Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of PLGA and PLGA/Collagen Scaffolds Using the Electrospinning Technique
2.3. Characterization of PLGA and PLGA/Collagen Scaffolds
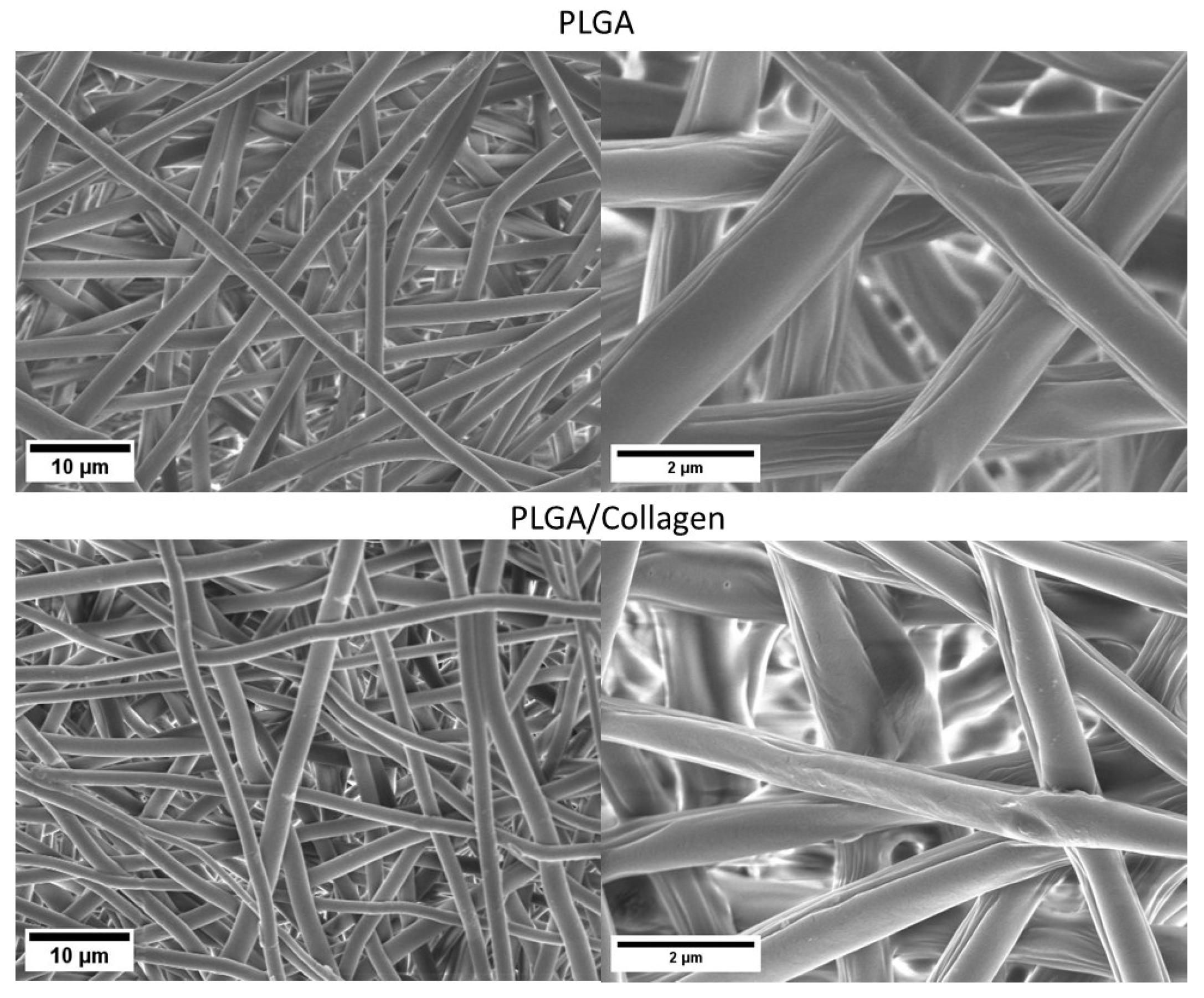
2.3.1. Morphological Analysis
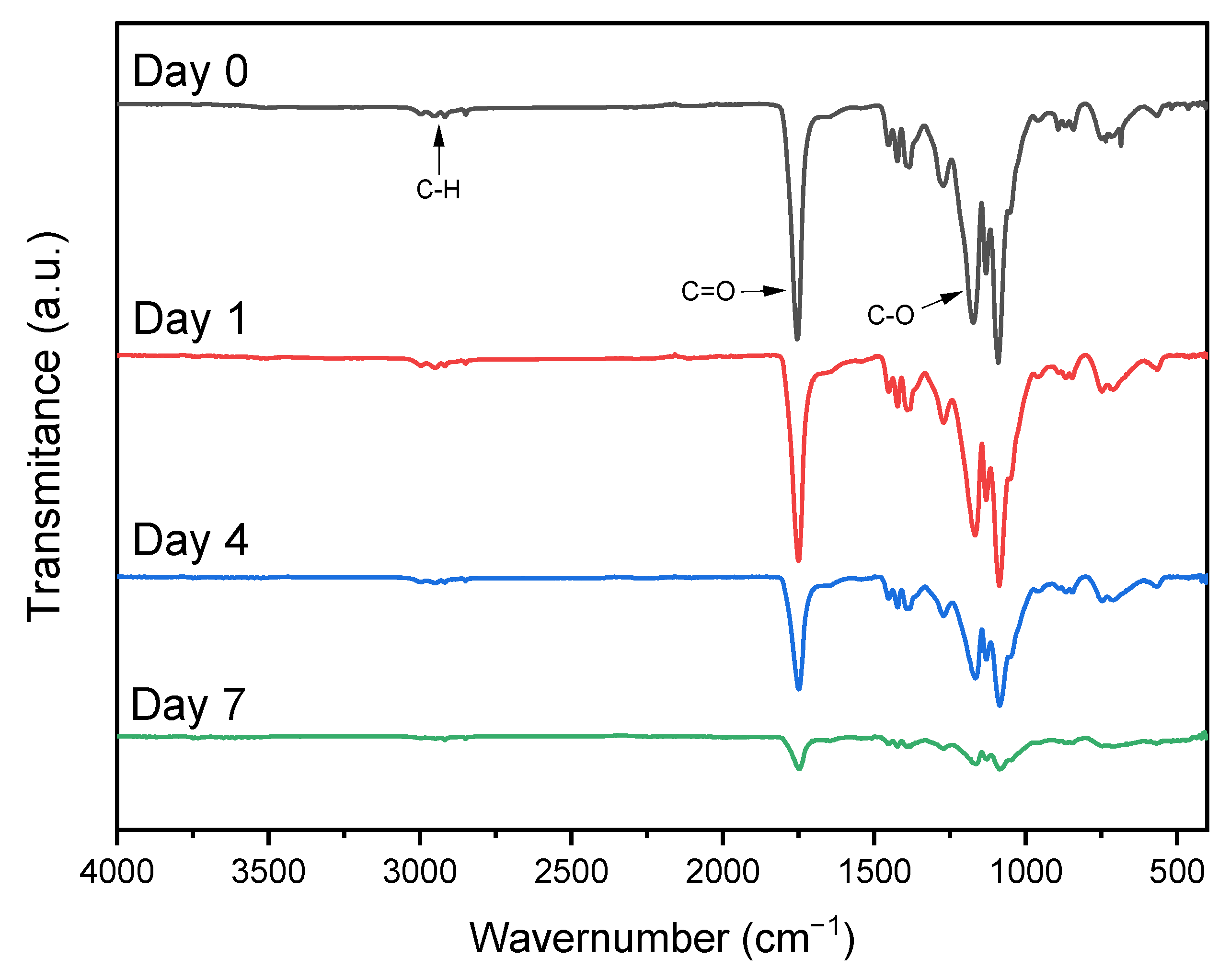
2.3.2. Characterization by ATR-FTIR Spectroscopy
2.3.3. Contact Angle Measurements
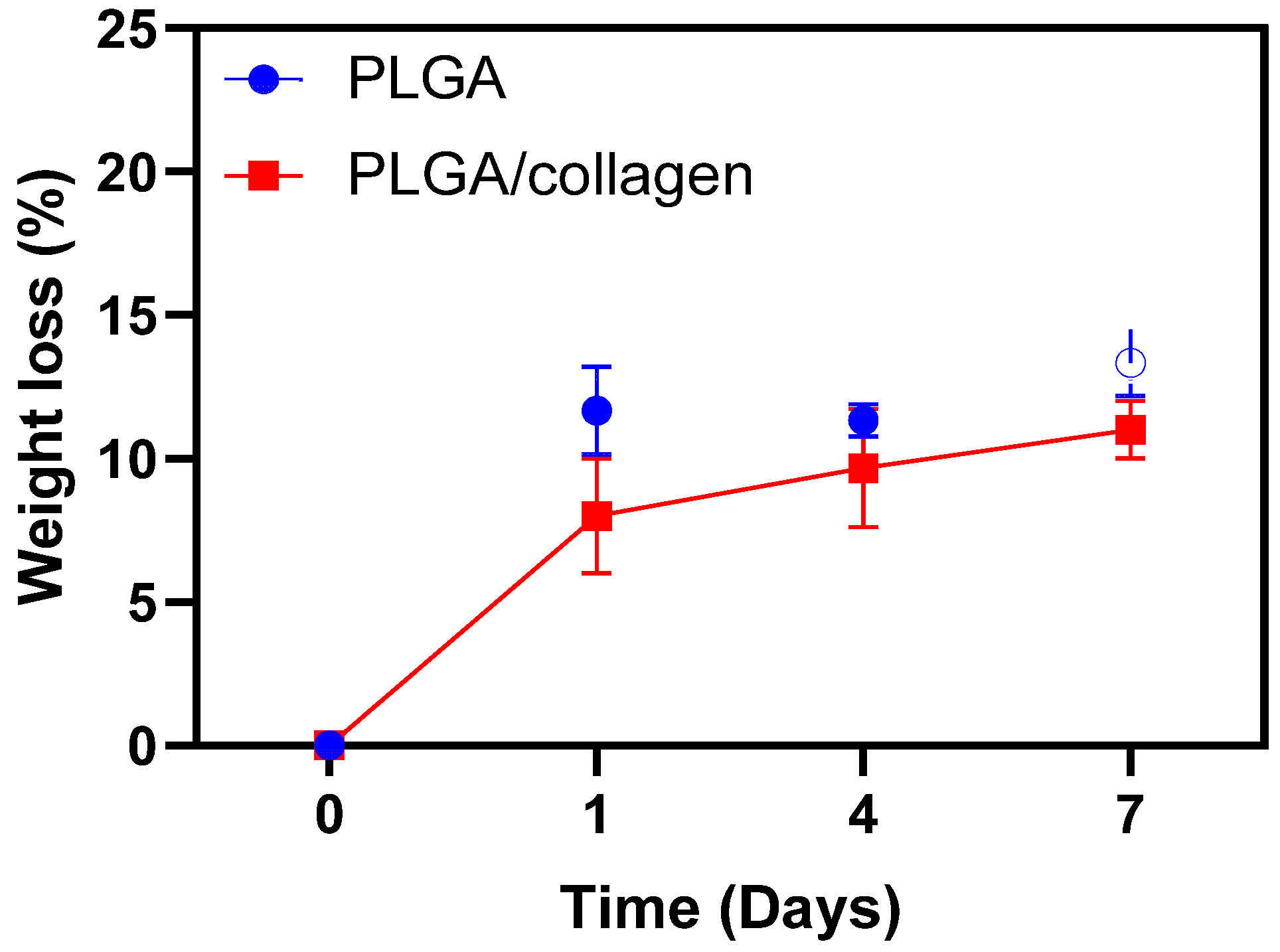
2.3.4. Enzymatic Degradation Test
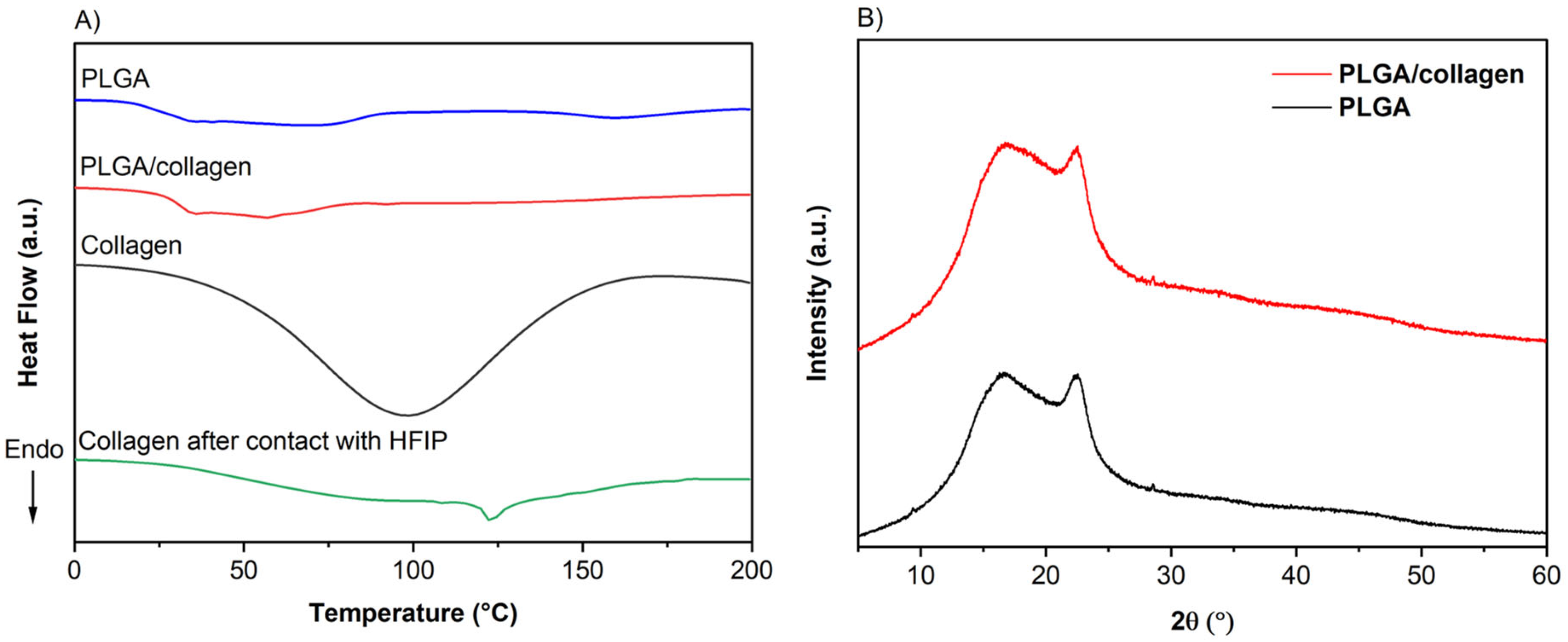
2.3.5. Thermal Properties and XRD Diffraction
2.3.6. Mechanical Properties of Fibers
2.4. Cell Culture
2.5. Cell Adhesion and Viability
2.6. Collagen Release Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis
3.2. Characterization by ATR-FTIR Spectroscopy
3.3. Contact Angle
3.4. Enzymatic Degradation Test
3.5. Thermal Properties and XRD Analysis
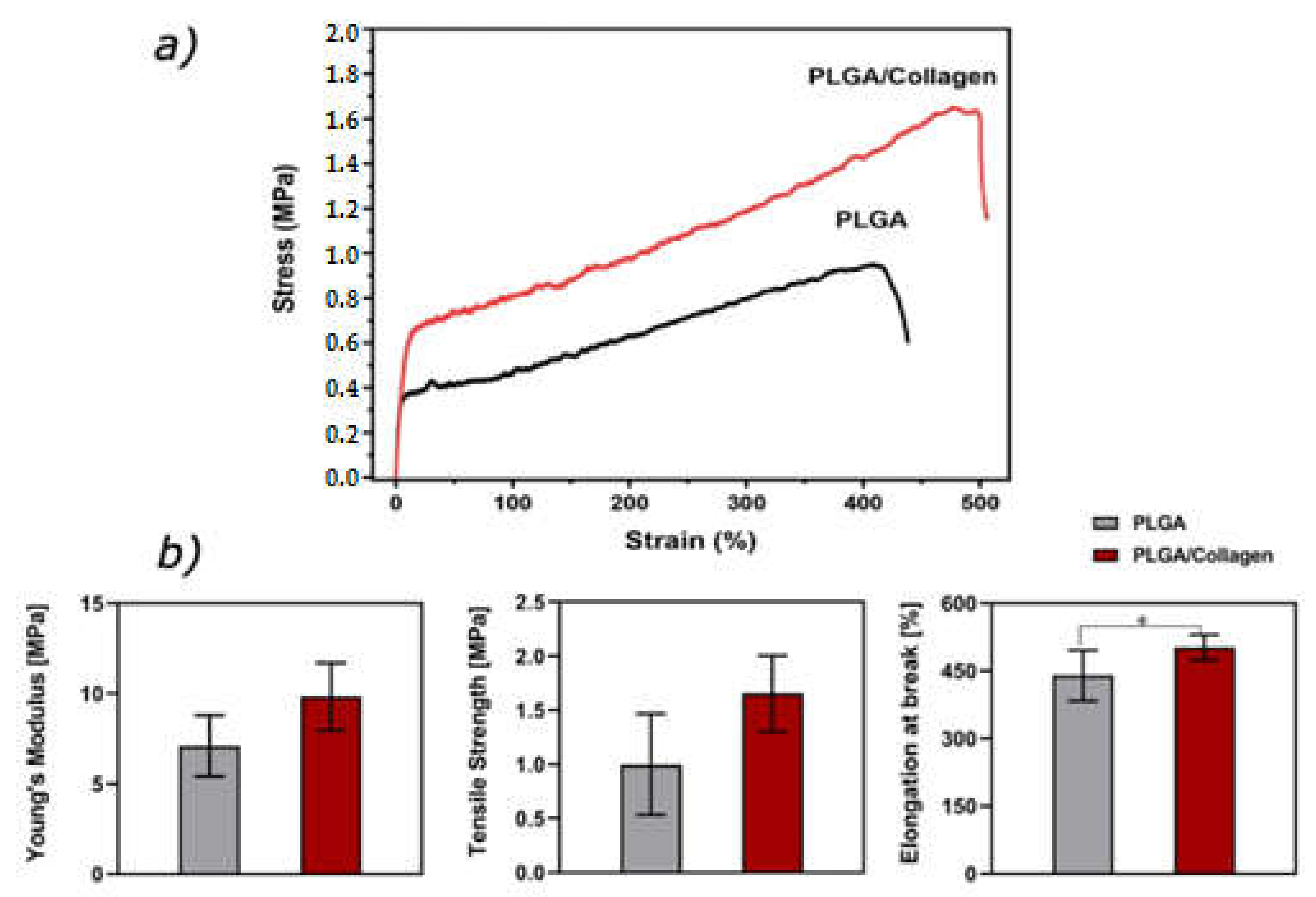
3.6. Mechanical Properties
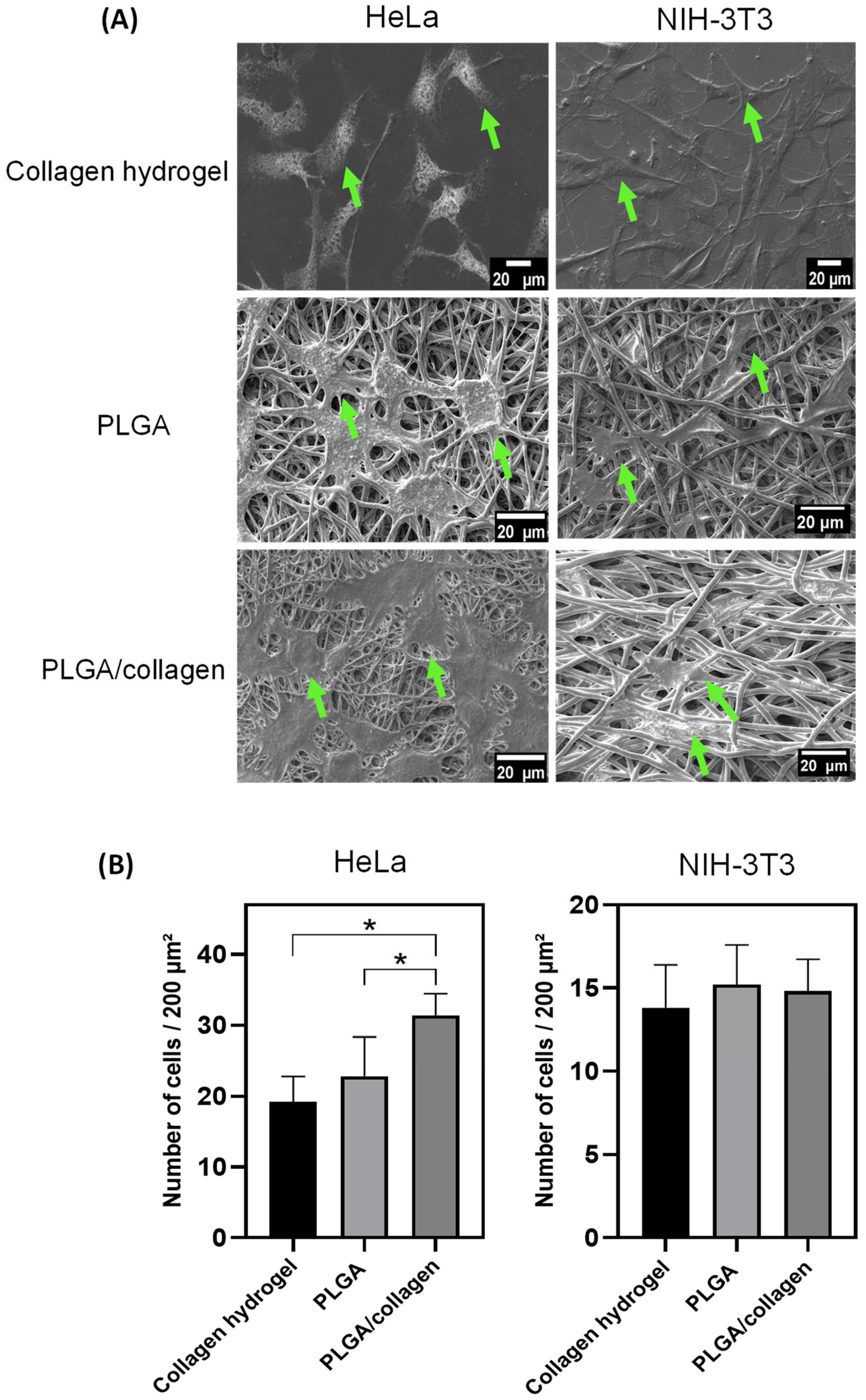
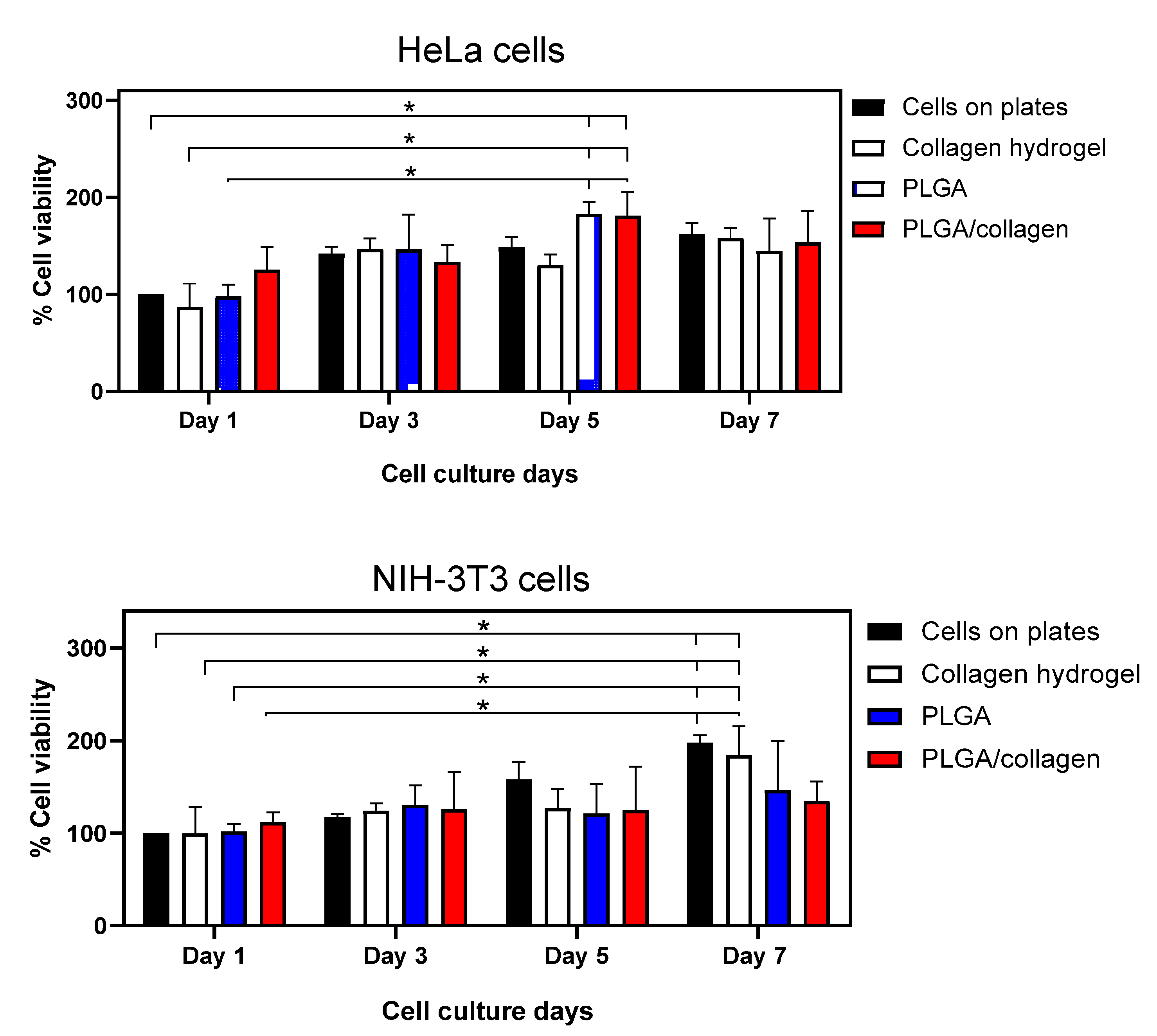
3.7. Cell Adhesion and Viability
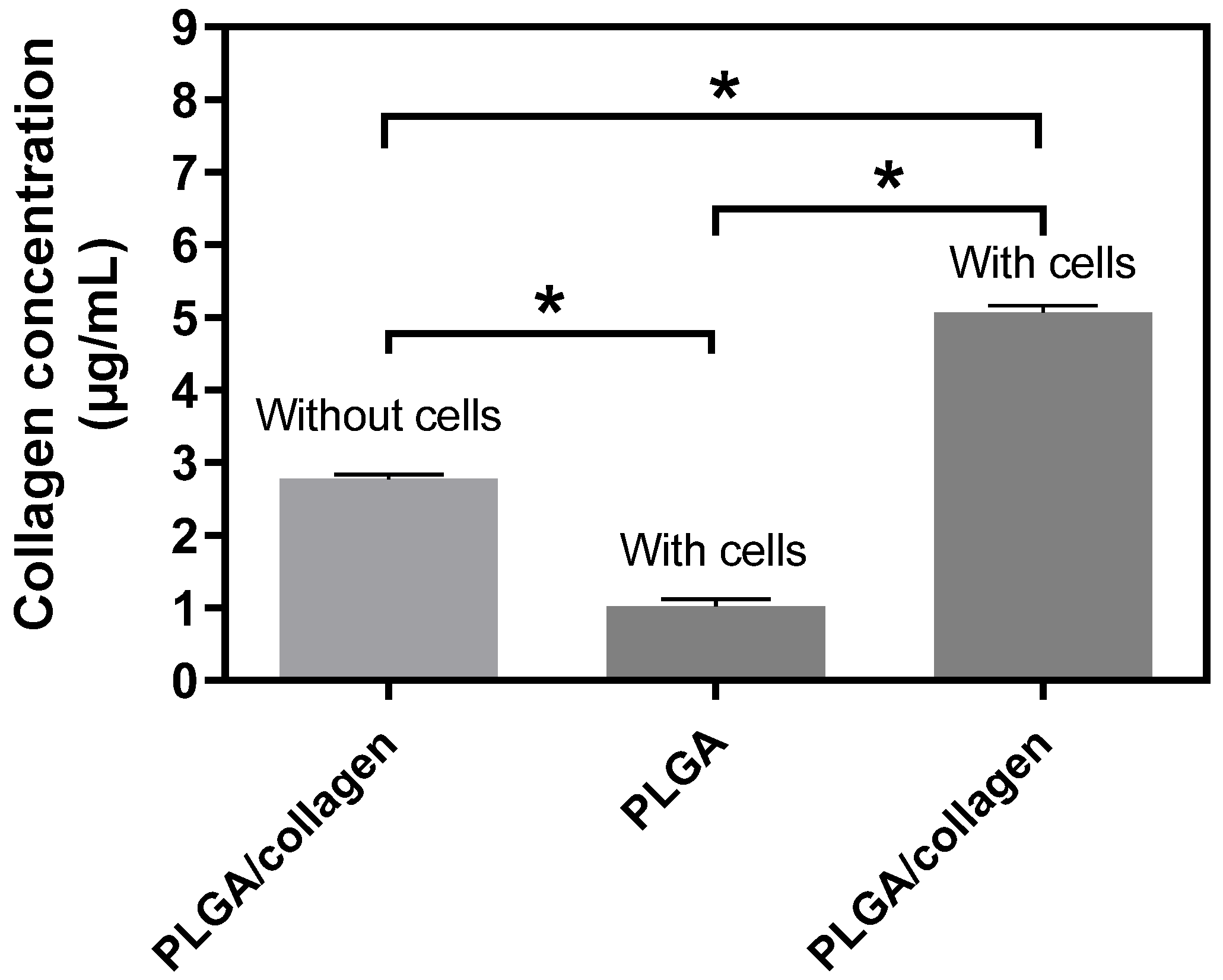
3.8. Collagen Release Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Sadeghi, A.; Moztarzadeh, F.; Aghazadeh Mohandesi, J. Investigating the Effect of Chitosan on Hydrophilicity and Bioactivity of Conductive Electrospun Composite Scaffold for Neural Tissue Engineering. Int. J. Biol. Macromol. 2019, 121, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A Review of Key Challenges of Electrospun Scaffolds for Tissue-Engineering Applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Haider, A.; Gupta, K.C.; Kim, S.; Kang, I.K. Micro/Nano Multilayered Scaffolds of PLGA and Collagen by Alternately Electrospinning for Bone Tissue Engineering. Nanoscale Res. Lett. 2016, 11, 323. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent Advances in PLGA-Based Biomaterials for Bone Tissue Regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Xie, Y.; Lee, K.; Wang, X.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Interconnected Collagen Porous Scaffolds Prepared with Sacrificial PLGA Sponge Templates for Cartilage Tissue Engineering. J. Mater. Chem. B 2021, 9, 8491–8500. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun Medical Sutures for Wound Healing: A Review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Irawan, V.; Sung, T.C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Mahdavi-Shahri, M. Synthesis and Characterization of Collagen/PLGA Biodegradable Skin Scaffold Fibers. Regen. Biomater. 2017, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Kau, Y.C.; Chou, C.Y.; Chen, J.K.; Wu, R.C.; Yeh, W.L. Electrospun PLGA/Collagen Nanofibrous Membrane as Early-Stage Wound Dressing. J. Memb. Sci. 2010, 355, 53–59. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, C.H.; Cho, I.H.; Kim, Y.J.; Lee, Y.J.; Kim, I.A.; Park, K.D.; Yui, N.; Shin, J.W. Electrospun PLGA Nanofiber Scaffolds for Articular Cartilage Reconstruction: Mechanical Stability, Degradation and Cellular Responses under Mechanical Stimulation in Vitro. J. Biomater. Sci. Polym. Ed. 2006, 17, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sato, T.; Ohgushi, H.; Ushida, T.; Tateishi, T.; Tanaka, J. Culturing of Skin Fibroblasts in a Thin PLGA–Collagen Hybrid Mesh. Biomaterials 2005, 26, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Das, P.; DiVito, M.D.; Ivancic, D.; Tan, L.P.; Wertheim, J.A. Nanofibrous PLGA Electrospun Scaffolds Modified with Type I Collagen Influence Hepatocyte Function and Support Viability in Vitro. Acta Biomater. 2018, 73, 217–227. [Google Scholar] [CrossRef]
- Carlson, B.M. Some Principles of Regeneration in Mammalian Systems. Anat. Rec. Part B New Anat. 2005, 287B, 4–13. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Tsivintzelis, I.; Panayiotou, C.; Papageorgiou, V.P. Electrospun Fiber Mats Containing Shikonin and Derivatives with Potential Biomedical Applications. Int. J. Pharm. 2011, 409, 216–228. [Google Scholar] [CrossRef]
- Khan, A.; Hadano, Y.; Takehara, H.; Ichiki, T. Effects of Physico-Chemical Treatments on PLGA 50:50 Electrospun Nanofibers. Polymer 2022, 261, 125400. [Google Scholar] [CrossRef]
- Hong, K.H.; Woo, S.H.; Kang, T.J. In Vitro Degradation and Drug-Release Behavior of Electrospun, Fibrous Webs of Poly(Lactic-Co-Glycolic Acid). J. Appl. Polym. Sci. 2012, 124, 209–214. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. Role of Electrospinning Parameters on Poly(Lactic-Co-Glycolic Acid) and Poly(Caprolactone-Co-Glycolic Acid) Membranes. Polymers 2021, 13, 695. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, K.; Khaleel Basha, S.; Sugantha Kumari, V.; Kaviyarasu, K.; Maaza, M. In Vitro Cytocompatibility of Chitosan/PVA/Methylcellulose—Nanocellulose Nanocomposites Scaffolds Using L929 Fibroblast Cells. Appl. Surf. Sci. 2018, 449, 574–583. [Google Scholar] [CrossRef]
- Boncler, M.; Rózalski, M.; Krajewska, U.; Podswdek, A.; Watala, C. Comparison of PrestoBlue and MTT Assays of Cellular Viability in the Assessment of Anti-Proliferative Effects of Plant Extracts on Human Endothelial Cells. J. Pharmacol. Toxicol. Methods 2014, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Granados, M.V.; Montesinos-Montesinos, J.J.; Álvarez-Pérez, M.A.; Granados, M.V.; Montesinos-Montesinos, J.J.; Álvarez-Pérez, M.A. Adhesion and Proliferation of Bone Marrow Mesenchymal Stem Cells on Poly(L-Lactic Acid) (PLA) Fibrillar Scaffolds. Rev. Mex. Ing. Biomédica 2016, 38, 288–296. [Google Scholar]
- Jafari, H.; Delporte, C.; Bernaerts, K.V.; de Leener, G.; Luhmer, M.; Nie, L.; Shavandi, A. Development of Marine Oligosaccharides for Potential Wound Healing Biomaterials Engineering. Chem. Eng. J. Adv. 2021, 7, 100113. [Google Scholar] [CrossRef]
- Quan, Z.; Wang, Y.; Zu, Y.; Qin, X.; Yu, J. A Rotary Spinneret for High Output of Electrospun Fibers with Bimodal Distribution. Eur. Polym. J. 2021, 159, 110707. [Google Scholar] [CrossRef]
- Zhao, L.; He, C.; Gao, Y.; Cen, L.; Cui, L.; Cao, Y. Preparation and Cytocompatibility of PLGA Scaffolds with Controllable Fiber Morphology and Diameter Using Electrospinning Method. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87B, 26–34. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Dean, D.R.; Nyairo, E. Fabrication and Characterization of Aligned Nanofibrous PLGA/Collagen Blends as Bone Tissue Scaffolds. Polymer 2009, 50, 3778–3785. [Google Scholar] [CrossRef]
- Kwon, I.K.; Matsuda, T. Co-Electrospun Nanofiber Fabrics of Poly(L-Lactide-Co-ε-Caprolactone) with Type I Collagen or Heparin. Biomacromolecules 2005, 6, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Mosallanezhad, P.; Nazockdast, H.; Ahmadi, Z.; Rostami, A. Fabrication and Characterization of Polycaprolactone/Chitosan Nanofibers Containing Antibacterial Agents of Curcumin and ZnO Nanoparticles for Use as Wound Dressing. Front. Bioeng. Biotechnol. 2022, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Qi, N. Controllable Structure, Properties, and Degradation of the Electrospun PLGA/PLA-Blended Nanofibrous Scaffolds. J. Appl. Polym. Sci. 2012, 125, E468–E476. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR Analysis of Natural and Synthetic Collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- De Campos Vidal, B.; Mello, M.L.S. Collagen Type I Amide I Band Infrared Spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Bürck, J.; Aras, O.; Bertinetti, L.; Ilhan, C.A.; Ermeydan, M.A.; Schneider, R.; Ulrich, A.S.; Kazanci, M. Observation of Triple Helix Motif on Electrospun Collagen Nanofibers and Its Effect on the Physical and Structural Properties. J. Mol. Struct. 2018, 1151, 73–80. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of Collagens from the Swim Bladders of Yellowfin Tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Castro, M.P.J.; Travália, B.M.; Forte, M.B.S. Trends in Lipase Immobilization: Bibliometric Review and Patent Analysis. Process. Biochem. 2021, 110, 37–51. [Google Scholar] [CrossRef]
- Nagai, T.; Tanoue, Y.; Kai, N.; Suzuki, N. Characterization of Collagen from Emu (Dromaius novaehollandiae) Skins. J. Food Sci. Technol. 2015, 52, 2344–2351. [Google Scholar] [CrossRef]
- Hall Barrientos, I.J.; Paladino, E.; Szabó, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun Collagen-Based Nanofibres: A Sustainable Material for Improved Antibiotic Utilisation in Tissue Engineering Applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA Nanofibrous Nanocomposite Scaffolds for Bone Tissue Engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Raja, I.S.; Lee, S.H.; Kang, M.S.; Hyon, S.-H.; Selvaraj, A.R.; Prabakar, K.; Han, D.-W. The Predominant Factor Influencing Cellular Behavior on Electrospun Nanofibrous Scaffolds: Wettability or Surface Morphology? Mater. Des. 2022, 216, 110580. [Google Scholar] [CrossRef]
- Agrawal, G.; Negi, Y.S.; Pradhan, S.; Dash, M.; Samal, S.K. Wettability and Contact Angle of Polymeric Biomaterials. In Characterization of Polymeric Biomaterials; Woodhead Publishing: Sawston, UK, 2017; pp. 57–81. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Karimi, Y.; Naghieh, S.; Alizadeh Sardroud, H.; Gorji, M.; Chen, X. Electrospinning of Scaffolds from the Polycaprolactone/Polyurethane Composite with Graphene Oxide for Skin Tissue Engineering. Appl. Biochem. Biotechnol. 2020, 191, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface Modification of Electrospun PLGA Scaffold with Collagen for Bioengineered Skin Substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef]
- Mozafari, M.; Kargozar, S.; de Santiago, G.T.; Mohammadi, M.R.; Milan, P.B.; Foroutan Koudehi, M.; Aghabarari, B.; Nourani, M.R. Synthesis and Characterisation of Highly Interconnected Porous Poly(ε-Caprolactone)-Collagen Scaffolds: A Therapeutic Design to Facilitate Tendon Regeneration. Mater. Technol. 2017, 33, 29–37. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Vallecillo-Rivas, M.; Toledano-Osorio, M.; Vallecillo, C.; Osorio, R.; Toledano, M. The Collagen Origin Influences the Degradation Kinetics of Guided Bone Regeneration Membranes. Polymers 2021, 13, 3007. [Google Scholar] [CrossRef]
- Said, S.S.; Aloufy, A.K.; El-Halfawy, O.M.; Boraei, N.A.; El-Khordagui, L.K. Antimicrobial PLGA Ultrafine Fibers: Interaction with Wound Bacteria. Eur. J. Pharm. Biopharm. 2011, 79, 108–118. [Google Scholar] [CrossRef]
- Peh, P.; Lim, N.S.J.; Blocki, A.; Chee, S.M.L.; Park, H.C.; Liao, S.; Chan, C.; Raghunath, M. Simultaneous Delivery of Highly Diverse Bioactive Compounds from Blend Electrospun Fibers for Skin Wound Healing. Bioconjug. Chem. 2015, 26, 1348–1358. [Google Scholar] [CrossRef]
- Vey, E.; Rodger, C.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation Kinetics of Poly(Lactic-Co-Glycolic) Acid Block Copolymer Cast Films in Phosphate Buffer Solution as Revealed by Infrared and Raman Spectroscopies. Polym. Degrad. Stab. 2011, 96, 1882–1889. [Google Scholar] [CrossRef]
- Haghighat, F.; Ravandi, S.A.H. Mechanical Properties and in Vitro Degradation of PLGA Suture Manufactured via Electrospinning. Fibers Polym. 2014, 15, 71–77. [Google Scholar] [CrossRef]
- Fouad, H.; Elsarnagawy, T.; Almajhdi, F.N.; Khalil, K.A. Preparation and In Vitro Thermo-Mechanical Characterization of Electrospun PLGA Nanofibers for Soft and Hard Tissue Replacement. Int. J. Electrochem. Sci. 2013, 8, 2293–2304. [Google Scholar]
- Liu, F.; Guo, R.; Shen, M.; Wang, S.; Shi, X. Effect of Processing Variables on the Morphology of Electrospun Poly[(Lactic Acid)-Co-(Glycolic Acid)] Nanofibers. Macromol. Mater. Eng. 2009, 294, 666–672. [Google Scholar] [CrossRef]
- Silva, A.T.C.R.; Cardoso, B.C.O.; e Silva, M.E.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 6, 8–19. [Google Scholar] [CrossRef]
- Seju, U.; Kumar, A.; Sawant, K.K. Development and Evaluation of Olanzapine-Loaded PLGA Nanoparticles for Nose-to-Brain Delivery: In Vitro and in Vivo Studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef]
- Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-tetraphenylporphyrin Metal Complex-loaded Plga Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef]
- Ivankin, A.; Boldirev, V.; Fadeev, G.; Baburina, M.; Kulikovskii, A.; Vostrikova, N. Denaturation of Collagen Structures and Their Transformation under the Physical and Chemical Effects. J. Phys. Conf. Ser. 2017, 918, 012010. [Google Scholar] [CrossRef]
- Badylak, S.F. Xenogeneic Extracellular Matrix as a Scaffold for Tissue Reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of Type I Collagen and PCL Nanofibers Using Acetic Acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal Denaturation Studies of Collagen by Microthermal Analysis and Atomic Force Microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, M.J.; Ryu, S.J.; Ki, C.S.; Park, Y.H. Effect of Fiber Diameter on Surface Morphology, Mechanical Property, and Cell Behavior of Electrospun Poly(ε-Caprolactone) Mat. Fibers Polym. 2016, 17, 1033–1042. [Google Scholar] [CrossRef]
- Morel, A.; Domaschke, S.; Urundolil Kumaran, V.; Alexeev, D.; Sadeghpour, A.; Ramakrishna, S.N.; Ferguson, S.J.; Rossi, R.M.; Mazza, E.; Ehret, A.E.; et al. Correlating Diameter, Mechanical and Structural Properties of Poly(l-Lactide) Fibres from Needleless Electrospinning. Acta Biomater. 2018, 81, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Cunniffe, G.M.; O’Brien, F.J. Collagen-Based Biomaterials for Tissue Regeneration and Repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Woodhead Publishing: Sawston, UK, 2018; pp. 127–150. [Google Scholar] [CrossRef]
- Ramanathan, G.; Singaravelu, S.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Ruban, K.; Kaveri, K.; Natarajan, T.S.; Sivagnanam, U.T.; Perumal, P.T. Fabrication and Characterization of a Collagen Coated Electrospun Poly(3-Hydroxybutyric Acid)–Gelatin Nanofibrous Scaffold as a Soft Bio-Mimetic Material for Skin Tissue Engineering Applications. RSC Adv. 2016, 6, 7914–7922. [Google Scholar] [CrossRef]
- Xu, H.; Bihan, D.; Chang, F.; Huang, P.H.; Farndale, R.W.; Leitinger, B. Discoidin Domain Receptors Promote A1β1- and A2β1-Integrin Mediated Cell Adhesion to Collagen by Enhancing Integrin Activation. PLoS ONE 2012, 7, e52209. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.; Griffith, L.; Wells, A. Extracellular Matrix Signaling through Growth Factor Receptors during Wound Healing. Wound Repair Regen. 2004, 12, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lee, K.; Kawazoe, N.; Yang, Y.; Chen, G. PLGA–Collagen–ECM Hybrid Scaffolds Functionalized with Biomimetic Extracellular Matrices Secreted by Mesenchymal Stem Cells during Stepwise Osteogenesis-Co-Adipogenesis. J. Mater. Chem. B 2019, 7, 7195–7206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lee, K.; Yang, Y.; Kawazoe, N.; Chen, G. PLGA-Collagen-ECM Hybrid Meshes Mimicking Stepwise Osteogenesis and Their Influence on the Osteogenic Differentiation of HMSCs. Biofabrication 2020, 12, 025027. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Warner, S.B.; Bhowmick, S. Role of Fiber Diameter in Adhesion and Proliferation of NIH 3T3 Fibroblast on Electrospun Polycaprolactone Scaffolds. Tissue Eng. 2007, 13, 579–587. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Bhaw-Luximon, A.; Jhurry, D. Cell-Matrix Mechanical Interaction in Electrospun Polymeric Scaffolds for Tissue Engineering: Implications for Scaffold Design and Performance. Acta Biomater. 2017, 50, 41–55. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Lovett, M.L.; Kaplan, D.L.; Bhowmick, S. Role of Electrospun Fibre Diameter and Corresponding Specific Surface Area (SSA) on Cell Attachment. J. Tissue Eng. Regen. Med. 2009, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Asaga, H.; Yoshizato, K. Recognition of Collagen by Fibroblasts through Cell Surface Glycoproteins Reactive with Phaseolus Vulgaris Agglutinin. J. Cell Sci. 1992, 101, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.C.; Rohman, G.; Southgate, J.; Cameron, N.R. The Relationship between the Mechanical Properties and Cell Behaviour on PLGA and PCL Scaffolds for Bladder Tissue Engineering. Biomaterials 2009, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Pauly, H.M.; Kelly, D.J.; Popat, K.C.; Trujillo, N.A.; Dunne, N.J.; McCarthy, H.O.; Haut Donahue, T.L. Mechanical Properties and Cellular Response of Novel Electrospun Nanofibers for Ligament Tissue Engineering: Effects of Orientation and Geometry. J. Mech. Behav. Biomed. Mater. 2016, 61, 258–270. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking Older: Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef]
- Katayoshi, T.; Kusano, Y.; Shibata, T.; Uchida, K.; Tsuji-Naito, K. Low-Molecular-Weight Whey Proteins Promote Collagen Production in Dermal Fibroblasts via the TGF-β Receptor/Smad Pathway. Biosci. Biotechnol. Biochem. 2021, 85, 2232–2240. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Soria, A.; Moreno-Serna, V.; Canales, D.A.; García-Herrera, C.; Zapata, P.A.; Orihuela, P.A. Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering. Polymers 2023, 15, 1079. https://doi.org/10.3390/polym15051079
Guzmán-Soria A, Moreno-Serna V, Canales DA, García-Herrera C, Zapata PA, Orihuela PA. Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering. Polymers. 2023; 15(5):1079. https://doi.org/10.3390/polym15051079
Chicago/Turabian StyleGuzmán-Soria, Aldo, Viviana Moreno-Serna, Daniel A. Canales, Claudio García-Herrera, Paula A. Zapata, and Pedro A. Orihuela. 2023. "Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering" Polymers 15, no. 5: 1079. https://doi.org/10.3390/polym15051079
APA StyleGuzmán-Soria, A., Moreno-Serna, V., Canales, D. A., García-Herrera, C., Zapata, P. A., & Orihuela, P. A. (2023). Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering. Polymers, 15(5), 1079. https://doi.org/10.3390/polym15051079





