Effect of GO on the Structure and Properties of PEG/Biochar Phase Change Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
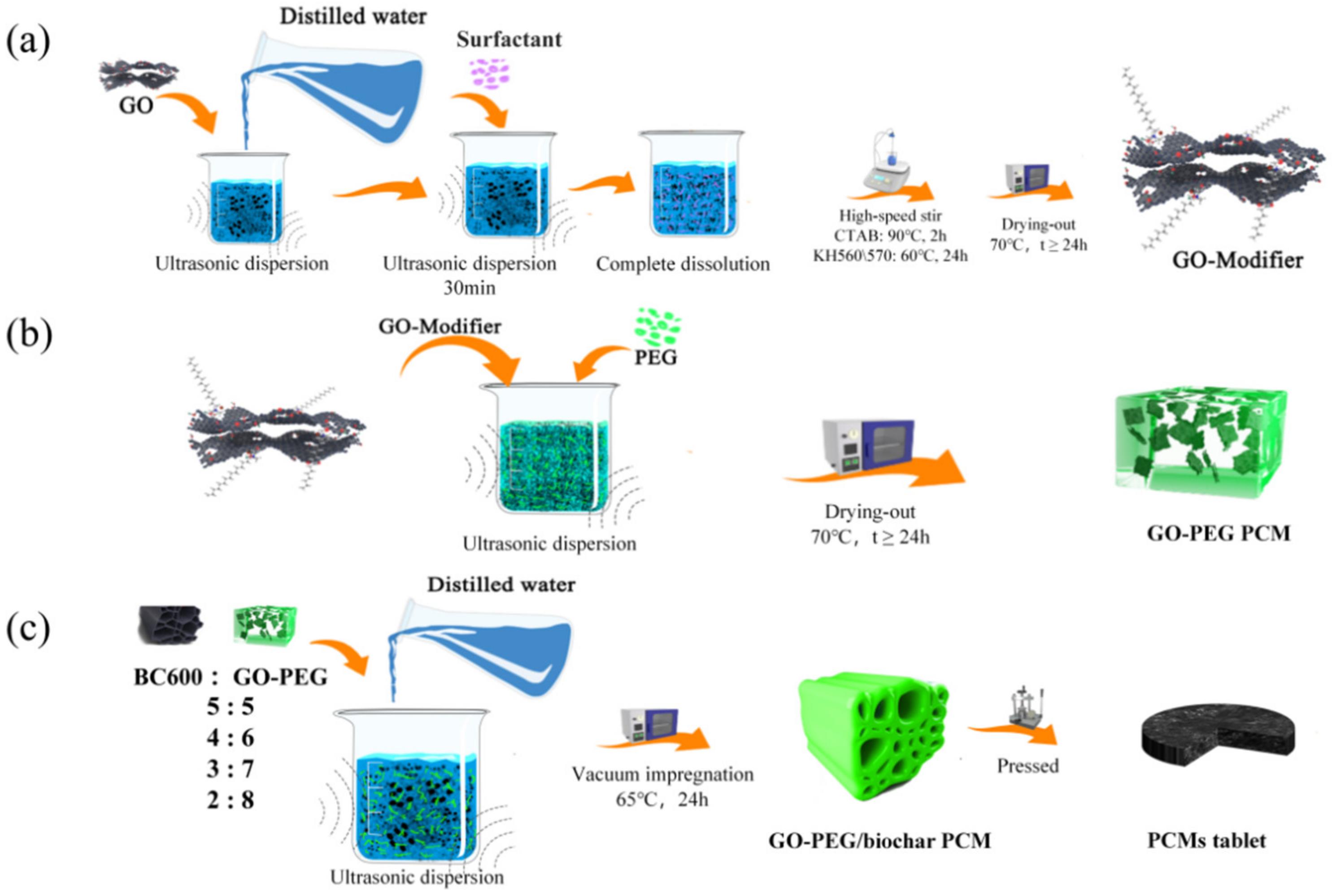
2.2.1. GO-Modifiers
2.2.2. GO-PEG PCMs
2.2.3. GO-PEG/Biochar PCMs
2.3. Material Characterizations
3. Results and Discussion
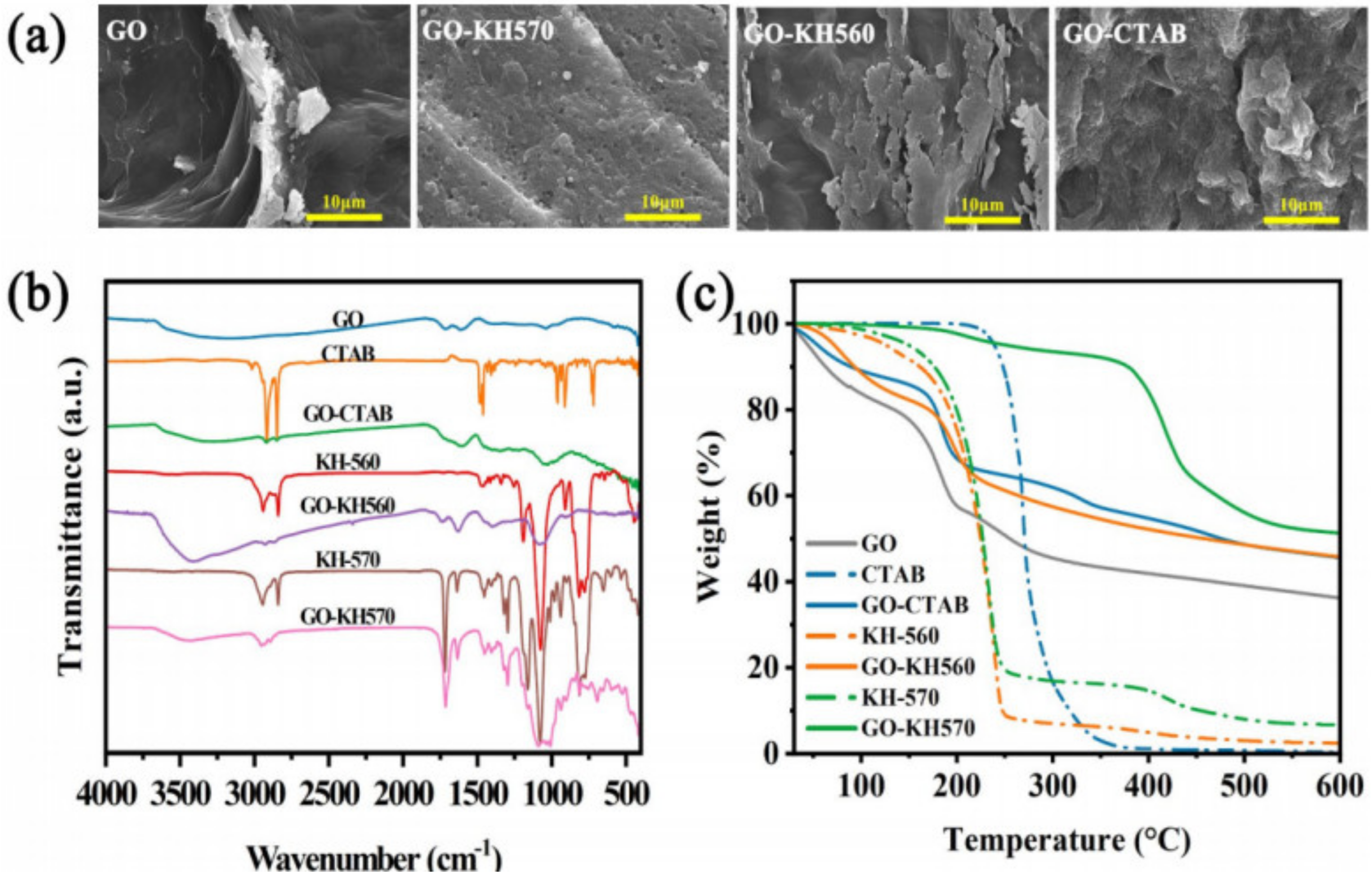
3.1. Microstructure and Thermal Stability of GO-Modifiers
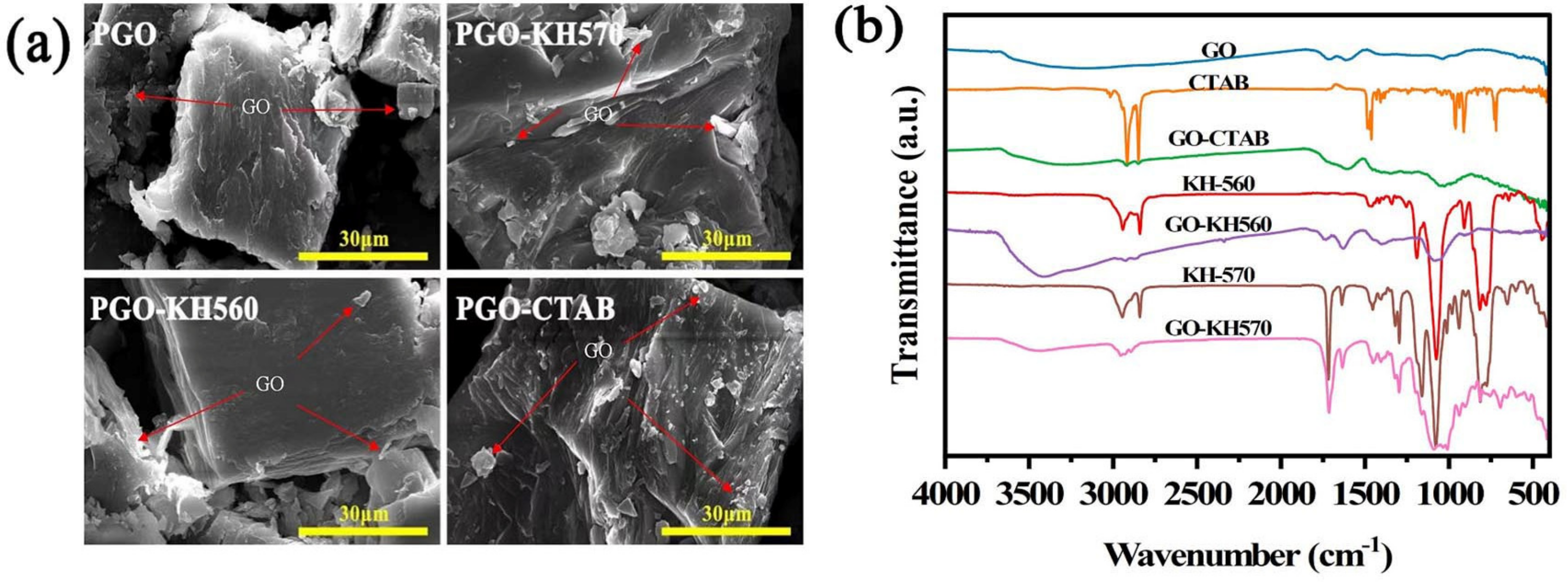
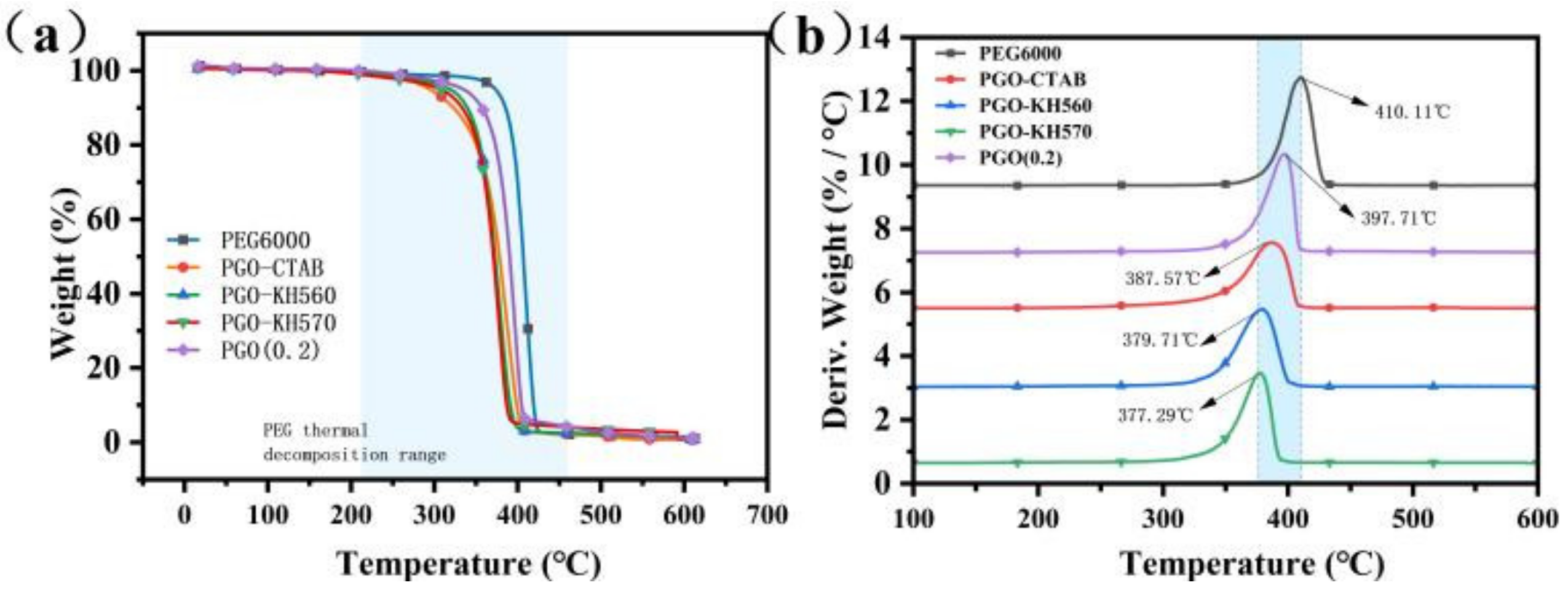
3.2. Microstructure and Thermal Property of GO-PEG PCMs
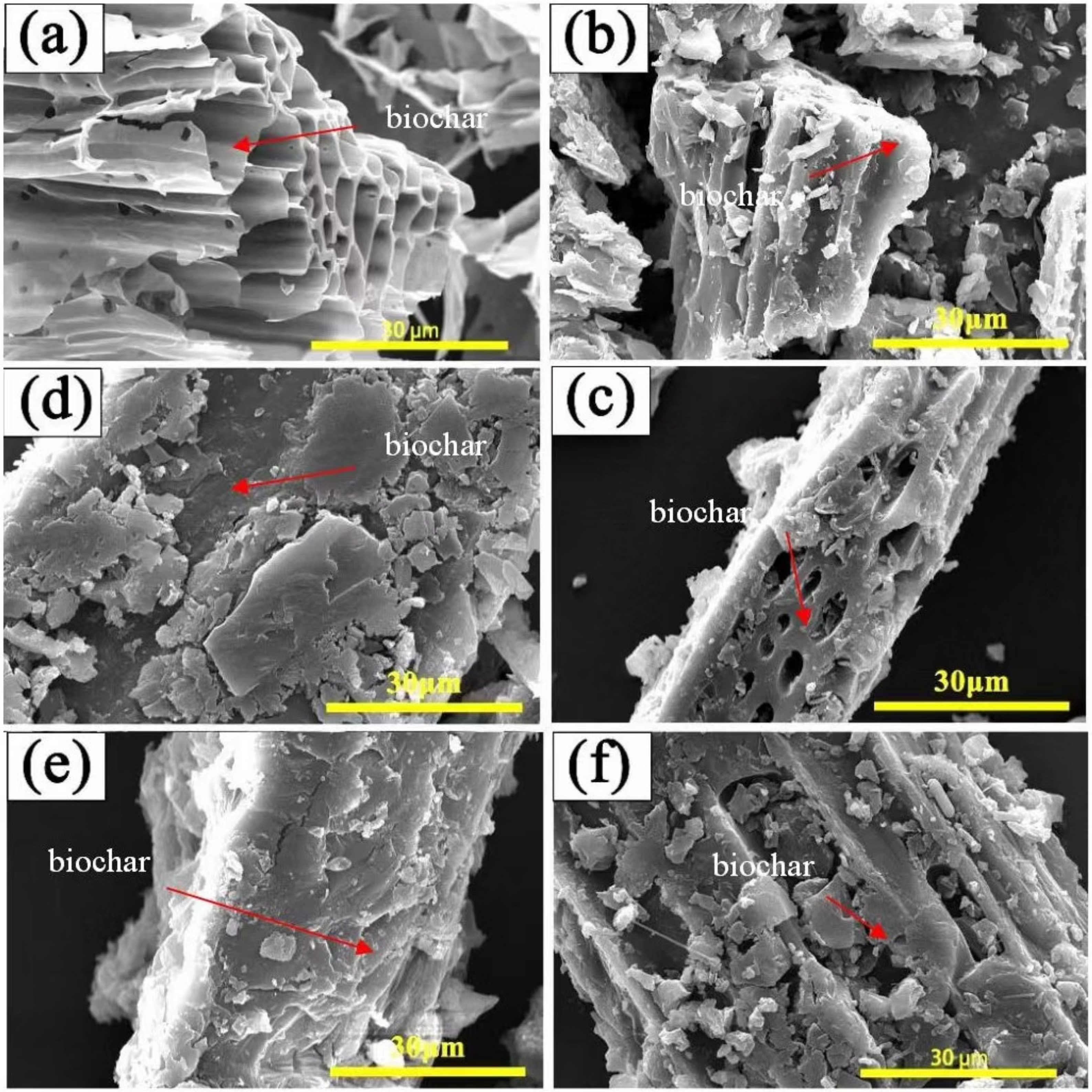
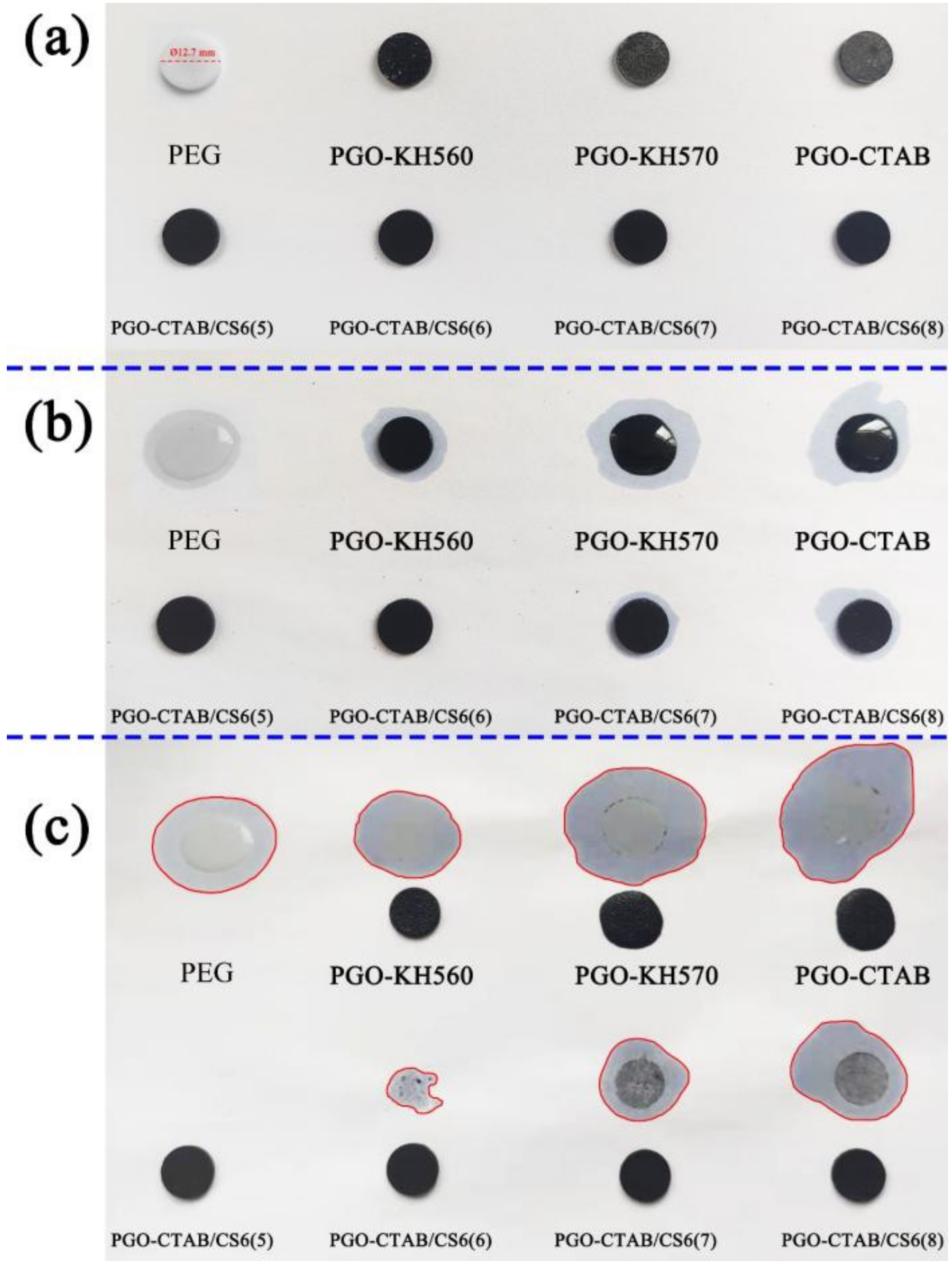
3.3. Microstructure and Thermal Performance of GO-PEG/Biochar PCMs
3.4. Thermal Conductivity and Photo-Thermal Conversion Performance of GO-PEG/Biochar PCMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, X.; Xu, Q.; Zhang, Y.; Zhang, Y.; Yan, Y.; Liu, T. Fabrication and Properties of Micro-Nanoencapsulated Phase Change Materials for Internally-Cooled Liquid Desiccant Dehumidification. Nanomaterials 2017, 7, 96. [Google Scholar] [CrossRef]
- Min, P.; Liu, J.; Li, X.F.; An, F.; Liu, P.F.; Shen, Y.X.; Koratkar, N.; Yu, Z.Z. Thermally Conductive Phase Change Composites Featuring Anisotropic Graphene Aerogels for Real-Time and Fast-Charging Solar-Thermal Energy Conversion. Adv. Funct. Mater 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Amaral, C.; Vicente, R.; Marques, P.A.A.P.; Barros-Timmons, A. Phase change materials and carbon nanostructures for thermal energy storage: A literature review. Renew. Sustain. Energy Rev. 2017, 79, 1212–1228. [Google Scholar] [CrossRef]
- Khairy, M.; Yehia, M.; Kamar, E.M.; Masoud, E.M. High Removal Efficiency of Methyl Orange Dye by Pure and (Cu, N) Doped TiO2/Polyaniline Nanocomposites. Biointerface Res. Appl. Chem. 2021, 12, 893–909. [Google Scholar]
- Du, X.S.; Xu, J.I.; Deng, S.; Du, Z.L.; Cheng, X.; Wang, H.B. Amino-Functionalized Single-Walled Carbon Nanotubes-Integrated Polyurethane Phase Change Composites with Superior Photothermal Conversion Efficiency and Thermal Conductivity. Acs Sustain. Chem. Eng. 2019, 7, 17682–17690. [Google Scholar] [CrossRef]
- Liu, H.; Niu, J.F.; Wang, X.D.; Wu, D.Z. Design and construction of mesoporous silica/n-eicosane phase-change nanocomposites for supercooling depression and heat transfer enhancement. Energy 2019, 188, 1–15. [Google Scholar] [CrossRef]
- Yi, H.; Zhan, W.Q.; Zhao, Y.L.; Qu, S.G.; Wang, W.; Chen, P.; Song, S.X. A novel core-shell structural montmorillonite nanosheets/stearic acid composite PCM for great promotion of thermal energy storage properties. Sol. Energy Mat. Sol. Cells 2019, 192, 57–64. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage–A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A.; Biwole, P.H. Heat transfer study of phase change materials with graphene nano particle for thermal energy storage. Sol. Energy 2017, 146, 453–463. [Google Scholar] [CrossRef]
- Aadmi, M.; Karkri, M.; El Hammouti, M. Heat transfer characteristics of thermal energy storage for PCM (phase change material) melting in horizontal tube: Numerical and experimental investigations. Energy 2015, 85, 339–352. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Z.; Li, A.; Zhang, Y.-F. RGO-Coated Polyurethane Foam/Segmented Polyurethane Composites as Solid–Solid Phase Change Thermal Interface Material. Polymers 2020, 12, 3004. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Pei, D.F.; Chen, S.; Cao, R.R.; Zhang, X.X. Fabrication and characterization of diethylene glycol hexadecyl ether-grafted graphene oxide as a form-stable phase change material. Thermochim. Acta 2018, 661, 166–173. [Google Scholar] [CrossRef]
- Cao, Y.F.; Fan, D.L.; Lin, S.H.; Mu, L.Y.; Ng, F.T.T.; Pan, Q.M. Phase change materials based on comb-like polynorbornenes and octadecylamine-functionalized graphene oxide nanosheets for thermal energy storage. Chem. Eng. J. 2020, 389, 1–10. [Google Scholar] [CrossRef]
- Suter, J.L.; Sinclair, R.C.; Coveney, P.V. Principles Governing Control of Aggregation and Dispersion of Graphene and Graphene Oxide in Polymer Melts. Adv. Mater. 2020, 32, 2003213. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Li, H.; Cui, Y.; Li, X. Enhanced thermal properties for nanoencapsulated phase change materials with functionalized graphene oxide (FGO) modified PMMA. Nanotechnology 2020, 31, 295704. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, Q.; Xu, F.; Sun, L.; Xia, Y.; Guan, Y.; Liao, L.; Zhou, T.; Lao, J.; Wang, Y.; et al. Fabricated Polyethylene glycol/ hydroxylated carbon nanotubes shape-stabilized phase change materials with improving thermal conductivity. Thermochim. Acta 2022, 718, 179363. [Google Scholar] [CrossRef]
- Chauhan, S.; Singh, R.; Sharma, K. Volumetric, compressibility, surface tension and viscometric studies of CTAB in aqueous solutions of polymers (PEG and PVP) at different temperatures. J. Chem. Thermodyn. 2016, 103, 381–394. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Lu, W.; Tian, Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs). Sol. Energy 2010, 84, 1402–1412. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Wi, S.; Yang, S.; Ok, Y.S.; Kim, S. Latent heat storage biocomposites of phase change material-biochar as feasible eco-friendly building materials. Environ. Res. 2019, 172, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zou, M.; Xie, Y.; Chen, W.; Hu, X.; Ma, Y.; Zu, S.; Che, Y.; Jiang, X. Nanosilver modified navel orange peel foam/polyethylene glycol composite phase change materials with improved thermal conductivity and photo-thermal conversion efficiency. J. Energy Storage 2022, 56, 105976. [Google Scholar] [CrossRef]
- He, S.; Xin, B.; Chen, Z.; Liu, Y. Functionalization of cotton by reduced graphene oxide for improved electrical conductivity. Text. Res. J. 2018, 89, 1038–1050. [Google Scholar] [CrossRef]
- Albojamal, A.; Hamzah, H.; Vafai, K. Energy storage analysis of phase change materials (PCMs) integrated with thermal conductivity enhancers (TCEs). Numer. Heat Transfer Part A Appl. 2022, 83, 1–18. [Google Scholar] [CrossRef]
- Ma, A.; Cai, C.; Peng, S.; Zhou, T. Combined measurement and calibration method for the thermal conductivity of phase change materials. Int. J. Therm. Sci. 2023, 184, 107987. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Khalid, M.; Goyal, R.; Sarı, A.; Tyagi, V.V. Evaluation of carbon based-supporting materials for developing form-stable organic phase change materials for thermal energy storage: A review. Sol. Energy Mat. Sol. Cells 2022, 246, 111896. [Google Scholar] [CrossRef]
- Liu, S.W.; Peng, S.G.; Zhang, B.B.; Xue, B.; Yang, Z.; Wang, S.; Xu, G.M. Effects of biochar pyrolysis temperature on thermal properties of polyethylene glycol/biochar composites as shape-stable biocomposite phase change materials. RSC Adv. 2022, 12, 9587–9598. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhang, D. Preparation and Thermal Properties of Graphene Oxide–Microencapsulated Phase Change Materials. Nanoscale Microscale Thermophys. Eng. 2016, 20, 147–157. [Google Scholar] [CrossRef]
- Qian, T.T.; Li, J.H.; Ma, H.W.; Yang, J. Adjustable thermal property of polyethylene glycol/diatomite shape-stabilized composite phase change material. Polym. Compos. 2016, 37, 854–860. [Google Scholar] [CrossRef]
- Wang, C.Y.; Feng, L.L.; Li, W.; Zheng, J.; Tian, W.H.; Li, X.G. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Wang, X.D.; Wu, D.Z. Silica encapsulation of n-octadecane via sol-gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Yu, S.Y.; Wang, X.D.; Wu, D.Z. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Voronin, D.V.; Mendgaziev, R.I.; Rubtsova, M.I.; Cherednichenko, K.A.; Demina, P.A.; Abramova, A.M.; Shchukin, D.G.; Vinokurov, V. Facile synthesis of shape-stable phase-change composites via the adsorption of stearic acid onto cellulose microfibers. Mater. Chem. Front. 2022, 6, 1033–1045. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Huang, H.; Dong, D.; Zhang, X.; Zhang, L.; Chen, Y.; Sheng, X.; Lu, X. Bio-Based Radish@PDA/PEG Sandwich Composite with High Efficiency Solar Thermal Energy Storage. Acs Sustain. Chem. Eng. 2020, 8, 8448–8457. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Engineering Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Yang, X.; Zhang, X.; Ma, B. Simple in situ synthesis of SiC nanofibers on graphite felt as a scaffold for improving performance of paraffin-based composite phase change materials. RSC Adv. 2022, 12, 878–887. [Google Scholar] [CrossRef]
- Maleki, M.; Karimian, H.; Shokouhimehr, M.; Ahmadi, R.; Valanezhad, A.; Beitollahi, A. Development of graphitic domains in carbon foams for high efficient electro/photo-to-thermal energy conversion phase change composites. Chem. Eng. J. 2019, 362, 469–481. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Chang, S.J.; Kim, K.-H.; Dong, W.; Kim, S. A novel enhancement of shape/thermal stability and energy-storage capacity of phase change materials through the formation of composites with 3D porous (3,6)-connected metal–organic framework. Chem. Eng. J. 2020, 389, 124430. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Tafrishi, H.; Sadeghzadeh, S.; Ahmadi, R. Molecular dynamics simulations of phase change materials for thermal energy storage: A review. RSC Adv. 2022, 12, 14776–14807. [Google Scholar] [CrossRef]
- Cao, Q.Y.; He, F.F.; Xie, C.Q.; Fan, J.H.; Wu, J.Y.; Zhang, K.; Yang, Z.J.; Yang, W.B. Paraffin-based shape-stable phase change materials with graphene/carbon nanotube three-dimensional network structure. Fuller. Nanotub. Carbon Nanostructures 2019, 27, 492–497. [Google Scholar] [CrossRef]
- Qi, G.; Yang, J.; Bao, R.; Xia, D.; Cao, M.; Yang, W.; Yang, M.; Wei, D. Hierarchical graphene foam-based phase change materials with enhanced thermal conductivity and shape stability for efficient solar-to-thermal energy conversion and storage. Nano Res. 2017, 10, 802–813. [Google Scholar] [CrossRef]
- Tan, Y.; Du, X.; Du, Z.; Wang, H.; Cheng, X. Form-stable phase change composites based on nanofibrillated cellulose/polydopamine hybrid aerogels with extremely high energy storage density and improved photothermal conversion efficiency. RSC Adv. 2021, 11, 5712–5721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Juan, S.; Yi, W.; Chen, Z. A novel form-stable phase change material based on halloysite nanotube for thermal energy storage. J. Energy Storage 2022, 45, 103703. [Google Scholar] [CrossRef]
- Xu, Y.F.; Wang, X.X.; Zhou, J.W.; Song, B.; Jiang, Z.; Lee, E.M.Y.; Huberman, S.; Gleason, K.K.; Chen, G. Molecular engineered conjugated polymer with high thermal conductivity. Sci. Adv. 2018, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]


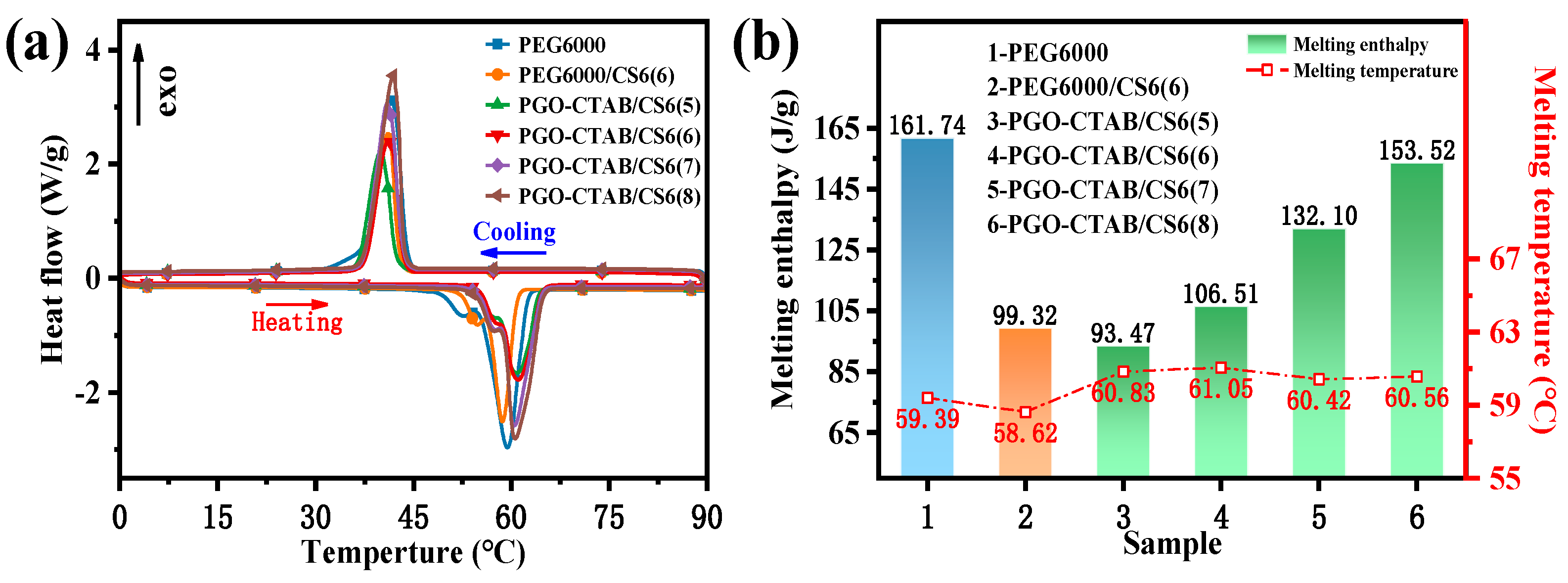
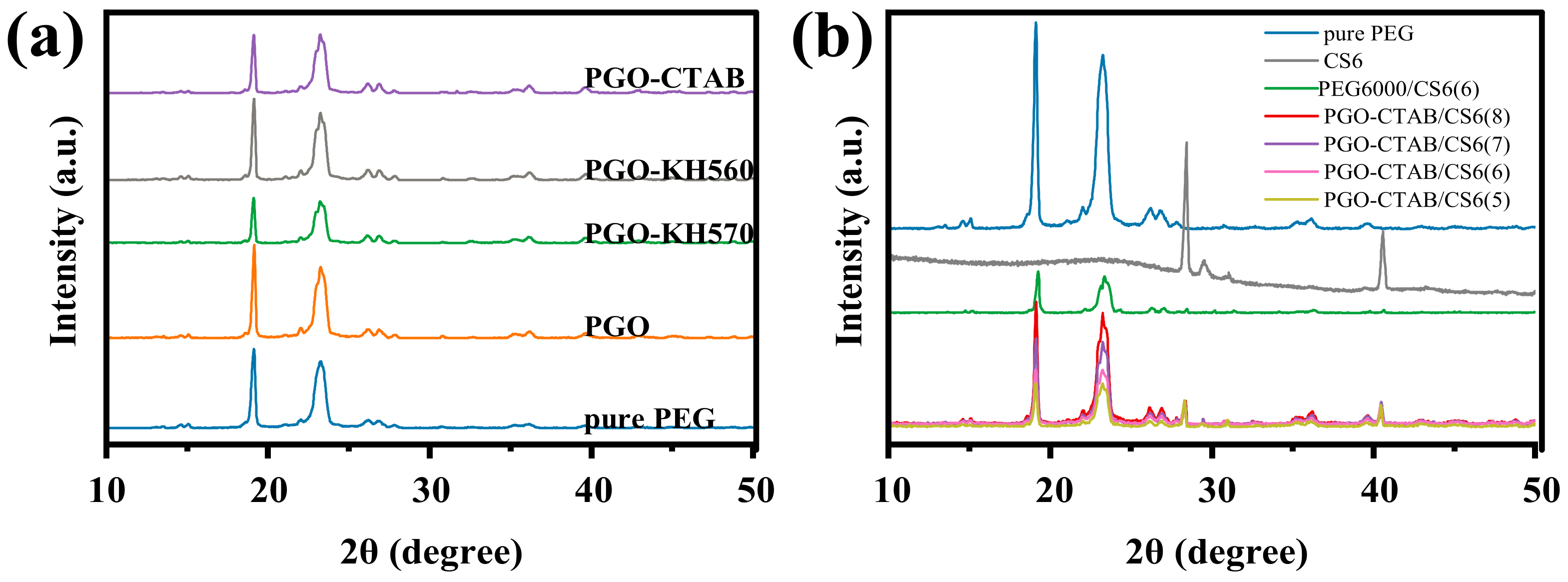


| Sample | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) |
|---|---|---|---|---|
| PEG6000 | 59.39 | 161.74 | 41.57 | 158.14 |
| PGO | 59.58 | 168.72 | 39.80 | 161.49 |
| PGO-KH570 | 59.44 | 173.04 | 40.02 | 163.40 |
| PGO-KH560 | 60.22 | 186.01 | 41.21 | 176.85 |
| PGO-CTAB | 60.69 | 191.36 | 40.99 | 181.87 |
| Sample | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) |
|---|---|---|---|---|
| PEG6000 | 59.39 | 161.74 | 41.57 | 158.14 |
| PEG6000/CS6(6) | 58.62 | 99.32 | 41.10 | 93.91 |
| PGO-CTAB/CS6(5) | 60.83 | 93.47 | 40.02 | 89.97 |
| PGO-CTAB/CS6(6) | 61.05 | 106.51 | 41.20 | 102.70 |
| PGO-CTAB/CS6(7) | 60.42 | 132.10 | 40.95 | 127.05 |
| PGO-CTAB/CS6(8) | 60.56 | 153.52 | 41.89 | 147.83 |
| Sample | PEG wt% | E | Fc |
|---|---|---|---|
| PEG6000 | 100% | 100% | 100% |
| PGO | 99.4% | 103.2% | 98.96% |
| PGO-KH570 | 99.4% | 105.2% | 98.31% |
| PGO-KH560 | 99.4% | 113.4% | 98.64% |
| PGO-CTAB | 99.4% | 116.7% | 98.62% |
| PEG6000/CS6(6) | 60% | 60.4% | 98.37% |
| PGO-CTAB/CS6(5) | 50% | 57.3% | 99.23% |
| PGO-CTAB/CS6(6) | 60% | 65.4% | 99.32% |
| PGO-CTAB/CS6(7) | 70% | 81.0% | 99.19% |
| PGO-CTAB/CS6(8) | 80% | 94.2% | 99.25% |
| Sample | Crystal Size (nm) |
|---|---|
| Pure PEG6000 | 12.04 |
| PGO | 11.83 |
| PEG6000/CS6(6) | 11.63 |
| PGO-KH570 | 11.30 |
| PGO-KH560 | 11.68 |
| PGO-CTAB | 11.64 |
| PGO-CTAB/CS6(5) | 11.22 |
| PGO-CTAB/CS6(6) | 11.17 |
| PGO-CTAB/CS6(7) | 11.24 |
| PGO-CTAB/CS6(8) | 11.54 |
| Sample | Mass (g) | Diameter (mm) | Leakage Area (mm2) | Leakage Rate Φ (%) |
|---|---|---|---|---|
| Pure PEG | 0.235 | 12.7 | 579.42 | 100.00 |
| PGO-KH560 | 0.236 | 12.7 | 447.05 | 77.15 |
| PGO-KH570 | 0.230 | 12.7 | 835.66 | 144.22 |
| PGO-CTAB | 0.255 | 12.7 | 894.28 | 154.34 |
| PGO-CTAB/CS6(5) | 0.246 | 12.7 | 0.00 | 0.00 |
| PGO-CTAB/CS6(6) | 0.242 | 12.7 | 99.15 | 17.11 |
| PGO-CTAB/CS6(7) | 0.235 | 12.7 | 353.70 | 61.04 |
| PGO-CTAB/CS6(8) | 0.239 | 12.7 | 543.91 | 93.87 |
| Sample | Thermal Conductivity Value K (W/m·K) |
|---|---|
| PEG6000 | 0.325 |
| PEG6000/CS6(6) | 0.299 |
| PGO | 0.628 |
| PGO-KH570 | 0.398 |
| PGO-KH560 | 0.424 |
| PGO-CTAB | 0.450 |
| PGO-CTAB/CS6(5) | 0.291 |
| PGO-CTAB/CS6(6) | 0.337 |
| PGO-CTAB/CS6(7) | 0.293 |
| PGO-CTAB/CS6(8) | 0.284 |
| Sample | m/(g) | ∆Hm/(J/g) | P/(mW/cm2) | ∆t/s | η/% |
|---|---|---|---|---|---|
| PEG6000 | 0.2309 | 161.74 | 200 | 633.0 | 44.63 |
| PEG6000/CS6(6) | 0.2540 | 86.57 | 200 | 543.0 | 56.36 |
| PGO-CTAB/CS6(5) | 0.2414 | 93.47 | 200 | 100.3 | 88.78 |
| PGO-CTAB/CS6(6) | 0.2428 | 106.51 | 200 | 131.8 | 77.43 |
| PGO-CTAB/CS6(7) | 0.2474 | 132.10 | 200 | 144.6 | 89.19 |
| PGO-CTAB/CS6(8) | 0.2503 | 153.53 | 200 | 176.0 | 86.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, B.; Wang, S.; Xue, B.; Liu, S.; An, M.; Yang, Z.; Xu, G. Effect of GO on the Structure and Properties of PEG/Biochar Phase Change Composites. Polymers 2023, 15, 963. https://doi.org/10.3390/polym15040963
Chen W, Zhang B, Wang S, Xue B, Liu S, An M, Yang Z, Xu G. Effect of GO on the Structure and Properties of PEG/Biochar Phase Change Composites. Polymers. 2023; 15(4):963. https://doi.org/10.3390/polym15040963
Chicago/Turabian StyleChen, Weijie, BingBing Zhang, Sheng Wang, Bin Xue, ShiWang Liu, MingZhe An, Zhao Yang, and Guomin Xu. 2023. "Effect of GO on the Structure and Properties of PEG/Biochar Phase Change Composites" Polymers 15, no. 4: 963. https://doi.org/10.3390/polym15040963
APA StyleChen, W., Zhang, B., Wang, S., Xue, B., Liu, S., An, M., Yang, Z., & Xu, G. (2023). Effect of GO on the Structure and Properties of PEG/Biochar Phase Change Composites. Polymers, 15(4), 963. https://doi.org/10.3390/polym15040963






