Advances in Peptide-Based Hydrogel for Tissue Engineering
Abstract
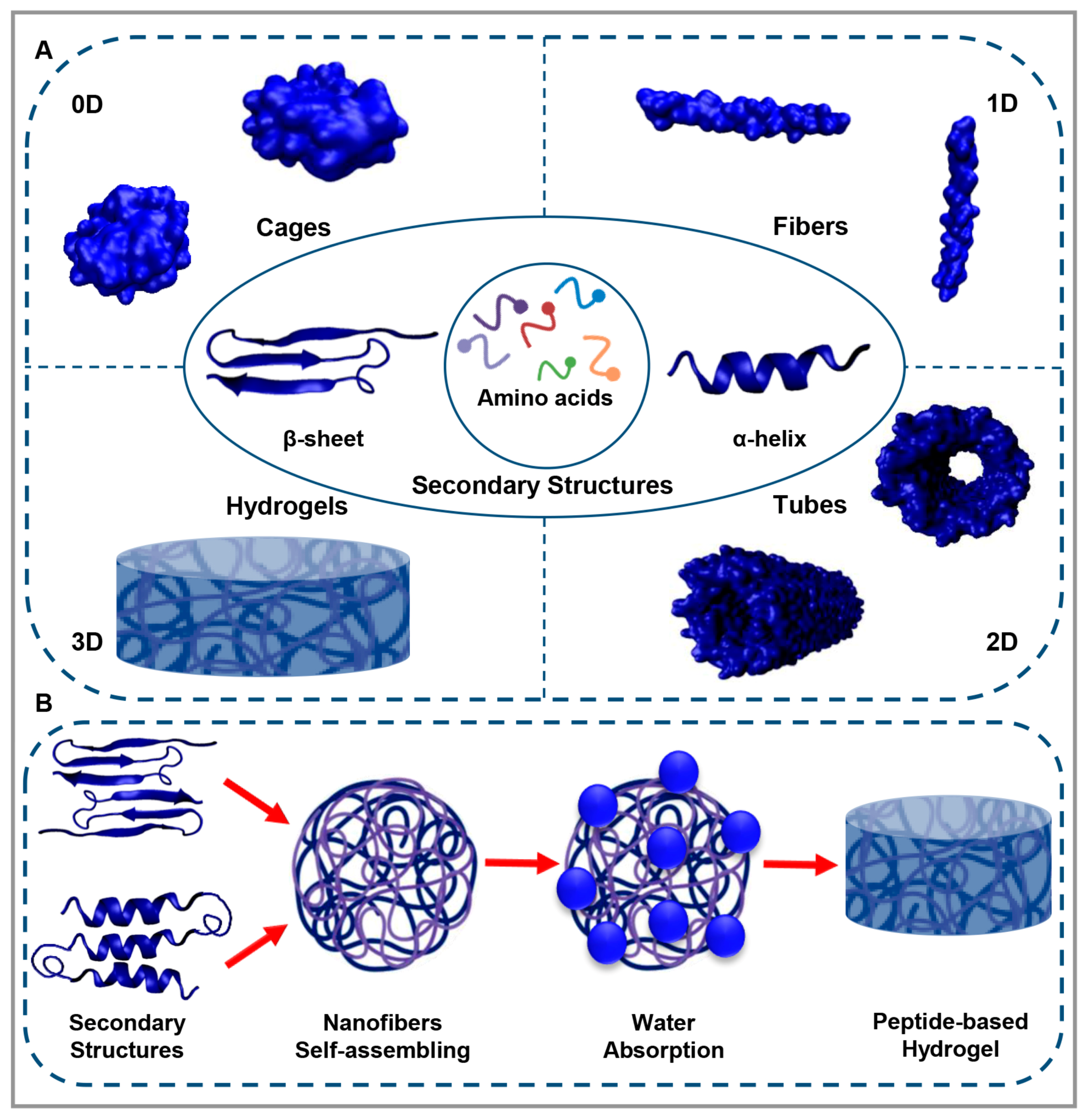
1. Peptide-Based Materials
2. Peptide-Based Hydrogels
3. Peptide-Based Hydrogel in Tissue Engineering
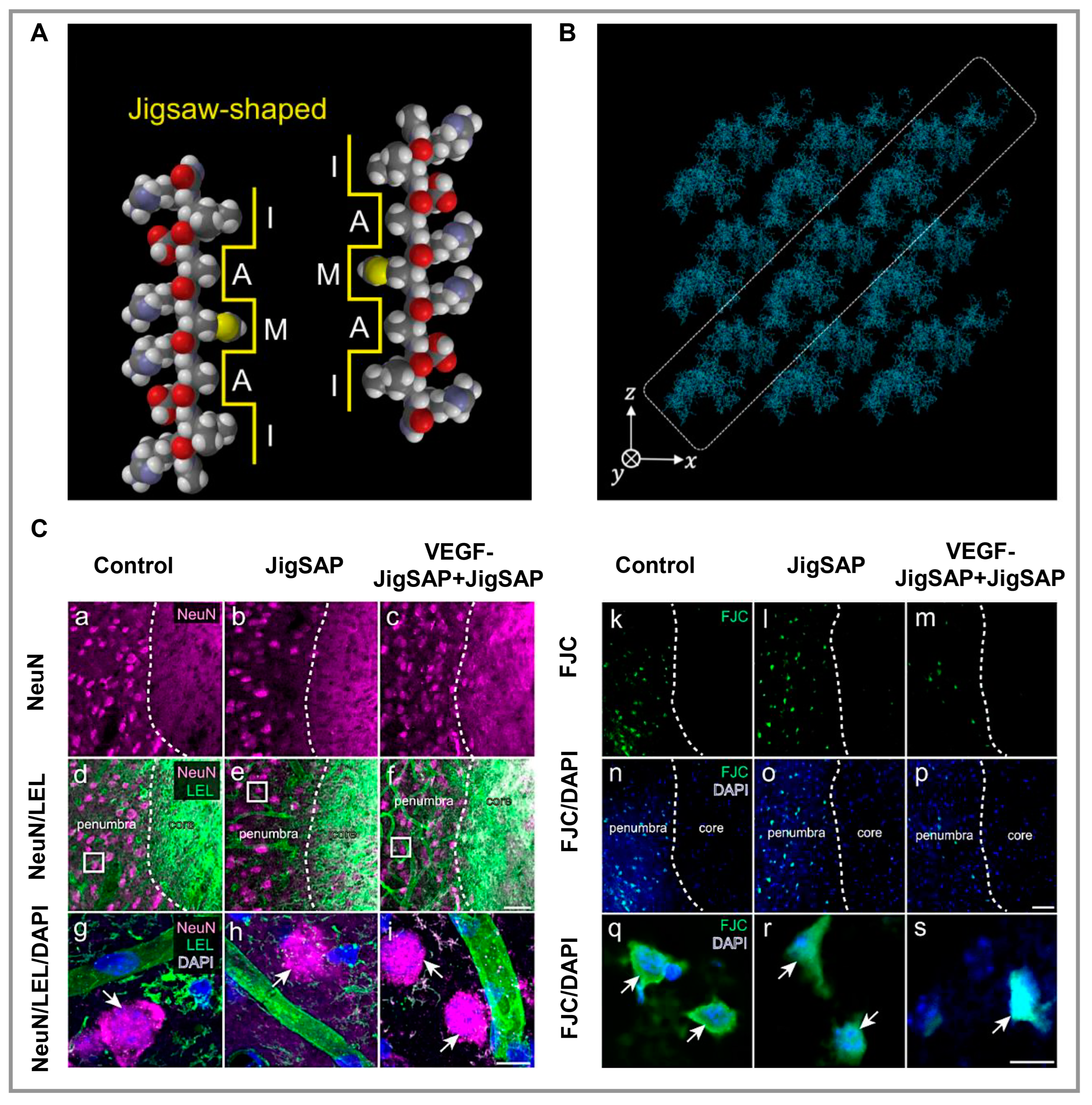
3.1. Nerve
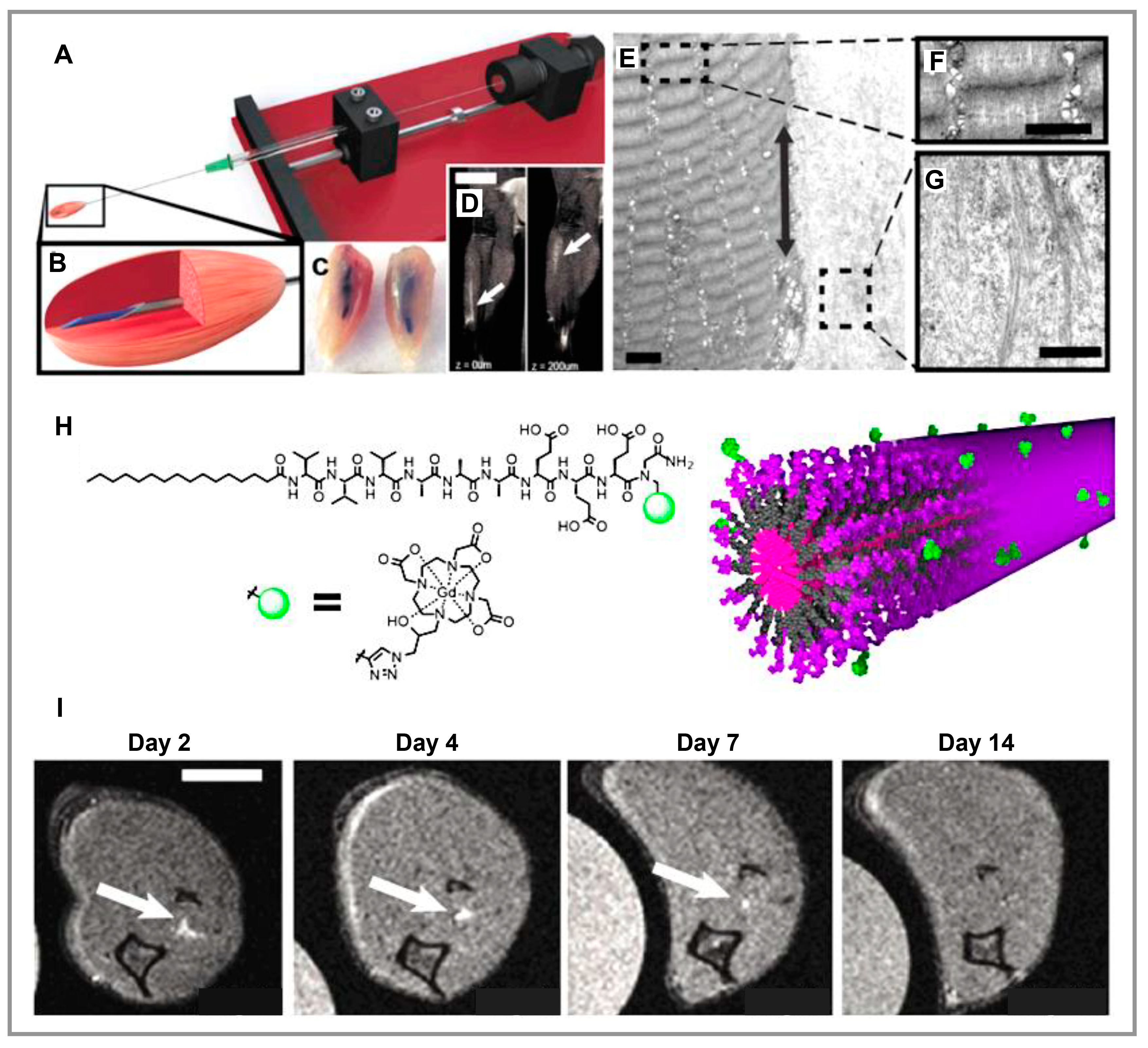
3.2. Muscle
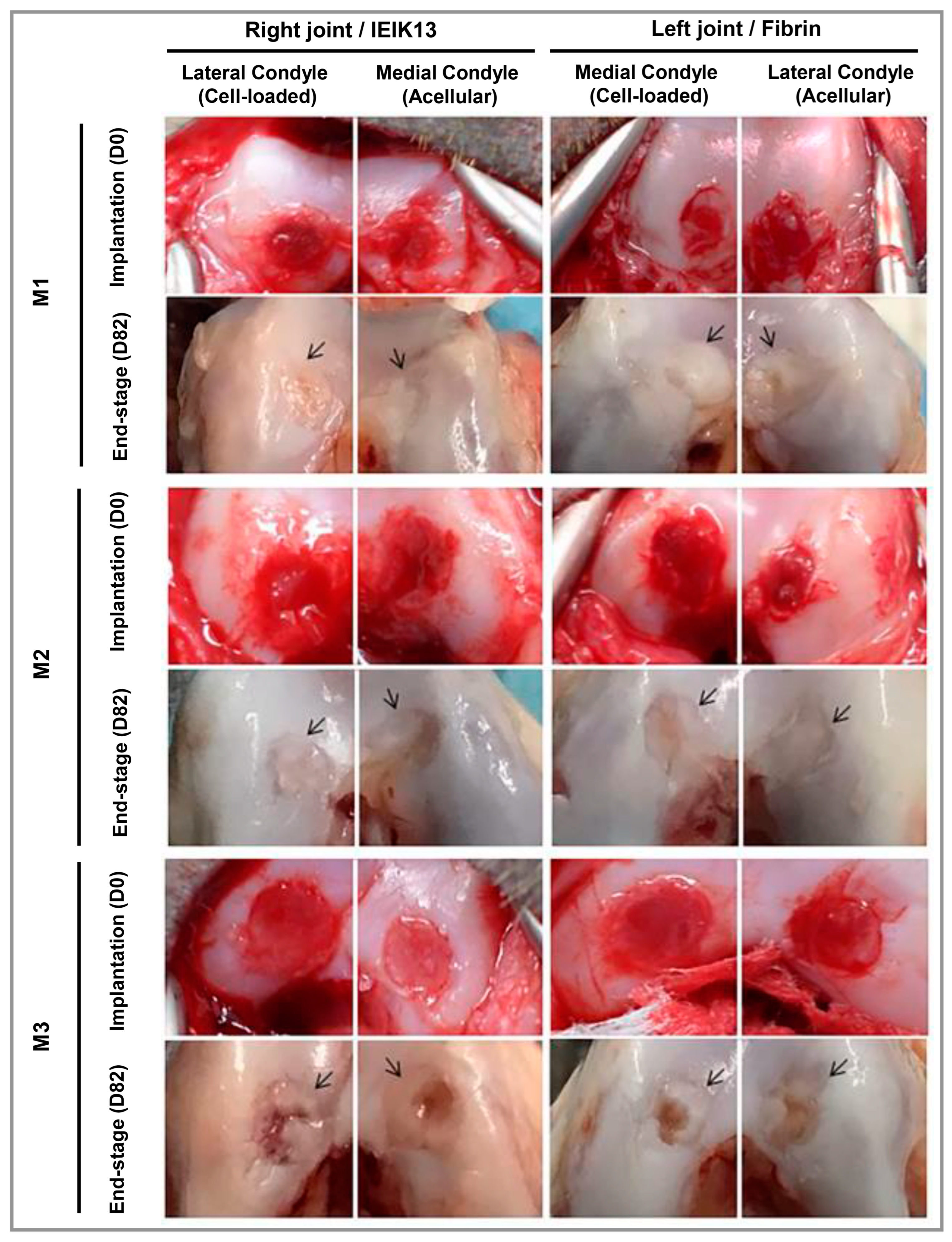
3.3. Cartilage
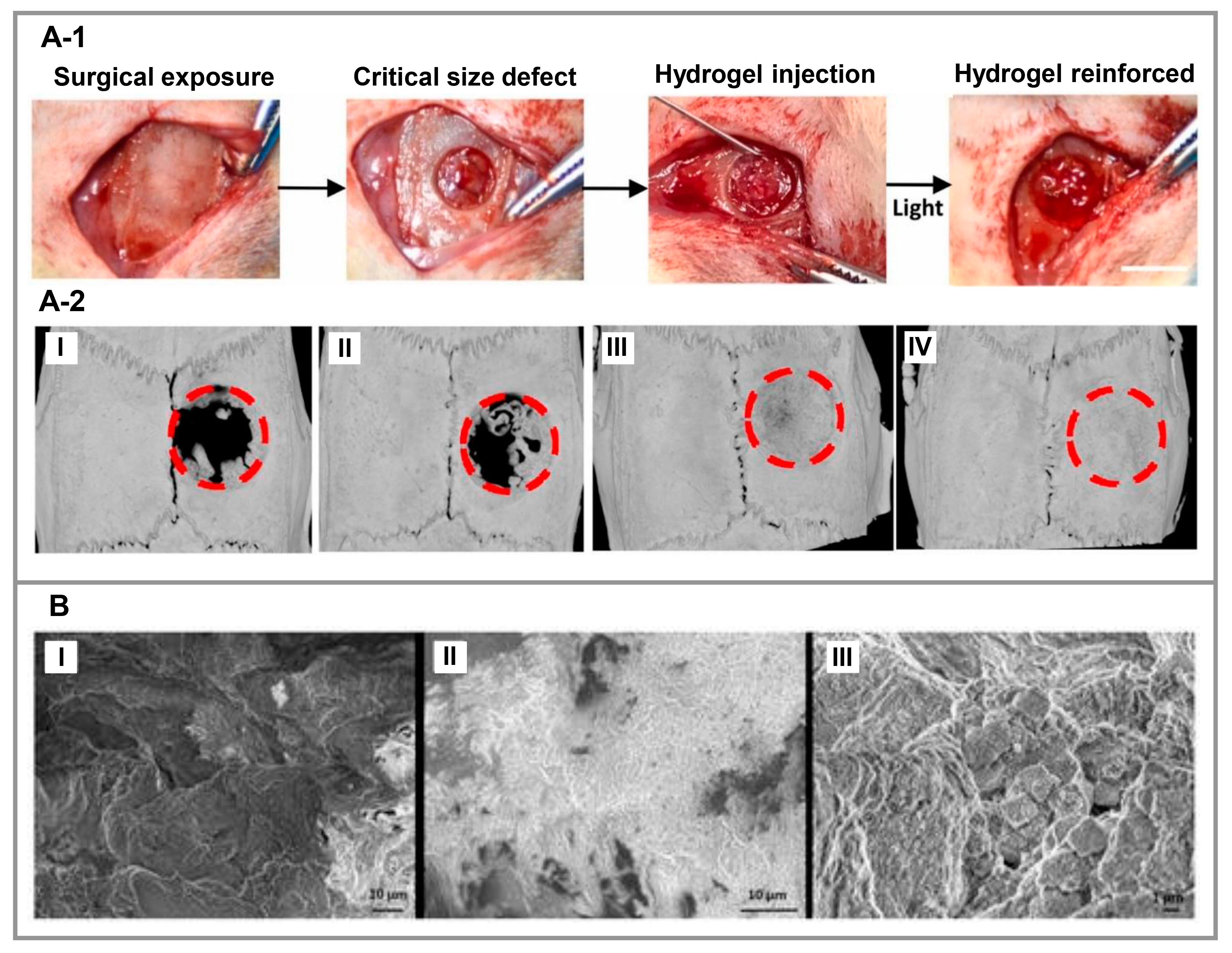
3.4. Bone
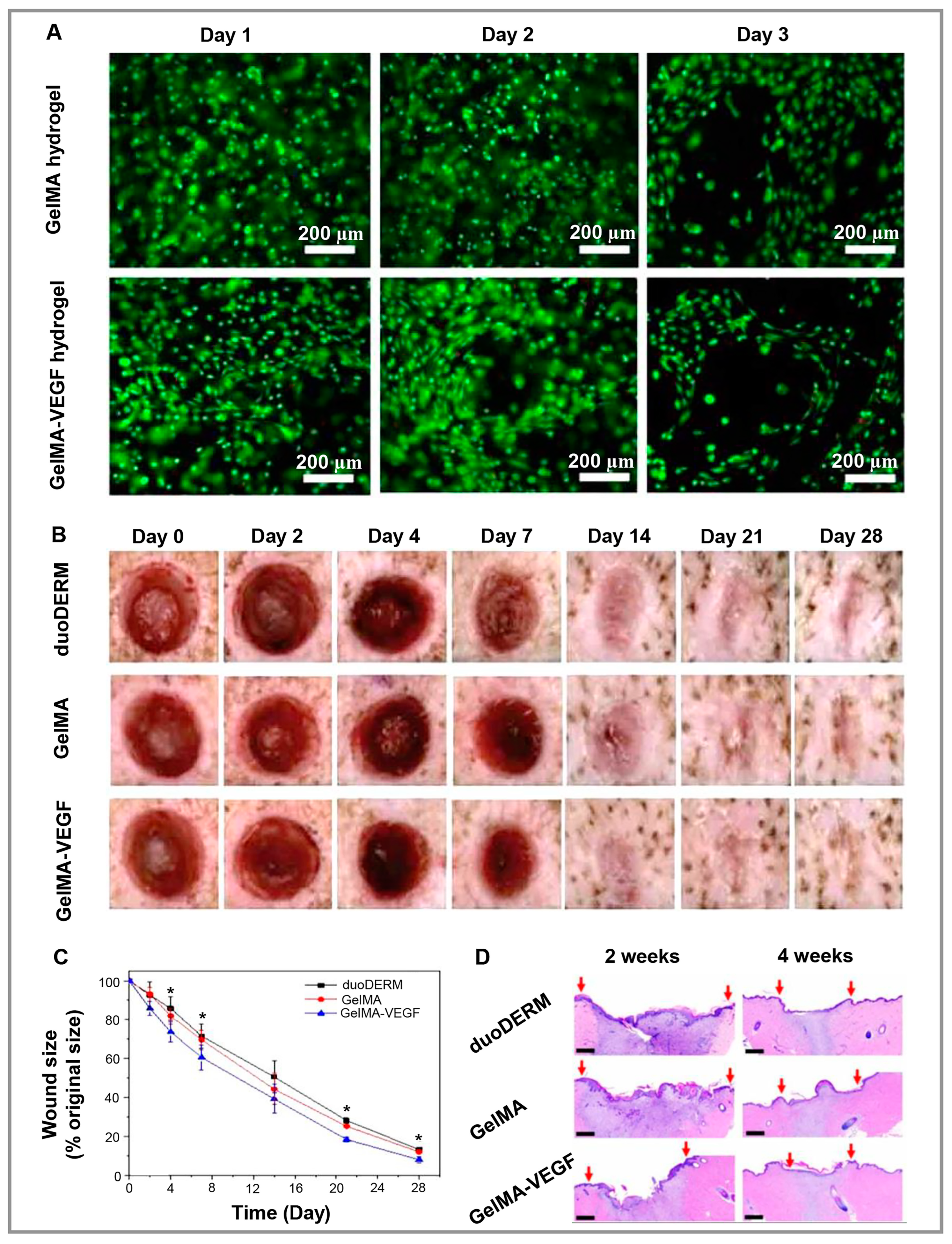
3.5. Skin
4. Future Perspective and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariga, K.; Lvov, Y.; Decher, G. There Is Still Plenty of Room for Layer-by-Layer Assembly for Constructing Nanoarchitectonics-Based Materials and Devices. Phys. Chem. Chem. Phys. 2022, 24, 4097–4115. [Google Scholar] [CrossRef] [PubMed]
- de la Rica, R.; Matsui, H. Applications of Peptide and Protein-Based Materials in Bionanotechnology. Chem. Soc. Rev. 2010, 39, 3499. [Google Scholar] [CrossRef]
- Stephanopoulos, N.; Ortony, J.H.; Stupp, S.I. Self-Assembly for the Synthesis of Functional Biomaterials. Acta Mater. 2013, 61, 912–930. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-G.; Pan, F.; Xia, X.-X. Synthetic Biology for Protein-Based Materials. Curr. Opin. Biotechnol. 2020, 65, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.; Gazit, E.; Knowles, T.P.J. Biomimetic Peptide Self-Assembly for Functional Materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Bicho, D.; Ajami, S.; Liu, C.; Reis, R.L.; Oliveira, J.M. Peptide-Biofunctionalization of Biomaterials for Osteochondral Tissue Regeneration in Early Stage Osteoarthritis: Challenges and Opportunities. J. Mater. Chem. B 2019, 7, 1027–1044. [Google Scholar] [CrossRef]
- Okesola, B.O.; Mata, A. Multicomponent Self-Assembly as a Tool to Harness New Properties from Peptides and Proteins in Material Design. Chem. Soc. Rev. 2018, 47, 3721–3736. [Google Scholar] [CrossRef]
- Bhatia, S.; Udgaonkar, J.B. Heterogeneity in Protein Folding and Unfolding Reactions. Chem. Rev. 2022, 122, 8911–8935. [Google Scholar] [CrossRef]
- Zhao, L.; Qiao, S.; Liu, J. Protein Self-Assembly: Technology and Strategy. Sci. China Chem. 2016, 59, 1531–1540. [Google Scholar] [CrossRef]
- Bai, Y.; Luo, Q.; Liu, J. Protein Self-Assembly via Supramolecular Strategies. Chem. Soc. Rev. 2016, 45, 2756–2767. [Google Scholar] [CrossRef]
- Hainline, K.M.; Fries, C.N.; Collier, J.H. Progress Toward the Clinical Translation of Bioinspired Peptide and Protein Assemblies. Adv. Healthc. Mater. 2018, 7, 1700930. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent Advances of Self-Assembling Peptide-Based Hydrogels for Biomedical Applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.; Zhang, S.; Hauser, C.A.E. Short Self-Assembling Peptides as Building Blocks for Modern Nanodevices. Trends Biotechnol. 2012, 30, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Vilela, C.; Marrucho, I.M.; Freire, C.S.R.; Pascoal Neto, C.; Silvestre, A.J.D. Protein-Based Materials: From Sources to Innovative Sustainable Materials for Biomedical Applications. J. Mater. Chem. B 2014, 2, 3715. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Wang, J.; Li, Z.; Zhang, J.; Li, J. In Silico Design of Photoresponsive Peptide-Based Hydrogel with Controllable Structural and Rheological Properties. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 663, 131020. [Google Scholar] [CrossRef]
- Qing, R.; Hao, S.; Smorodina, E.; Jin, D.; Zalevsky, A.; Zhang, S. Protein Design: From the Aspect of Water Solubility and Stability. Chem. Rev. 2022, 122, 14085–14179. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Yu, S.; Liu, J. Hierarchical Self-Assembly of Proteins Through Rationally Designed Supramolecular Interfaces. Front. Bioeng. Biotechnol. 2020, 8, 295. [Google Scholar] [CrossRef]
- Duro-Castano, A.; Moreira Leite, D.; Forth, J.; Deng, Y.; Matias, D.; Noble Jesus, C.; Battaglia, G. Designing Peptide Nanoparticles for Efficient Brain Delivery. Adv. Drug Deliv. Rev. 2020, 160, 52–77. [Google Scholar] [CrossRef]
- Ghosh, U.; Ghosh, G. Supramolecular Self-Assembled Peptide-Based Nanostructures and Their Applications in Biomedicine. In Pharmaceutical Applications of Supramolecules; Springer International Publishing: Cham, Switzerland, 2022; pp. 241–271. [Google Scholar]
- Pieters, B.J.G.E.; van Eldijk, M.B.; Nolte, R.J.M.; Mecinović, J. Natural Supramolecular Protein Assemblies. Chem. Soc. Rev. 2016, 45, 24–39. [Google Scholar] [CrossRef]
- Schröder, H.V.; Zhang, Y.; Link, A.J. Dynamic Covalent Self-Assembly of Mechanically Interlocked Molecules Solely Made from Peptides. Nat. Chem. 2021, 13, 850–857. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wan, K.; Zhou, N.; Wei, G.; Su, Z. Supramolecular Peptide Nano-Assemblies for Cancer Diagnosis and Therapy: From Molecular Design to Material Synthesis and Function-Specific Applications. J. Nanobiotechnol. 2021, 19, 253. [Google Scholar] [CrossRef]
- Shen, Y.; Levin, A.; Kamada, A.; Toprakcioglu, Z.; Rodriguez-Garcia, M.; Xu, Y.; Knowles, T.P.J. From Protein Building Blocks to Functional Materials. ACS Nano 2021, 15, 5819–5837. [Google Scholar] [CrossRef]
- Qiao, S.; Liu, J. Protein Self-Assembly: Strategies and Applications. In Handbook of Macrocyclic Supramolecular Assembly; Springer: Singapore, 2019; pp. 1–41. [Google Scholar]
- Yao, S.; Brahmi, R.; Bouschon, A.; Chen, J.; Halila, S. Supramolecular Carbohydrate-Based Hydrogels from Oxidative Hydroxylation of Amphiphilic β- C -Glycosylbarbiturates and α-Glucosidase-Induced Hydrogelation. Green Chem. 2023, 25, 330–335. [Google Scholar] [CrossRef]
- Sun, H.; Luo, Q.; Hou, C.; Liu, J. Nanostructures Based on Protein Self-Assembly: From Hierarchical Construction to Bioinspired Materials. Nano Today 2017, 14, 16–41. [Google Scholar] [CrossRef]
- Zeng, R.; Lv, C.; Wang, C.; Zhao, G. Bionanomaterials Based on Protein Self-Assembly: Design and Applications in Biotechnology. Biotechnol. Adv. 2021, 52, 107835. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhang, X.; Liu, Y.; Zhang, T.; Chen, H.; Zang, J.; Zheng, B.; Zhao, G. Redesign of Protein Nanocages: The Way from 0D, 1D, 2D to 3D Assembly. Chem. Soc. Rev. 2021, 50, 3957–3989. [Google Scholar] [CrossRef]
- Edwardson, T.G.W.; Levasseur, M.D.; Tetter, S.; Steinauer, A.; Hori, M.; Hilvert, D. Protein Cages: From Fundamentals to Advanced Applications. Chem. Rev. 2022, 122, 9145–9197. [Google Scholar] [CrossRef]
- Matsubara, S.; Okamoto, Y.; Yoshikawa, M.; Tsukiji, S.; Higuchi, M. A Peptide Nanocage Constructed by Self-Assembly of Oligoproline Conjugates. Bioconjug. Chem. 2022, 33, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Korpi, A.; Ma, C.; Liu, K.; Nonappa; Herrmann, A.; Ikkala, O.; Kostiainen, M.A. Self-Assembly of Electrostatic Cocrystals from Supercharged Fusion Peptides and Protein Cages. ACS Macro Lett. 2018, 7, 318–323. [Google Scholar] [CrossRef]
- Mandal, D.; Nasrolahi Shirazi, A.; Parang, K. Self-Assembly of Peptides to Nanostructures. Org. Biomol. Chem. 2014, 12, 3544–3561. [Google Scholar] [CrossRef]
- Shang, L.; Yu, Y.; Liu, Y.; Chen, Z.; Kong, T.; Zhao, Y. Spinning and Applications of Bioinspired Fiber Systems. ACS Nano 2019, 13, 2749–2772. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Li, S.; Lang, J.C.; Mao, C.; Kroll, P.; Tang, L.; Dong, H. Self-Assembled Peptide Nanofibers Display Natural Antimicrobial Peptides to Selectively Kill Bacteria without Compromising Cytocompatibility. ACS Appl. Mater. Interfaces 2019, 11, 28681–28689. [Google Scholar] [CrossRef]
- Loo, Y.; Zhang, S.; Hauser, C.A.E. From Short Peptides to Nanofibers to Macromolecular Assemblies in Biomedicine. Biotechnol. Adv. 2012, 30, 593–603. [Google Scholar] [CrossRef]
- Hu, T.; Xu, Y.; Xu, G.; Pan, S. Sequence-Selected C 13 -Dipeptide Self-Assembled Hydrogels for Encapsulation of Lemon Essential Oil with Antibacterial Activity. J. Agric. Food Chem. 2022, 70, 7148–7157. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Gazit, E. Self-Assembly of Functional Nanostructures by Short Helical Peptide Building Blocks. Protein Pept. Lett. 2019, 26, 88–97. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to Ultrashort Peptide-Based Hydrogels as a Platform for Biomedical Applications. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Smart Soft-Matter Nanotubes; Nanostructure Science and Technology; Springer: Singapore, 2021; ISBN 978-981-16-2684-5. [Google Scholar]
- Conley, K.M.; Whitehead, M.A.; van de Ven, T.G.M. Linear Growth of Self-Assembled Alternating Oligopeptide Nanotubes with Self-Locking Building Blocks. Mol. Simul. 2019, 45, 549–555. [Google Scholar] [CrossRef]
- Lamas, A.; Guerra, A.; Amorín, M.; Granja, J.R. New Self-Assembling Peptide Nanotubes of Large Diameter Using δ-Amino Acids. Chem. Sci. 2018, 9, 8228–8233. [Google Scholar] [CrossRef]
- Wang, F.; Gnewou, O.; Modlin, C.; Beltran, L.C.; Xu, C.; Su, Z.; Juneja, P.; Grigoryan, G.; Egelman, E.H.; Conticello, V.P. Structural Analysis of Cross α-Helical Nanotubes Provides Insight into the Designability of Filamentous Peptide Nanomaterials. Nat. Commun. 2021, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Katyal, P.; Meleties, M.; Montclare, J.K. Self-Assembled Protein- and Peptide-Based Nanomaterials. ACS Biomater. Sci. Eng. 2019, 5, 4132–4147. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals 2022, 15, 1048. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-H.; Liaw, J. Applications of Cyclic Peptide Nanotubes (CPNTs). J. Food Drug Anal. 2019, 27, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Cheng, Z.; Kariuki, M.; Hall, S.C.L.; Hill, S.K.; Rho, J.Y.; Perrier, S. Molecular Self-Assembly and Supramolecular Chemistry of Cyclic Peptides. Chem. Rev. 2021, 121, 13936–13995. [Google Scholar] [CrossRef]
- Reja, A.; Afrose, S.P.; Das, D. Aldolase Cascade Facilitated by Self-Assembled Nanotubes from Short Peptide Amphiphiles. Angew. Chemie Int. Ed. 2020, 59, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.A.E.; Zhang, S. Designer Self-Assembling Peptide Nanofiber Biological Materials. Chem. Soc. Rev. 2010, 39, 2780. [Google Scholar] [CrossRef]
- Sunna, A.; Care, A.; Bergquist, P.L. (Eds.) Peptides and Peptide-Based Biomaterials and Their Biomedical Applications; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 1030, ISBN 978-3-319-66094-3. [Google Scholar]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef]
- Debele, T.A.; Su, W.-P. Polysaccharide and Protein-Based Functional Wound Dressing Materials and Applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 87–108. [Google Scholar] [CrossRef]
- Sui, Z.; King, W.J.; Murphy, W.L. Protein-Based Hydrogels with Tunable Dynamic Responses. Adv. Funct. Mater. 2008, 18, 1824–1831. [Google Scholar] [CrossRef]
- Bian, Q.; Fu, L.; Li, H. Engineering Shape Memory and Morphing Protein Hydrogels Based on Protein Unfolding and Folding. Nat. Commun. 2022, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Yamada, Y.; Patel, N.L.; Kalen, J.D.; Schneider, J.P. Design of a Peptide-Based Electronegative Hydrogel for the Direct Encapsulation, 3D Culturing, in Vivo Syringe-Based Delivery, and Long-Term Tissue Engraftment of Cells. ACS Appl. Mater. Interfaces 2019, 11, 34688–34697. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Self-Assembling Peptide Nanofiber Hydrogels in Tissue Engineering and Regenerative Medicine: Progress, Design Guidelines, and Applications. J. Biomed. Mater. Res. Part A 2016, 104, 1002–1016. [Google Scholar] [CrossRef]
- Liu, C.; Gong, S.; Du, X.; Liu, Y. Micelle Morphology Phase Diagram in a Phospholipid, PEGylated Lipid, and Peptide Amphiphiles Ternary System. Chem. Eng. Res. Des. 2022, 181, 354–362. [Google Scholar] [CrossRef]
- Gray, V.P.; Amelung, C.D.; Duti, I.J.; Laudermilch, E.G.; Letteri, R.A.; Lampe, K.J. Biomaterials via Peptide Assembly: Design, Characterization, and Application in Tissue Engineering. Acta Biomater. 2022, 140, 43–75. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. The Application of Natural Polymer–Based Hydrogels in Tissue Engineering. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–307. [Google Scholar]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Hamidi, M.; Valentine Okoro, O.; Eskandani, M.; Jaymand, M. Polysaccharide-Based Hydrogels: Properties, Advantages, Challenges, and Optimization Methods for Applications in Regenerative Medicine. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1319–1333. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Liu, C.; Ghorbani, F. Bioactive Inks Development for Osteochondral Tissue Engineering: A Mini-Review. Gels 2021, 7, 274. [Google Scholar] [CrossRef]
- Schloss, A.C.; Williams, D.M.; Regan, L.J. Protein-Based Hydrogels for Tissue Engineering. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 940, pp. 167–177. [Google Scholar]
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous Protein-Based Hydrogels for Cell Encapsulation. Biomaterials 2014, 35, 6727–6738. [Google Scholar] [CrossRef]
- Frederix, P.W.J.M.; Scott, G.G.; Abul-Haija, Y.M.; Kalafatovic, D.; Pappas, C.G.; Javid, N.; Hunt, N.T.; Ulijn, R.V.; Tuttle, T. Exploring the Sequence Space for (Tri-)Peptide Self-Assembly to Design and Discover New Hydrogels. Nat. Chem. 2015, 7, 30–37. [Google Scholar] [CrossRef]
- Liyanage, W.; Ardoña, H.A.M.; Mao, H.-Q.; Tovar, J.D. Cross-Linking Approaches to Tuning the Mechanical Properties of Peptide π-Electron Hydrogels. Bioconjug. Chem. 2017, 28, 751–759. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Mondal, J.H.; Das, D. Peptide Hydrogels. RSC Adv. 2013, 3, 9117. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, M.; Miravet, J.F.; Ulijn, R.V.; Escuder, B. Peptide-Based Molecular Hydrogels as Supramolecular Protein Mimics. Chem.—Eur. J. 2017, 23, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.D.; Roloson, E.B.; Amelung, C.D.; Lampe, K.J. Rapidly Assembling Pentapeptides for Injectable Delivery (RAPID) Hydrogels as Cytoprotective Cell Carriers. ACS Biomater. Sci. Eng. 2019, 5, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-Assembly of Bioactive Peptides, Peptide Conjugates, and Peptide Mimetic Materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.F.; Saiani, A. Engineering Peptide Based Biomaterials. Struct. Prop. Appl. Chem. Today 2010, 28, 34–38. [Google Scholar]
- Priftis, D.; Leon, L.; Song, Z.; Perry, S.L.; Margossian, K.O.; Tropnikova, A.; Cheng, J.; Tirrell, M. Self-Assembly of α-Helical Polypeptides Driven by Complex Coacervation. Angew. Chemie Int. Ed. 2015, 54, 11128–11132. [Google Scholar] [CrossRef]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent Advances in Self-Assembled Peptides: Implications for Targeted Drug Delivery and Vaccine Engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef]
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-Engineered Functional Materials. Adv. Healthc. Mater. 2019, 8, 1801374. [Google Scholar] [CrossRef]
- Xing, R.; Yuan, C.; Li, S.; Song, J.; Li, J.; Yan, X. Charge-Induced Secondary Structure Transformation of Amyloid-Derived Dipeptide Assemblies from β-Sheet to α-Helix. Angew. Chemie Int. Ed. 2018, 57, 1537–1542. [Google Scholar] [CrossRef]
- Saiani, A.; Mohammed, A.; Frielinghaus, H.; Collins, R.; Hodson, N.; Kielty, C.M.; Sherratt, M.J.; Miller, A.F. Self-Assembly and Gelation Properties of α-Helix versus β-Sheet Forming Peptides. Soft Matter 2009, 5, 193–202. [Google Scholar] [CrossRef]
- Ha, M.Y.; Yang, D.H.; You, S.J.; Kim, H.J.; Chun, H.J. In-Situ Forming Injectable GFOGER-Conjugated BMSCs-Laden Hydrogels for Osteochondral Regeneration. npj Regen. Med. 2023, 8, 2. [Google Scholar] [CrossRef]
- Duan, T.; Li, H. In Situ Phase Transition of Elastin-Like Polypeptide Chains Regulates Thermoresponsive Properties of Elastomeric Protein-Based Hydrogels. Biomacromolecules 2020, 21, 2258–2267. [Google Scholar] [CrossRef]
- Behravesh, E.; Zygourakis, K.; Mikos, A.G. Adhesion and Migration of Marrow-Derived Osteoblasts on Injectable in situ Crosslinkable Poly(Propylene Fumarate-Co-Ethylene Glycol)-Based Hydrogels with a Covalently Linked RGDS Peptide. J. Biomed. Mater. Res. 2003, 65A, 260–270. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and Cisplatin Co-Encapsulated Thermosensitive Polypeptide Hydrogels for Combined Immuno-Chemotherapy. J. Control. Release 2017, 255, 81–93. [Google Scholar] [CrossRef]
- Ren, K.; He, C.; Cheng, Y.; Li, G.; Chen, X. Injectable Enzymatically Crosslinked Hydrogels Based on a Poly(l-Glutamic Acid) Graft Copolymer. Polym. Chem. 2014, 5, 5069–5076. [Google Scholar] [CrossRef]
- Toledano, S.; Williams, R.J.; Jayawarna, V.; Ulijn, R.V. Enzyme-Triggered Self-Assembly of Peptide Hydrogels via Reversed Hydrolysis. J. Am. Chem. Soc. 2006, 128, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Debnath, S.; Knapp, C.W.; Ulijn, R.V. Antimicrobial Properties of Enzymatically Triggered Self-Assembling Aromatic Peptide Amphiphiles. Biomater. Sci. 2013, 1, 1138. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Javid, N.; Sefcik, J.; Halling, P.J.; Ulijn, R.V. Salt-Induced Control of Supramolecular Order in Biocatalytic Hydrogelation. Langmuir 2012, 28, 16664–16670. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, W.; Kong, D.; Yang, Z. Dual Enzymes Regulate the Molecular Self-Assembly of Tetra-Peptide Derivatives. Soft Matter 2011, 7, 10443. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, D.; Shi, F.; Xu, T.; Yang, C.; Liu, J.; Wang, L.; Yang, Z. Enzyme-Assisted Peptide Folding, Assembly and Anti-Cancer Properties. Nanoscale 2017, 9, 11987–11993. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gan, S.; Guo, Q.; Zhang, H.; Chen, Q.; Li, H.; Shi, J.; Sun, H. Stimuli-Controlled Peptide Self-Assembly with Secondary Structure Transitions and Its Application in Drug Release. Mater. Chem. Front. 2021, 5, 4664–4671. [Google Scholar] [CrossRef]
- Liang, C.; Yan, X.; Zhang, R.; Xu, T.; Zheng, D.; Tan, Z.; Chen, Y.; Gao, Z.; Wang, L.; Li, X.; et al. Enhanced Cellular Uptake and Nuclear Accumulation of Drug-Peptide Nanomedicines Prepared by Enzyme-Instructed Self-Assembly. J. Control. Release 2020, 317, 109–117. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Qin, M.; Cao, Y.; Wang, W. Photo-Cross-Linking Approach to Engineering Small Tyrosine-Containing Peptide Hydrogels with Enhanced Mechanical Stability. Langmuir 2013, 29, 13299–13306. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, M.; Liao, C.; Wu, Q.; Liu, M.; Zhang, Y.; Wu, C.; Cheng, L.; Wang, Q. Printable Hybrid Hydrogel by Dual Enzymatic Polymerization with Superactivity. Chem. Sci. 2016, 7, 2748–2752. [Google Scholar] [CrossRef]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Xing, R.; Li, S.; Zhang, N.; Shen, G.; Möhwald, H.; Yan, X. Self-Assembled Injectable Peptide Hydrogels Capable of Triggering Antitumor Immune Response. Biomacromolecules 2017, 18, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, X. Self-Assemble Peptide Biomaterials and Their Biomedical Applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Dube, T.; Panda, J.J. Anti-Glioma Activity Achieved by Dual Blood–Brain Barrier/Glioma Targeting Naive Chimeric Peptides-Based Co-Assembled Nanophototheranostics. Pharmaceutics 2023, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Rao, P.; Jadhav, G.S.; Kulkarni, B.; Kanakavalli, N.; Kirad, S.; Salunke, S.; Tanpure, V.; Sahu, B. Emerging Role of Injectable Dipeptide Hydrogels in Biomedical Applications. ACS Omega 2023, 8, 3551–3570. [Google Scholar] [CrossRef]
- Chai, Y.; Long, Y.; Dong, X.; Liu, K.; Wei, W.; Chen, Y.; Qiu, T.; Dai, H. Improved Functional Recovery of Rat Transected Spinal Cord by Peptide-Grafted PNIPAM Based Hydrogel. Colloids Surfaces B Biointerfaces 2022, 210, 112220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chai, Y.; Zhao, H.; Yang, S.; Liu, W.; Yang, Z.; Ye, W.; Wang, C.; Gao, X.; Kong, X.; et al. Crosstalk between PC12 Cells and Endothelial Cells in an Artificial Neurovascular Niche Constructed by a Dual-Functionalized Self-Assembling Peptide Nanofiber Hydrogel. Nano Res. 2022, 15, 1433–1445. [Google Scholar] [CrossRef]
- Hivare, P.; Gangrade, A.; Swarup, G.; Bhavsar, K.; Singh, A.; Gupta, R.; Thareja, P.; Gupta, S.; Bhatia, D. Peptide Functionalized DNA Hydrogel Enhances Neuroblastoma Cell Growth and Differentiation. Nanoscale 2022, 14, 8611–8620. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, A.; Oshikawa, M.; Watanabe, G.; Hiramatsu, H.; Uchida, N.; Hara, C.; Kaneko, N.; Sawamoto, K.; Muraoka, T.; Ajioka, I. Efficient Protein Incorporation and Release by a Jigsaw-Shaped Self-Assembling Peptide Hydrogel for Injured Brain Regeneration. Nat. Commun. 2021, 12, 6623. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, T.M.; Baron-Heeris, D.; Houwers, I.G.J.; Keenan, R.; Williams, R.J.; Nisbet, D.R.; Harvey, A.R.; Hodgetts, S.I. Peptide Hydrogel Scaffold for Mesenchymal Precursor Cells Implanted to Injured Adult Rat Spinal Cord. Tissue Eng. Part A 2021, 27, 993–1007. [Google Scholar] [CrossRef]
- Ji, W.; Álvarez, Z.; Edelbrock, A.N.; Sato, K.; Stupp, S.I. Bioactive Nanofibers Induce Neural Transdifferentiation of Human Bone Marrow Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 41046–41055. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, Z.; Zhong, F.; Wang, B.; Liu, L.; Ma, W.; Yu, C.; Wei, H. A Dual Crosslinked Hydrogel-Mediated Integrated Peptides and BMSC Therapy for Myocardial Regeneration. J. Control. Release 2022, 347, 127–142. [Google Scholar] [CrossRef]
- Zhan, J.; Liao, X.; Fan, X.; Zhang, J.; Li, H.; Cai, Y.; Qiu, X. An Injectable and Conductive TEMPOL/Polypyrrole Integrated Peptide Co-Assembly Hydrogel Promotes Functional Maturation of Cardiomyocytes for Myocardial Infarction Repair. Compos. Part B Eng. 2022, 236, 109794. [Google Scholar] [CrossRef]
- Alheib, O.; da Silva, L.P.; Caballero, D.; Pires, R.A.; Kundu, S.C.; Correlo, V.M.; Reis, R.L. Micropatterned Gellan Gum-Based Hydrogels Tailored with Laminin-Derived Peptides for Skeletal Muscle Tissue Engineering. Biomaterials 2021, 279, 121217. [Google Scholar] [CrossRef] [PubMed]
- Sleep, E.; Cosgrove, B.D.; McClendon, M.T.; Preslar, A.T.; Chen, C.H.; Sangji, M.H.; Pérez, C.M.R.; Haynes, R.D.; Meade, T.J.; Blau, H.M.; et al. Injectable Biomimetic Liquid Crystalline Scaffolds Enhance Muscle Stem Cell Transplantation. Proc. Natl. Acad. Sci. USA 2017, 114, E7919–E7928. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Yang, Z.; Cao, F.; Li, H.; Zhao, T.; Zhang, H.; Zhang, Z.; Yang, S.; Zhu, J.; Liu, Z.; et al. Articular Cartilage Reconstruction with TGF-Β1-Simulating Self-Assembling Peptide Hydrogel-Based Composite Scaffold. Acta Biomater. 2022, 146, 94–106. [Google Scholar] [CrossRef]
- Thomas, J.; Gupta, N.; Joseph, J.P.; Chopra, V.; Pal, A.; Ghosh, D. Mechanical Integrity in a Dynamic Interpenetrating Hydrogel Network of Supramolecular Peptide–Polysaccharide Supports Enhanced Chondrogenesis. ACS Biomater. Sci. Eng. 2021, 7, 5798–5809. [Google Scholar] [CrossRef]
- Dufour, A.; Lafont, J.E.; Buffier, M.; Verset, M.; Cohendet, A.; Contamin, H.; Confais, J.; Sankar, S.; Rioult, M.; Perrier-Groult, E.; et al. Repair of Full-Thickness Articular Cartilage Defects Using IEIK13 Self-Assembling Peptide Hydrogel in a Non-Human Primate Model. Sci. Rep. 2021, 11, 4560. [Google Scholar] [CrossRef]
- Zanotto, G.M.; Liesbeny, P.; Barrett, M.; Zlotnick, H.; Frank, E.; Grodzinsky, A.J.; Frisbie, D.D. Microfracture Augmentation with Trypsin Pretreatment and Growth Factor–Functionalized Self-Assembling Peptide Hydrogel Scaffold in an Equine Model. Am. J. Sports Med. 2021, 49, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Stüdle, C.; Vallmajó-Martín, Q.; Haumer, A.; Guerrero, J.; Centola, M.; Mehrkens, A.; Schaefer, D.J.; Ehrbar, M.; Barbero, A.; Martin, I. Spatially Confined Induction of Endochondral Ossification by Functionalized Hydrogels for Ectopic Engineering of Osteochondral Tissues. Biomaterials 2018, 171, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, C.; Chen, J.; Luo, H.; Li, R.; Chen, D.; Zou, X.; Wang, W. Synergistic Osteogenic and Angiogenic Effects of KP and QK Peptides Incorporated with an Injectable and Self-Healing Hydrogel for Efficient Bone Regeneration. Bioact. Mater. 2022, 18, 267–283. [Google Scholar] [CrossRef]
- Panek, M.; Antunović, M.; Pribolšan, L.; Ivković, A.; Gotić, M.; Vukasović, A.; Caput Mihalić, K.; Pušić, M.; Jurkin, T.; Marijanović, I. Bone Tissue Engineering in a Perfusion Bioreactor Using Dexamethasone-Loaded Peptide Hydrogel. Materials 2019, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Qi, Y.; Zhao, Y.; Nie, G.; Wang, X.; Zheng, S. Self-assembled Peptide Hydrogel Scaffolds with VEGF and BMP-2 Enhanced in Vitro Angiogenesis and Osteogenesis. Oral Dis. 2022, 28, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.; Susapto, H.H.; Hauser, C.A.E. Scaffolds from Self-Assembling Tetrapeptides Support 3D Spreading, Osteogenic Differentiation, and Angiogenesis of Mesenchymal Stem Cells. Biomacromolecules 2021, 22, 2094–2106. [Google Scholar] [CrossRef]
- Halperin-Sternfeld, M.; Pokhojaev, A.; Ghosh, M.; Rachmiel, D.; Kannan, R.; Grinberg, I.; Asher, M.; Aviv, M.; Ma, P.X.; Binderman, I.; et al. Immunomodulatory fibrous hyaluronic acid-Fmoc-diphenylalanine-based hydrogel induces bone regeneration. J. Clin. Periodontol. 2023, 50, 200–219. [Google Scholar] [CrossRef]
- Jang, M.J.; Bae, S.K.; Jung, Y.S.; Kim, J.C.; Kim, J.S.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced Wound Healing Using a 3D Printed VEGF-Mimicking Peptide Incorporated Hydrogel Patch in a Pig Model. Biomed. Mater. 2021, 16, 045013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ouyang, Q.; Hu, Z.; Lu, S.; Quan, W.; Li, P.; Chen, Y.; Li, S. Catechol Functionalized Chitosan/Active Peptide Microsphere Hydrogel for Skin Wound Healing. Int. J. Biol. Macromol. 2021, 173, 591–606. [Google Scholar] [CrossRef]
- Roy, T.; James, B.D.; Allen, J.B. Anti-VEGF-R2 Aptamer and RGD Peptide Synergize in a Bifunctional Hydrogel for Enhanced Angiogenic Potential. Macromol. Biosci. 2021, 21, 2000337. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Buffier, M.; Vertu-Ciolino, D.; Disant, F.; Mallein-Gerin, F.; Perrier-Groult, E. Combination of Bioactive Factors and IEIK13 Self-Assembling Peptide Hydrogel Promotes Cartilage Matrix Production by Human Nasal Chondrocytes. J. Biomed. Mater. Res. Part A 2019, 107, 893–903. [Google Scholar] [CrossRef]
- Liebesny, P.H.; Mroszczyk, K.; Zlotnick, H.; Hung, H.-H.; Frank, E.; Kurz, B.; Zanotto, G.; Frisbie, D.; Grodzinsky, A.J. Enzyme Pretreatment plus Locally Delivered HB-IGF-1 Stimulate Integrative Cartilage Repair In Vitro. Tissue Eng. Part A 2019, 25, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, L.D.; Iaccarino, G.; Fattorusso, R.; Sorriento, D.; Carannante, C.; Capasso, D.; Trimarco, B.; Pedone, C. Targeting Angiogenesis: Structural Characterization and Biological Properties of a de Novo Engineered VEGF Mimicking Peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 14215–14220. [Google Scholar] [CrossRef]
| Main Material | Advantages | Disadvantages |
|---|---|---|
| Polymers | Biocompatible, Available, Abundance, Easy interaction with cells [63,64] | Need to be manipulated to achieve favorable properties, The synthesis process can affect the final properties, Achieving desired features takes time, Limitations to single-component using [63] |
| Proteins | Biocompatible, Bioactive [65]- Easy interaction with cells, Similar mechanical, chemical, and structural properties to the ECM, Easy degradation by proteolytic enzymes [66,67] | Proteins must be unfolded to become a hydrogel, Some of physicochemical properties are lost during hydrogelation [56] |
| Peptides | Biocompatible, Bioactive, The properties of the final hydrogel can be predicted by arranging the appropriate sequence, Easy interaction with cells [68] | Low mechanical properties [69] |
| Target Tissue | Peptides | Other Components | Effects of Peptide | Ref. |
|---|---|---|---|---|
| Nerve | IKVAV | PNIPAM-b-poly(AC-PEG-COOH) and PNIPAM-b-poly(AC-PEG-IKVAV) | Angiogenic factors expression, Decreasing pro-inflammatory factors, Inhibition of glial scar tissue regrowth | [100] |
| RGI and KLT | RADA | Outgrow PC12 cells, Improving the tube-like formation of HUVECs, Facilitation neurovascular crosstalk | [101] | |
| IKVAV | ssDNA | Cytoskeleton and microtubules differentiation, Longer neurites and modified endocytic mechanisms | [102] | |
| Ac-RIDARMRADIR-NH2 | Glycophorin A (GYPA) | Controlled release of VEGF, Cell transplantation-free regenerative capabilities | [103] | |
| Fmoc-DIKVAV | - | Promoting cell adhesion and growth, angiogenesis, and neurite outgrowth, Reducing cell death, astrogliosis, Enhancing function in SCI mice. | [104] | |
| IKVAV-PA and VKIVA-PA | - | Enhancing the function of endogenous or transplanted BMSCs | [105] | |
| Muscle | GGR-KLT | MaHA and Gelatin | Adequate prevention of uncontrolled matrix degradation, Improvements in angiogenesis, Improving myocardial regeneration over 28 days | [106] |
| TEMPOL | Polypyrrole | Enhancing cardiomyocytes’ contractile and electrical properties | [107] | |
| KNRLTIELEVRTC, CIKVAVS, and RKRLQVQLSIRTC | GeG | Differentiation and spreading of C2C12 | [108] | |
| PA | - | Providing myogenic progenitor cells with a suitable biological environment for the survival, maturation, and regeneration of muscle tissue | [109] | |
| Cartilage | RAD-CM | Decellularized Cartilage ECM | Chondrogenic gene expression and ECM deposition | [110] |
| NVFFAC | carboxymethyl cellulose dialdehyde and carboxymethyl chitosan | Human chondrocytes were stimulated to undergo in vitro chondrogenesis using hydrogels, and the authors observed enhanced cell growth and cartilage-specific ECM secretion | [111] | |
| IEIK13 | BMP-2, T3 | Regeneration of full-thickness cartilage injuries | [112] | |
| [KLDL]3 | PDGF-BB, HB-IGF-1 | Better integration of newly produced cartilage compared with microfracture alone | [113] | |
| FKGG-GPQGIWGQ-ERCG | PEG, BMP-2, TGF-3 | Formation of osteoblast-like structures and cartilage | [114] | |
| Bone | QK and KP | GelMA, ODex | Considerable increase in the osteogenic differentiation of BMSCs and the angiogenesis capability of HUVECs | [115] |
| [COCH3]-RADARADARADARADA-[CONH2] | DEX | Bone tissue regeneration for 21 days with the DEX perfusion rate of 0.1 mL/min | [116] | |
| RATEA16 | rhVEGF165 and BMP-2 | Able to deliver growth factors quickly and precisely, Bone regeneration | [117] | |
| IVFK and IVZK | - | Cell adhesion and spreading of hMSCs | [118] | |
| FmocFF | HA | Facilitating osteogenic differentiation of MC3T3-E1 preosteoblasts and calcium deposition, Regeneration of ~93% of bone mass | [119] | |
| Skin | Ac-KVKFMDVYQRSYCHP-amide | GelMA, VEGF | Cell proliferation, Viability, Tubular structure formation | [120] |
| Oyster | Chitosan, Glycerin | Inhibiting the formation of a variety of inflammatory cells, Stimulating collagen fiber formation and new blood vessel formation, Regulating Ki-67 and VEGF expression in the wound, Enhancing the biosynthesis of total protein in the granulation tissue | [121] | |
| RGD | tHA-PEGDA, anti-VEGF-R2 DNA | Enhancing cell growth | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtiary, N.; Ghalandari, B.; Ghorbani, F.; Varma, S.N.; Liu, C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers 2023, 15, 1068. https://doi.org/10.3390/polym15051068
Bakhtiary N, Ghalandari B, Ghorbani F, Varma SN, Liu C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers. 2023; 15(5):1068. https://doi.org/10.3390/polym15051068
Chicago/Turabian StyleBakhtiary, Negar, Behafarid Ghalandari, Farnaz Ghorbani, Swastina Nath Varma, and Chaozong Liu. 2023. "Advances in Peptide-Based Hydrogel for Tissue Engineering" Polymers 15, no. 5: 1068. https://doi.org/10.3390/polym15051068
APA StyleBakhtiary, N., Ghalandari, B., Ghorbani, F., Varma, S. N., & Liu, C. (2023). Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers, 15(5), 1068. https://doi.org/10.3390/polym15051068








