Self-Supported Biopolymeric Films Based on Onion Bulb (Allium cepa L.): Gamma-Radiation Effects in Sterilizing Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Onion-Based Films Production
2.2. Co-60 γ-Radiation
2.3. Film Characterizations
3. Results
3.1. Film Morphology—Field Emission Scanning Electron Microscopy (FE-SEM)
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. X-ray Diffraction (XRD)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Thermogravimetry (TG/DTG)
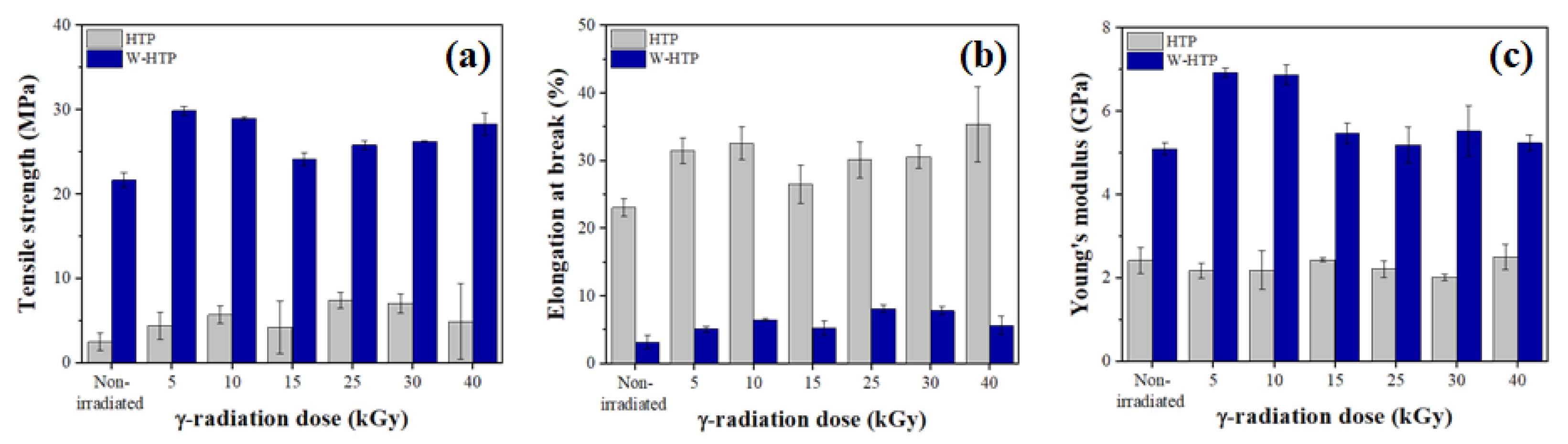
3.6. Mechanical Properties
3.7. Wettability—Apparent Contact Angle Measurements
3.8. Barrier Properties
3.9. Mutagenicity Assays
3.10. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewster, J.L. Onion and other vegetable Alliums; CABI: Wallingford, UK, 2008; pp. 85–170. [Google Scholar]
- Bell, A.D. Plant Form: An Illustrated Guide to Flowering Plant Morphology; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- dos Santos Dias, D.; Otoni, C.G.; da Silva, R.R.; Meneguin, A.B.; Mattoso, L.H.C.; da Silva Barud, H.; Ribeiro, C.A. Large scale manufacturing of puree-only edible films from onion bulb (Allium cepa L.): Probing production and structure–processing–property correlations. Ind. Crops Prod. 2020, 145, 111847. [Google Scholar] [CrossRef]
- Peirce, L.C.V. Vegetables Characteristics, Production and Marketing; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Benkeblia, N.; Shiomi, N. Hydrolysis kinetic parameters of DP 6, 7, 8, and 9− 12 fructooligosaccharides (FOS) of onion bulb tissues. Effect of temperature and storage time. J. Agric. Food Chem. 2006, 54, 2587–2592. [Google Scholar] [CrossRef]
- Jaime, L.; Martínez, F.; A Martín-Cabrejas, M.; Mollá, E.; López-Andréu, F.J.; Waldron, K.W.; Esteban, R.M. Study of total fructan and fructooligosaccharide content in different onion tissues. J. Sci. Food Agric. 2000, 81, 177–182. [Google Scholar] [CrossRef]
- Rhodes, M.J.C.; Price, K.R. Analytical problems in the study of flavonoid compounds in onions. Food Chem. 1996, 57, 113–117. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Li, Z.; An, X.; Gao, Z.; Tian, S. Preparation and performance characterization of a composite film based on corn starch, κ-carrageenan, and ethanol extract of onion skin. Polymers 2022, 14, 2986. [Google Scholar] [CrossRef]
- Jones, M.G.; Hughes, J.; Tregova, A.; Milne, J.; Tomsett, A.B.; Collin, H.A. Biosynthesis of the flavour precursors of onion and garlic. J. Exp. Bot. 2004, 55, 1903–1918. [Google Scholar] [CrossRef]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef]
- Liguori, L.; Califano, R.; Albanese, D.; Raimo, F.; Crescitelli, A.; Di Matteo, M. Chemical composition and antioxidant properties of five white onion (Allium cepa L.) landraces. J. Food Qual. 2017, 2017, 6873651. [Google Scholar] [CrossRef]
- Ueda, Y.; Tsubuku, T.; Miyajima, R. Composition of Sulfur-Containing Components in Onion and Their Flavor Characters. Biosci. Biotechnol. Biochem. 1994, 58, 108–110. [Google Scholar] [CrossRef]
- Golovchenko, V.V.; Khramova, D.S.; Ovodova, R.G.; Shashkov, A.S.; Ovodov, Y.S. Structure of pectic polysaccharides isolated from onion Allium cepa L. using a simulated gastric medium and their effect on intestinal absorption. Food Chem. 2012, 134, 1813–1822. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Robert, R.S. Structural features of cell-wall polysaccharides of onion Allium cepa. Carbohydr. Res. 1986, 157, 183–199. [Google Scholar] [CrossRef]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive Polymeric Films Based on Red Onion Skins Extract for Wound Treatment: An Innovative and Eco-Friendly Formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.d.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Faraco, T.A.; Silva, H.O.X.; Barud, H.S.; Maciel, I.O.; da Silva, R.R.; Quirino, W.G.; Fragneaud, B.; Ribeiro, C.A.; Pandoli, O.G.; Cremona, M.; et al. Ecological biosubstrates obtained from onion pulp (Allium cepa L.) for flexible organic light-emitting diodes. ACS Appl. Mater. Interfaces 2019, 11, 42420–42428. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.R.; Aleixo, N.A.; Silvestre, R.B.; Fregonezi, N.F.; da Silva Barud, H.; Dias, D.S.; Ribeiro, C.A.; Resende, F.A. Genotoxicological safety assessment of puree-only edible films from onion bulb (Allium cepa L.) for use in food packaging-related applications. J. Food Sci. 2020, 85, 201–208. [Google Scholar] [CrossRef]
- Soares, K.S.; Souza, M.P.; Silva-Filho, E.C.; Barud, H.S.; Ribeiro, C.A.; Santos, D.D.; Rocha, K.N.S.; de Moura, J.F.P.; Oliveira, R.L.; Bezerra, L.R. Effect of Edible Onion (Allium cepa L.) Film on Quality, Sensory Properties and Shelf Life of Beef Burger Patties. Molecules 2021, 26, 7202. [Google Scholar] [CrossRef]
- Gopal, N.G.S. Radiation sterilization of pharmaceuticals and polymers. Radiat. Phys. Chem. 1977, 12, 35–50. [Google Scholar] [CrossRef]
- Lenfeld, P.; Brdlík, P.; Borůvka, M.; Běhálek, L.; Habr, J. Effect of radiation crosslinking and surface modification of cellulose fibers on properties and characterization of biopolymer composites. Polymers 2020, 12, 3006. [Google Scholar] [CrossRef]
- Van Cauwenbergh, T.; Theys, E.; Stroeykens, D.; Croonenborghs, B.; Gillet, A.; DeMent, A.; Van Schepdael, A.; Haghedooren, E. The effect of Gamma and Ethylene Oxide Sterilization on a Selection of Active Pharmaceutical Ingredients for Ophthalmics. J. Pharm. Sci. 2022, 111, 2011–2017. [Google Scholar] [CrossRef]
- Vessoni Penna, T.C.; Machoshvili, I.A. Esterilização térmica: Conceitos básicos da cinética de morte microbiana. Rev. Farm. Bioquím. Univ. São Paulo 1997, 34 (Suppl. 1), 1–5. [Google Scholar]
- Clark, N.V.; Endicott, S.P.; Jorgensen, E.M.; Hur, H.C.; Lockrow, E.G.; Kern, M.E.; Jones-Cox, C.E.; Dunlow, S.G.; Einarsson, J.I.; Cohen, S.L. Review of sterilization techniques and clinical updates. J. Minim. Invasive Gynecol. 2018, 25(7), 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Aquino, K.A. Sterilization by gamma irradiation. Gamma Radiat. 2012, 9, 172–202. [Google Scholar]
- US Food and Drug Administration. Radiation in the production, processing, and handling of food. Fed. Regist. Final. Rule 2001, 7, 10574. [Google Scholar]
- Artandi, C. Successful and promising applications of radiation processing-sterilization. Radiat. Phys. Chem. 1977, 9, 183–191. [Google Scholar] [CrossRef]
- Rutala, W.A. (Ed.) Disinfection, Sterilization, and Antisepsis. In Proceedings of the International Symposium on Disinfection, Sterilization and Antisepsis in Healt Care, New Orleans, LA, USA, 12–13 June 1997. [Google Scholar]
- Güven, O. Radiation-assisted synthesis of polymer-based nanomaterials. Appl. Sci. 2021, 11, 7913. [Google Scholar] [CrossRef]
- Woo, C.H. Theory of irradiation deformation in non-cubic metals: Effects of anisotropic diffusion. J. Nucl. Mater. 1988, 159, 237–256. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Radiation in food production, processing, and handling. Fed. Regist. Final Rule 2005, 70, 48057–48073. [Google Scholar]
- Benyathiar, P.; Selke, S.E.; Harte, B.R.; Mishra, D.K. The effect of irradiation sterilization on poly (lactic) acid films. J. Polym. Environ. 2021, 29, 460–471. [Google Scholar] [CrossRef]
- Sant, N.J.; Proffen, B.L.; Murray, M.M. Effects of radiation dose and nitrogen purge on collagen scaffold properties. J. Biomater. Appl. 2022, 36, 1011–1018. [Google Scholar] [CrossRef]
- Davila, S.P.; Rodríguez, L.G.; Chiussi, S.; Serra, J.; González, P. How to sterilize polylactic acid based medical devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Mchugh, T.H.; Avena-Bustillos, R.; Krochta, J. Hydrophilic Edible Films: Modified Procedure for Water Vapor Permeability and Explanation of Thickness Effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard test methods for water vapor transmission of materials (E96/E96M-16). In Annual Book of American Standard Testing Methods; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2016; pp. 719–725. [Google Scholar]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Environ. Mutagenesis Relat. Subj. 1983, 113, 173–215. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Brussels, Belgium, 2021.
- Page, B.; Page, M.; Noel, C. A New Fluorometric Assay for Cytotoxicity Measurements In-Vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.; Sarbon, N. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Isotton, F.; Bernardo, G.; Baldasso, C.; Rosa, L.; Zeni, M. The plasticizer effect on preparation and properties of etherified corn starchs films. Ind. Crop. Prod. 2015, 76, 717–724. [Google Scholar] [CrossRef]
- Shahbazi, M.; Rajabzadeh, G.; Ahmadi, S.J. Characterization of nanocomposite film based on chitosan intercalated in clay platelets by electron beam irradiation. Carbohydr. Polym. 2017, 157, 226–235. [Google Scholar] [CrossRef]
- Sionkowska, A.; Płanecka, A. Surface properties of thin films based on the mixtures of chitosan and silk fibroin. J. Mol. Liq. 2013, 186, 157–162. [Google Scholar] [CrossRef]
- Soliman, E.A.; Eldin, M.S.M.; Furuta, M. Biodegradable Zein-Based Films: Influence of γ-Irradiation on Structural and Functional Properties. J. Agric. Food Chem. 2009, 57, 2529–2535. [Google Scholar] [CrossRef]
- Yang, E.I.; Lee, E.M.; Kim, Y.S.; Chung, B.Y. The role of gamma irradiation on the extraction of phenolic compounds in onion (Allium cepa L.). Radiat. Phys. Chem. 2012, 81, 1025–1028. [Google Scholar] [CrossRef]
- Pati, S.; Chatterji, A.; Dash, B.P.; Nelson, B.R.; Sarkar, T.; Shahimi, S.; Edinur, H.A.; Manan, T.S.B.A.; Jena, P.; Mohanta, Y.K.; et al. Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers 2020, 12, 2361. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym. Degrad. Stab. 2003, 79, 465–474. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Sagar, N.A.; Khar, A.; Vikas; Tarafdar, A.; Pareek, S. Physicochemical and Thermal Characteristics of Onion Skin from Fifteen Indian Cultivars for Possible Food Applications. J. Food Qual. 2021, 2021, 7178618. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Hinterstoisser, B.; Salmén, L. Application of dynamic 2D FTIR to cellulose. Vib. Spectrosc. 2000, 22, 111–118. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Popescu, M.-C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral Characterization of Eucalyptus Wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Monsoor, M.A.; Kalapathy, U.; Proctor, A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2001, 74, 233–238. [Google Scholar] [CrossRef]
- Mihoubi, W.; Sahli, E.; Gargouri, A.; Amiel, C. FTIR spectroscopy of whole cells for the monitoring of yeast apoptosis mediated by p53 over-expression and its suppression by Nigella sativa extracts. PLoS ONE 2017, 12, e0180680. [Google Scholar] [CrossRef] [PubMed]
- Tsiaganis, M.C.; Laskari, K.; Melissari, E. Fatty acid composition of Allium species lipids. J. Food Compos. Anal. 2006, 19, 620–627. [Google Scholar] [CrossRef]
- Wilson, R.H.; Smith, A.C.; Kacuráková, M.; Saunders, P.K.; Wellner, N.; Waldron, K.W. The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Plant Physiol. 2000, 124, 397–405. [Google Scholar] [CrossRef]
- Zaouak, A.; Jebali, S.; Chouchane, H.; Jelassi, H. Impact of gamma-irradiation on the degradation and mineralization of hydroxychloroquine aqueous solutions. Int. J. Environ. Sci. Technol. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sivasangar, S.; Taufiq-Yap, Y.H.; Zainal, Z.; Kitagawa, K. Thermal behavior of lignocellulosic materials under aerobic/anaerobic environments. Int. J. Hydrog. Energy 2013, 38, 16011–16019. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Busu, T.N.Z.T.M.; Noor, A.M.M.; Shaari, N.; Mat, H. An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohydr. Polym. 2017, 166, 291–299. [Google Scholar] [CrossRef]
- de Cassan, D.; Hoheisel, A.L.; Glasmacher, B.; Menzel, H. Impact of sterilization by electron beam, gamma radiation and X-rays on electrospun poly-(ε-caprolactone) fiber mats. J. Mater. Sci. Mater. Med. 2019, 30, 42. [Google Scholar] [CrossRef]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef]
- Lee, J.W.; Thomas, L.C.; Schmidt, S.J. Investigation of the heating rate dependency associated with the loss of crystalline structure in sucrose, glucose, and fructose using a thermal analysis approach (Part I). J. Agric. Food Chem. 2011, 59, 684–701. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Effects of chemical treatments on hemp fibre structure. Appl. Surf. Sci. 2013, 276, 13–23. [Google Scholar] [CrossRef]
- Kabir, M.M.; Alhaik, M.Y.; Aldajah, S.H.; Lau, K.T.; Wang, H.; Islam, M.M. Effect of Hemp Fibre Surface Treatment on the Fibre-Matrix Interface and the Influence of Cellulose, Hemicellulose, and Lignin Contents on Composite Strength Properties. Adv. Mater. Sci. Eng. 2021, 2021, 9753779. [Google Scholar] [CrossRef]
- Henniges, U.; Okubayashi, S.; Rosenau, T.; Potthast, A. Irradiation of cellulosic pulps: Understanding its impact on cellulose oxidation. Biomacromolecules 2012, 13, 4171–4178. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kaminski, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Açıkalın, K.; Gözke, G. Thermogravimetric pyrolysis of onion skins: Determination of kinetic and thermodynamic parameters for devolatilization stages using the combinations of isoconversional and master plot methods. Bioresour. Technol. 2021, 342, 125936. [Google Scholar] [CrossRef]
- Açıkalın, K. Pyrolytic characteristics and kinetics of pistachio shell by thermogravimetric analysis. J. Therm. Anal. Calorim. 2012, 109, 227–235. [Google Scholar] [CrossRef]
- Zhang, S.I.; Deng, P.; Xu, Y.C.; Lü, S.W.; Wang, J.J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef]
- Darbyshire, B.; Henry, R.J. The association of fructans with high percentage dry weight in onion cultivars suitable for dehydrating. J. Sci. Food Agric. 1979, 30, 1035–1038. [Google Scholar] [CrossRef]
- O’Donoghue, E.M.; Somerfield, S.D.; Shaw, M.; Bendall, M.; Hedderly, D.; Eason, J.; Sims, I. Evaluation of carbohydrates in pukekohe longkeeper and grano cultivars of Allium cepa. J. Agric. Food Chem. 2004, 52, 5383–5390. [Google Scholar] [CrossRef]
- Almeida do Nascimento, H.; Amorim, J.D.P.; as Silva Júnior, C.J.G.; de Medeiros, A.D.M.; Costa, A.F.S.; Napoleão, D.C.; Vinhas, G.M.; Sarubbo, L.A. Influence of gamma irradiation on the properties of bacterial cellulose produced with concord grape and red cabbage extracts. Curr. Res. Biotechnol. 2022, 4, 119–128. [Google Scholar] [CrossRef]
- Ito, V.C.; Bet, C.D.; Wojeicchowski, J.P.; Demiate, I.M.; Spoto, M.H.F.; Schnitzler, E.; Lacerda, L.G. Effects of gamma radiation on the thermoanalytical, structural and pasting properties of black rice (Oryza sativa L.) flour. J. Therm. Anal. Calorim. 2018, 133, 529–537. [Google Scholar] [CrossRef]
- Huang, Q. When Polymer Chains Are Highly Aligned: A Perspective on Extensional Rheology. Macromolecules 2022, 55, 715–727. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Li, Y.; Lu, H.; Zhang, N.; Mingming, M. Bioinspired ultra-stretchable and anti-freezing conductive hydrogel fibers with ordered and reversible polymer chain alignment. Nat. Commun. 2018, 9, 3579. [Google Scholar] [CrossRef]
- Song, F.; Wang, Q.; Wang, T. The effects of crystallinity on the mechanical properties and the limiting PV (pressure×velocity) value of PTFE. Tribol. Int. 2016, 93, 1–10. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers on the crystallinity and mechanical properties of starch-based films at different relative humidity values. Carbohydr. Polym. 2009, 77, 293–299. [Google Scholar] [CrossRef]
- Erbil, H.Y. Practical Applications of Superhydrophobic Materials and Coatings: Problems and Perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef]
- Himma, N.F.; Prasetya, N.; Anisah, S.; Wenten, I.G. Superhydrophobic membrane: Progress in preparation and its separation properties. Rev. Chem. Eng. 2019, 35, 211–238. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Van den Hoek, I.A.F.; Renzetti, S. Sugar replacement with zwitterionic plasticizers like amino acids. Food Hydrocoll. 2020, 109, 106113. [Google Scholar] [CrossRef]
- Sain, S.; Åkesson, D.; Skrifvars, M.; Roy, S. Hydrophobic Shape-Memory Biocomposites from Tung-Oil-Based Bioresin and Onion-Skin-Derived Nanocellulose Networks. Polymers 2020, 12, 2470. [Google Scholar] [CrossRef]
- Parparita, E.; Zaharescu, T.; Darie, R.N.; Vasile, C. Biomass effect on γ-irradiation behavior of some polypropylene biocomposites. Ind. Eng. Chem. Res. 2015, 54, 2404–2413. [Google Scholar] [CrossRef]
- Tayel, A.; Zaki, M.F.; El Basaty, A.B.; Hegazy, T.M. Modifications induced by gamma irradiation to Makrofol polymer nuclear track detector. J. Adv. Res. 2015, 6, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chenab, K.K.; Sohrabi, B.; Rahmanzadeh, A. Superhydrophobicity: Advanced biological and biomedical applications. Biomater. Sci. 2019, 7, 3110–3137. [Google Scholar] [CrossRef] [PubMed]
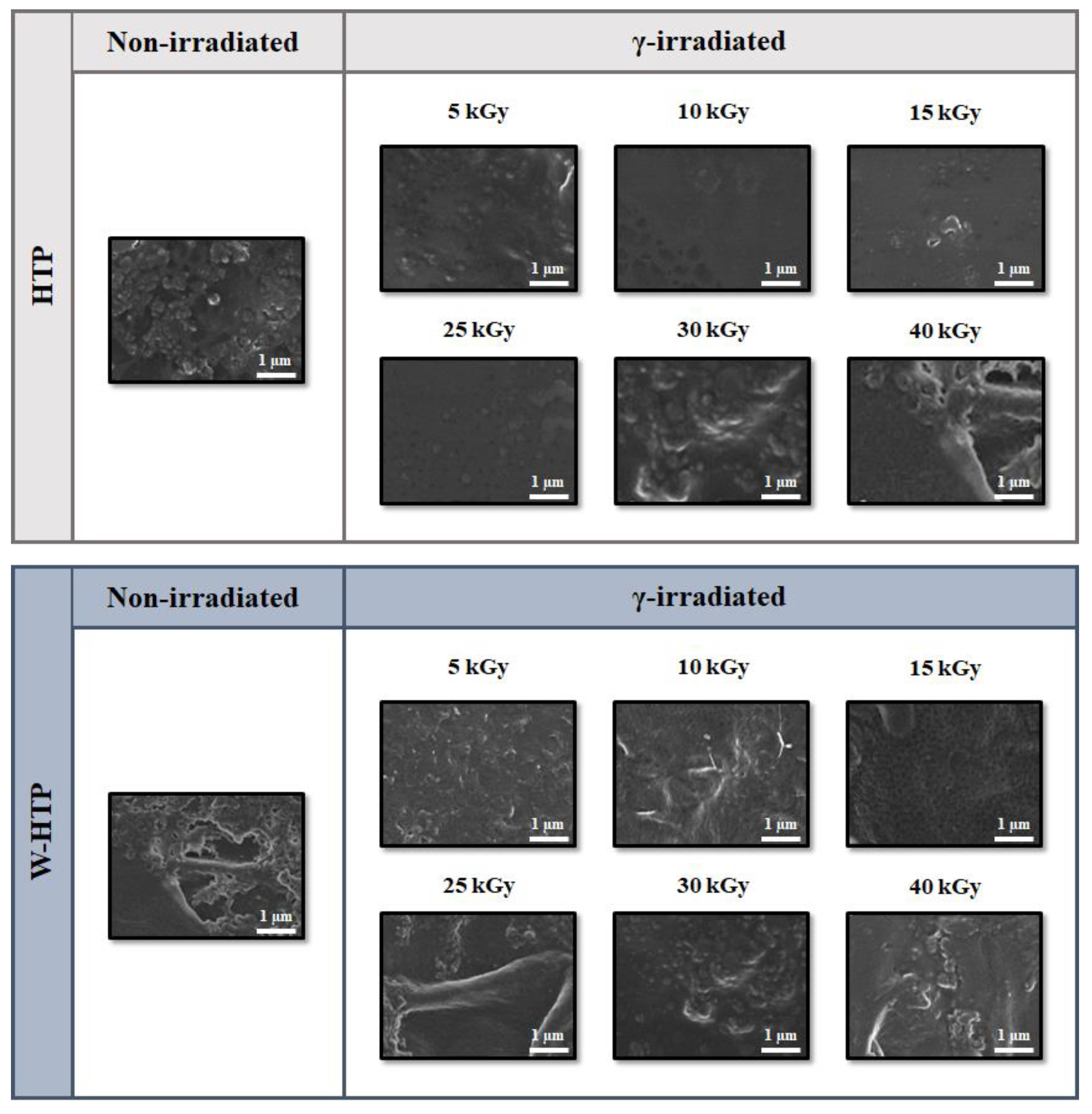
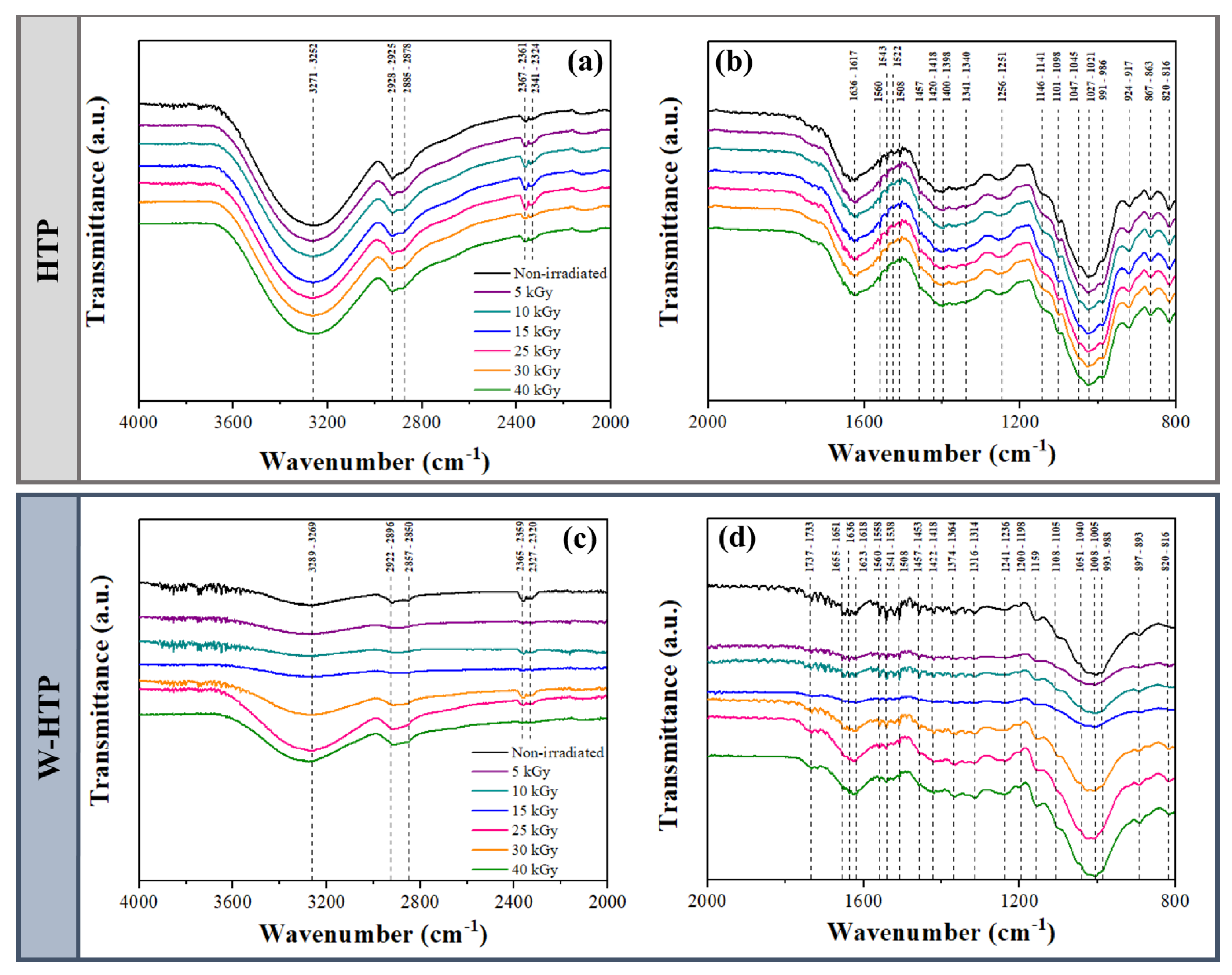






| Sample | γ-Radiation Dose (kGy) | I(200) (2Ɵ ≈ 22°) | Iam (2Ɵ ≈ 18°) | CrI (%) |
|---|---|---|---|---|
| HTP | 5 | 966 | 914 | 5.38 |
| 10 | 1068 | 988 | 7.40 | |
| 15 | 910 | 866 | 4.80 | |
| 25 | 926 | 890 | 4.00 | |
| 30 | 1014 | 946 | 6.70 | |
| 40 | 1074 | 966 | 10.0 | |
| W-HTP | 5 | 2138 | 1084 | 49.0 |
| 10 | 1696 | 826 | 51.2 | |
| 15 | 1656 | 972 | 41.3 | |
| 25 | 1636 | 964 | 41.7 | |
| 30 | 1760 | 902 | 48.7 | |
| 40 | 2712 | 1236 | 54.4 |
| Sample | γ-Radiation Dose (kGy) | Tdehyd | Td | ||||
|---|---|---|---|---|---|---|---|
| Tdehyd,onset * | Tdehyd,endset * | ΔHdehyd ** | Td,onset * | Td,endset * | ΔHd ** | ||
| HTP | Non-irradiated | 132 | 154 | 8 | 229/342 | 188/318 | −53/−9 |
| 5 | 121 | 173 | 124 | 228/336 | 195/310 | −18/−9 | |
| 10 | 121 | 169 | 97 | 225/338 | 192/317 | −17/−5 | |
| 15 | 122 | 166 | 79 | 224/333 | 197/312 | −16/−7 | |
| 25 | 119 | 159 | 78 | 225/335 | 195/315 | −13/−7 | |
| 30 | 120 | 179 | 85 | 222/334 | 193/313 | −24/−10 | |
| 40 | 118 | 171 | 42 | 227/332 | 193/312 | −12/−6 | |
| W-HTP | Non-irradiated | - | - | - | 357 | 326 | −5 |
| 5 | - | - | - | 354 | 320 | −14 | |
| 10 | - | - | - | 368 | 324 | −15 | |
| 15 | - | - | - | 369 | 300 | −190 | |
| 25 | - | - | - | 371 | 317 | −230 | |
| 30 | - | - | - | 356 | 302 | −171 | |
| 40 | - | - | - | 359 | 316 | −158 | |
| Revertants Number (M ± SD)/Plate and MI | ||||
|---|---|---|---|---|
| TA98 | TA102 | |||
| C− | 23 ± 2 | 245 ± 49 | ||
| C+ | 862 ± 64 a,* | 1259 ± 102 b,* | ||
| γ-radiation dose (kGy) | ||||
| HTP (100 µL/plate) | 5 | 23 ± 7 (1.0) | 244 ± 23 (1.00) | |
| 10 | 22 ± 0 (0.96) | 249 ± 30 (1.02) | ||
| 15 | 22 ± 3 (0.96) | 230 ± 14 (0.94) | ||
| 25 | 25 ± 4 (1.09) | 265± 44 (1.08) | ||
| 30 | 31 ± 1 (1.35) | 284 ± 12 (1.16) | ||
| 40 | 25 ± 4 (1.09) | 260 ± 28 (1.06) | ||
| W-HTP (100 µL/plate) | 5 | 30 ± 1 (1.28) | 266 ± 23 (1.08) | |
| 10 | 25 ± 1 (1.09) | 236 ± 18 (0.96) | ||
| 15 | 24 ± 6 (1.04) | 252 ± 40 (1.03) | ||
| 25 | 27 ± 4 (1.17) | 257 ± 26 (1.05) | ||
| 30 | 26 ± 6 (1.13) | 234 ± 37 (0.96) | ||
| 40 | 24 ± 6 (1.04) | 230 ± 14 (0.94) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa Borges, M.A.; Sorigotti, A.R.; Paschoalin, R.T.; Júnior, J.A.P.; da Silva, L.H.D.; Dias, D.S.; Ribeiro, C.A.; de Araújo, E.S.; Resende, F.A.; da Silva Barud, H. Self-Supported Biopolymeric Films Based on Onion Bulb (Allium cepa L.): Gamma-Radiation Effects in Sterilizing Doses. Polymers 2023, 15, 914. https://doi.org/10.3390/polym15040914
da Costa Borges MA, Sorigotti AR, Paschoalin RT, Júnior JAP, da Silva LHD, Dias DS, Ribeiro CA, de Araújo ES, Resende FA, da Silva Barud H. Self-Supported Biopolymeric Films Based on Onion Bulb (Allium cepa L.): Gamma-Radiation Effects in Sterilizing Doses. Polymers. 2023; 15(4):914. https://doi.org/10.3390/polym15040914
Chicago/Turabian Styleda Costa Borges, Marco Antonio, Amanda Rinaldi Sorigotti, Rafaella Takehara Paschoalin, José Alberto Paris Júnior, Lucas Henrique Domingos da Silva, Diógenes Santos Dias, Clóvis Augusto Ribeiro, Elmo Silvano de Araújo, Flávia Aparecida Resende, and Hernane da Silva Barud. 2023. "Self-Supported Biopolymeric Films Based on Onion Bulb (Allium cepa L.): Gamma-Radiation Effects in Sterilizing Doses" Polymers 15, no. 4: 914. https://doi.org/10.3390/polym15040914
APA Styleda Costa Borges, M. A., Sorigotti, A. R., Paschoalin, R. T., Júnior, J. A. P., da Silva, L. H. D., Dias, D. S., Ribeiro, C. A., de Araújo, E. S., Resende, F. A., & da Silva Barud, H. (2023). Self-Supported Biopolymeric Films Based on Onion Bulb (Allium cepa L.): Gamma-Radiation Effects in Sterilizing Doses. Polymers, 15(4), 914. https://doi.org/10.3390/polym15040914









