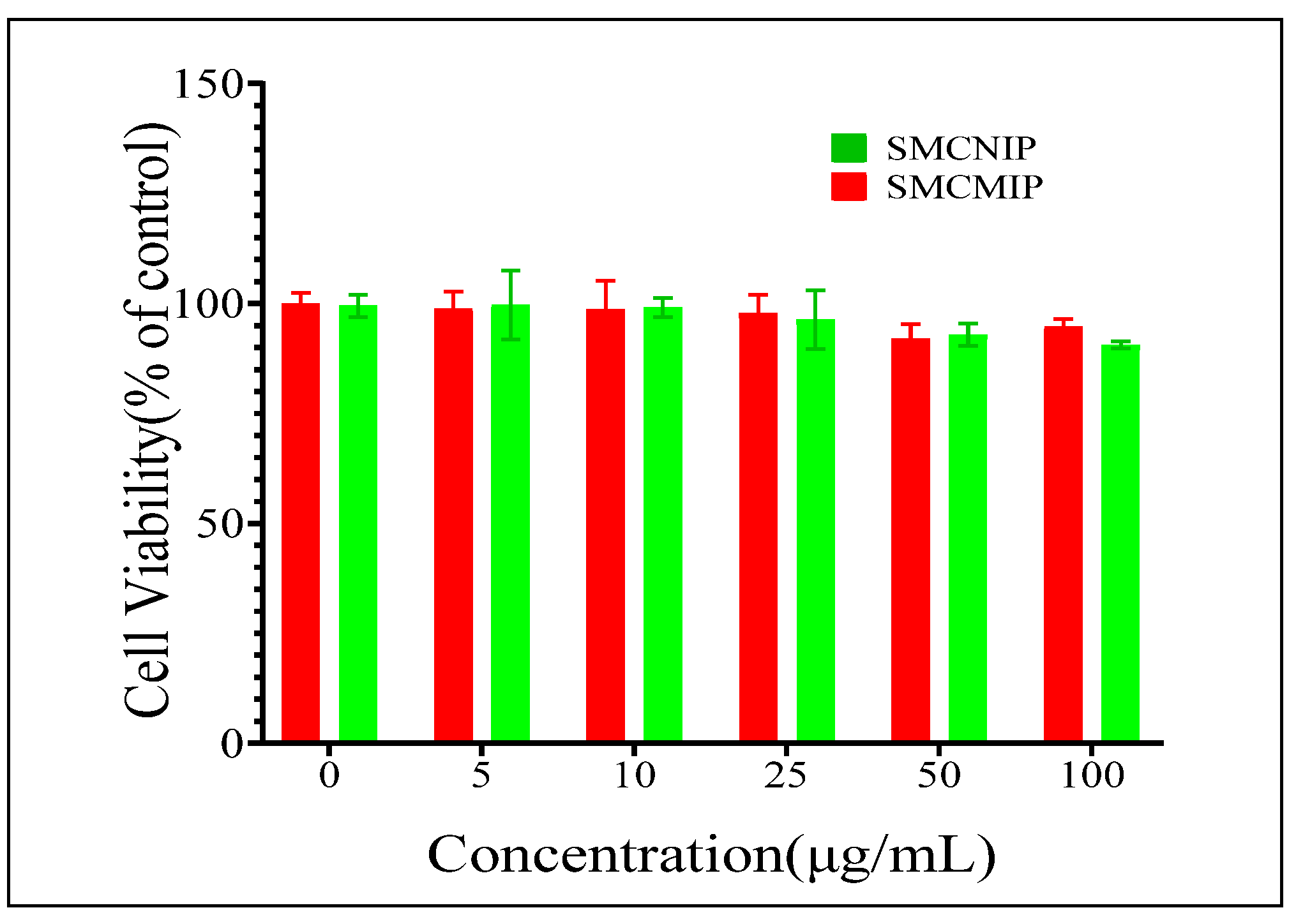
3.6. Determination of the Effect of SMCMIP and SMCNIP on IPEC-J2 Cell Viability by CCK8
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; An, D.; Zhang, R.; Huang, Y.; Liu, Z. Preparation of carbon nanotubes and polyhedral oligomeric-reinforced molecularly imprinted polymer composites for drug delivery of gallic acid. Int. J. Pharm. 2022, 615, 121476. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.I.; Ruffo, M.; Malivindi, R.; Vattimo, A.F.; Pezzi, V.; Puoci, F. Molecularly Imprinted Polymers (MIPs) as Theranostic Systems for Sunitinib Sustained Release and Self-Monitoring in Cancer Therapy. Pharmaceutics 2020, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.S.B.; Akhtar, N.; Minhas, M.U.; Mahmood, A.; Khan, K.U. Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement. Gels 2022, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.H. Study on pharmacokinetics and tissue distribution characteristics of salidroside in mice. China J. Chin. Mater. Med. 2020, 45, 4466–4471. [Google Scholar] [CrossRef]
- Chen, W.; Xu, W.; Wang, A.; Yin, L. Methodology Exploration for Multi-component Chinese Medicine Based on Protein Signal Transduction Network. World Sci. Technol. Mod. Tradit. Chin. Med. 2017, 19, 725–729. [Google Scholar] [CrossRef]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous Determination of 78 Compounds of Rhodiola rosea Extract by Supercritical CO(2)-Extraction and HPLC-ESI-MS/MS Spectrometry. Biochem. Res. Int. 2021, 2021, 9957490. [Google Scholar] [CrossRef]
- Seczyk, L.; Sugier, D.; Dervisoglu, G.; Ozdemir, F.A.; Kolodziej, B. Phytochemical profile, in vitro bioaccessibility, and anticancer potential of golden root (Rhodiola rosea L.) extracts. Food Chem. 2022, 404, 134779. [Google Scholar] [CrossRef]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Webster, T.J. The promising use of nano-molecular imprinted templates for improved SARS-CoV-2 detection, drug delivery and research. J. Nanobiotechnol. 2021, 19, 305. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Yang, N.; Chen, C.; Zhang, M. Chitosan-Based Surface Molecularly Imprinted Polymer Microspheres for Sustained Release of Sinomenine Hydrochloride in Aqueous Media. Appl. Biochem. Biotechnol. 2018, 185, 370–384. [Google Scholar] [CrossRef]
- Jing, T.; Wang, Y.; Dai, Q.; Xia, H.; Niu, J.; Hao, Q.; Mei, S.; Zhou, Y. Preparation of mixed-templates molecularly imprinted polymers and investigation of the recognition ability for tetracycline antibiotics. Biosens. Bioelectron. 2010, 25, 2218–2224. [Google Scholar] [CrossRef]
- Mo, C.E.; Chai, M.H.; Zhang, L.P.; Ran, R.X.; Huang, Y.P.; Liu, Z.S. Floating molecularly imprinted polymers based on liquid crystalline and polyhedral oligomeric silsesquioxanes for capecitabine sustained release. Int. J. Pharm. 2019, 557, 293–303. [Google Scholar] [CrossRef]
- Kioomars, S.; Heidari, S.; Malaekeh-Nikouei, B.; Shayani Rad, M.; Khameneh, B.; Mohajeri, S.A. Ciprofloxacin-imprinted hydrogels for drug sustained release in aqueous media. Pharm. Dev. Technol. 2017, 22, 122–129. [Google Scholar] [CrossRef]
- Parisi, O.I.; Scrivano, L.; Candamano, S.; Ruffo, M.; Vattimo, A.F.; Spanedda, M.V.; Puoci, F. Molecularly Imprinted Microrods via Mesophase Polymerization. Molecules 2017, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.Y.; Lei, X.X.; Hu, J.J.; Jiang, Y.L.; Li, Q.J.; Song, Y.T.; Zhang, Q.Y.; Li-Ling, J.; Xie, H.Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, G.; Ferreira, I.C.; Viveiros, R.; Casimiro, T. Development of itaconic acid-based molecular imprinted polymers using supercritical fluid technology for pH-triggered drug delivery. Int. J. Pharm. 2018, 542, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Iemma, F.; Muzzalupo, R.; Spizzirri, U.G.; Trombino, S.; Cassano, R.; Picci, N. Spherical molecularly imprinted polymers (SMIPs) via a novel precipitation polymerization in the sustained delivery of sulfasalazine. Macromol. Biosci. 2004, 4, 22–26. [Google Scholar] [CrossRef]
- Azodi-Deilami, S.; Abdouss, M.; Kordestani, D.; Shariatinia, Z. Preparation of N,N-p-phenylene bismethacryl amide as a novel cross-link agent for synthesis and characterization of the core-shell magnetic molecularly imprinted polymer nanoparticles. J. Mater. Sci. Mater. Med. 2014, 25, 645–656. [Google Scholar] [CrossRef]
- Azodi-Deilami, S.; Abdouss, M.; Kordestani, D. Synthesis and characterization of the magnetic molecularly imprinted polymer nanoparticles using N, N-bis methacryloyl ethylenediamine as a new cross-linking agent for sustained release of meloxicam. Appl. Biochem. Biotechnol. 2014, 172, 3271–3286. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Zhang, H.; Yang, Y.; Liu, X.; Chen, Y. Temperature and magnetism bi-responsive molecularly imprinted polymers: Preparation, adsorption mechanism and properties as drug delivery system for sustained release of 5-fluorouracil. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 158–168. [Google Scholar] [CrossRef]
- Wu, C.; Zhi, Z.; Duan, M.; Sun, J.; Jiang, H.; Pang, J. Insights into the formation of carboxymethyl chitosan-nisin nanogels for sustainable antibacterial activity. Food Chem. 2023, 402, 134260. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, H.; Wang, J.; Wang, J.; Yan, L.; Liu, C.; Zheng, L. Pickering emulsion hydrogel based on alginate-gellan gum with carboxymethyl chitosan as a pH-responsive sustained release delivery system. Int. J. Biol. Macromol. 2022, 216, 850–859. [Google Scholar] [CrossRef]
- Zheng, Q.; Qin, D.; Wang, R.; Yan, W.; Zhao, W.; Shen, S.; Huang, S.; Cheng, D.; Zhao, C.; Zhang, Z. Novel application of biodegradable chitosan in agriculture: Using green nanopesticides to control Solenopsis invicta. Int. J. Biol. Macromol. 2022, 220, 193–203. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, H.; Dong, M.; Cheng, B.; Jin, Y.; Tong, Z.; Li, P.; Li, S.; Yang, Z. Preparation of hydroxylated lecithin complexed iodine/carboxymethyl chitosan/sodium alginate composite membrane by microwave drying and its applications in infected burn wound treatment. Carbohydr. Polym. 2019, 206, 435–445. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Row, K.H. Magnetic Solid-phase Extraction with Fe(3)O(4)/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea. Molecules 2017, 22, 1061. [Google Scholar] [CrossRef]
- Babu, C.M.; Palanisamy, B.; Sundaravel, B.; Palanichamy, M.; Murugesan, V. A novel magnetic Fe3O4/SiO2 core-shell nanorods for the removal of arsenic. J. Nanosci. Nanotechnol. 2013, 13, 2517–2527. [Google Scholar] [CrossRef]
- Li, X.; Zeng, D.; Ke, P.; Wang, G.; Zhang, D. Synthesis and characterization of magnetic chitosan microspheres for drug delivery. RSC Adv. 2020, 10, 7163–7169. [Google Scholar] [CrossRef]
- Milojevic, S.; Newton, J.M.; Cummings, J.H.; Gibson, G.R.; Botham, R.L.; Ring, S.G.; Stockham, M.; Allwood, M.C. Amylose as a coating for drug delivery to the colon: Preparation and in vitro evaluation using 5-aminosalicylic acid pellets. J. Sustain. Release 1996, 38, 75–84. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Lin, H.; Elty, A.; Laba, C.; Liu, X.; Yu, Z.; Yong, Y.; Ju, X.; She, Y. Magnetic molecularly imprinted specific solid-phase extraction for determination of dihydroquercetin from Larix griffithiana using HPLC. J. Sep. Sci. 2020, 43, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Vidinha, H.; Branco, R.; Neto, M.A.; Amaro, A.M.; Reis, P. Numerical Modeling of Damage Caused by Seawater Exposure on Mechanical Strength in Fiber-Reinforced Polymer Composites. Polymers 2022, 14, 3955. [Google Scholar] [CrossRef] [PubMed]
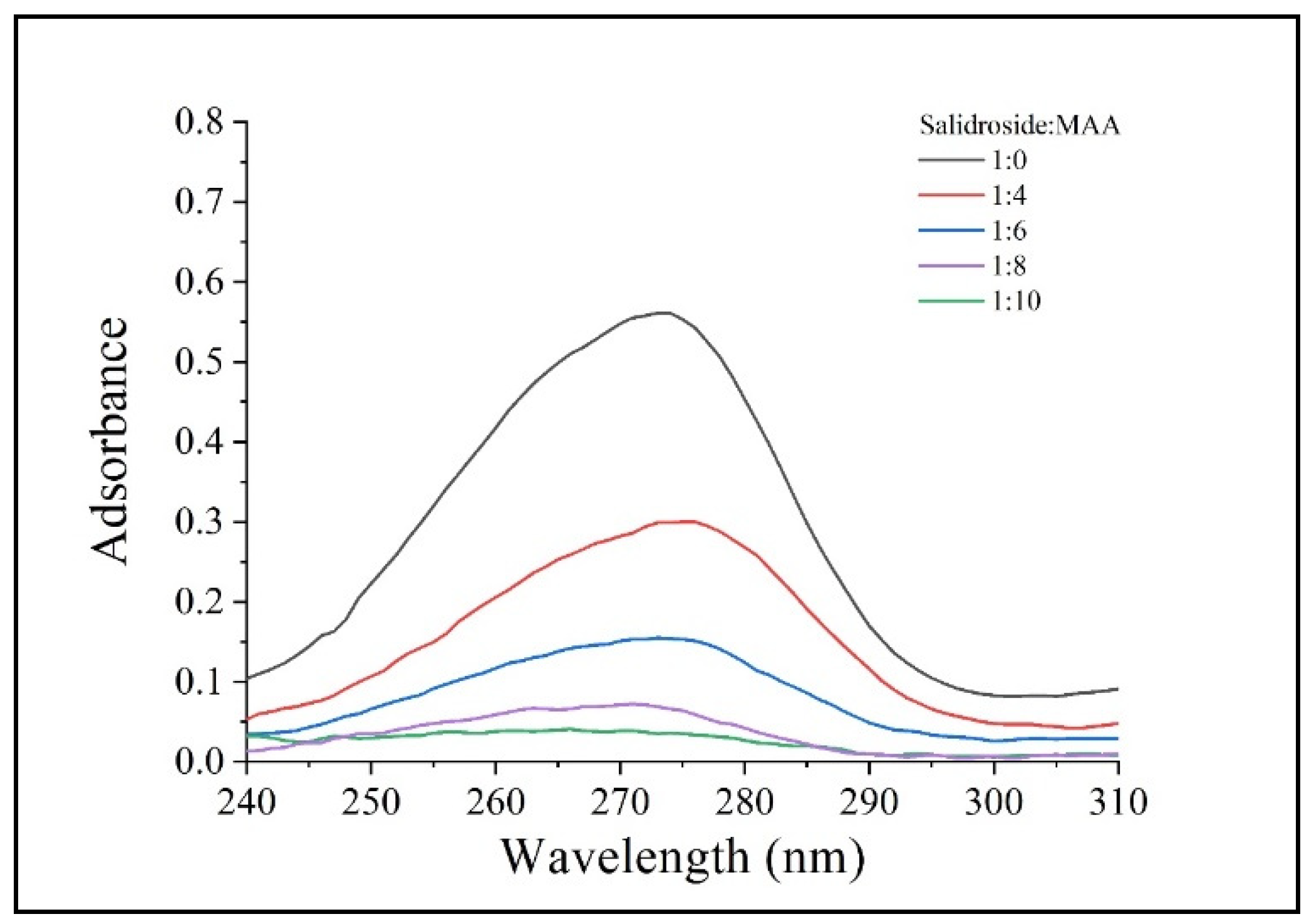
| Microspheres Name | Template (Salidroside) | Functional Monomers (MAA) | Functional Scaffold | Cross-Linker | Salidroside: MAA Molar Ratio | Yield (%) | Saturated Swelling (%) |
|---|---|---|---|---|---|---|---|
| SMCNIP | - | 34.44 mg | MCC (108.7 mg) | EGDMA (396.4 mg) | - | 62 | 44 ± 6 |
| SMCMIP | 30.03 mg | 34.44 mg | MCC (108.7 mg) | EGDMA (396.4 mg) | 1/4 | 74 | 67 ± 2 |
| SMCMIP1 | 30.03 mg | 34.44 mg | MCC (108.7 mg) | EGDMA (396.4 mg) | 1/2 | 12 | 33 ± 3 |
| SMCMIP2 | 30.03 mg | 34.44 mg | MCC (108.7 mg) | EGDMA (396.4 mg) | 1/6 | 24 | 30 ± 4 |
| SMCMIP3 | 30.03 mg | 34.44 mg | MCC (217.4 mg) | TRIM (396.4 mg) | 1/4 | 24 | 29 ± 4 |
| Sample | pH | Higuchi | Korsmeyer-Peppas | Release Mechanism | ||
|---|---|---|---|---|---|---|
| Fits Equation | R2 | Fits Equation | R2 | |||
| SMCMIP | 1.5 | Mt = 21.47 t1/2 − 7.69 | 0.9422 | Mt = 11.19 t0.77 | 0.9840 | Anomalous |
| 7.4 | Mt = 12.72 t1/2 − 0.13 | 0.9959 | Mt = 12.56 t0.43 | 0.9941 | Fickian | |
| SMCNIP | 1.5 | Mt = 4.33 t1/2 − 2.17 | 0.9053 | Mt = 1.54 t0.94 | 0.9890 | Anomalous |
| 7.4 | Mt = 4.15 t1/2 − 2.14 | 0.9646 | Mt = 2.01 t0.32 | 0.9650 | Fickian | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, S.; Qiu, J.; Liu, Z.; Liu, S.; Huang, Z.; Yong, Y.; Li, Y.; Yu, Z.; Liu, X.; et al. Development of an Fe3O4 Surface-Grafted Carboxymethyl Chitosan Molecularly Imprinted Polymer for Specific Recognition and Sustained Release of Salidroside. Polymers 2023, 15, 1187. https://doi.org/10.3390/polym15051187
Ma X, Li S, Qiu J, Liu Z, Liu S, Huang Z, Yong Y, Li Y, Yu Z, Liu X, et al. Development of an Fe3O4 Surface-Grafted Carboxymethyl Chitosan Molecularly Imprinted Polymer for Specific Recognition and Sustained Release of Salidroside. Polymers. 2023; 15(5):1187. https://doi.org/10.3390/polym15051187
Chicago/Turabian StyleMa, Xingbin, Shuyu Li, Jiajie Qiu, Zijie Liu, Siyu Liu, Zhifeng Huang, Yanhong Yong, Youquan Li, Zhichao Yu, Xiaoxi Liu, and et al. 2023. "Development of an Fe3O4 Surface-Grafted Carboxymethyl Chitosan Molecularly Imprinted Polymer for Specific Recognition and Sustained Release of Salidroside" Polymers 15, no. 5: 1187. https://doi.org/10.3390/polym15051187
APA StyleMa, X., Li, S., Qiu, J., Liu, Z., Liu, S., Huang, Z., Yong, Y., Li, Y., Yu, Z., Liu, X., Lin, H., Ju, X., & Abd El-Aty, A. M. (2023). Development of an Fe3O4 Surface-Grafted Carboxymethyl Chitosan Molecularly Imprinted Polymer for Specific Recognition and Sustained Release of Salidroside. Polymers, 15(5), 1187. https://doi.org/10.3390/polym15051187










