Accelerated Shelf-Life and Stability Testing of Hydrolyzed Corn Starch Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Corn Starch Hydrolysate (CSH)
2.2. Preparation of CSH Films
2.3. Physical and Hygroscopic Properties: Film Thickness, Moisture Content (Mc), Swelling Degree (Sd), and Total Soluble Matter (Tsm)
2.3.1. Film Thickness
2.3.2. Moisture Content (Mc), Swelling Degree (Sd), and Total Soluble Matter (Tsm)
2.3.3. Water Vapor Permeability (WVP)
2.4. Accelerated Shelf Life and Stability Testing
- -
- Time compression (high usage rate, testing is performed more intensively than actual usage);
- -
- Accelerated degradation testing (the same conditions that degrade the product under normal storage conditions).
2.4.1. Moisture Absorption (Ma)
2.4.2. Folding Strength
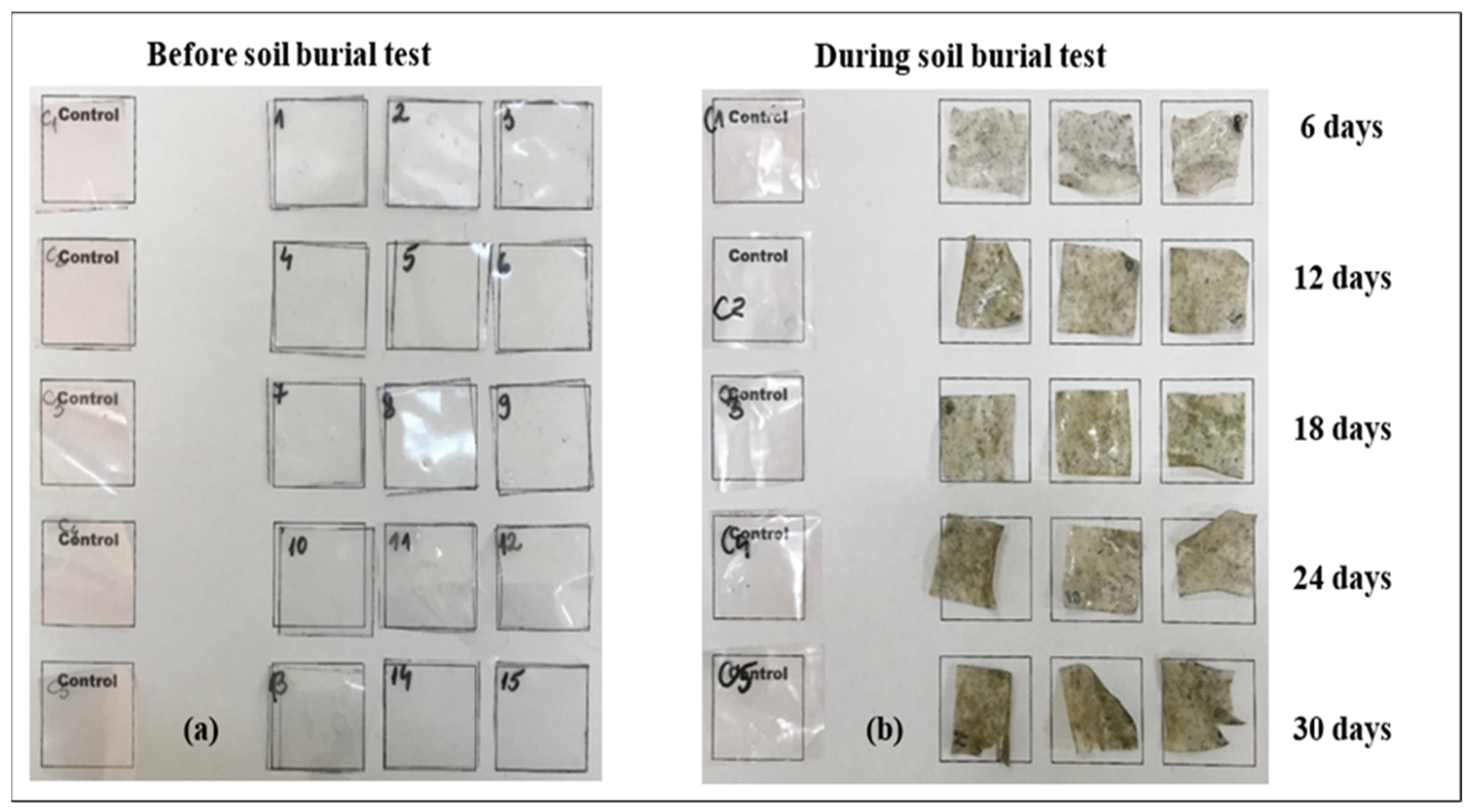
2.5. Biodegradability Property: Soil Burial Test
2.5.1. Visual Appearance
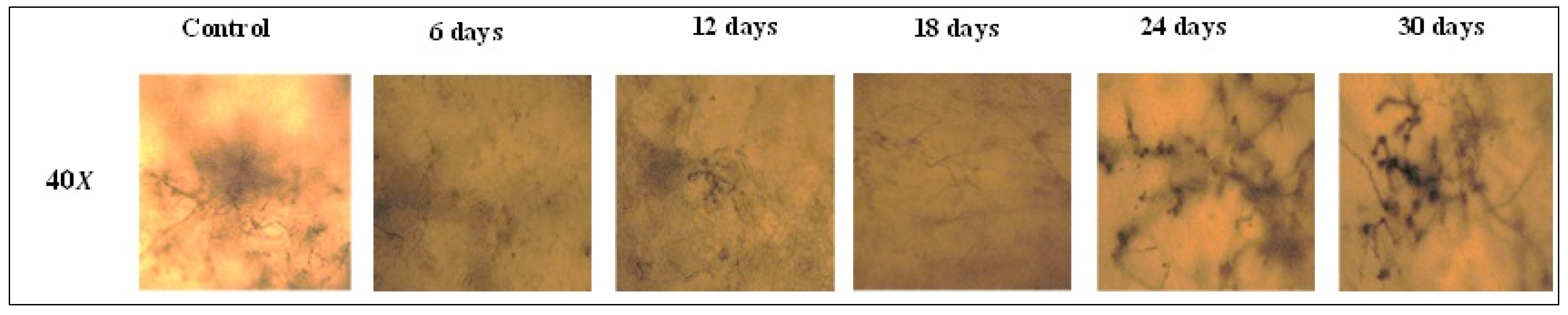
2.5.2. Polarized Light Microscopy
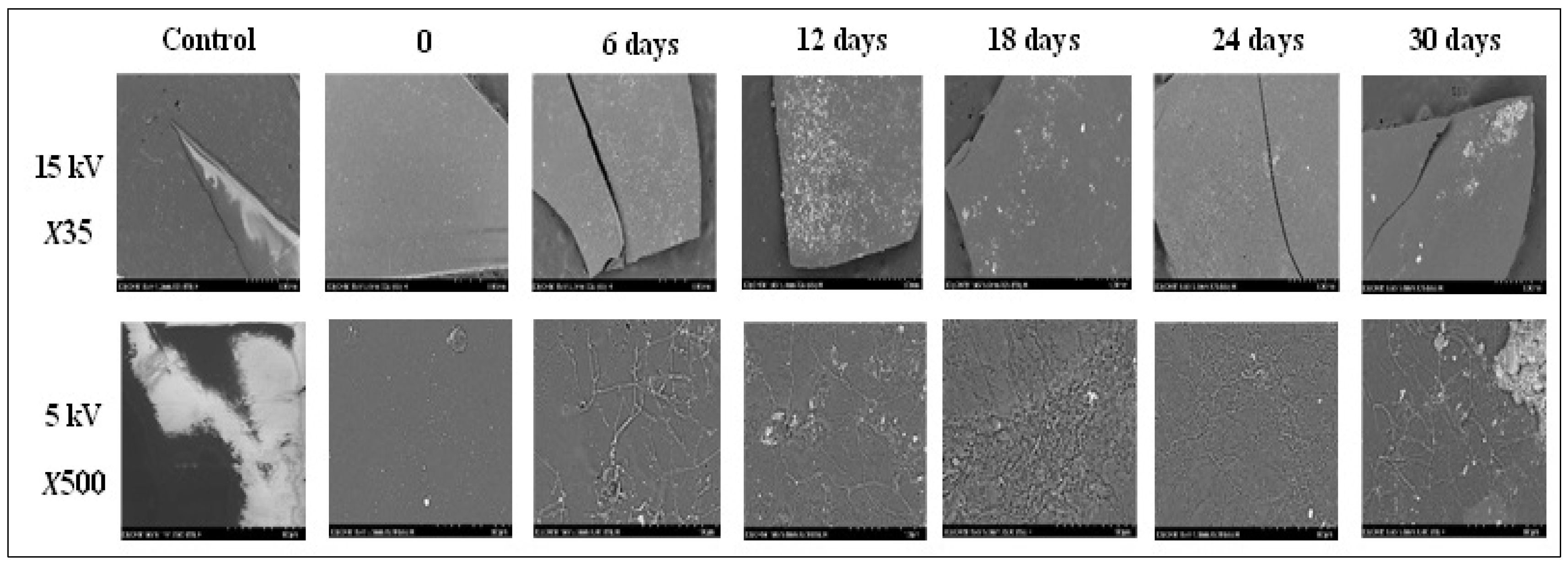
2.5.3. SEM Characterization
2.5.4. FTIR Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physical and Hygroscopic Properties
3.1.1. Film Thickness, Mc, Sd, and Tsm
3.1.2. WVP
3.2. Accelerated Shelf Life and Stability Testing
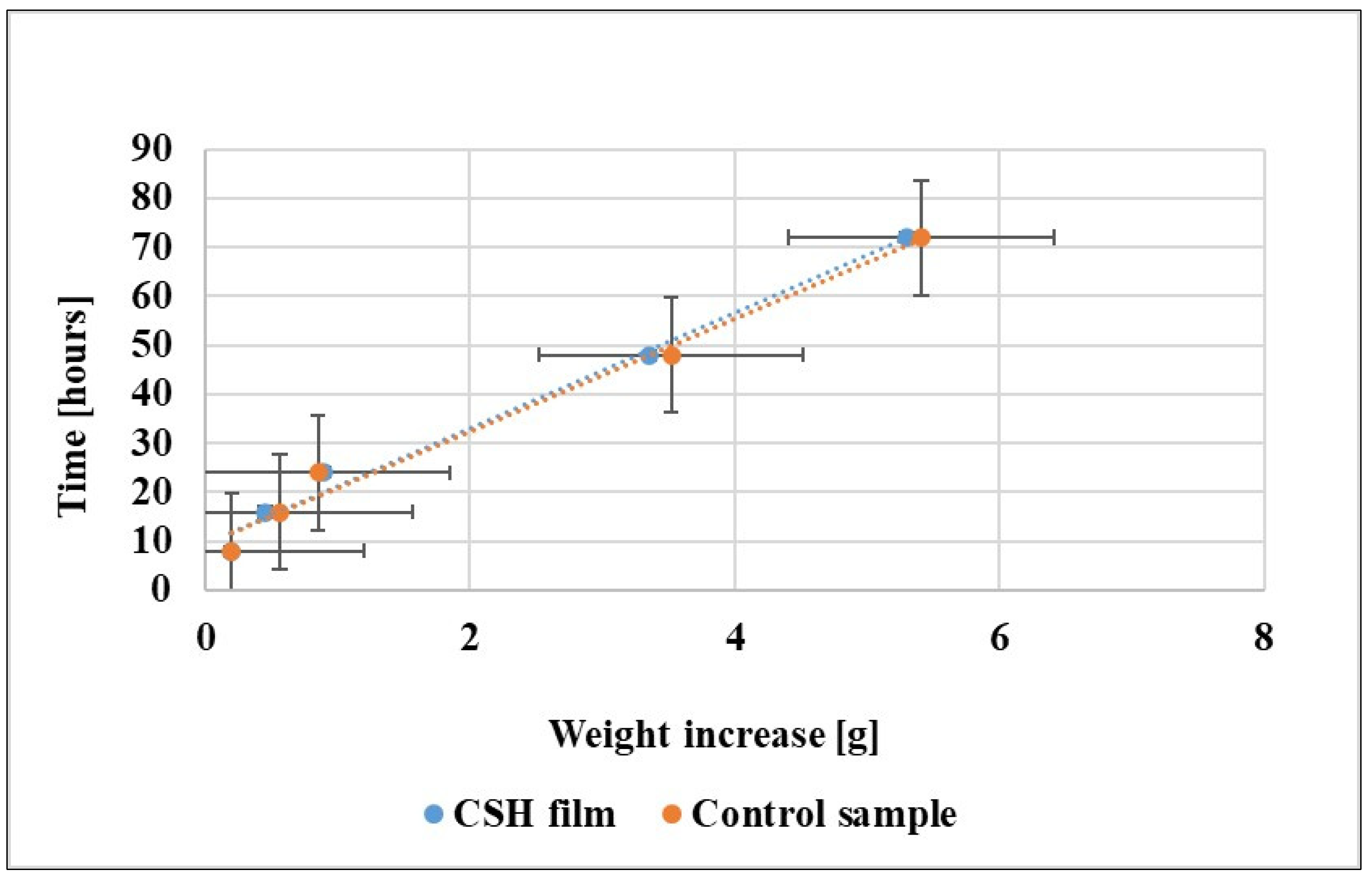
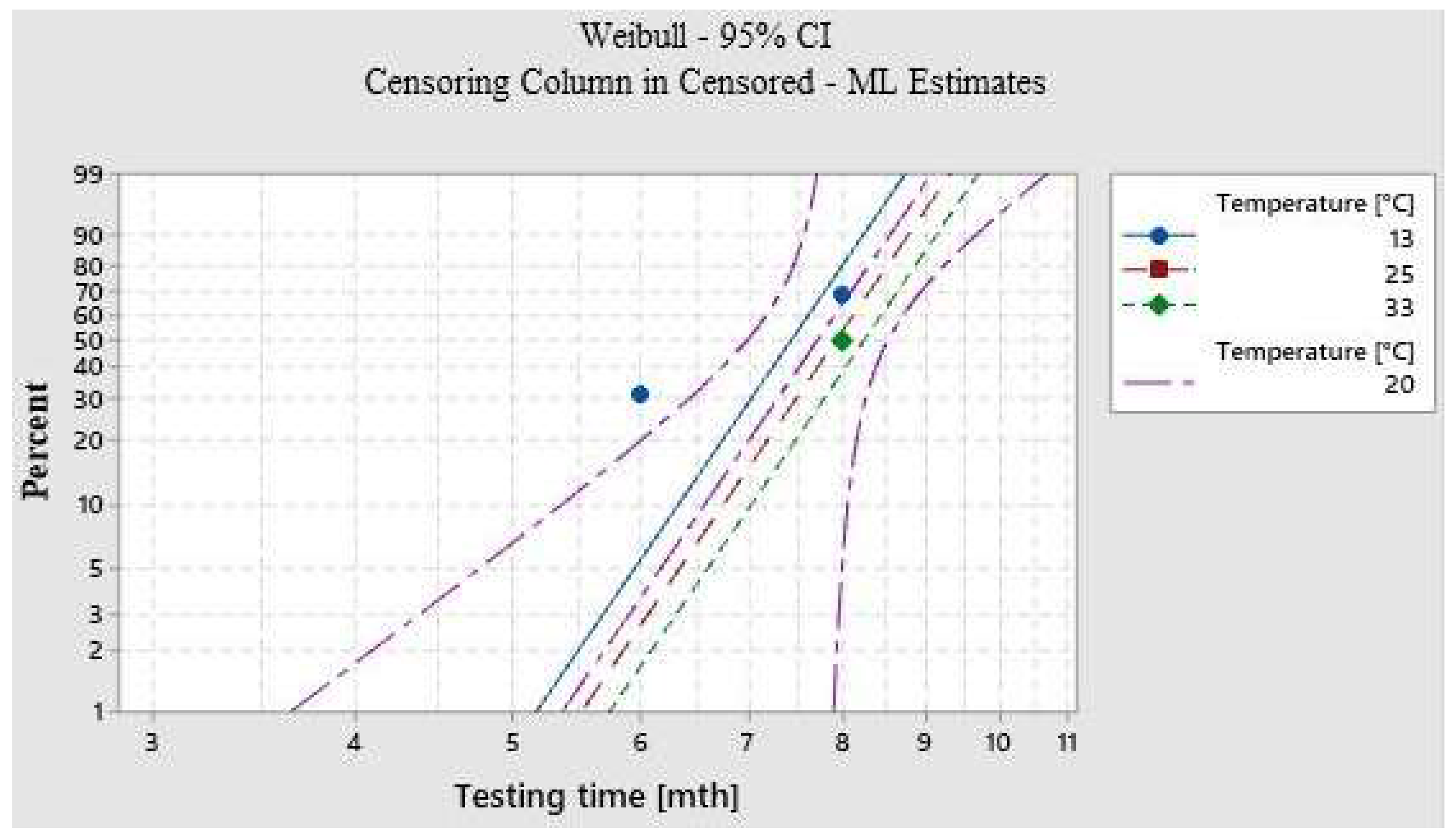
3.2.1. ASLT
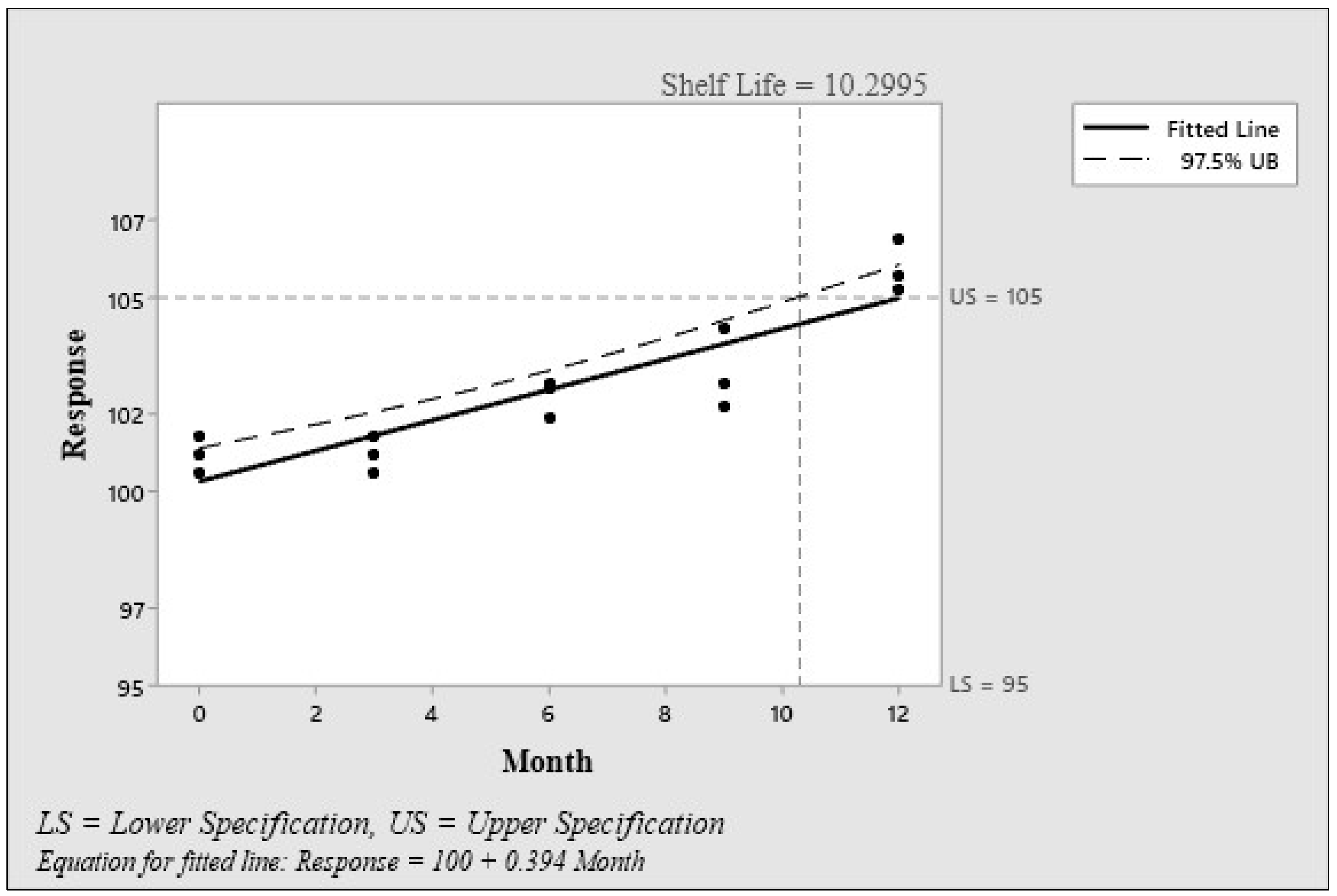
3.2.2. Stability Test
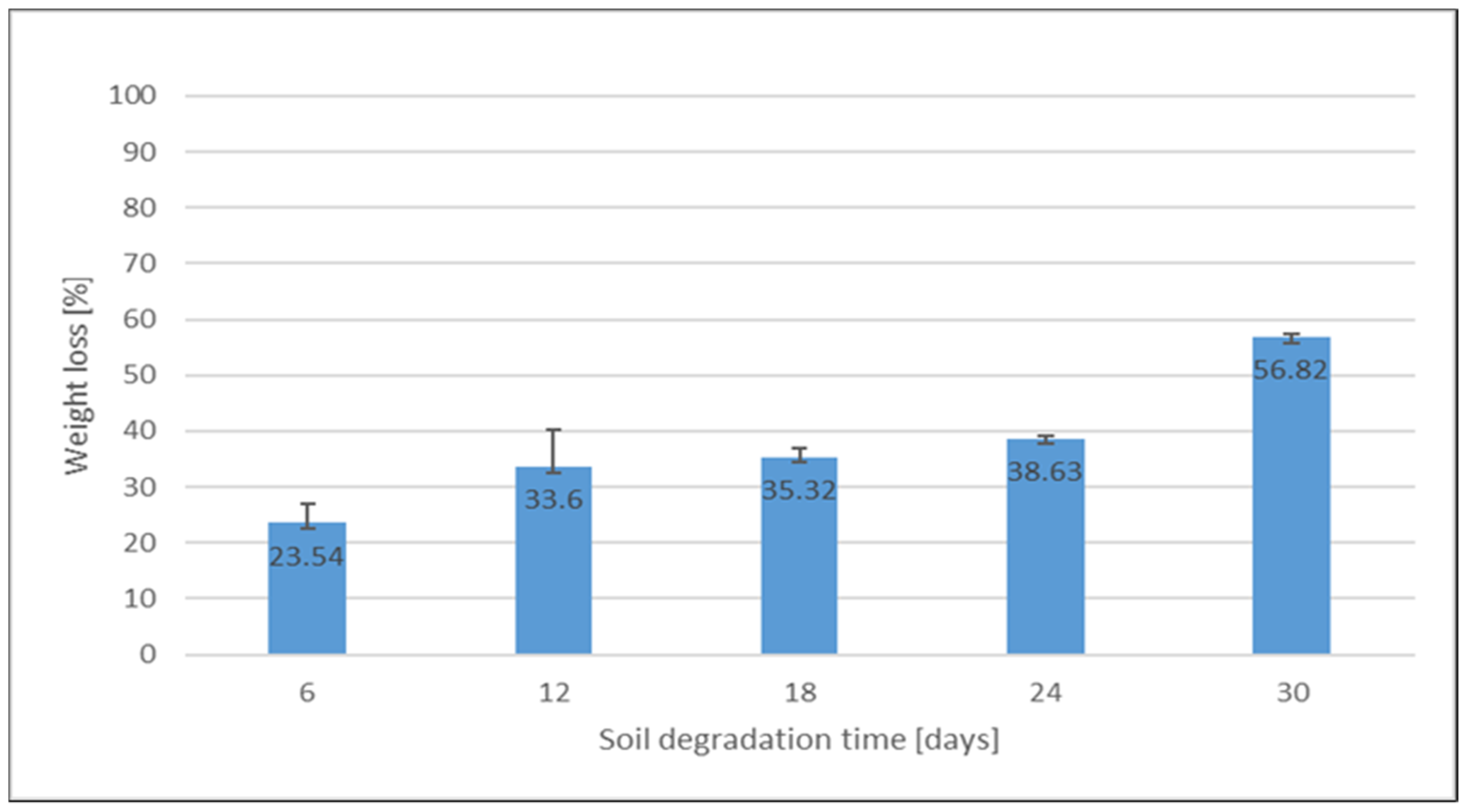
3.3. Biodegradability Property
3.3.1. Visual Appearance
3.3.2. Polarized Light Microscopy
3.3.3. SEM Characterization
3.3.4. FTIR Analysis
4. Conclusions
Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nissa, R.C.; Fikriyyah, A.K.; Abdullah, A.H.D. Preliminary study of biodegradability of starch-based bioplastics using ASTM G21-70, dip-hanging, and Soil Burial Test methods. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 277, No. 1. [Google Scholar] [CrossRef]
- Costa, A.; Encarnação, T.; Tavares, R.; Todo Bom, T.; Mateus, A. Bioplastics: Innovation for Green Transition. Polymers 2023, 15, 517. [Google Scholar] [CrossRef]
- Guliyev, V.; Tanunchai, B.; Udovenko, M.; Menyailo, O.; Glaser, B.; Purahong, W.; Buscot, F.; Blagodatskaya, E. Degradation of Bio-Based and Biodegradable Plastic and Its Contribution to Soil Organic Carbon Stock. Polymers 2023, 15, 660. [Google Scholar] [CrossRef]
- Luchese, C.L.; Benelli, P.; Spada, J.C.; Tessaro, I.C. Impact of the starch source on the physicochemical properties and biodegradability of different starch-based films. J. Appl. Polym. Sci. 2018, 135, 46564. [Google Scholar] [CrossRef]
- Liu, J.; Ni, R.; Chau, Y. A self-assembled peptidic nanomillipede to fabricate a tuneable hybrid hydrogel. Chem. Commun. 2019, 55, 7093–7096. [Google Scholar] [CrossRef]
- Liu, J.; Zhorabek, F.; Chau, Y. Biomaterial design inspired by membraneless organelles. Matter 2022, 5, 2787–2812. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Song, H.; Young, M.; Fan, Y.; Xu, F.J.; Qu, X.; Lei, X.; Liu, Y.; Cheng, G. Peptide-grafted dextran vectors for efficient and high-loading gene delivery. Biomater. Sci.-UK 2019, 7, 1543–1553. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Hou, X.; Wu, H.; Li, Q.; Li, S.; Luo, Q.; Li, M.; Liu, X.; Shen, G.; et al. Physicochemical, antibacterial, and biodegradability properties of green Sichuan pepper (Zanthoxylum armatum DC.) essential oil incorporated starch films. LWT 2022, 161, 113392. [Google Scholar] [CrossRef]
- Othman, S.H.; Wane, B.M.; Nordin, N.; Noor Hasnan, N.Z.; Talib, A.; Karyadi, J.N.W.R. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers 2021, 13, 4060. [Google Scholar] [CrossRef]
- Thuppahige, V.T.W.; Moghaddam, L.; Whelsh, Z.G.; Karim, A. Investigation of Morphological, Chemical, and Thermal Properties of Biodegradable Food Packaging Films Synthesised by Direct Utilisation of Cassava (Monihot esculanta) Bagasse. Polymers 2023, 15, 767. [Google Scholar] [CrossRef]
- Debnath, B.; Duarah, P.; Haldar, D.; Purkait, M.K. Improving the properties of corn starch films for application as packaging material via reinforcement with microcrystalline cellulose synthesized from elephant grass. Food Packag. Shelf Life 2022, 34, 100937. [Google Scholar] [CrossRef]
- Boeira, C.P.; Flores, D.C.B.; dos Santos Alves, J.; de Moura, M.R.; Melo, P.T.S.; Rolim, C.M.B.; Nogueira-Librelotto, D.R.; da Rosa, C.S. Effect of corn stigma extract on physical and antioxidant properties of biodegradable and edible gelatin and corn starch films. Int. J. Biol. Macromol. 2022, 208, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.M.; Pavan, S.; Phanipriya, P. Production of rice bran wax-based biodegradable film. Indian J. Ecol. 2022, 49, 905–909. [Google Scholar]
- Sommerfeld, H.; Blume, R. Biodegradable films. Based on partially hydrolyzed corn starch or potato starch. J. Chem. Educ. 1992, 69, A151. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, H.; Liu, P.; Wang, W.; Dong, H. Effects of acid hydrolysis on the physicochemical properties of pea starch and its film forming capacity. Food Hydrocolloid. 2019, 87, 173–179. [Google Scholar] [CrossRef]
- Martins, P.C.; Latorres, J.M.; Martins, V.G. Impact of starch nanocrystals on the physicochemical, thermal and structural characteristics of starch-based films. LWT 2022, 156, 113041. [Google Scholar] [CrossRef]
- Wawro, D.; Kazimierczak, J. Forming conditions and mechanical properties of potato starch films. Fibres Text. East. Eur. 2008, 16, 71. [Google Scholar]
- Rosseto, M.; Krein, D.D.; Balbé, N.P.; Dettmer, A. Starch–gelatin film as an alternative to the use of plastics in agriculture: A review. J. Sci. Food. Agr. 2019, 99, 6671–6679. [Google Scholar] [CrossRef]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohyd. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep.-UK 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Vidaurre, E.C.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch-based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Chang, Y.P.; Cheah, P.B.; Seow, C.C. Plasticizing—Antiplasticizing effects of water on physical properties of tapioca starch films in the glassy state. J. Food Sci. 2000, 65, 445–451. [Google Scholar] [CrossRef]
- Macedo, I.S.M.; Sousa-Gallagher, M.J.; Oliveira, J.C.; Byrne, E.P. Quality by design for packaging of granola breakfast product. Food Control. 2013, 29, 438–443. [Google Scholar] [CrossRef]
- Bilbie, C.; Ghizdareanu, A. Comparative analysis of estimated shelf life, approaching accelerated aging methods. Sci. Bull. Ser. F Biotechnol. 2021, 25, 104–111. [Google Scholar]
- Macedo, I.S.M.; Sousa-Gallagher, M.J.; Mahajan, P.V. Kinetic modelling of quality decay of granulated breakfast cereal during the storage. In Proceedings of the 7th International Conference on Predictive Modelling of Food Quality and Safety, Dublin, Ireland, 12 September 2011; pp. 402–405. [Google Scholar]
- Kong, H.; Yang, X.; Gu, Z.; Li, Z.; Cheng, L.; Hong, Y.; Li, C. Heat pretreatment improves the enzymatic hydrolysis of granular corn starch at high concentration. Process. Biochem. 2018, 64, 193–199. [Google Scholar] [CrossRef]
- Beer-Lech, K.J.; Skic, A.; Skic, K.; Stropek, Z. Characterization of the Structural and Physical Properties of the Thermoplastic Starch Film with Kaolinite and Beeswax Addition. Adv. Sci. Technol. Res. J. 2022, 16, 312–323. [Google Scholar] [CrossRef]
- Janik, W.; Nowotarski, M.; Shyntum, D.Y.; Banaś, A.; Krukiewicz, K.; Kudła, S.; Dudek, G. Antibacterial and Biodegradable Polysaccharide-Based Films for Food Packaging Applications: Comparative Study. Materials 2022, 15, 3236. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Majid, N.A.; Tawakkal, I.S.M.A.; Basha, R.K.; Nordin, N.; Shapi’I, R.A. Tapioca starch films reinforced with microcrystalline cellulose for potential food packaging application. Food Sci. Tech-Braz. 2019, 39, 605–612. [Google Scholar] [CrossRef]
- Adjouman, Y.D.; Nindjin, C.; Tetchi, F.A.; Dalcq, A.C.; Amani, N.G.; Sindic, M. Water vapor permeability of edible films based on improved Cassava (Manihot esculenta Crantz) native starches. J. Food Process. Technol. 2017, 8. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Synthesis and functional properties of gelatin/CA–starch composite film: Excellent food packaging material. J. Food Sci. Technol. Mys. 2019, 56, 1954–1965. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Moth bean starch (Vigna aconitifolia): Isolation, characterization, and development of edible/biodegradable films. J. Food Sci. Technol. Mys. 2019, 56, 4891–4900. [Google Scholar] [CrossRef]
- Astm, E. Standard test methods for water vapour transmission of material. In E96-95. Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 1995; Volume 4, pp. 697–704. [Google Scholar]
- Mizrahi, S. The stability and shelf-life of food. In Accelerated Shelf-Life Tests; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2000; pp. 107–125. [Google Scholar]
- Kilcast, D.; Subramaniam, P. The Stability and Shelf-Life of Food; Woodhead Publishing Limited: Cambridge, UK, 2000. [Google Scholar]
- Mizrahi, S. Understanding and measuring the shelf life of food. In Accelerated Shelf-Life Tests; Steele, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2004. [Google Scholar]
- McMahon, T.J. Accelerated testing and failure of thin-film PV modules. Prog. Photovolt. Res. Appl. 2004, 12, 235–248. [Google Scholar] [CrossRef]
- Tarantili, P.A.; Kiose, V. Effect of accelerated aging on the structure and properties of monolayer and multilayer packaging films. J. Appl. Polym. Sci. 2008, 109, 674–682. [Google Scholar] [CrossRef]
- Niaounakis, M.; Kontou, E.; Pispas, S.; Kafetzi, M.; Giaouzi, D. Aging of packaging films in the marine environment. Polym. Eng. Sci. 2019, 59, E432–E441. [Google Scholar] [CrossRef]
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics 25–28. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Sharmila, S.; Ravi Teja, P.; Vijay Chandra Gangadhar Gupta, D. Bioplastic production using corn starch with natural fillers and its seem-eds report. Plant Arch. 2021, 21. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and Solubility; IntechOpen: London, UK, November 2017. [Google Scholar]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocoll. 2020, 98, 105007. [Google Scholar] [CrossRef]
- E.N. 13432:2000; Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Committee for Standardization: Brussels, Belgium, 2000.

| Sample | Property | |||
|---|---|---|---|---|
| Film Thickness [mm] | Mc [%] | Sd [%] | Tsm [%] | |
| Control | 0.1756 ± 0.0004 a | 23.30 ± 0.025 a | 56.2 ± 0.050 a | 5.65 ± 0.030 a |
| CSH film | 0.1563 ± 0.0007 b | 19.15 ± 0.020 b | 60.3 ± 0.025 b | 4.75 ± 0.030 b |
| Sample | Property | ||
|---|---|---|---|
| R2 | Y | WVP | |
| [g × mm/m2 × h × kPa] | |||
| Control | 0.9852 | 11.469x + 9.4239 | 0.3596 ± 0.0013 a |
| CSH film | 0.9884 | 11.751x + 9.6306 | 0.3296 ± 0.0003 b |
| Unchanged Samples [%] | Time until Degradation [mth] | Expected Durability [mth] | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| 1 | 5.36 | 3.64 | 7.89 |
| 2 | 5.69 | 4.09 | 7.93 |
| 3 | 5.90 | 4.59 | 7.95 |
| 4 | 6.05 | 4.76 | 7.97 |
| 5 | 6.17 | 4.91 | 7.99 |
| 6 | 6.27 | 5.03 | 8.00 |
| 7 | 6.35 | 5.15 | 8.02 |
| 8 | 6.43 | 5.25 | 8.03 |
| 9 | 6.50 | 5.34 | 8.04 |
| 10 | 7.00 | 6.00 | 8.05 |
| 20 | 7.29 | 6.42 | 8.16 |
| 30 | 7.51 | 6.73 | 8.39 |
| 40 | 7.77 | 6.98 | 8.53 |
| 50 | 7.90 | 7.17 | 8.77 |
| 60 | 8.09 | 7.32 | 8.94 |
| 70 | 8.29 | 7.45 | 9.23 |
| 80 | 8.55 | 7.57 | 9.67 |
| 90 | 8.75 | 7.63 | 10.03 |
| 95 | 8.89 | 7.66 | 10.25 |
| 99 | 9.08 | 7.71 | 10.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghizdareanu, A.-I.; Pasarin, D.; Banu, A.; Ionita, A.; Enascuta, C.E.; Vlaicu, A. Accelerated Shelf-Life and Stability Testing of Hydrolyzed Corn Starch Films. Polymers 2023, 15, 889. https://doi.org/10.3390/polym15040889
Ghizdareanu A-I, Pasarin D, Banu A, Ionita A, Enascuta CE, Vlaicu A. Accelerated Shelf-Life and Stability Testing of Hydrolyzed Corn Starch Films. Polymers. 2023; 15(4):889. https://doi.org/10.3390/polym15040889
Chicago/Turabian StyleGhizdareanu, Andra-Ionela, Diana Pasarin, Alexandra Banu, Andreea Ionita (Afilipoaei), Cristina Emanuela Enascuta, and Alexandru Vlaicu. 2023. "Accelerated Shelf-Life and Stability Testing of Hydrolyzed Corn Starch Films" Polymers 15, no. 4: 889. https://doi.org/10.3390/polym15040889
APA StyleGhizdareanu, A.-I., Pasarin, D., Banu, A., Ionita, A., Enascuta, C. E., & Vlaicu, A. (2023). Accelerated Shelf-Life and Stability Testing of Hydrolyzed Corn Starch Films. Polymers, 15(4), 889. https://doi.org/10.3390/polym15040889





