Self-Assembly of a Two-Dimensional Coordination Polymer Based on Silver and Lanthanide Tetrakis-Acylpyrazolonates: An Efficient New Strategy for Suppressing Ligand-to-Metal Charge Transfer Quenching of Europium Luminescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Spectroscopic Characterization of the Complexes
2.2. Apparatus
3. Results
3.1. Synthesis
3.2. Single Crystal Structures
3.3. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Li, J. Luminescent Metal–Organic Frameworks and Coordination Polymers as Alternative Phosphors for Energy Efficient Lighting Devices. Coord. Chem. Rev. 2018, 373, 116–147. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nakanishi, T. Luminescent Lanthanide Coordination Polymers for Photonic Applications. RSC Adv. 2015, 5, 338–353. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Cui, Y.; Qian, G.; Chen, B. Multifunctional Lanthanide Coordination Polymers. Prog. Polym. Sci. 2015, 48, 40–84. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide Azolecarboxylate Compounds: Structure, Luminescent Properties and Applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Piguet, C. Lanthanide-Containing Molecular and Supramolecular Polymetallic Functional Assemblies. Chem. Rev. 2002, 102, 1897–1928. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd: Chichester, UK, 2006; ISBN 9780470010082. [Google Scholar]
- Xu, H.; Sun, Q.; An, Z.; Wei, Y.; Liu, X. Electroluminescence from Europium(III) Complexes. Coord. Chem. Rev. 2015, 293–294, 228–249. [Google Scholar] [CrossRef]
- Álvarez, Á.L.; Coya, C. OLEDs Based on Ln(III) Complexes for near-Infrared Emission. In Lanthanide-Based Multifunctional Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–170. ISBN 9780128138403. [Google Scholar]
- Wang, L.; Zhao, Z.; Wei, C.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Review on the Electroluminescence Study of Lanthanide Complexes. Adv. Opt. Mater. 2019, 7, 1801256. [Google Scholar] [CrossRef]
- Zinna, F.; Pasini, M.; Galeotti, F.; Botta, C.; Di Bari, L.; Giovanella, U. Design of Lanthanide-Based OLEDs with Remarkable Circularly Polarized Electroluminescence. Adv. Funct. Mater. 2017, 27, 1603719. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 1, 189–227. [Google Scholar] [CrossRef]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide Probes for Bioresponsive Imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Zobel, M.; Tsymbarenko, D. Partially Ordered Lanthanide Carboxylates with a Highly Adaptable 1D Polymeric Structure. Polymers 2022, 14, 3328. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. A Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef]
- Lee, S.; Lin, M.; Lee, A.; Park, Y. Lanthanide-Doped Nanoparticles for Diagnostic Sensing. Nanomaterials 2017, 7, 411. [Google Scholar] [CrossRef]
- Aulsebrook, M.L.; Graham, B.; Grace, M.R.; Tuck, K.L. Lanthanide Complexes for Luminescence-Based Sensing of Low Molecular Weight Analytes. Coord. Chem. Rev. 2018, 375, 191–220. [Google Scholar] [CrossRef]
- Aletti, A.B.; Gillen, D.M.; Gunnlaugsson, T. Luminescent/Colorimetric Probes and (Chemo-) Sensors for Detecting Anions Based on Transition and Lanthanide Ion Receptor/Binding Complexes. Coord. Chem. Rev. 2018, 354, 98–120. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.S.; Kang, X.M.; Zhao, B. Lanthanide-Based Metal-Organic Frameworks as Luminescent Probes. Dalt. Trans. 2016, 45, 18003–18017. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef]
- Lunev, A.M.; Belousov, Y.A. Luminescent Sensor Materials Based on Rare-Earth Element Complexes for Detecting Cations, Anions, and Small Molecules. Russ. Chem. Bull. 2022, 71, 825–857. [Google Scholar] [CrossRef]
- Crosby, G.A.; Whan, R.E.; Freeman, J.J. Spectroscopic Studies of Rare Earth Chelates. J. Phys. Chem. 1962, 66, 2493–2499. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking Advantage of Luminescent Lanthanide Ions. Chem. Soc. Rev. 2005, 34, 1048. [Google Scholar] [CrossRef]
- Bünzli, J.; Eliseeva, S.V. Basics of Lanthanides Photophysics. In Lanthanide Luminescence; Hänninen, P., Härmä, H., Eds.; Springer Series on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2010; Volume 7, pp. 1–45. [Google Scholar] [CrossRef]
- Nehra, K.; Dalal, A.; Hooda, A.; Bhagwan, S.; Saini, R.K.; Mari, B.; Kumar, S.; Singh, D. Lanthanides β-Diketonate Complexes as Energy-Efficient Emissive Materials: A Review. J. Mol. Struct. 2022, 1249, 131531. [Google Scholar] [CrossRef]
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of Rare Earth Elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- Taidakov, I.V.; Lobanov, A.N.; Vitukhnovskii, A.G.; Starikova, Z.A. Synthesis and Unusual Crystal Structure of the Eu(III) Complex with 1-(1,5-Dimethyl-1H-Pyrazol-4-Yl)-4,4,4-Trifluorobutane-1,3-Dione. Russ. J. Coord. Chem. 2013, 39, 437–441. [Google Scholar] [CrossRef]
- Yang, P. Synthesis, Structure, and Luminescence Properties of Rare Earth Complexes with β-Diketone Containing Imidazole Group. Z. Anorg. Allg. Chem. 2018, 644, 838–843. [Google Scholar] [CrossRef]
- Burrows, A.D.; Mahon, M.F.; Renouf, C.L.; Richardson, C.; Warren, A.J.; Warren, J.E. Dipyridyl β-Diketonate Complexes and Their Use as Metalloligands in the Formation of Mixed-Metal Coordination Networks. Dalt. Trans. 2012, 41, 4153. [Google Scholar] [CrossRef]
- Hui, Y.-C.; Meng, Y.-S.; Li, Z.; Chen, Q.; Sun, H.-L.; Zhang, Y.-Q.; Gao, S. Construction and Theoretical Study of a New Dy-β-Diketone Chain Featuring Slow Magnetic Relaxation. CrystEngComm 2015, 17, 5620–5624. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Y. Polymeric [Eu(L)3(H2O)] (HL = 1-(Pyridin-4-Yl)Butane- 1,3-Dione and Dimeric [Eu2(L)6(H2O)2] (HL = 1,3-Di(Pyridin-3-Yl)Propane-1,3-Dione) as Selective and Sensitive Chemosensors for Hg(II) Ion. Inorg. Chem. Commun. 2010, 13, 1410–1413. [Google Scholar] [CrossRef]
- Guettas, D.; Montigaud, V.; Garcia, G.F.; Larini, P.; Cador, O.; Le Guennic, B.; Pilet, G. Fine Control of the Metal Environment within Dysprosium-Based Mononuclear Single-Molecule Magnets. Eur. J. Inorg. Chem. 2018, 2018, 333–339. [Google Scholar] [CrossRef]
- Andrews, P.C.; Deacon, G.B.; Frank, R.; Fraser, B.H.; Junk, P.C.; MacLellan, J.G.; Massi, M.; Moubaraki, B.; Murray, K.S.; Silberstein, M. Formation of Ho III Trinuclear Clusters and Gd III Monodimensional Polymers Induced by Ortho and Para Regioisomers of Pyridyl-Functionalised Β-Diketones: Synthesis, Structure, and Magnetic Properties. Eur. J. Inorg. Chem. 2009, 2009, 744–751. [Google Scholar] [CrossRef]
- Semenov, S.N.; Rogachev, A.Y.; Eliseeva, S.V.; Pettinari, C.; Marchetti, F.; Drozdov, A.A.; Troyanov, S.I. First Direct Assembly of Molecular Helical Complexes into a Coordination Polymer. Chem. Commun. 2008, 17, 1992–1994. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pizzabiocca, A.; Drozdov, A.A.; Troyanov, S.I.; Zhuravlev, C.O.; Semenov, S.N.; Belousov, Y.A.; Timokhin, I.G. Syntheses, Structures, and Spectroscopy of Mono- and Polynuclear Lanthanide Complexes Containing 4-Acyl-Pyrazolones and Diphosphineoxide. Inorg. Chim. Acta 2010, 363, 4038–4047. [Google Scholar] [CrossRef]
- Miyata, K.; Ohba, T.; Kobayashi, A.; Kato, M.; Nakanishi, T.; Fushimi, K.; Hasegawa, Y. Thermostable Organo-Phosphor: Low-Vibrational Coordination Polymers That Exhibit Different Intermolecular Interactions. Chempluschem 2012, 77, 277–280. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Miura, Y.; Kitagawa, Y.; Wada, S.; Nakanishi, T.; Fushimi, K.; Seki, T.; Ito, H.; Iwasa, T.; Taketsugu, T.; et al. Spiral Eu(iii) Coordination Polymers with Circularly Polarized Luminescence. Chem. Commun. 2018, 54, 10695–10697. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Naito, A.; Fushimi, K.; Hasegawa, Y. Bright Sky-Blue Fluorescence with High Color Purity: Assembly of Luminescent Diphenyl-Anthracene Lutetium-Based Coordination Polymer. RSC Adv. 2021, 11, 6604–6606. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, P.; Li, H.-F.; Tian, Y.-M.; Yan, P.-F.; Sun, W.-B. From Zero-Dimensional to One-Dimensional Chain N -Oxide Bridged Compounds with Enhanced Single-Molecule Magnetic Performance. Dalt. Trans. 2019, 48, 4324–4332. [Google Scholar] [CrossRef]
- Hirai, Y.; Ferreira Da Rosa, P.P.; Nakanishi, T.; Kitagawa, Y.; Fushimi, K.; Seki, T.; Ito, H.; Hasegawa, Y. Structural Manipulation of Triboluminescent Lanthanide Coordination Polymers by Side-Group Alteration. Inorg. Chem. 2018, 57, 14653–14659. [Google Scholar] [CrossRef]
- Armelao, L.; Belli Dell’Amico, D.; Bellucci, L.; Bottaro, G.; Labella, L.; Marchetti, F.; Samaritani, S. A Convenient Synthesis of Highly Luminescent Lanthanide 1D-Zigzag Coordination Chains Based Only on 4,4′-Bipyridine as Connector. Polyhedron 2016, 119, 371–376. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Sun, J.; Li, H.; Liu, J.; Wang, Q.; Li, L.; Ma, Y.; Zhao, B.; Cheng, P. Enhancing the Energy Barrier of Dysprosium(iii) Single-Molecule Magnets by Tuning the Magnetic Interactions through Different N -Oxide Bridging Ligands. CrystEngComm 2019, 21, 6219–6225. [Google Scholar] [CrossRef]
- Flores Gonzalez, J.; Cador, O.; Ouahab, L.; Norkov, S.; Kuropatov, V.; Pointillart, F. Field-Induced Dysprosium Single-Molecule Magnet Involving a Fused o-Semiquinone-Extended-Tetrathiafulvalene-o-Semiquinone Bridging Triad. Inorganics 2018, 6, 45. [Google Scholar] [CrossRef]
- Fratini, A.; Richards, G.; Larder, E.; Swavey, S. Neodymium, Gadolinium, and Terbium Complexes Containing Hexafluoroacetylacetonate and 2,2‘-Bipyrimidine: Structural and Spectroscopic Characterization. Inorg. Chem. 2008, 47, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.H.C.; Motevalli, M.; Abrahams, I.; Wyatt, P.B.; Gillin, W.P. Quenching of IR Luminescence of Erbium, Neodymium, and Ytterbium β-Diketonate Complexes by Ligand C−H and C−D Bonds. J. Phys. Chem. B 2006, 110, 24476–24479. [Google Scholar] [CrossRef] [PubMed]
- Mara, D.; Artizzu, F.; Laforce, B.; Vincze, L.; Van Hecke, K.; Van Deun, R.; Kaczmarek, A.M. Novel Tetrakis Lanthanide β-Diketonate Complexes: Structural Study, Luminescence Properties and Temperature Sensing. J. Lumin. 2019, 213, 343–355. [Google Scholar] [CrossRef]
- Abad Galán, L.; Sobolev, A.N.; Zysman-Colman, E.; Ogden, M.I.; Massi, M. Lanthanoid Complexes Supported by Retro-Claisen Condensation Products of β-Triketonates. Dalt. Trans. 2018, 47, 17469–17478. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone Ligands: Synthesis, Structures, Metal Coordination Chemistry and Applications. Coord. Chem. Rev. 2005, 249, 2909–2945. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, R.; Pettinari, C. Recent Advances in Acylpyrazolone Metal Complexes and Their Potential Applications. Coord. Chem. Rev. 2015, 303, 1–31. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A. Lanthanide Acylpyrazolonates: Synthesis, Properties and Structural Features. Russ. Chem. Rev. 2012, 81, 1159–1169. [Google Scholar] [CrossRef]
- Capecchi, S.; Renault, O.; Moon, D.G.; Halim, M.; Etchells, M.; Dobson, P.J.; Salata, O.V.; Christou, V. High-Efficiency Organic Electroluminescent Devices Using an Organoterbium Emitter. Adv. Mater. 2000, 12, 1591–1594. [Google Scholar] [CrossRef]
- Thorne, J.R.G.; Rey, J.M.; Denning, R.G.; Watkins, S.E.; Etchells, M.; Green, M.; Christou, V. Excited State Dynamics of Organo-Lanthanide Electroluminescent Phosphors: The Properties of Tb(Tb-Pmp)3 and Gd(Tb-Pmp)3. J. Phys. Chem. A 2002, 106, 4014–4021. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, F.; Hao, F.; Bian, Z.; Ding, B.; Zhu, Y.; Chen, F.; Huang, C. A Highly Efficient OLED Based on Terbium Complexes. Org. Electron. 2009, 10, 939–947. [Google Scholar] [CrossRef]
- Girotto, E.; Pereira, A.; Arantes, C.; Cremona, M.; Bortoluzzi, A.J.; Salla, C.A.M.; Bechtold, I.H.; Gallardo, H. Efficient Terbium Complex Based on a Novel Pyrazolone Derivative Ligand Used in Solution-Processed OLEDs. J. Lumin. 2019, 208, 57–62. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Korshunov, V.M.; Metlin, M.T.; Metlina, D.A.; Kiskin, M.A.; Aminev, D.F.; Datskevich, N.P.; Drozdov, A.A.; Pettinari, C.; Marchetti, F.; et al. Towards Bright Dysprosium Emitters: Single and Combined Effects of Environmental Symmetry, Deuteration, and Gadolinium Dilution. Dye. Pigment. 2022, 199, 110078. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhou, L.; Yu, J.B.; Zhang, H.J.; Deng, R.P.; Peng, Z.P.; Guo, Z.Y. Synthesis, Structure, Photoluminescence, and Electroluminescence Properties of a New Dysprosium Complex. J. Phys. Chem. C 2007, 111, 2295–2300. [Google Scholar] [CrossRef]
- Li, X.L.; Li, J.; Zhu, C.; Han, B.; Liu, Y.; Yin, Z.; Li, F.; Liu, C.M. An Intense Luminescent Dy(iii) Single-Ion Magnet with the Acylpyrazolonate Ligand Showing Two Slow Magnetic Relaxation Processes. New J. Chem. 2018, 42, 16992–16998. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Pettinari, R.; Drozdov, A.; Troyanov, S.; Voloshin, A.I.; Shavaleev, N.M. Synthesis, Structure and Luminescence Properties of New Rare Earth Metal Complexes with 1-Phenyl-3-Methyl-4-Acylpyrazol-5-Ones. J. Chem. Soc. Dalt. Trans. 2002, 7, 1409–1415. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, M.; Liu, Z.; Li, F.; Yi, T.; Huang, C. Luminescence Modulation of a Terbium Complex with Anions and Its Application as a Reagent. Eur. J. Inorg. Chem. 2006, 3, 2277–2284. [Google Scholar] [CrossRef]
- Shen, L.; Shi, M.; Li, F.; Zhang, D.; Li, X.; Shi, E.; Yi, T.; Du, Y.; Huang, C. Polyaryl Ether Dendrimer with a 4-Phenylacetyl-5-Pyrazolone-Based Terbium(III) Complex as Core: Synthesis and Photopysical Properties. Inorg. Chem. 2006, 45, 6188–6197. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Q.; He, Y.; Fu, G.; Li, W.; Miao, T.; Lü, X. Single-Molecule White-Light of Tris-Pyrazolonate-Dy3+ Complexes. Inorg. Chem. Commun. 2019, 109, 107573. [Google Scholar] [CrossRef]
- Xin, H.; Shi, M.; Zhang, X.M.; Li, F.Y.; Bian, Z.Q.; Ibrahim, K.; Liu, F.Q.; Huang, C.H. Carrier-Transport, Photoluminescence, and Electroluminescence Properties Comparison of a Series of Terbium Complexes with Different Structures. Chem. Mater. 2003, 15, 3728–3733. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Belousov, Y.A.; Lyssenko, K.A.; Varaksina, E.; Drozdov, A.A.; Marchetti, F.; Pettinari, R.; Pettinari, C. Synthesis, Phosphorescence and Luminescence Properties of Novel Europium and Gadolinium Tris-Acylpyrazolonate Complexes. Inorg. Chim. Acta 2020, 502, 119279. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Liu, L.; Liu, G.; Jia, D.; Xu, G. A Series of Pyrazolone Lanthanide (III) Complexes: Synthesis, Crystal Structures and Fluorescence. Inorg. Chim. Acta 2007, 360, 1995–2001. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Pettinari, R.; Drozdov, A.; Semenov, S.; Troyanov, S.I.; Zolin, V. A New Rare-Earth Metal Acylpyrazolonate Containing the Zundel Ion H5O2+ Stabilized by Strong Hydrogen Bonding. Inorg. Chem. Commun. 2006, 9, 634–637. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Pettinari, R.; Natanti, P.; Drozdov, A.; Semenov, S.; Troyanov, S.I.; Zolin, V. Syntheses, spectroscopic characterization and X-ray structural studies of lanthanide complexes with adamantyl substituted 4-acylpyrazol-5-one. Inorg. Chim. Acta 2006, 359, 4063–4070. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Cingolani, A.; Drozdov, A.; Troyanov, S.; Timokhin, I.; Vertlib, V. Lanthanide Metal Complexes Containing the First Structurally Characterized β-Diketonate Acid Stabilized by Hydrogen Bonding. Inorg. Chem. Commun. 2003, 6, 48–51. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Kolybalov, D.S.; Pylova, E.K.; Bashirov, D.A.; Komarov, V.Y.; Kuratieva, N.V.; Smolentsev, A.I.; Fitch, A.N.; Konchenko, S.N. A Fresh Look at the Structural Diversity of Dibenzoylmethanide Complexes of Lanthanides. New J. Chem. 2019, 43, 9934–9942. [Google Scholar] [CrossRef]
- Safronova, A.V.; Bochkarev, L.N.; Baranov, E.V. Synthesis of Lanthanide Pyrazolonate Complexes by the Reactions of 1-Phenyl-3-Methyl-4-(2,2-Dimethylpropan-1-Oyl)Pyrazol-5-One with Metallic Lanthanides. Crystal Structures of [Ln(Bu t -PMP)3] 2 (Ln = Gd, Tb, and Tm). Russ. J. Coord. Chem. Khimiya 2013, 39, 537–543. [Google Scholar] [CrossRef]
- Safronova, A.V.; Bochkarev, L.N.; Malysheva, I.P.; Baranov, E. V Inorganica Chimica Acta Facile Synthesis of Rare-Earth Pyrazolonates by the Reaction of Rare-Earth Metals with 1-Phenyl-3-Methyl-4-Isobutyryl-5-Pyrazolone. Crystal Structures of [Ln(PMIP)3]2 (Ln = Y, Gd, Tb, Er, Tm). Inorg. Chim. Acta 2012, 392, 454–458. [Google Scholar] [CrossRef]
- Cingolani, A.; Effendy; Marchetti, F.; Pettinari, C.; Pettinari, R.; Skelton, B.W.; White, A.H. First Structurally Characterized Silver(I) Derivatives with Nonfluorinated β-Diketones. Inorg. Chem. 2002, 41, 1151–1161. [Google Scholar] [CrossRef]
- Marchetti, F.; Palmucci, J.; Pettinari, C.; Pettinari, R.; Condello, F.; Ferraro, S.; Marangoni, M.; Crispini, A.; Scuri, S.; Grappasonni, I.; et al. Novel Composite Plastics Containing Silver(I) Acylpyrazolonato Additives Display Potent Antimicrobial Activity by Contact. Chem. A Eur. J. 2015, 21, 836–850. [Google Scholar] [CrossRef]
- Bui, A.T.; Roux, A.; Grichine, A.; Duperray, A.; Andraud, C.; Maury, O. Twisted Charge-Transfer Antennae for Ultra-Bright Terbium(III) and Dysprosium(III) Bioprobes. Chem. A Eur. J. 2018, 24, 3408–3412. [Google Scholar] [CrossRef]
- Lo, W.-S.; Zhang, J.; Wong, W.-T.; Law, G.-L. Highly Luminescent Sm III Complexes with Intraligand Charge-Transfer Sensitization and the Effect of Solvent Polarity on Their Luminescent Properties. Inorg. Chem. 2015, 54, 3725–3727. [Google Scholar] [CrossRef]
- Barge, A.; Cravotto, G.; Gianolio, E.; Fedeli, F. How to Determine Free Gd and Free Ligand in Solution of Gd Chelates. A Technical Note. Contrast Media Mol. Imaging 2006, 1, 184–188. [Google Scholar] [CrossRef]
- Hillebrand, W.F.; Lundell, G.E.F. Applied Inorganic Analysis; John Wiley & Sons, Ltd: New York City, NY, USA, 1929. [Google Scholar]
- SMART (Control) and SAINT (Integration) Software, Version 5.0; Bruker AXS Inc.: Madison, WI, USA, 1997.
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- van der Sluis, P.; Spek, A.L. BYPASS: An Effective Method for the Refinement of Crystal Structures Containing Disordered Solvent Regions. Acta Crystallogr. Sect. A Found. Crystallogr. Adv. 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Kordeyro Magrino, D.A.; Korshunov, V.M.; Lyssenko, K.A.; Gontcharenko, V.E.; Belousov, Y.A.; Pettinari, C.; Taydakov, I. V Luminescent Complexes of Eu3+,Tb3+ and Gd3+ Nitrates with Polytopic Ligand 2,4,6-Tris(1H-Pyrazol-1-Yl)-1,3,5-Triazine. Inorg. Chim. Acta 2020, 510, 119764. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Proserpio, D.M. How 2-Periodic Coordination Networks Are Interweaved: Entanglement Isomerism and Polymorphism. CrystEngComm 2017, 19, 1993–2006. [Google Scholar] [CrossRef]
- Kovacs, D.; Borbas, K.E. The Role of Photoinduced Electron Transfer in the Quenching of Sensitized Europium Emission. Coord. Chem. Rev. 2018, 364, 1–9. [Google Scholar] [CrossRef]
- Sizov, V.S.; Komissar, D.A.; Metlina, D.A.; Aminev, D.F.; Ambrozevich, S.A.; Nefedov, S.E.; Varaksina, E.A.; Metlin, M.T.; Mislavskií, V.V.; Taydakov, I.V. Effect of Ancillary Ligands on Visible and NIR Luminescence of Sm3+ β-Diketonate Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117503. [Google Scholar] [CrossRef] [PubMed]
- Gontcharenko, V.E.; Kiskin, M.A.; Dolzhenko, V.D.; Korshunov, V.M.; Taydakov, I.V.; Belousov, Y.A. Mono- and Mixed Metal Complexes of Eu3+, Gd3+, and Tb3+ with a Diketone, Bearing Pyrazole Moiety and Chf2-Group: Structure, Color Tuning, and Kinetics of Energy Transfer between Lanthanide Ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Interpretation of Europium (III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Vicentini, G.; Zinner, L.B.; Zukerman-Schpector, J.; Zinner, K. Luminescence and Structure of Europium Compounds. Coord. Chem. Rev. 2000, 196, 353–382. [Google Scholar] [CrossRef]
- Sato, S.; Wada, M. Relations between Intramolecular Energy Transfer Efficiencies and Triplet State Energies in Rare Earth β-Diketone Chelates. Bull. Chem. Soc. Jpn. 1970, 43, 1955–1962. [Google Scholar] [CrossRef]
- Latva, M.; Takalob, H.; Mukkala, V.M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the Lowest Triplet State Energy Level of the Ligand and Lanthanide(III) Luminescence Quantum Yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Lewis, G.N.; Kasha, M. Phosphorescence and the Triplet State. J. Am. Chem. Soc. 1944, 66, 2100–2116. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Opelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Kozma, I.Z.; Krok, P.; Riedle, E. Direct Measurement of the Group-Velocity Mismatch and Derivation of the Refractive-Index Dispersion for a Variety of Solvents in the Ultraviolet. J. Opt. Soc. Am. B 2005, 22, 1479. [Google Scholar] [CrossRef]
- Liang, H.; Xie, F. Optical Investigation of Sm(III)-β-Diketonate Complexes with Different Neutral Ligands. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2009, 73, 309–312. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. A Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef]

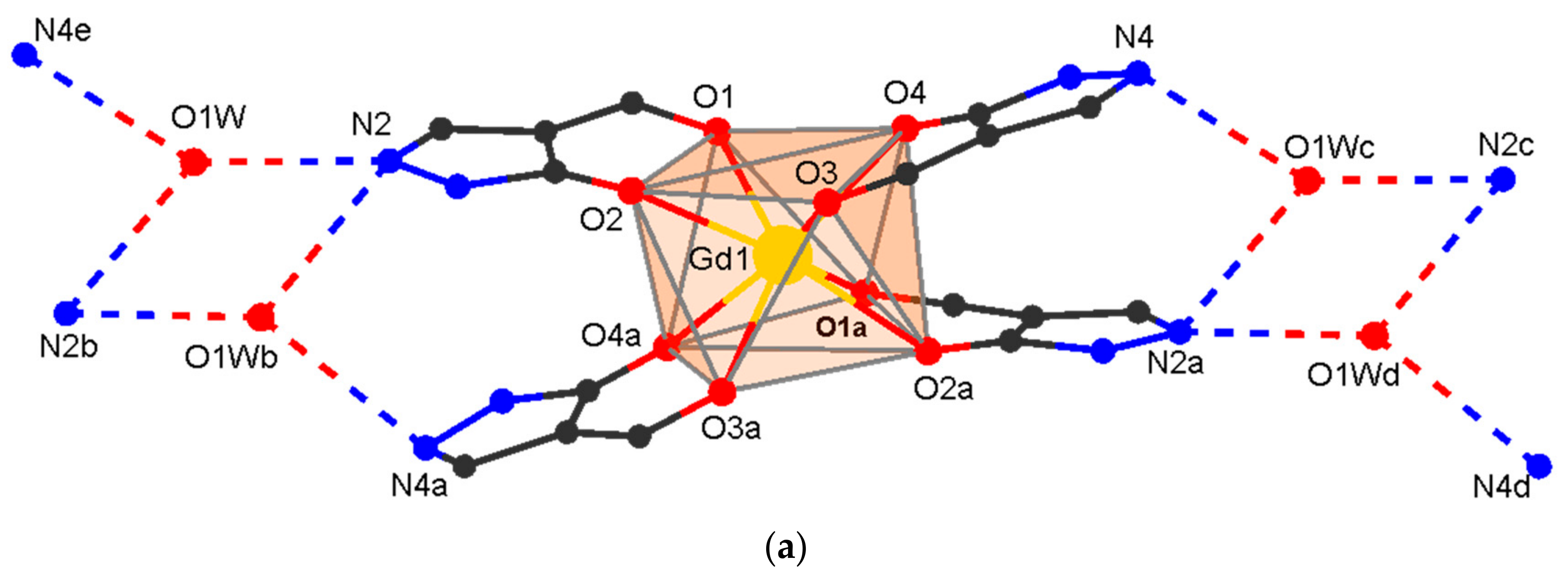
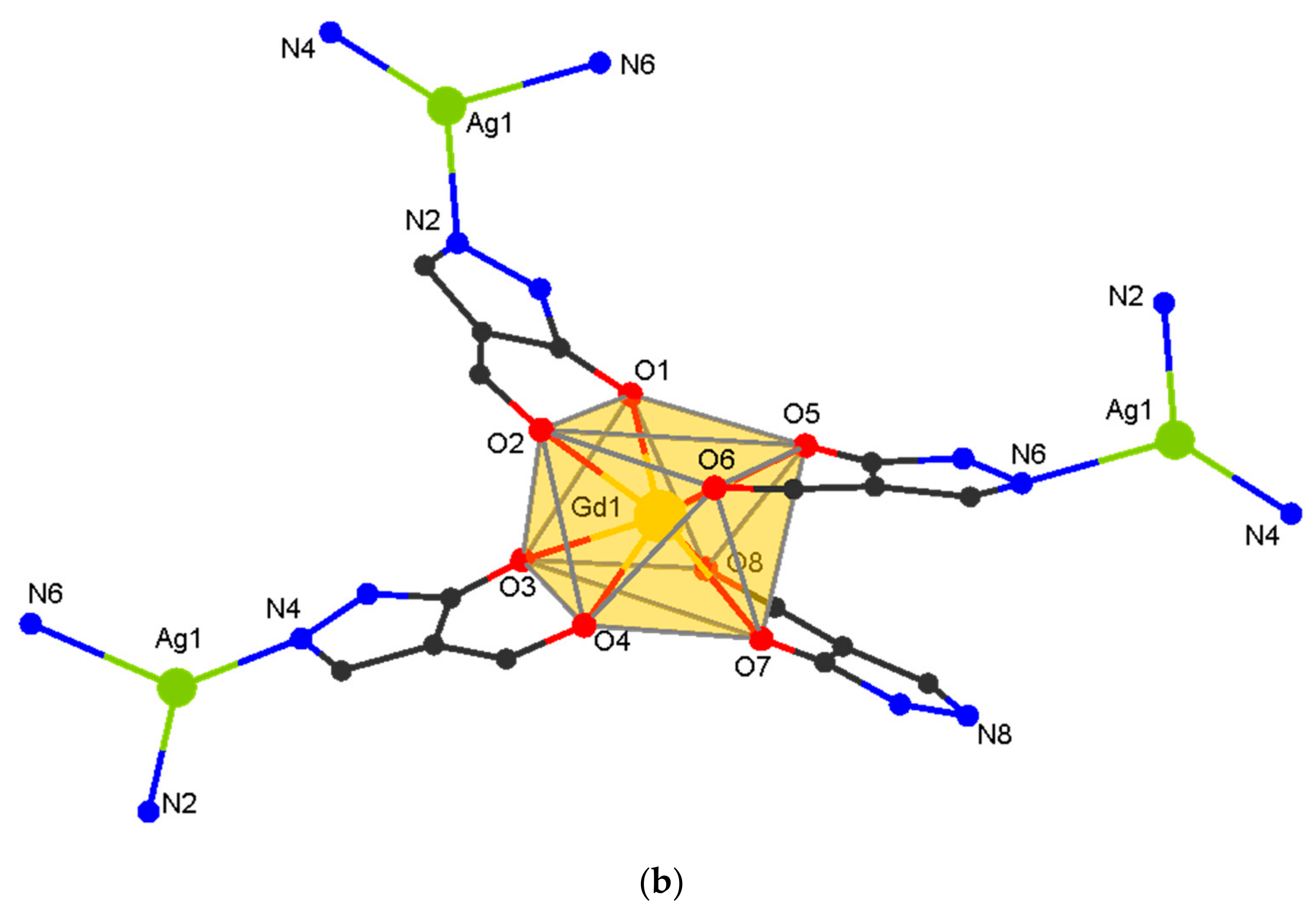

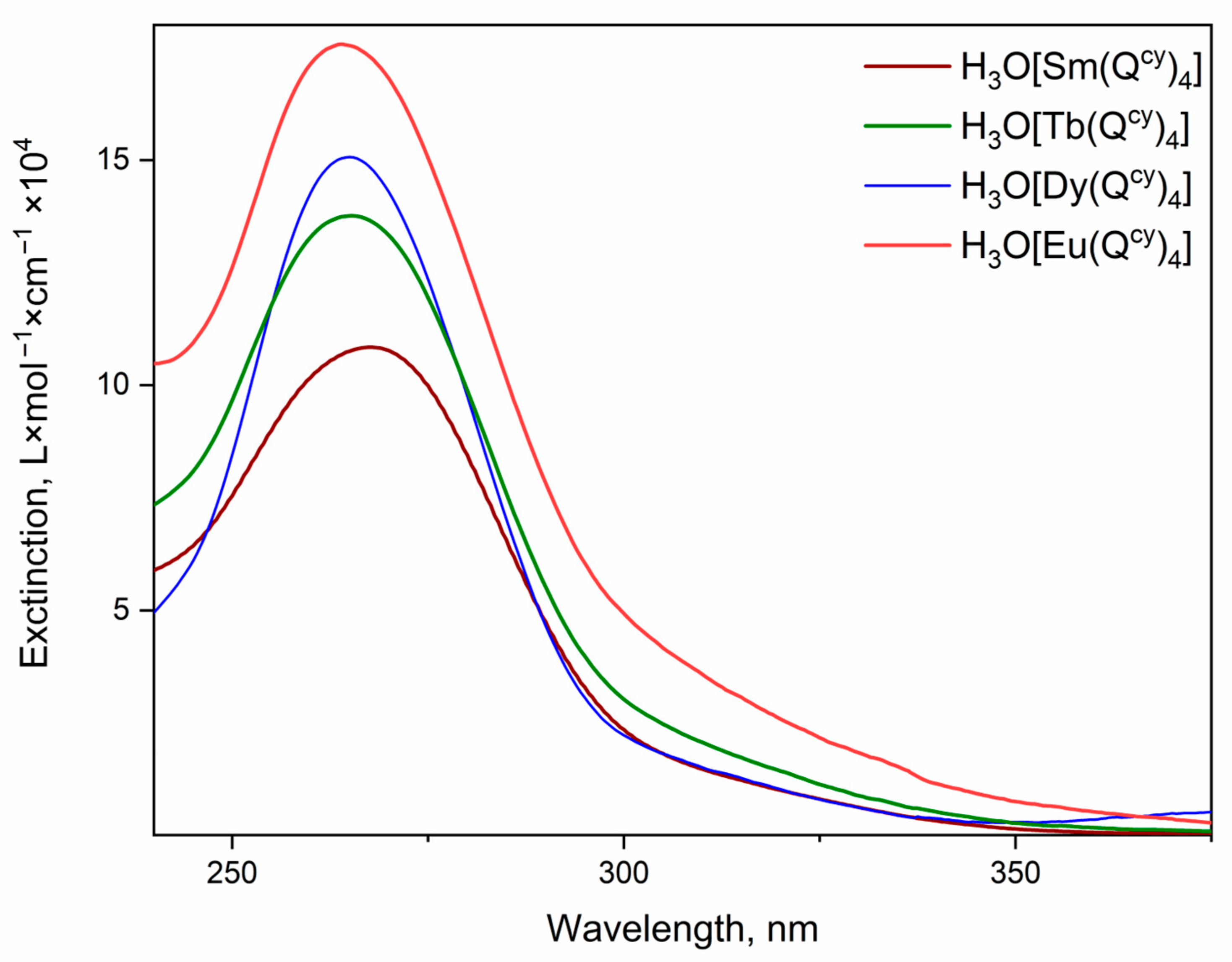



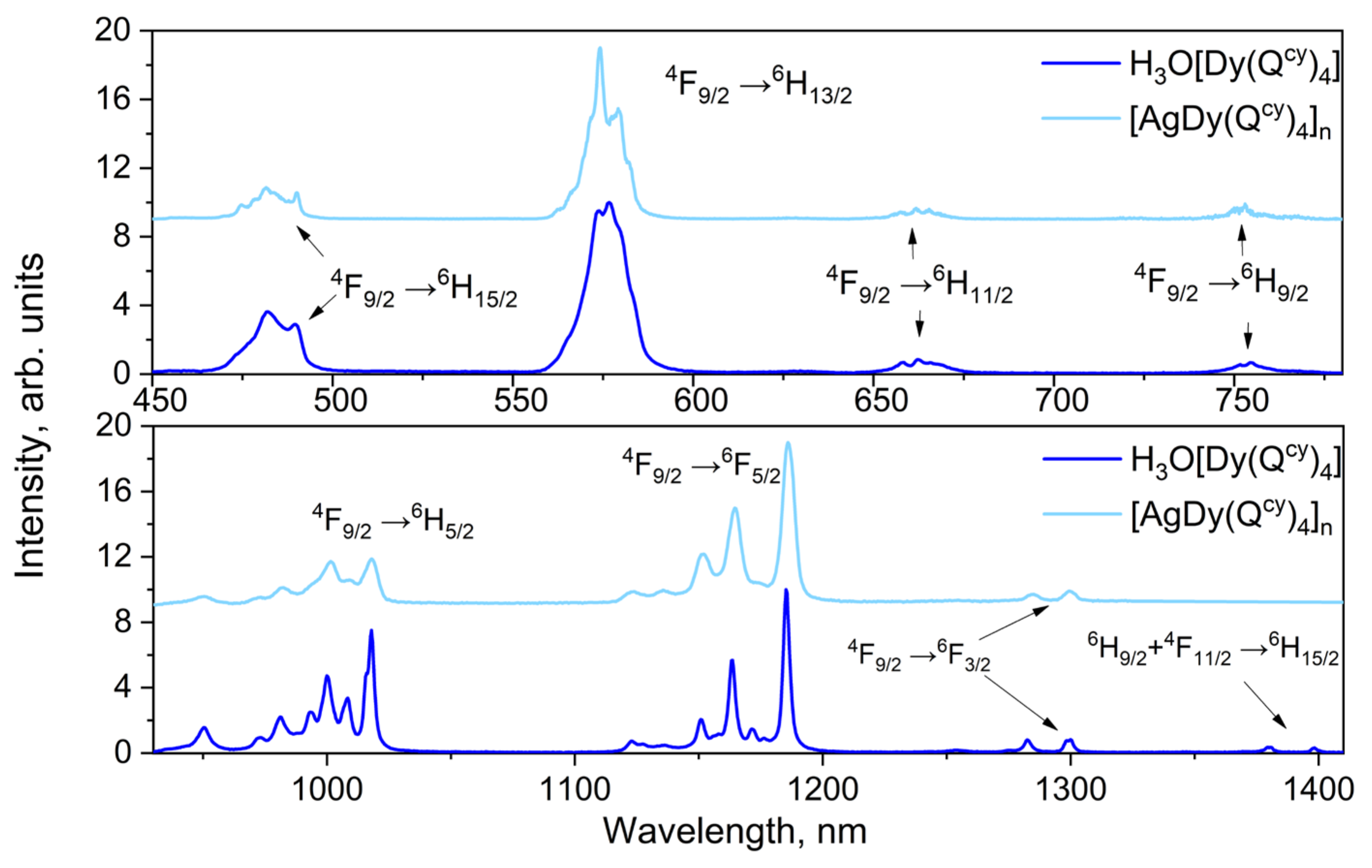

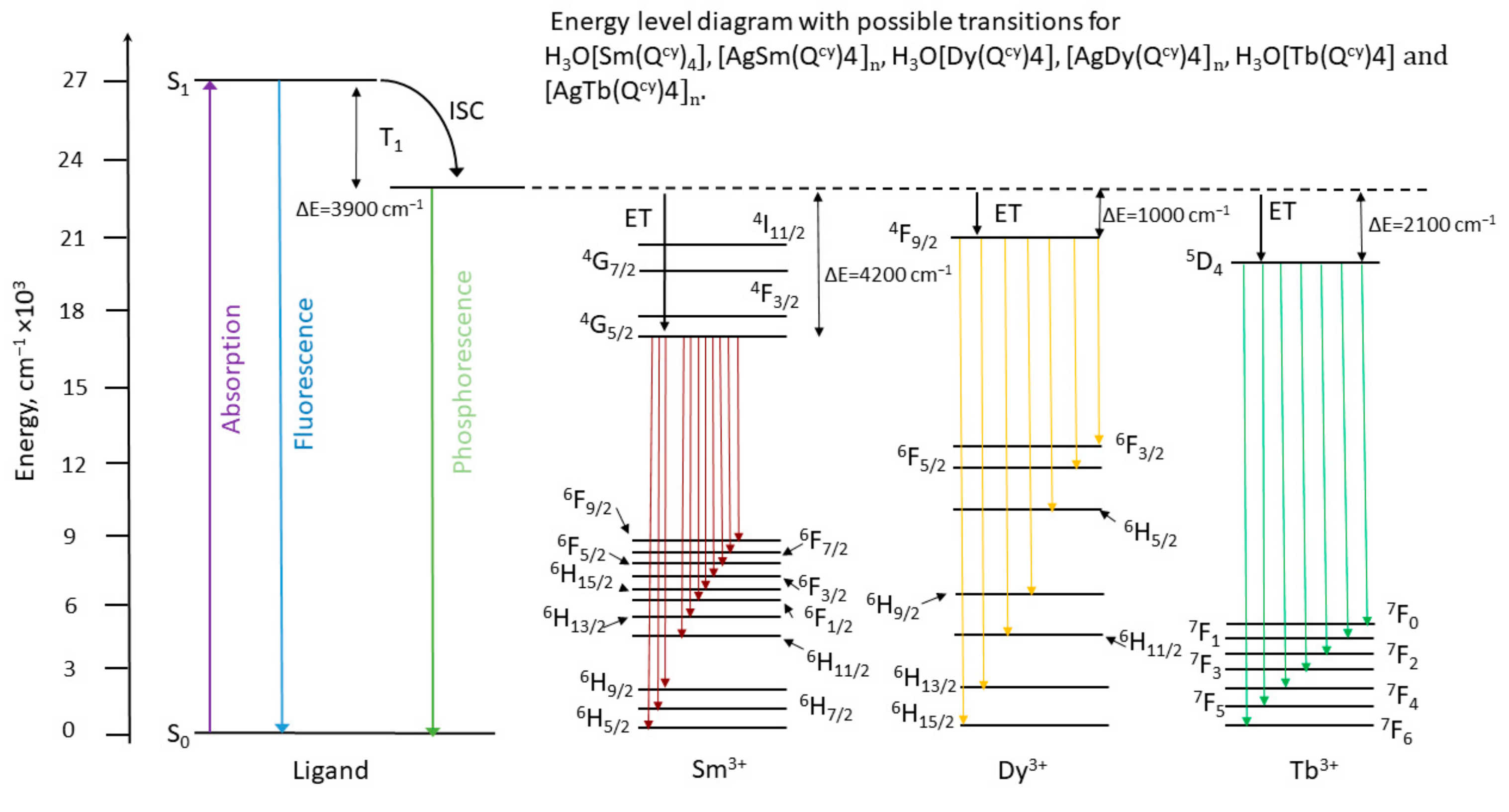
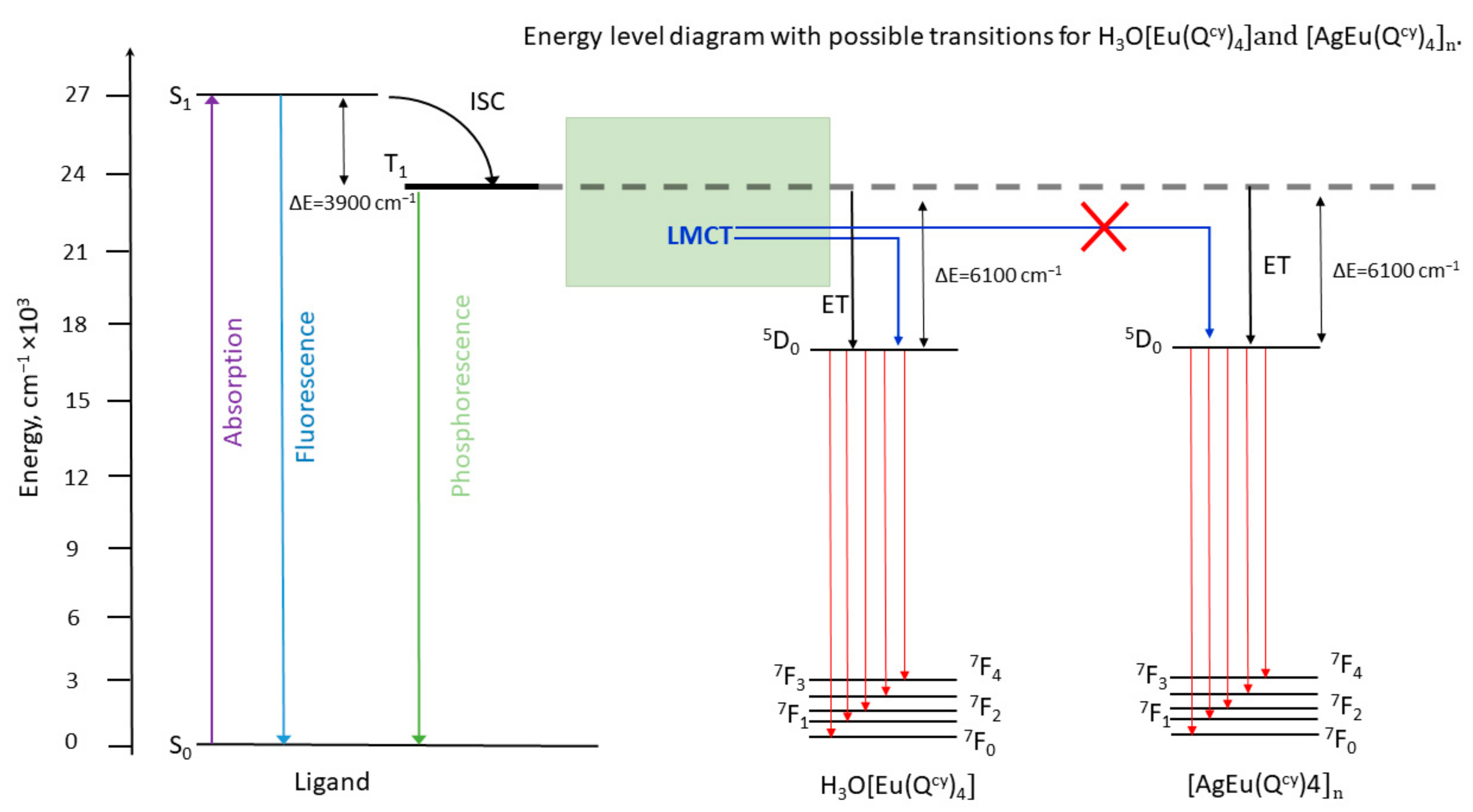

| Complex/Parameters | 1 a | 2 | 3 | 4 | 8 |
|---|---|---|---|---|---|
| Empirical formula | C68H79EuN8O9 | C68H79GdN8O9 | C68H79N8O9Tb | C68H76AgGdN8O8 | |
| Formula weight | 1304.35 | 1309.64 | 1311.31 | 1398.48 | |
| Crystal system | Monoclinic | ||||
| Space group | C | C2/c | P21/n | ||
| T, K | 296 | 296 | 150 | 150 | 100 |
| a, Å | 14.907 (8) | 14.8703 (6) | 14.8096 (5) | 14.7960 (5) | 15.0083 (11) |
| b, Å | 25.244 (16) | 25.2111 (14) | 25.1474 (9) | 25.1641 (11) | 17.7224 (16) |
| c, Å | 16.773 (10) | 16.9977 (11) | 16.7390 (9) | 16.7208 (9) | 25.1710 (19) |
| β, deg. | 93.87 (2) | 94.7700 (10) | 94.1050 (10) | 94.3260 (10) | 92.623 (3) |
| V, Å3 | 6297 (2) | 6350.3 (6) | 6218.0 (5) | 6207.9 (5) | 6688.0 (9) |
| Z | 4 | 4 | 4 | 4 | |
| Dcalc, g cm3 | 1.364 | 1.399 | 1.403 | 1.389 | |
| μ, mm −1 | 1.051 | 1.131 | 1.204 | 1.782 | |
| θmax, deg. | 27.10 | 30.52 | 26.61 | 29.716 | |
| Index ranges | −18 ≤ h ≤ 19 −32 ≤ k ≤ 32 −19 ≤ l ≤ 21 | −20 ≤ h ≤ 21 −35 ≤ k ≤ 35 −23 ≤ l ≤ 23 | −18 ≤ h ≤ 18 −31 ≤ k ≤ 31 −19 ≤ l ≤ 21 | −18 ≤ h ≤ 18 −22 ≤ k ≤ 22 −31 ≤ l ≤ 31 | |
| F (000) | 2712 | 2716 | 2720 | 2860 | |
| Rint | 0.1612 | 0.1319 | 0.0697 | 0.2473 | |
| Number of collected/total reflections | 34,077/7002 | 37,516/9476 | 28,718/6414 | 60,971/13,654 | |
| Number of reflections (I > 2σ(I)) | 5265 | 6920 | 5570 | 4961 | |
| Number of parameters | 393 | 395 | 395 | 779 | |
| GooF | 1.060 | 1.077 | 1.095 | 0.881 | |
| R1, wR2 (I > 2σ(I)) | 0.0699, 0.1542 | 0.0875, 0.1535 | 0.0467, 0.0980 | 0.0911/0.1939 | |
| Δρmax, ρmin(e/Å3) | 0.895, −1.774 | 5.325, −2.815 | 1.467, −1.238 | 0.783/−2.114 | |
| Complex/Parameter | 2 | 3 | 4 | 8 |
|---|---|---|---|---|
| Ln-O | 2.354 (4)–2.436 (4) | 2.349 (4)–2.414 (4) | 2.328 (3)–2.412 (3) | 2.287 (8)–2.448 (9) |
| O-Ln-O (1,3-diketone) | 71.14 (13), 72.13 (14) | 71.75 (13), 72.28 (14) | 72.22 (9), 72.66 (9) | 68.1 (3)–70.8 (3) |
| Ag-N | - | - | - | 2.229 (11)–2.403 (9) |
| N-Ag-N | - | - | - | 99.4 (4), 129.3 (4), 131.2 (4) |
| Symmetry of the LnO8 polyhedron with SQ (p) value a | Square antiprism, D4d, 0.322 | Square antiprism, D4d, 0.254 | Square antiprism, D4d, 0.246 | Square antiprism, D4d, 0.322 |
| Complex | λexc, nm | PLQY, % | λreg, nm | ||
|---|---|---|---|---|---|
| 1 | 350 | 2.0 | 650 | 44.1 ± 0.5 | 73.6 ± 1.2 |
| 6 | 350 | 0.8 | 650 | 66.7 ± 3.1 | 88.2 ± 7.6 |
| 4 | 350 | 55.6 | 550 | 593.9 ± 4.8 | 1403.9 ± 1.9 |
| 9 | 350 | 14.8 | 550 | 292.9 ± 2.9 | 805.7 ± 1.6 |
| 5 | 350 | 2.8 [57] | 570 | 13.0 ± 0.1 | 42.7 ± 0.1 |
| 10 | 350 | 1.0 | 570 | 25.3 ± 1.3 | 49.8 ± 0.1 |
| 2 | 370 | n.a. | 615 | 97.9 ± 0.5 | 486.9 ± 2.0 |
| 7 | 350 | 0.3 | 615 | 179.8 ± 2.2 | 592.5 ± 1.4 |
| 6H5/2→2S+1LJ | Wavelength, nm | fexp × 108 | fcalc × 108 |
|---|---|---|---|
| 6F7/2 | 1237 | 99.4 | 99.4 |
| 6F9/2 | 1086 | 49.0 | 49.2 |
| 6F11/2 | 948 | 8.5 | 7.4 |
| 4F5/2 | 454 | 1.6 | 1.8 |
| Ω2, cm2 | 8.1 × 10−20 | ||
| Ω4, cm2 | 1.8 × 10−20 | ||
| Ω6, cm2 | 0.5 × 10−20 | ||
| RMS = 1.3 × 10−8 | |||
| 4G5/2→2S+1LJ | Wavelength, nm | Arad, s−1 | βcalc, % |
|---|---|---|---|
| 6H5/2 | 565 | 8.65 | 2.7 |
| 6H7/2 | 610 | 46.21 | 14.9 |
| 6H9/2 | 650 | 178.67 | 57.6 |
| 6H11/2 | 715 | 14.27 | 4.6 |
| 6H13/2 | 800 | 0.85 | 0.27 |
| 6F3/2 | 936 | 6.14 | 1.98 |
| 6F5/2 | 949 | 34.99 | 11.2 |
| 6F7/2 | 1036 | 1.47 | 0.47 |
| 6F9/2 | 1180 | 18.67 | 6.02 |
| τrad = 3.2 ms; RMS = 1.3 × 10−8 | |||
| Complex | n | Itot/IMD | krad, s−1 | knrad, s−1 | τobs−1, s−1 | PLQYin, % | PLQY, % | η |
|---|---|---|---|---|---|---|---|---|
| 2 | 1.5 | 8.87 | 438 | 2000 | 2439 | 17.90 | — | — |
| 7 | 19.03 | 941 | 748 | 1689 | 55.70 | 0.3 | 0.05385 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belousov, Y.A.; Metlin, M.T.; Metlina, D.A.; Kiskin, M.A.; Yakushev, I.A.; Polikovskiy, T.A.; Taydakov, I.V.; Drozdov, A.A.; Marchetti, F.; Pettinari, C. Self-Assembly of a Two-Dimensional Coordination Polymer Based on Silver and Lanthanide Tetrakis-Acylpyrazolonates: An Efficient New Strategy for Suppressing Ligand-to-Metal Charge Transfer Quenching of Europium Luminescence. Polymers 2023, 15, 867. https://doi.org/10.3390/polym15040867
Belousov YA, Metlin MT, Metlina DA, Kiskin MA, Yakushev IA, Polikovskiy TA, Taydakov IV, Drozdov AA, Marchetti F, Pettinari C. Self-Assembly of a Two-Dimensional Coordination Polymer Based on Silver and Lanthanide Tetrakis-Acylpyrazolonates: An Efficient New Strategy for Suppressing Ligand-to-Metal Charge Transfer Quenching of Europium Luminescence. Polymers. 2023; 15(4):867. https://doi.org/10.3390/polym15040867
Chicago/Turabian StyleBelousov, Yury A., Mikhail T. Metlin, Darya A. Metlina, Mikhail A. Kiskin, Ilya A. Yakushev, Trofim A. Polikovskiy, Ilya V. Taydakov, Andrei A. Drozdov, Fabio Marchetti, and Claudio Pettinari. 2023. "Self-Assembly of a Two-Dimensional Coordination Polymer Based on Silver and Lanthanide Tetrakis-Acylpyrazolonates: An Efficient New Strategy for Suppressing Ligand-to-Metal Charge Transfer Quenching of Europium Luminescence" Polymers 15, no. 4: 867. https://doi.org/10.3390/polym15040867
APA StyleBelousov, Y. A., Metlin, M. T., Metlina, D. A., Kiskin, M. A., Yakushev, I. A., Polikovskiy, T. A., Taydakov, I. V., Drozdov, A. A., Marchetti, F., & Pettinari, C. (2023). Self-Assembly of a Two-Dimensional Coordination Polymer Based on Silver and Lanthanide Tetrakis-Acylpyrazolonates: An Efficient New Strategy for Suppressing Ligand-to-Metal Charge Transfer Quenching of Europium Luminescence. Polymers, 15(4), 867. https://doi.org/10.3390/polym15040867









