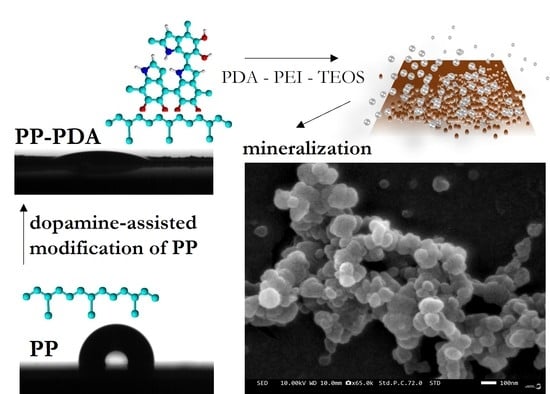
Dopamine-Assisted Modification of Polypropylene Film to Attain Hydrophilic Mineral-Rich Surfaces
Abstract
1. Introduction
2. Experimental
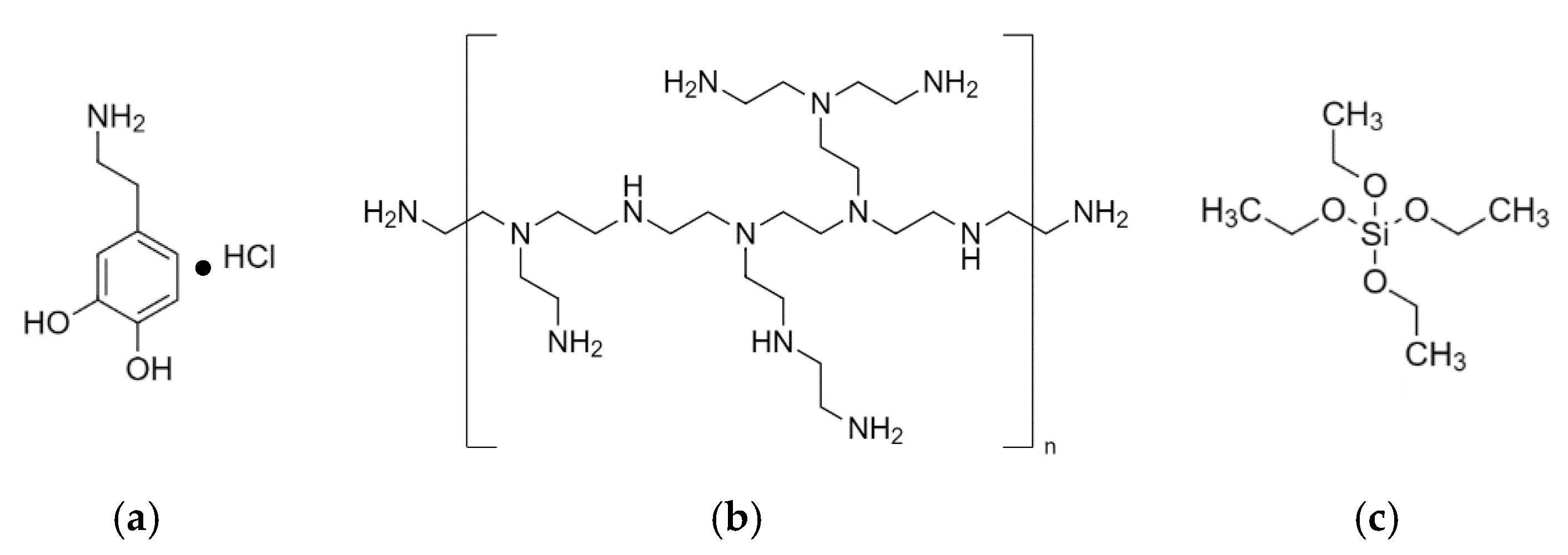
2.1. Materials
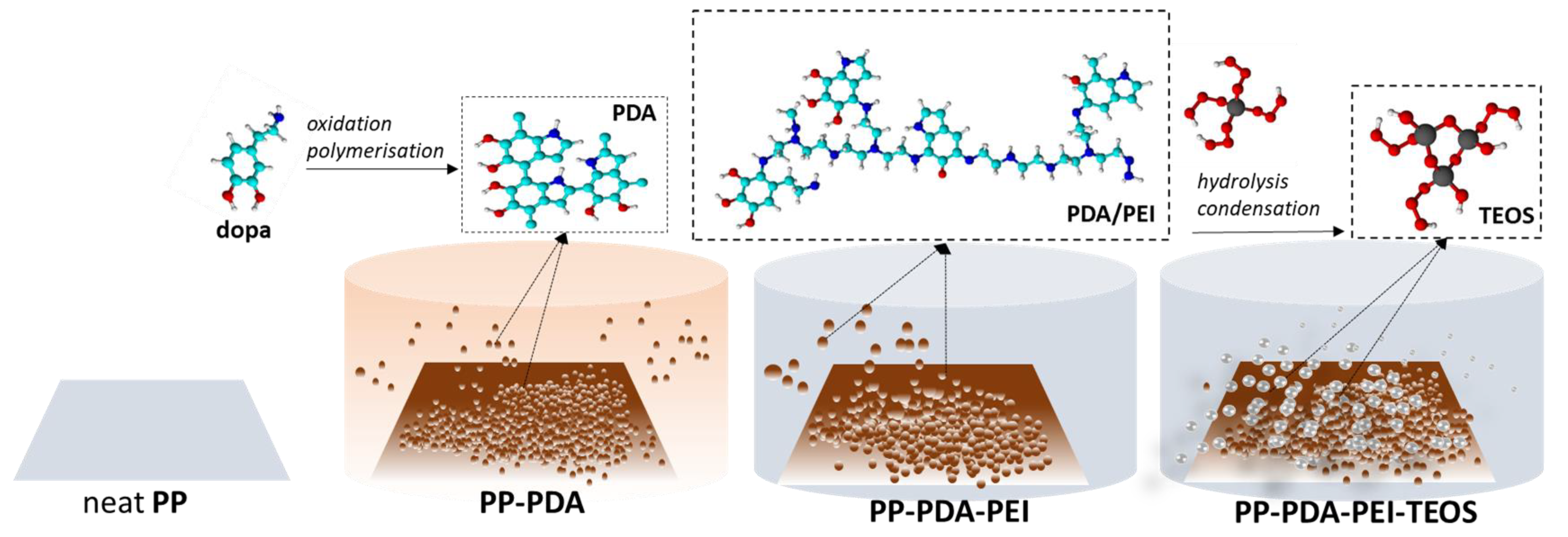
2.2. Modification of PP Film
- Preparation of films without dopa-HCl:
- 2.
- Preparation of films with dopa-HCl:
2.3. Bioactivity Assessment
2.4. Analytical Methods
2.4.1. Fourier Transform Infrared (FTIR) Analysis
2.4.2. Water Contact Angle (WCA) Measurement
2.4.3. Streaming Potential Measurement
2.4.4. Atomic Force Microscopy (AFM) Analysis
2.4.5. Scanning Electron Microscopy (SEM) Analysis
3. Results and Discussion
3.1. Modification of PP Film
3.2. Bioactivity
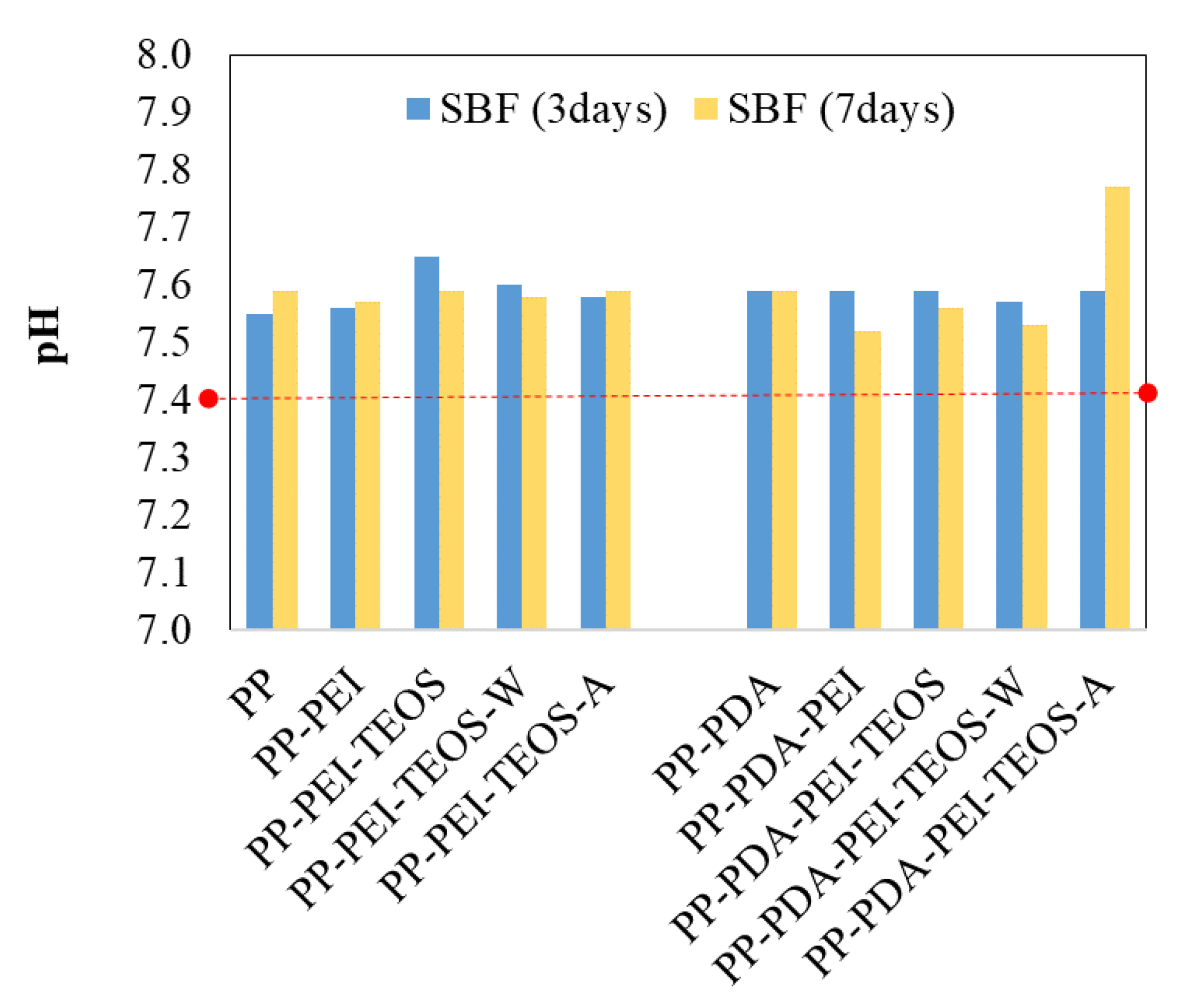
3.2.1. pH
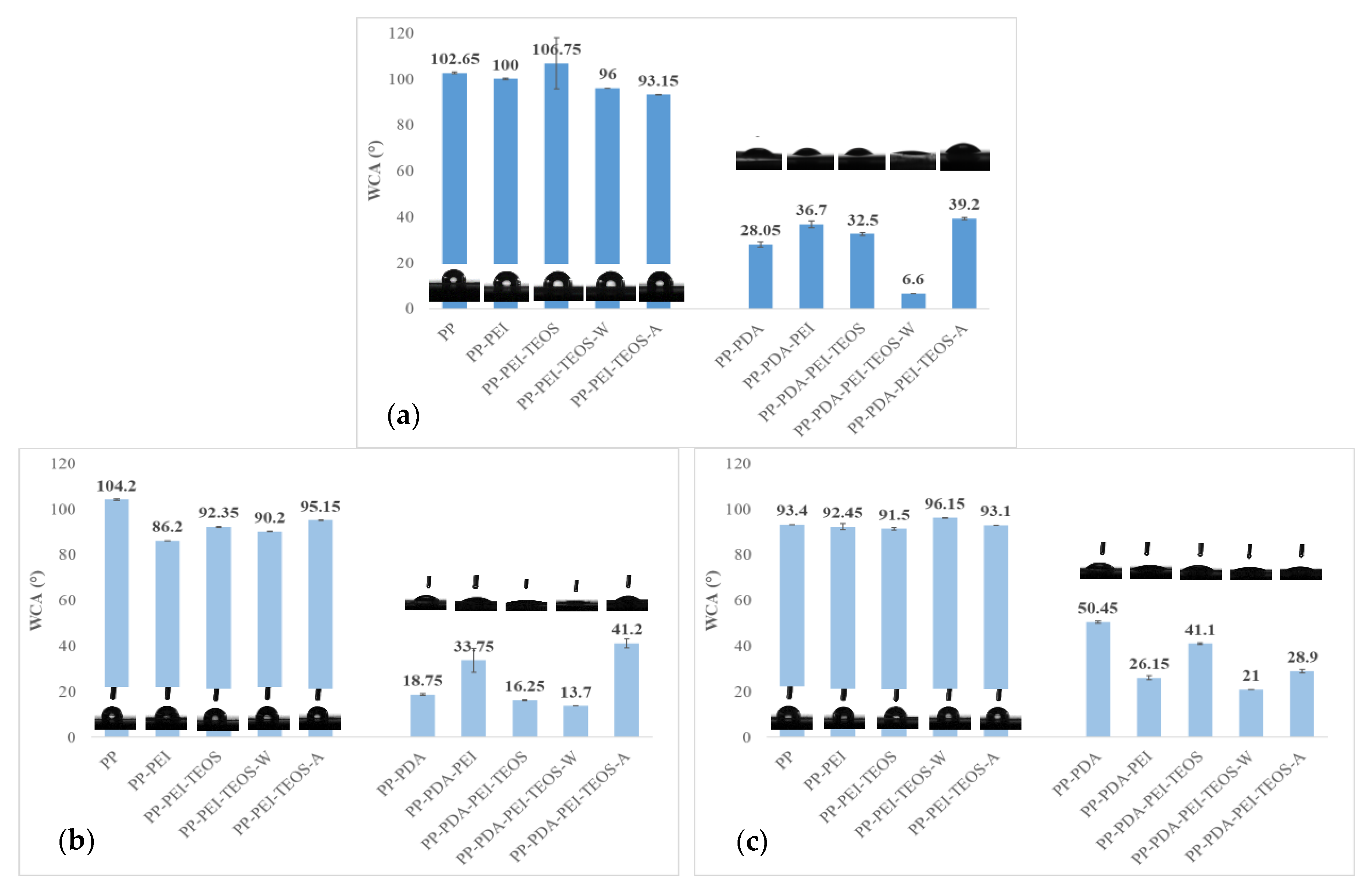
3.2.2. Surface Wettability
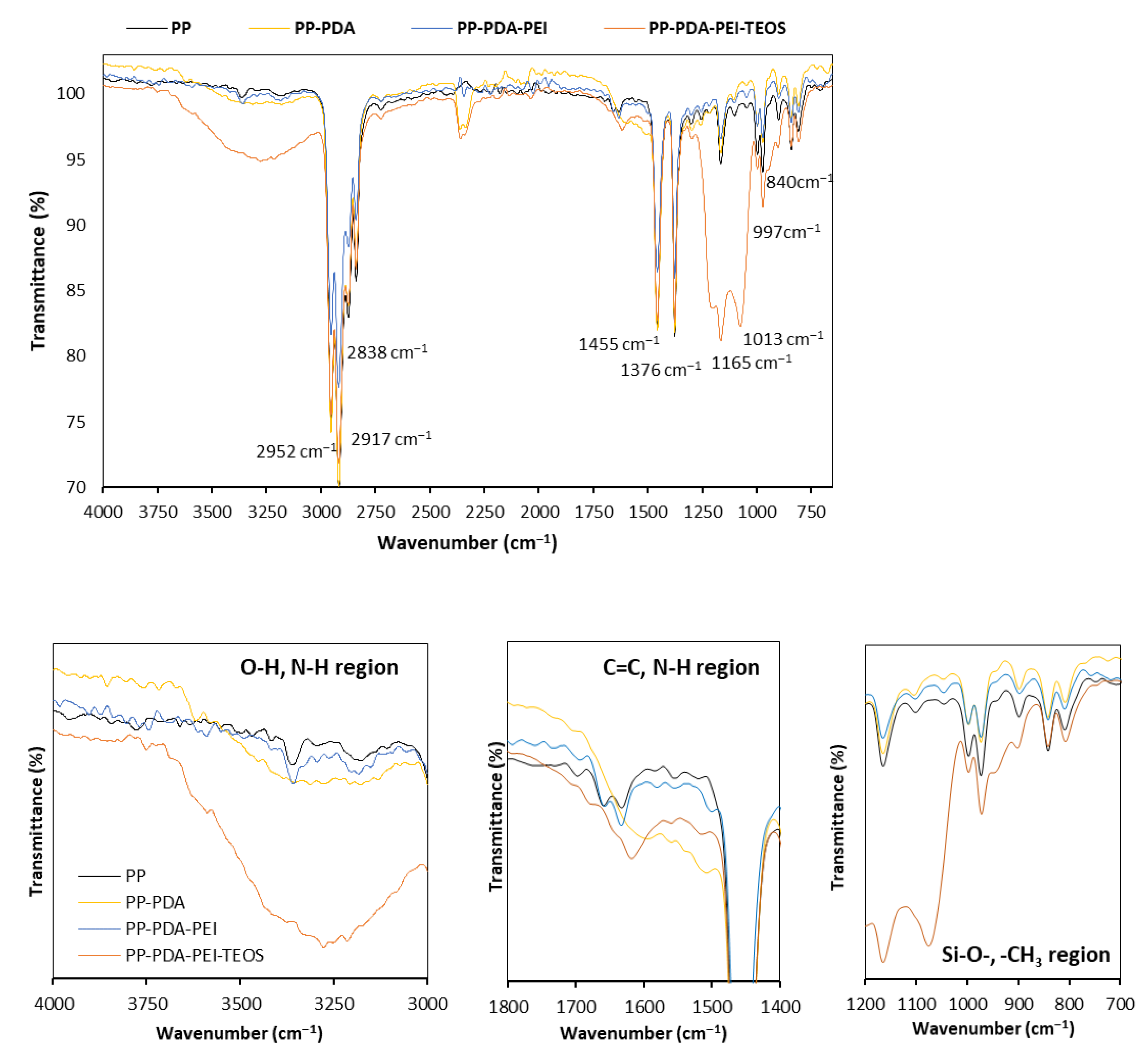
3.2.3. FTIR Spectroscopy
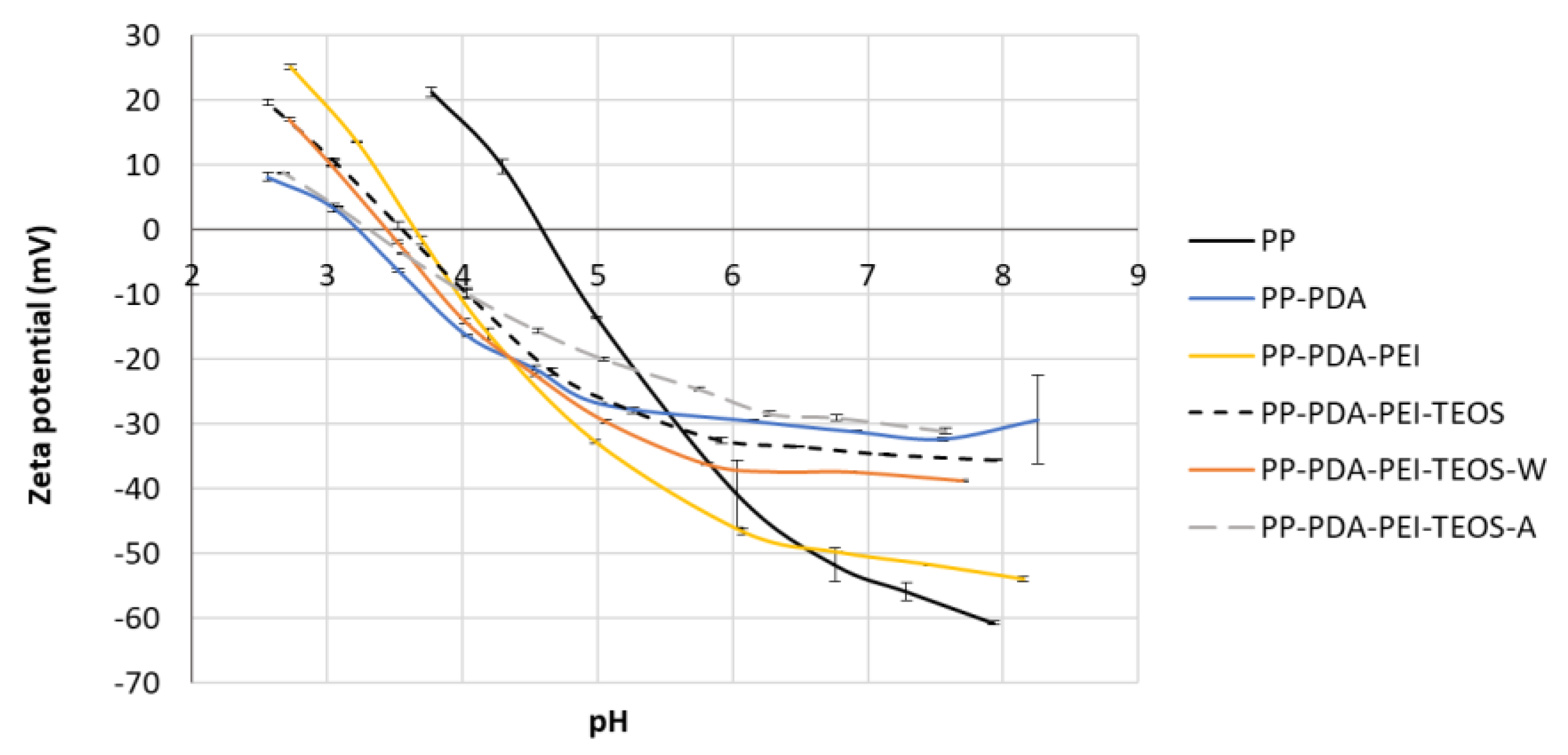
3.2.4. Zeta Potential
3.2.5. Surface Morphology and Topography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef]
- Kepa, K.; Coleman, R.; Grøndahl, L. In vitro mineralization of functional polymers. Biosurface Biotribology 2015, 1, 214–227. [Google Scholar] [CrossRef]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azais, T. Bone mineral: New insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef]
- Lee, M.; Ku, S.H.; Ryu, J.; Park, C.B. Mussel-inspired functionalization of carbon nanotubes for hydroxyapatite mineralization. J. Mater. Chem. 2010, 20, 1339. [Google Scholar] [CrossRef]
- De Oliveira, E.L.; De Carvalho, P.S.P.; Da Silva, T.B. Histological and histomorphometric evaluation of efficacy of a polypropylene barrier in guided bone regeneration and modified guided bone regeneration in critical defects in rodent cranial vaults. J. Indian Soc. Periodontol. 2019, 23, 351–355. [Google Scholar] [CrossRef]
- Ejeian, F.; Razmjou, A.; Nasr-Esfahani, M.H.; Mohammad, M.; Karamali, F.; Warkiani, M.E.; Asadnia, M.; Chen, V. ZIF-8 Modified Polypropylene Membrane: A Biomimetic Cell Culture Platform with a View to the Improvement of Guided Bone Regeneration. Int. J. Nanomed. 2020, 15, 10029–10043. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, L.; da Costa Marques, M.; Weinfeld, I. Guided bone regeneration with polypropylene barrier in rabbit’s calvaria: A preliminary experimental study. Heliyon 2018, 4, e00651. [Google Scholar] [CrossRef]
- Dos Santos, C.C.V.; Tonini, K.R.; Silva, M.A.A.; de Carvalho, P.S.P.; Ponzoni, D. Short-term use of an exposed polypropylene barrier in the preservation of alveolar bone after extraction: Randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2021, 50, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.; Martinez, E.F. Topographic characterization and in vitro biofilm adhesion to titanium and polypropylene membranes used for alveolar preservation. J. Indian Soc. Periodontol. 2020, 24, 316. [Google Scholar] [CrossRef]
- Ariono, D.; Wardani, A.K. Modification and Applications of Hydrophilic Polypropylene Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 214, 012014. [Google Scholar] [CrossRef]
- Migneco, C.; Fiume, E.; Verne, E.; Baino, F. A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef]
- Gotz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Iwata, T.; Maeda, H.; Hirata, H.; Kasuga, T. Preparation of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)-based composites releasing soluble silica for bone regeneration. J. Ceram. Soc. Jpn. 2013, 121, 753–758. [Google Scholar] [CrossRef]
- Macon, A.L.B.; Li, S.; Chung, J.J.; Nommeots-Nomm, A.; Solanki, A.K.; Stevens, M.M.; Jones, J.R. Ductile silica/methacrylate hybrids for bone regeneration. J. Mater. Chem. B 2016, 4, 6032–6042. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Li, J.; Zhu, H.; Guo, Y. A catechol-based biomimetic strategy combined with surface mineralization to enhance hydrophilicity and anti-fouling property of PTFE flat membrane. J. Membr. Sci. 2017, 524, 409–418. [Google Scholar] [CrossRef]
- Qiu, W.Z.; Yang, H.C.; Xu, Z.K. Dopamine-assisted co-deposition: An emerging and promising strategy for surface modification. Adv. Colloid Interface Sci. 2018, 256, 111–125. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. A facile method to synthesize mussel-inspired polydopamine nanospheres as an active template for in situ formation of biomimetic hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 729–739. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, W.; Zhang, S.; Wang, X.; Jiang, Z. Synthesis of organic-inorganic hybrid microcapsules through in situ generation of an inorganic layer on an adhesive layer with mineralization-inducing capability. J. Mater. Chem. B 2015, 3, 465–474. [Google Scholar] [CrossRef]
- Gorgieva, S.; Hribernik, S. Microstructured and Degradable Bacterial Cellulose(-)Gelatin Composite Membranes: Mineralization Aspects and Biomedical Relevance. Nanomaterials 2019, 9, 303. [Google Scholar] [CrossRef]
- Plohl, O.; Zemljic, L.F.; Potrc, S.; Luxbacher, T. Applicability of electro-osmotic flow for the analysis of the surface zeta potential. RSC Adv. 2020, 10, 6777–6789. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Gopanna, A.; Mandapati, R.N.; Thomas, S.P.; Rajan, K.; Chavali, M. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polym. Bull. 2018, 76, 4259–4274. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, N.; Xie, C.; Mou, B.; Ai, Y. Design dopamine-modified polypropylene fibers towards removal of heavy metal ions from water. AIP Adv. 2017, 7, 045011. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, S.; Liu, H. Effect of surface chemistry of polyethyleneimine-grafted polypropylene fiber on its CO 2 adsorption. RSC Adv. 2014, 4, 27176–27183. [Google Scholar] [CrossRef]
- Liu, M.; Ji, J.; Zhang, X.; Zhang, X.; Yang, B.; Deng, F.; Li, Z.; Wang, K.; Yang, Y.; Wei, Y. Self-polymerization of dopamine and polyethyleneimine: Novel fluorescent organic nanoprobes for biological imaging applications. J. Mater. Chem. B 2015, 3, 3476–3482. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR Study of the Hydrolysis of Tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Ojstršek, A.; Fakin, D. Washing durability and photo-stability of nanoTiO2-SiO2 coatings exhausted onto cotton and cotton/polyester fabrics. Coatings 2019, 9, 545. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, K.; Blanford, C.F.; Francis, L.F.; Stein, A. In vitro hydroxycarbonate apatite mineralization of CaO− SiO2 sol− gel glasses with a three-dimensionally ordered macroporous structure. J. Chem. Mater. 2001, 13, 1374–1382. [Google Scholar] [CrossRef]
- Srinivasan, A.; Rajendran, N. Surface characteristics, corrosion resistance and MG63 osteoblast-like cells attachment behaviour of nano SiO2–ZrO2 coated 316L stainless steel. RSC Adv. 2015, 5, 26007–26016. [Google Scholar] [CrossRef]
- Rocton, N.; Oudadesse, H.; Lefeuvre, B.; Peisker, H.; Rbii, K. Fine analysis of interaction mechanism of bioactive glass surface after soaking in SBF solution: AFM and ICP-OES investigations. Appl. Surf. Sci. 2020, 505, 144076. [Google Scholar] [CrossRef]
- Lameiras, F.S.; Souza, A.L.d.; Melo, V.A.R.d.; Nunes, E.H.M.; Braga, I.D. Measurement of the zeta potential of planar surfaces with a rotating disk. Mater. Res. 2008, 11, 217–219. [Google Scholar] [CrossRef]
- Cai, K.; Frant, M.; Bossert, J.; Hildebrand, G.; Liefeith, K.; Jandt, K.D. Surface functionalized titanium thin films: Zeta-potential, protein adsorption and cell proliferation. Colloids Surf B Biointerfaces 2006, 50, e386–e395. [Google Scholar] [CrossRef] [PubMed]
- Lukowiak, M.C.; Wettmarshausen, S.; Hidde, G.; Landsberger, P.; Boenke, V.; Rodenacker, K.; Braun, U.; Friedrich, J.F.; Gorbushina, A.A.; Haag, R. Polyglycerol coated polypropylene surfaces for protein and bacteria resistance. Polym. Chem. 2015, 6, 1350–1359. [Google Scholar] [CrossRef]
- Szewczyk, A.; Skwira, A.; Ginter, M.; Tajer, D.; Prokopowicz, M. Microwave-Assisted Fabrication of Mesoporous Silica-Calcium Phosphate Composites for Dental Application. Polymers 2020, 13, 53. [Google Scholar] [CrossRef]
- Yadav, N.; Srivastava, P. Study on Gelatin/Hydroxyapatite/Chitosan Material Modified with Osteoblast for Bone Bioengineering. Arab. J. Sci. Eng. 2021, 47, 165–178. [Google Scholar] [CrossRef]


| Sample | Root-Mean-Square Height (nm) | Skewness | Kurtosis | Arithmetic Mean Height (nm) | Areal Height Difference (nm) |
|---|---|---|---|---|---|
| PP | 13.4 | 0.0124 | 5.62 | 9.79 | 24.5 |
| PP-PDA | 207 | 2.81 | 11.8 | 135 | 376 |
| PP-PDA-PEI | 19.2 | 4.20 | 24.5 | 9.99 | 19.2 |
| PP-PDA-PEI-TEOS | 59.0 | 6.01 | 48.3 | 25.2 | 36.5 |
| PP-PDA-PEI-TEOS-W | 26.5 | 4.70 | 35.7 | 14.1 | 31.4 |
| PP-PDA-PEI-TEOS-A | 19.6 | 3.36 | 22.8 | 12.0 | 29.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojstršek, A.; Chemelli, A.; Osmić, A.; Gorgieva, S. Dopamine-Assisted Modification of Polypropylene Film to Attain Hydrophilic Mineral-Rich Surfaces. Polymers 2023, 15, 902. https://doi.org/10.3390/polym15040902
Ojstršek A, Chemelli A, Osmić A, Gorgieva S. Dopamine-Assisted Modification of Polypropylene Film to Attain Hydrophilic Mineral-Rich Surfaces. Polymers. 2023; 15(4):902. https://doi.org/10.3390/polym15040902
Chicago/Turabian StyleOjstršek, Alenka, Angela Chemelli, Azra Osmić, and Selestina Gorgieva. 2023. "Dopamine-Assisted Modification of Polypropylene Film to Attain Hydrophilic Mineral-Rich Surfaces" Polymers 15, no. 4: 902. https://doi.org/10.3390/polym15040902
APA StyleOjstršek, A., Chemelli, A., Osmić, A., & Gorgieva, S. (2023). Dopamine-Assisted Modification of Polypropylene Film to Attain Hydrophilic Mineral-Rich Surfaces. Polymers, 15(4), 902. https://doi.org/10.3390/polym15040902