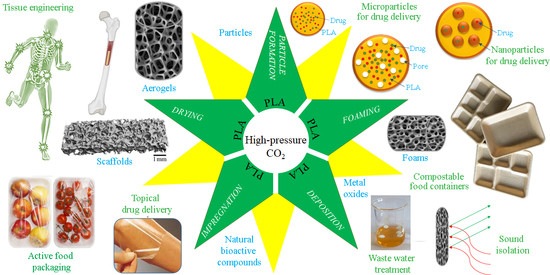
Green Processing of Neat Poly(lactic acid) Using Carbon Dioxide under Elevated Pressure for Preparation of Advanced Materials: A Review (2012–2022)
Abstract
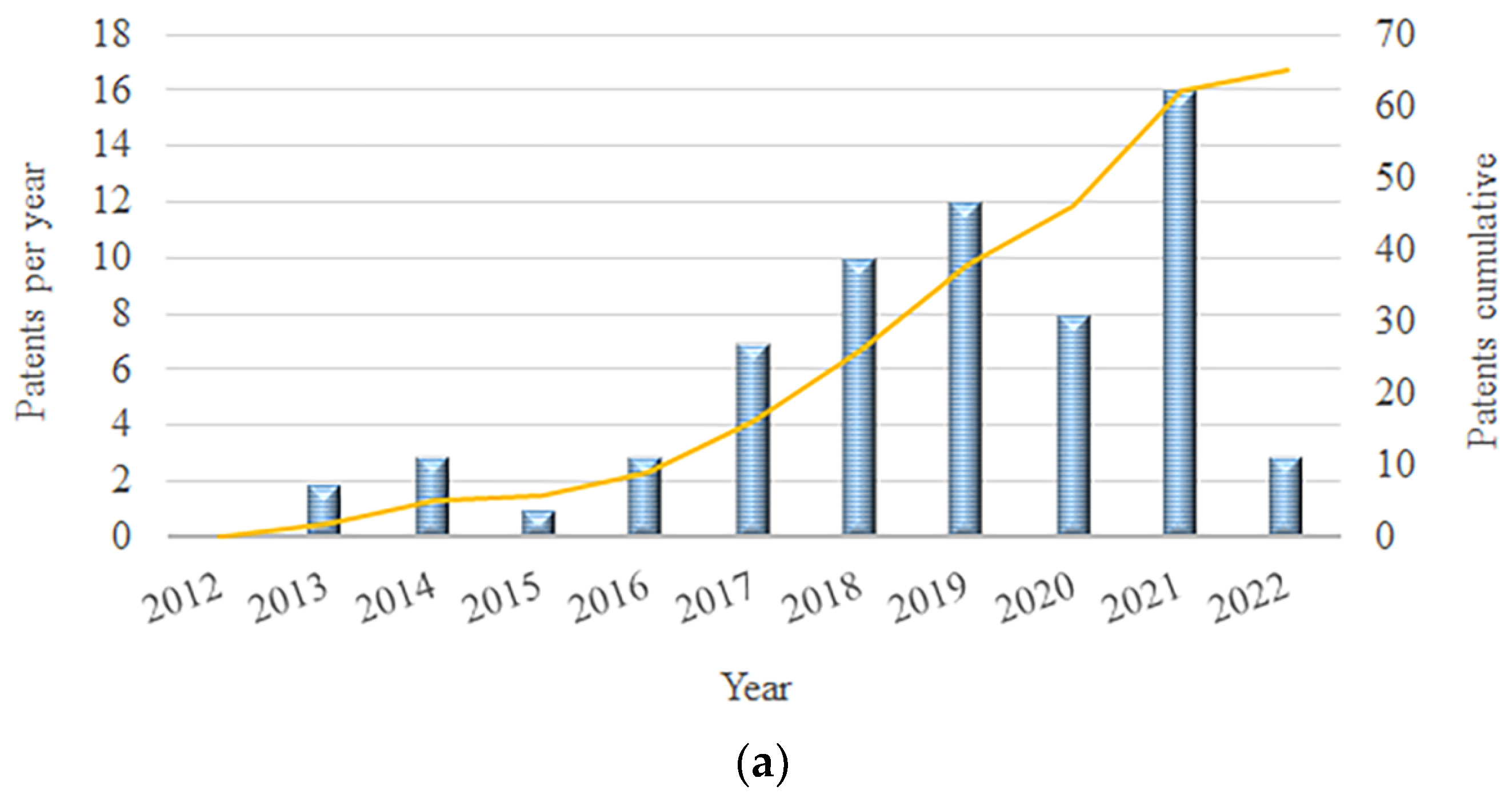
:1. Introduction
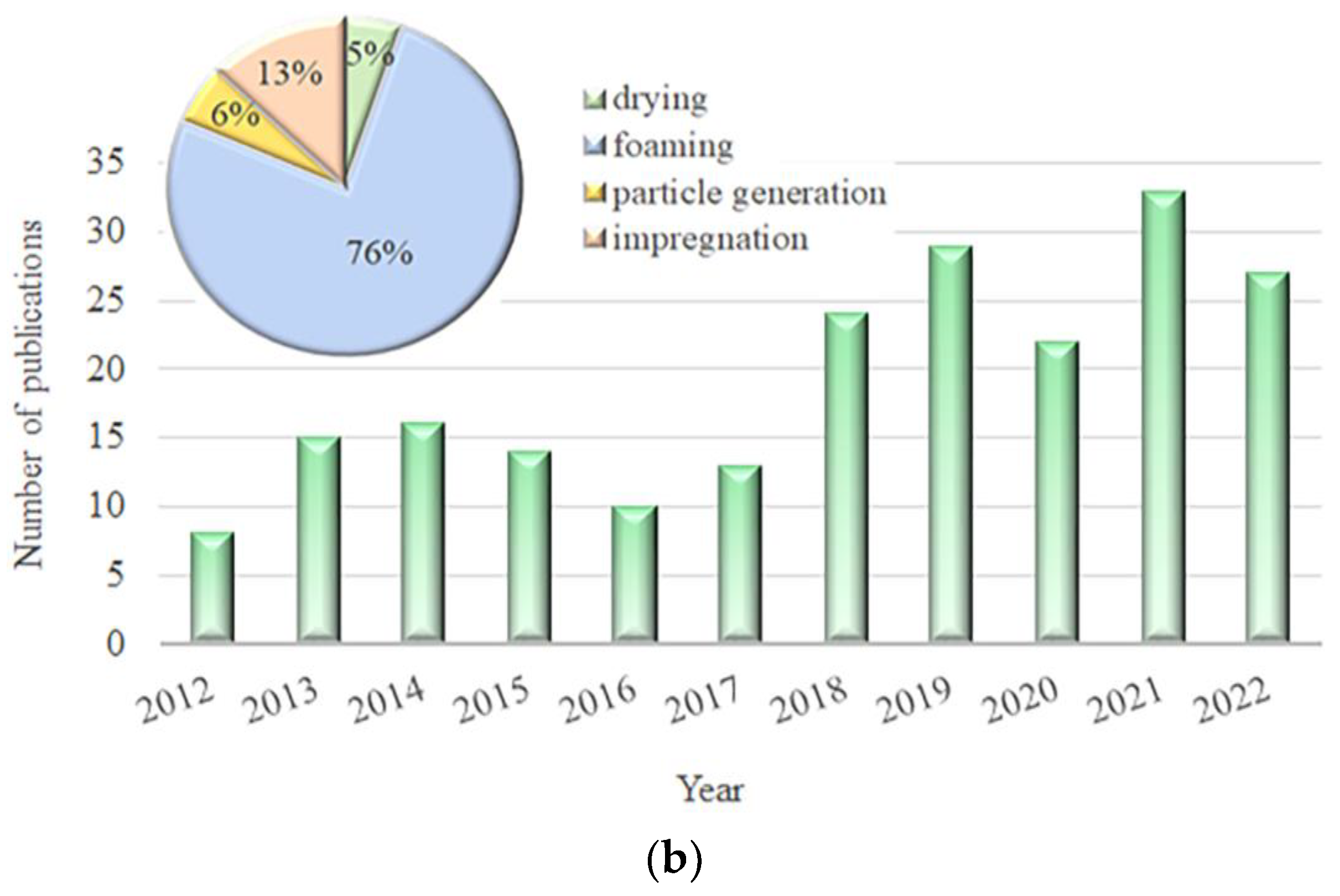
2. Drying of PLA Solutions, Gels, and Emulsions
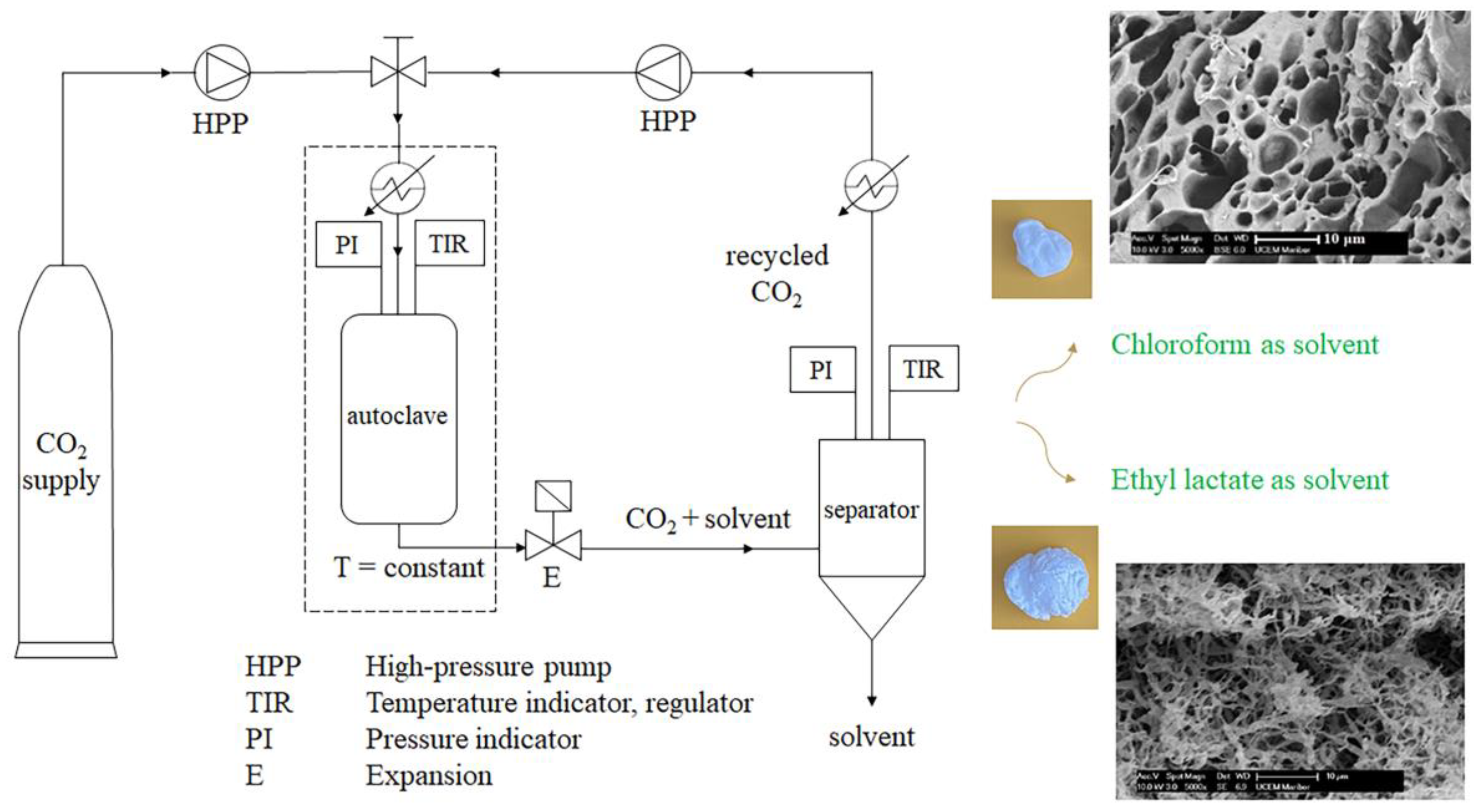
2.1. Methods for Sc-CO2-Assisted Drying of Wet PLA
| Material | Producer | Method | Regime | P (MPa) | T (°C) | t (h) | Material Properties | Material Application | Lit |
|---|---|---|---|---|---|---|---|---|---|
| PLLA | Purac Biochem | STIPS | Static | 19 | 39 | 1.5 | ε = 90–95% S = 70–95 m2/g | Tissue engineering scaffolds | [33] |
| 2500HP 4060D | Natureworks | STIPS | Dynamic | 10 | 40 | 1 | S = 29–85 m2/g | Pharmaceutical | [46] |
| PLLA | Purac Biochem | STIPS SPL | Static | 19 | 39 | 1.5 | ε = 79–98% Dave = 171–440 μm S = 29–39 m2/g | Tissue engineering scaffolds | [39] |
| PLLA with ibuprofen | Boehringer Ingelheim | SFEP | Static | 10–20 | 35–55 | 4 | ε = 78–89% Raver = 8–30 μm Daver = 8–30 μm | Tissue engineering scaffolds | [40] |
| PLLA | Boehringer Ingelheim | SFEP | Static | 10–25 | 35 | 4 | ε = 84–93% Daver = 3–15 μm | Tissue engineering scaffolds | [41] |
| 3052D | NatureWorks | SCD STIPS | Static and dynamic | 15, 19 | 35, 39 | 2.7 | ρ = 337–468 kg/m3 ε = 63–73% Daver = 0.08–5.1 μm | Wastewater treatment | [37,38] |
| PLLA | synthetized | STIPS | Dynamic | 15 | 35 | 4 | ρ = 75–310 kg/m3 ε = 75–94% Daver = 3–138 μm | Tissue engineering scaffolds | [36] |
| 203H | Evonik Industries | SEE | Dynamic | 8 | 38 | n.i.a. | Raver = 0.2–0.9 μm | Bactericidal nanocomposites | [42] |
| 708H | Evonik Industries | SEE | Dynamic | 8 | 38 | n.i.a. | Raver = 1.5 μm | Nutraceutical formulation | [43] |
| 203H | Evonik Industries | SEE | Dynamic | 8 | 39 | n.i.a. | Raver = 0.4–3 μm | Growth factor delivery | [44] |
2.2. Application of Dried PLA Materials
3. PLA Foaming Using CO2 under Elevated Pressure
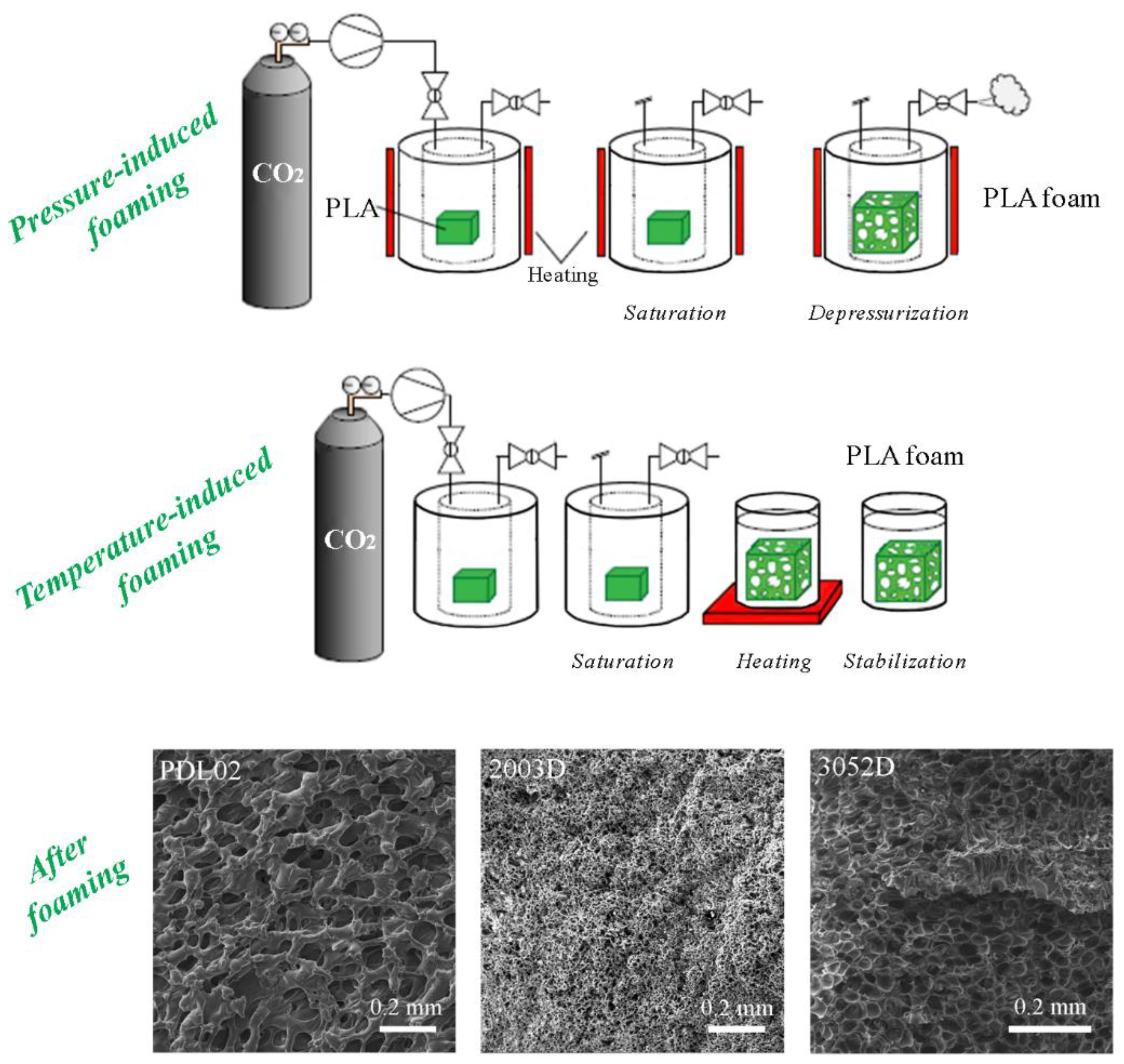
3.1. Methods and Parameters for PLA Foaming under High-Pressure CO2
| PLA | Producer | CO2 | Regime Method | P (MPa) | T (°C) | t (h) | Foam Properties | Foam Application | Lit |
|---|---|---|---|---|---|---|---|---|---|
| PDL02 | Corbion Purac | sc-CO2 | Static | 10, 15 | 35, 40, 50 | 2–24 | Tg = 31 °C Daver = 67 μm | Drug delivery | [75] |
| 2003D | NatureWorks | sc-CO2 | Static | 20–30 | 100–120 | 2, 24 | ρ = 427–685 kg/m3 ε = 45–66% | Food packaging | [15] |
| 2002D | NatureWorks | sc-CO2 | Static | 20–30 | 131,132 | 0.08–12 | Ec = 61–82 MPa σ60 = 9–11 MPa | n.i.a. | [70] |
| 2003D | NatureWorks | sc-CO2 | Static | 8–24 | 70–120 | 1 | Daver = 4–23 μm ε = 43–80% | n.i.a. | [65] |
| 2003D | NatureWorks | sc-CO2 | Static | 10–20 | 130, 190 | 1 | Daver = 0–200 μm | n.i.a. | [81] |
| L175 DO70 | Total Corbion | sc-CO2 | Static | 15 | 45 | 8 | Daver = 2–4 μm Nc ~2∙1010 cells/cm3 | n.i.a. | [82] |
| 4032D | NatureWorks | sc-CO2 | Extrusion and static | 10–31 | 190 115–155 | 0.25 | Daver = 0.7–22 μm Nc ~1011 cells/cm3 | Thermal insulation | [63] |
| 4032D | NatureWorks | sc-CO2 | Extrusion and static | 17–31 | 190 118–143 | 0.03–1 | Daver = 1–22 μm Nc = 108–1012 cells/cm3 | Oil sorption | [64] |
| 4060D | NatureWorks | sc-CO2 | Extrusion and static | 10–33 | 170–180 40 | 0.08–0.5 | Daver = 11–117 μm Nc = 103–109 cells/cm3 | n.i.a. | [71] |
| 2003D | NatureWorks | sc-CO2 | Heat pressing and static | 15 | 190 (85–117) | 2 | Daver = 31–1700 nm Nc = 1013–1015 cells/cm3 | Thermal insulation | [83] |
| 4032D | NatureWorks | d-CO2 sc-CO2 | Two-step static | 6.9–17.2 | 180 (85–125) | 0.67 | Daver = 25–287 μm Nc ~104–107 cells/cm3 | Oil adsorption; thermal insulation | [68] |
| 2003D | NatureWorks | d-CO2 sc-CO2 | Extrusion | 6–8 | 140–190 | n.i.a | Daver = 0.1–1 mm χ = 1.5–15.5% | n.i.a. | [84] |
| PLE001 | NaturePlast | sc-CO2 | Extrusion | 17.7–23.8 | 100–180 | n.i.a | ε = 55–96% Tm = 139.5–142.9 °C | Food packaging | [78] |
3.2. Application of PLA Foams
4. PLA Particle Generation
4.1. Methods for PLA Particle Generation Using CO2 under Elevated Pressure
4.2. Application of PLA Particles
5. PLA Impregnation with Bioactive Components Using Sc-CO2
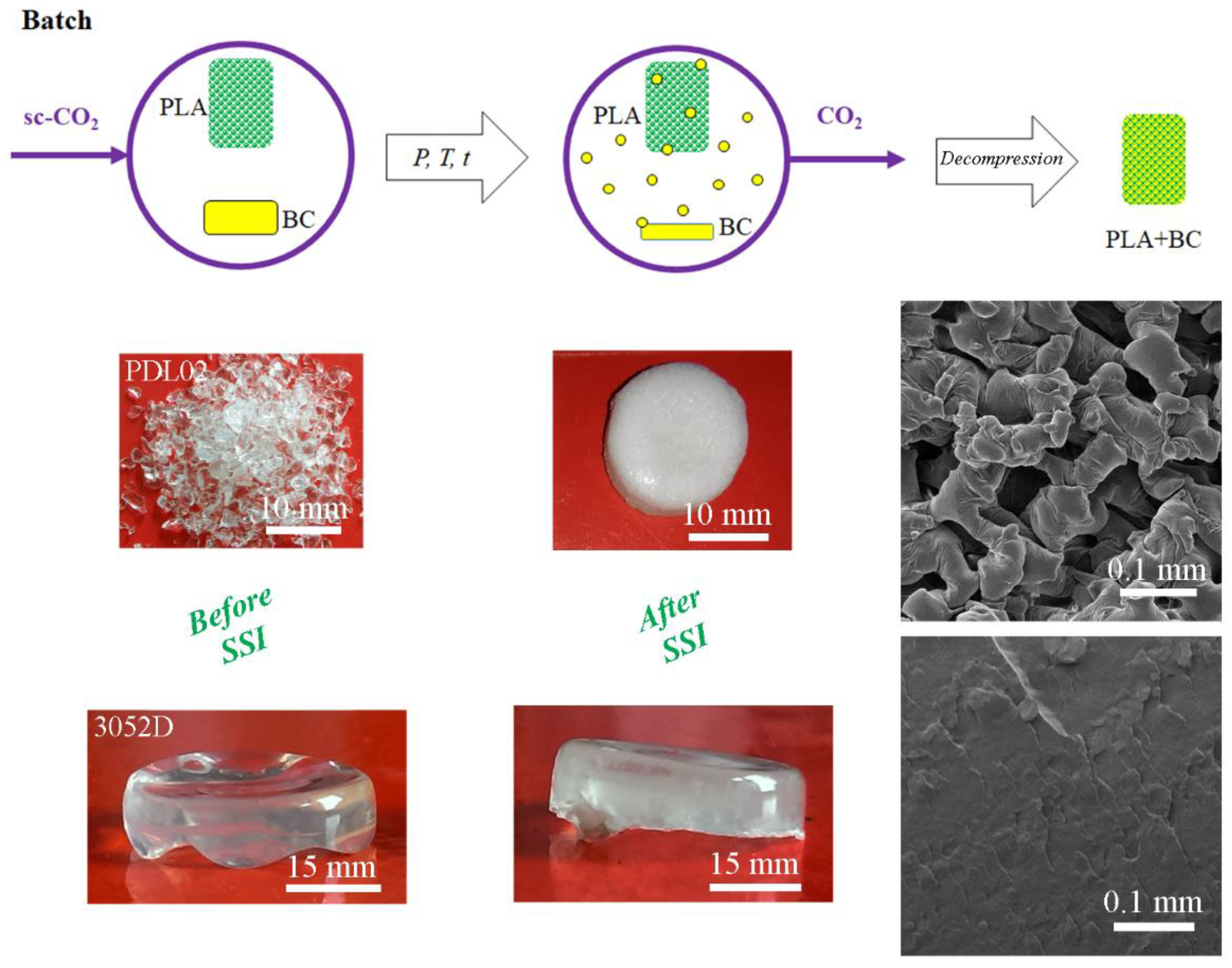
5.1. Methods for PLA Impregnation Using Sc-CO2
 —[138]; ▼—[137]; ♦—[137]; ►—[137]).
—[138]; ▼—[137]; ♦—[137]; ►—[137]).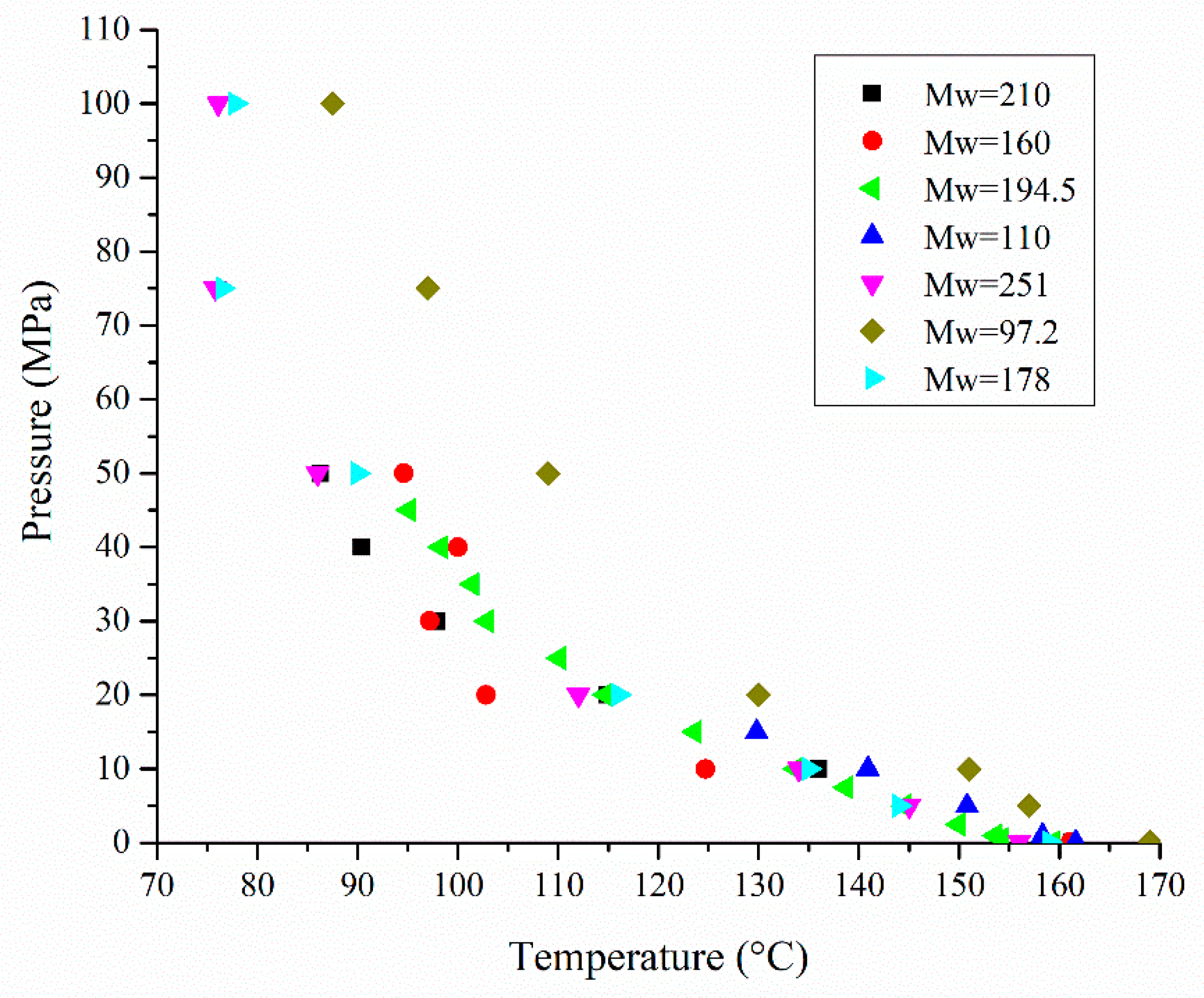
| PLA | PLA Form | Bioactive Component | P (MPa) | T (°C) | t (h) | Dec. (MPa/min) | Material Properties | Material Application | Lit |
|---|---|---|---|---|---|---|---|---|---|
| 2003D | Film | Thymol | 9, 12 | 40 | 3 | 0.1, 1, 10 | L = 13–20%; σ = 13–21 MPa Tm = 130–141 °C; χ = 1.2–3.3% | Active food packaging | [4] |
| PLA | Filament | Ketoprofen | 10–40 | 35–75 | 2 | Fast | L = 0.1–9.0% Sw = 4–30% | Biomedical applications | [135,136] |
| 2003D | Beads | Thymol | 20–30 | 100–120 | 2, 24 | 180 | L = 4.7–19.8%; Sw = 11–47% ρ ~470 kg/m3; ε~61% | Bioactive materials | [15] |
| PLLA | Suture fibers | Ketoprofen, aspirin | 10–35 | 40–140 | 3 | 0.06, 6, 360 | L = 0.5–32%; Tm = 155–170 °C | Bioactive sutures | [20] |
| PLA | Films | Multiflora extract | 15, 25 | 45, 65 | 2, 8 | 0.5 | L = 6.7–23.8%; Tm = 167.8 °C; IC50 = 19.9 μg/mL | Active food packaging | [126] |
| 2003D 3052D | Beads | Thyme extract | 30 | 110 | 2–7 | 180 | L = 0.2–0.7%; ε = 64–76% ρ = 299.4–433.2 kg/m3 | Bioactive materials | [15] |
| PLLA | Fibers | Ketoprofen | 30, 35 | 80, 90 | 3 | 0.06, fast | L = 12–33%; DR = 3 days–3 months | Bioactive sutures | [129] |
| PDL02 | Flakes | Thymol | 10, 15 | 35–50 | 2–24 | 30 | L = 2.3–18.8%; Tg = 32.4 °C; Daver ~51.4 μm; DR = 1 month | Biomedical devices | [75] |
| PLA | Filaments | Mango extract | 10.1, 25.3, 40.5 | 35–55 | 1–3 | 3 | L = 1–9%; OI = 21–90%; IC50 = 8.24 μg/mL; ADC = 4.3–25.0% | Bioactive materials | [134] |
| PLLA | Films | Aspirin, carvone, ketoprofen | 9, 30 | 60, 80 | 3 | Fast | L = 1.4–26.5%; DR = 20 days–2 months | Drug delivery | [128,133] |
| PLA | Films | R-carvone | 8–17.8 | 40, 60 | 2 | 0.6, 6.0 | L = 6.7–29.8%; Tg = 40.4 °C; Tm = 133.0 °C; χ = 25.7% | Active food packaging | [139] |
| 3052D | Disc, film | Thymol | 10 | 40 | 2–24 | n.i.a. | L = 6–30%; Sw = 0.1–7.5% | Active food packaging | [140] |
| 2003D | Film | Cinnamaldehyde | 9, 12 | 40 | 3 | 0.1, 1, 10 | L = 8–13%; χ = 0.6–3.1%; σ = 14.3–38.0 MPa | Active food packaging | [127] |
| PLA | Filaments discs | Mango leaf extract | 10, 40 | 35, 55 | 2 | 4, 10 | n.i.a | Endoprostheses | [141] |
5.2. Application of PLA Material Impregnated with Bioactive Substances
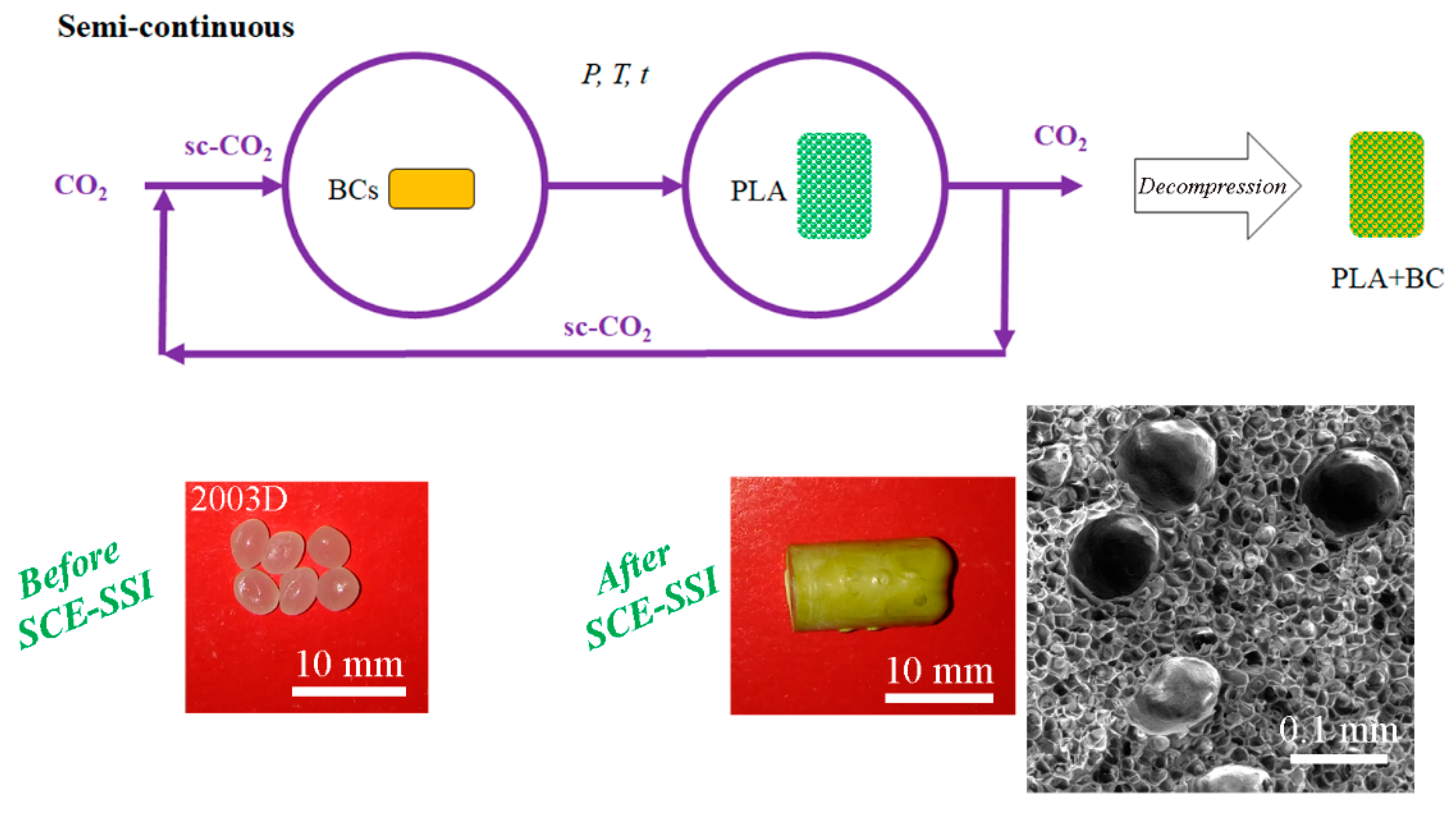
6. Emerging Sc-CO2 Techniques for PLA Processing
6.1. Integrated Processes of Supercritical Extraction of Bioactive Compounds and PLA Impregnation
6.2. Deposition of Metal Oxides onto PLA
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhvi, M.; Gokhale, D. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558–13568. [Google Scholar] [CrossRef]
- Peng, K.; Mubarak, S.; Diao, X.; Cai, Z.; Zhang, C.; Wang, J.; Wu, L. Progress in the Preparation, Properties, and Applications of PLA and Its Composite Microporous Materials by Supercritical CO2: A Review from 2020 to 2022. Polymers 2022, 14, 4320. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Zhang, L. Understanding self-accelerated water diffusion within poly-lactic acid via molecular dynamics simulation. Chin. J. Chem. Eng. 2019, 27, 759–764. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly(lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Reignier, J.; Gendron, R.; Champagne, M.F. Extrusion Foaming of Poly(Lactic acid) Blown with CO2: Toward 100% Green Material. Cell. Polym. 2007, 26, 83–115. [Google Scholar] [CrossRef]
- About NatureWorks. Available online: https://www.natureworksllc.com/About-NatureWorks (accessed on 22 October 2022).
- TotalEnergies Corbion. Available online: https://www.totalenergies-corbion.com/about-totalenergies-corbion/ (accessed on 22 October 2022).
- Evonik, Products & Solutions. Available online: https://corporate.evonik.com/en/evonik-launches-resomer-filaments-for-the-3d-printing-of-bioresorbable-medical-implants-114379.html (accessed on 22 October 2022).
- ThyssenKrupp, Industrial Solutions PLAneo® Polylactide Technology. Available online: https://ucpcdn.thyssenkrupp.com/_legacy/UCPthyssenkruppBAIS/assets.files/products___services/chemical_plants___processes/polymer_plants/brochure_pla.pdf (accessed on 22 October 2022).
- Djukić-Vuković, A.; Mladenović, D.; Ivanovic, J.; Pejin, J.; Mojović, L. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew. Sustain. Energy Rev. 2019, 108, 238–252. [Google Scholar] [CrossRef]
- Ioannidou, S.M.; Ladakis, D.; Moutousidi, E.; Dheskali, E.; Kookos, I.K.; Câmara-Salim, I.; Moreira, M.T.; Koutinas, A. Techno-economic risk assessment, life cycle analysis and life cycle costing for poly(butylene succinate) and poly(lactic acid) production using renewable resources. Sci. Total. Environ. 2021, 806, 150594. [Google Scholar] [CrossRef]
- Available online: https://businessanalytiq.com/procurementanalytics/index/polylactic-acid-pla-price-index/ (accessed on 2 January 2023).
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef]
- Kuska, R.; Milovanovic, S.; Frerich, S.; Ivanovic, J. Thermal analysis of polylactic acid under high CO2 pressure applied in supercritical impregnation and foaming process design. J. Supercrit. Fluids 2018, 144, 71–80. [Google Scholar] [CrossRef]
- Standau, T.; Zhao, C.J.; Castellón, S.M.; Bonten, C.; Altstädt, V. Chemical Modification and Foam Processing of Polylactide (PLA). Polymers 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Pajnik, J.; Lukic, I. Tailoring of advanced poly(lactic acid)-based materials: A review. J. Appl. Polym. Sci. 2021, 139. [Google Scholar] [CrossRef]
- Lora, M.; Kikic, I. Polymer Processing with Supercritical Fluids. Polymer Sci. 2000, 42, 78–101. [Google Scholar] [CrossRef]
- Barry, J.J.A.; Silva, M.M.C.G.; Popov, V.; Shakesheff, K.; Howdle, S.M. Supercritical carbon dioxide: Putting the fizz into biomaterials. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2005, 364, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jerome, C. Drug Loading of Sutures by Supercritical CO2 Impregnation: Effect of Polymer/Drug Interactions and Thermal Transitions. Macromol. Mater. Eng. 2015, 300, 596–610. [Google Scholar] [CrossRef]
- Lopez-Periago, A.M.; Vega, A.; Subra, P.; Argemí, A.; Saurina, J.; A García-González, C.; Domingo, C. Supercritical CO2 processing of polymers for the production of materials with applications in tissue engineering and drug delivery. J. Mater. Sci. 2008, 43, 1939–1947. [Google Scholar] [CrossRef]
- Milovanovic, S.; Djuris, J.; Dapčević, A.; Skoric, M.L.; Medarevic, D.; Pavlović, S.M.; Ibric, S. Preparation of floating polymer-valsartan delivery systems using supercritical CO2. J. Polym. Res. 2021, 28, 74. [Google Scholar] [CrossRef]
- Khanh, P.N.; Trung, N.T. Understanding Interaction Capacity of CO2 with Organic Compounds at Molecular Level: A Theoretical Approach. In Carbon Dioxide Chemistry, Capture and Oil Recovery; IntechOpen: London, UK, 2018; p. 105. [Google Scholar] [CrossRef]
- Aschenbrenner, O.; Styring, P. Comparative study of solvent properties for carbon dioxide absorption. Energy Environ. Sci. 2010, 3, 1106–1113. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A Review of CO2 Applications in the Processing of Polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Extrusion Foaming of PLA and Its Compounds. In Polylactide Foams: Fundamentals, Manufacturing, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 113–149. [Google Scholar] [CrossRef]
- Placin, F.; Desvergne, J.-P.; Cansell, F. Organic low molecular weight aerogel formed in supercritical fluids. J. Mater. Chem. 2000, 10, 2147–2149. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A. Production of porous polylactic acid monoliths via nonsolvent induced phase separation. Polymer 2014, 55, 6743–6753. [Google Scholar] [CrossRef]
- Chen, J.-S.; Tu, S.-L.; Tsay, R.-Y. A morphological study of porous polylactide scaffolds prepared by thermally induced phase separation. J. Taiwan Inst. Chem. Eng. 2010, 41, 229–238. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Zeng, X.; Quan, D.; Liao, S.; Zeng, Y.; Lu, J.; Ramakrishna, S. Fabrication and characterization of poly(l-lactic acid) 3D nanofibrous scaffolds with controlled architecture by liquid–liquid phase separation from a ternary polymer–solvent system. Polymer 2009, 50, 4128–4138. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Salerno, A.; Domingo, C. Making microporous nanometre-scale fibrous PLA aerogels with clean and reliable supercritical CO2 based approaches. Microporous Mesoporous Mater. 2014, 184, 162–168. [Google Scholar] [CrossRef]
- Şahin, I.; Özbakır, Y.; Inönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2018, 4, 4010003. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S.; Rapuano, C. A new supercritical fluid-based process to produce scaffolds for tissue replacement. J. Supercrit. Fluids 2008, 45, 365–373. [Google Scholar] [CrossRef]
- Gay, S.; Lefebvre, G.; Bonnin, M.; Nottelet, B.; Boury, F.; Gibaud, A.; Calvignac, B. PLA scaffolds production from Thermally Induced Phase Separation: Effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide. J. Supercrit. Fluids 2018, 136, 123–135. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Pantic, M.; Pavlovic, S.M.; Knapczyk-Korczak, J.; Stachewicz, U.; Novak, Z. Development of advanced floating poly(lactic acid)-based materials for colored wastewater treatment. J. Supercrit. Fluids 2021, 177, 105328. [Google Scholar] [CrossRef]
- Milovanović, S.L.; Marković, D.D.; Nikolić, I.D. Functionalization of PLA aerogels with TiO2 nanoparticles. Tehnika 2021, 76, 403–408. [Google Scholar] [CrossRef]
- Salerno, A.; Fernández-Gutiérrez, M.; del Barrio, J.S.R.; Domingo, C. Bio-safe fabrication of PLA scaffolds for bone tissue engineering by combining phase separation, porogen leaching and scCO2 drying. J. Supercrit. Fluids 2015, 97, 238–246. [Google Scholar] [CrossRef]
- Cardea, S.; Baldino, L.; Scognamiglio, M.; Reverchon, E. 3D PLLA/Ibuprofen composite scaffolds obtained by a supercritical fluids assisted process. J. Mater. Sci. Mater. Med. 2014, 25, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Cardea, S.; Baldino, L.; Pisanti, P.; Reverchon, E. 3-D PLLA scaffolds formation by a supercritical freeze extraction assisted process. J. Mater. Sci. Mater. Med. 2013, 25, 355–362. [Google Scholar] [CrossRef]
- Campardelli, R.; Della Porta, G.; Gomez, V.; Irusta, S.; Reverchon, E.; Santamaria, J. Encapsulation of titanium dioxide nanoparticles in PLA microspheres using supercritical emulsion extraction to produce bactericidal nanocomposites. J. Nanoparticle Res. 2013, 15, 1987. [Google Scholar] [CrossRef]
- Gimenez-Rota, C.; Palazzo, I.; Scognamiglio, M.; Mainar, A.; Reverchon, E.; Della Porta, G. β-Carotene, α-tocoferol and rosmarinic acid encapsulated within PLA/PLGA microcarriers by supercritical emulsion extraction: Encapsulation efficiency, drugs shelf-life and antioxidant activity. J. Supercrit. Fluids 2019, 146, 199–207. [Google Scholar] [CrossRef]
- Palazzo, I.; Lamparelli, E.P.; Ciardulli, M.C.; Scala, P.; Reverchon, E.; Forsyth, N.; Maffulli, N.; Santoro, A.; Della Porta, G. Supercritical emulsion extraction fabricated PLA/PLGA micro/nano carriers for growth factor delivery: Release profiles and cytotoxicity. Int. J. Pharm. 2021, 592, 120108. [Google Scholar] [CrossRef]
- Oliveira, J.; Brichi, G.S.; Marconcini, J.M.; Mattoso, L.H.C.; Glenn, G.M.; Medeiros, E.S. Effect of Solvent on the Physical and Morphological Properties of Poly(Lactic Acid) Nanofibers Obtained by Solution Blow Spinning. J. Eng. Fibers Fabr. 2014, 9, 155892501400900414. [Google Scholar] [CrossRef]
- Bueno, A.; Luebbert, C.; Enders, S.; Sadowski, G.; Smirnova, I. Production of polylactic acid aerogels via phase separation and supercritical CO2 drying: Thermodynamic analysis of the gelation and drying process. J. Mater. Sci. 2021, 56, 18926–18945. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.A.V.; Le Moigne, N.; Bénézet, J.-C.; Sauceau, M.; Sescousse, R.; Fages, J. Foaming of PLA Composites by Supercritical Fluid-Assisted Processes: A Review. Molecules 2020, 25, 3408. [Google Scholar] [CrossRef]
- Larsen, Å.; Neldin, C. Physical extruder foaming of poly(lactic acid)-processing and foam properties. Polym. Eng. Sci. 2013, 53, 941–949. [Google Scholar] [CrossRef]
- Molladavoodi, S.; Gorbet, M.; Medley, J.; Kwon, H.J. Investigation of microstructure, mechanical properties and cellular viability of poly(L-lactic acid) tissue engineering scaffolds prepared by different thermally induced phase separation protocols. J. Mech. Behav. Biomed. Mater. 2013, 17, 186–197. [Google Scholar] [CrossRef]
- Buzarovska, A.; Gualandi, C.; Parrilli, A.; Scandola, M. Effect of TiO2 nanoparticle loading on Poly(l-lactic acid) porous scaffolds fabricated by TIPS. Compos. Part B Eng. 2015, 81, 189–195. [Google Scholar] [CrossRef]
- Bernardes, B.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.; Oliveira, A. Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef]
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconjugate Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef]
- Mao, D.; Li, Q.; Bai, N.; Dong, H.; Li, D. Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohydr. Polym. 2018, 180, 104–111. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; López, C.; de Dicastillo, C.L.; Velásquez, E.; Villegas, C.; Faba, S.; Rivera, P.; Guarda, A.; Romero, J.; et al. Foaming with scCO2 and Impregnation with Cinnamaldehyde of PLA Nanocomposites for Food Packaging. Processes 2022, 10, 376. [Google Scholar] [CrossRef]
- Oliveira, N.S.; Dorgan, J.; Coutinho, J.A.P.; Ferreira, A.; Daridon, J.L.; Marrucho, I.M. Gas solubility of carbon dioxide in poly(lactic acid) at high pressures. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1010–1019. [Google Scholar] [CrossRef]
- Mahmood, S.; Keshtkar, M.; Park, C. Determination of carbon dioxide solubility in polylactide acid with accurate PVT properties. J. Chem. Thermodyn. 2014, 70, 13–23. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Turng, L.; Gong, S.; Zhang, C. Measurement of gas solubility and diffusivity in polylactide. Fluid Phase Equilib. 2006, 246, 158–166. [Google Scholar] [CrossRef]
- Yoon, Y.; Plummer, C.J.; Thoemen, H.; E Månson, J.-A. Liquid CO2 processing of solid polylactide foam precursors. J. Cell. Plast. 2016, 52, 153–174. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J. Fabrication of high-expansion microcellular PLA foams based on pre-isothermal cold crystallization and supercritical CO2 foaming. Polym. Degrad. Stab. 2018, 156, 75–88. [Google Scholar] [CrossRef]
- Li, B.; Ma, X.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J. Green fabrication method of layered and open-cell polylactide foams for oil-sorption via pre-crystallization and supercritical CO2-induced melting. J. Supercrit. Fluids 2020, 162, 104854. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhang, Q.; Xia, C.; Chen, C.; Chen, X.; Yu, P. Foaming of poly(lactic acid) with supercritical CO2: The combined effect of crystallinity and crystalline morphology on cellular structure. J. Supercrit. Fluids 2019, 145, 122–132. [Google Scholar] [CrossRef]
- López-Periago, A.; García-González, C.A.; Domingo, C. Solvent- and thermal-induced crystallization of poly-L-lactic acid in supercritical CO2 medium. J. Appl. Polym. Sci. 2009, 111, 291–300. [Google Scholar] [CrossRef]
- Plummer, C.J.G.; Yonghoon, Y.; Garin, L.; Månson, J.-A.E. Crystallization of polylactide during impregnation with liquid CO2. Polym. Bull. 2015, 72, 103–116. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J.; Shi, Z. Super High-Expansion Poly(Lactic Acid) Foams with Excellent Oil-Adsorption and Thermal-Insulation Properties Fabricated by Supercritical CO2 Foaming. Adv. Sustain. Syst. 2021, 5, 2000295. [Google Scholar] [CrossRef]
- Kiran, E. Foaming strategies for bioabsorbable polymers in supercritical fluid mixtures. Part II. Foaming of poly(ε-caprolactone-co-lactide) in carbon dioxide and carbon dioxide + acetone fluid mixtures and formation of tubular foams via solution extrusion. J. Supercrit. Fluids 2010, 54, 296–307. [Google Scholar] [CrossRef]
- Frerich, S.C. Biopolymer foaming with supercritical CO2—Thermodynamics, foaming behaviour and mechanical characteristics. J. Supercrit. Fluids 2015, 96, 349–358. [Google Scholar] [CrossRef]
- Haham, H.; Riscoe, A.; Frank, C.W.; Billington, S.L. Effect of bubble nucleating agents derived from biochar on the foaming mechanism of poly lactic acid foams. Appl. Surf. Sci. Adv. 2021, 3, 100059. [Google Scholar] [CrossRef]
- Yan, Z.; Liao, X.; He, G.-J.; Li, S.; Guo, F.; Li, G. Green Method to Widen the Foaming Processing Window of PLA by Introducing Stereocomplex Crystallites. Ind. Eng. Chem. Res. 2019, 58, 21466–21475. [Google Scholar] [CrossRef]
- Tai, H.; Mather, M.; Howard, D.; Wang, W.; White, L.; Crowe, J.; Morgan, S.; Chandra, A.; Williams, D.; Howdle, S.; et al. Control of pore size and structure of tissue engineering scaffolds produced by supercritical fluid processing—Discussion with reviewers. Eur. Cells Mater. 2007, 14, 76–77. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Iglesias-Mejuto, A.; García-González, C. Solvent-Free Approaches for the Processing of Scaffolds in Regenerative Medicine. Polymers 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2-assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Pavlidou, E.; Panayiotou, C. Porous scaffolds prepared by phase inversion using supercritical CO2 as antisolvent: I. Poly(l-lactic acid). J. Supercrit. Fluids 2007, 40, 317–322. [Google Scholar] [CrossRef]
- Stoclet, G.; Sclavons, M.; Lecouvet, B.; Devaux, J.; Van Velthem, P.; Boborodea, A.; Bourbigot, S.; Sallem-Idrissi, N. Elaboration of poly(lactic acid)/halloysite nanocomposites by means of water assisted extrusion: Structure, mechanical properties and fire performance. RSC Adv. 2014, 4, 57553–57563. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Baillon, F.; Fages, J. Mastering the structure of PLA foams made with extrusion assisted by supercritical CO2. J. Appl. Polym. Sci. 2017, 134, 45067. [Google Scholar] [CrossRef]
- Weishaupt, O.; Frerich, S. Empirical Modelling of Foam Extrusion Using Latin Hypercube Sampling and Machine Learning, ProcessNet High Pressure Technology. Available online: https://processnet.org/Veranstaltungen.html (accessed on 16 December 2022).
- Lee, S.; Kareko, L.; Jun, J. Study of Thermoplastic PLA Foam Extrusion. J. Cell. Plast. 2008, 44, 293–305. [Google Scholar] [CrossRef]
- Yu, K.; Ni, J.; Zhou, H.; Wang, X.; Mi, J. Effects of in-situ crystallization on poly (lactic acid) microcellular foaming: Density functional theory and experiment. Polymer 2020, 200, 122539. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Kang, Y.; Qin, J.; Li, J.; Zhang, H.; Fan, X.; Liu, Y.; Zhang, G. Effect of poly(butylenes succinate) on the microcellular foaming of polylactide using supercritical carbon dioxide. J. Polym. Res. 2018, 25, 229. [Google Scholar] [CrossRef]
- Ni, J.; Yu, K.; Zhou, H.; Mi, J.; Chen, S.; Wang, X. Morphological evolution of PLA foam from microcellular to nanocellular induced by cold crystallization assisted by supercritical CO2. J. Supercrit. Fluids 2020, 158, 104719. [Google Scholar] [CrossRef]
- Kuska, R.; Milovanovic, S.; Janowski, J.; Frerich, S.; Zizovic, I.; Ivanovic, J. Supercritical Foaming and Impregnation Processes Design based on Thermodynamic measurements of PLA under High Pressure CO2. In Proceedings of the 15th European Meeting on Supercritical Fluids (EMSF), Essen, Germany, 8–11 May 2016; pp. 9–11. [Google Scholar]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Hou, J.; Gong, J. A green strategy to regulate cellular structure and crystallization of poly(lactic acid) foams based on pre-isothermal cold crystallization and CO2 foaming. Int. J. Biol. Macromol. 2019, 129, 171–180. [Google Scholar] [CrossRef]
- Milovanović, S.; Lukić, I. An overview on the application of supercritical carbon dioxide for the processing of pharmaceuticals. Arh. za Farm. 2022, 72, 566–590. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Drug–polymer microparticles produced by supercritical assisted atomization. Biotechnol. Bioeng. 2007, 6, 1626–1637. [Google Scholar] [CrossRef]
- Girard, E.; Tassaing, T.; Marty, J.-D.; Destarac, M. Structure–Property Relationships in CO2-philic (Co)polymers: Phase Behavior, Self-Assembly, and Stabilization of Water/CO2 Emulsions. Chem. Rev. 2016, 116, 4125–4169. [Google Scholar] [CrossRef]
- Conway, S.E.; Byun, H.-S.; McHugh, M.A.; Wang, J.D.; Mandel, F.S. Poly(lactide-co-glycolide) solution behavior in supercritical CO2, CHF3, and CHClF2. J. Appl. Polym. Sci. 2001, 80, 1155–1161. [Google Scholar] [CrossRef]
- Gregorowicz, J.; Bernatowicz, P. Phase behaviour of l-lactic acid based polymers of low molecular weight in supercritical carbon dioxide at high pressures. J. Supercrit. Fluids 2009, 51, 270–277. [Google Scholar] [CrossRef]
- Fages, J.; Lochard, H.; Letourneau, J.-J.; Sauceau, M.; Rodier, E. Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technol. 2004, 141, 219–226. [Google Scholar] [CrossRef]
- Petermann, M. Supercritical fluid-assisted sprays for particle generation. J. Supercrit. Fluids 2018, 134, 234–243. [Google Scholar] [CrossRef]
- Weidner, E.; Knez, Z.; Novak, Z. A Process and Equipment for Production and Fractionation of Fine Particles from Gas Saturated Solutions. World Patent WO 95/21688, 1994. [Google Scholar]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.; Braga, M.E.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Nishijima, M.; Sano, K.; Demoto, K.; Sakabe, J.; Shimoyama, Y. Production of theophylline nanoparticles using rapid expansion of supercritical solutions with a solid cosolvent (RESS-SC) technique. J. Supercrit. Fluids 2015, 105, 128–135. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Daneshyan, S. Preparation of Aprepitant nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC). J. Supercrit. Fluids 2018, 140, 72–84. [Google Scholar] [CrossRef]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar] [CrossRef]
- Perinelli, D.; Bonacucina, G.; Cespi, M.; Naylor, A.; Whitaker, M.; Palmieri, G.; Giorgioni, G.; Casettari, L. Evaluation of P(L)LA-PEG-P(L)LA as processing aid for biodegradable particles from gas saturated solutions (PGSS) process. Int. J. Pharm. 2014, 468, 250–257. [Google Scholar] [CrossRef]
- Vergara-Mendoza, M.D.S.; Ortiz-Estrada, C.-H.; González-Martínez, J.; Quezada-Gallo, J.-A. Microencapsulation of Coenzyme Q10 in Poly(ethylene glycol) and Poly(lactic acid) with Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2012, 51, 5840–5846. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Izzo, L.; Pappalardo, D.; Reverchon, E. PLA–PEG copolymers micronization by supercritical assisted atomization. J. Supercrit. Fluids 2012, 72, 15–21. [Google Scholar] [CrossRef]
- Sacchetin, P.S.C.; Morales, A.R.; Moraes, M.; Rosa, P.D.T.V.E. Formation of PLA particles incorporating 17α-methyltestosterone by supercritical fluid technology. J. Supercrit. Fluids 2013, 77, 52–62. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical-Assisted Atomization to Produce Micro- and/or Nanoparticles of Controlled Size and Distribution. Ind. Eng. Chem. Res. 2002, 41, 2405–2411. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Micronization of antibiotics by supercritical assisted atomization. J. Supercrit. Fluids 2003, 26, 243–252. [Google Scholar] [CrossRef]
- Chen, A.-Z.; Li, Y.; Chau, F.-T.; Lau, T.-Y.; Hu, J.-Y.; Zhao, Z.; Mok, D.K.-W. Microencapsulation of puerarin nanoparticles by poly(l-lactide) in a supercritical CO2 process. Acta Biomater. 2009, 5, 2913–2919. [Google Scholar] [CrossRef]
- Chen, F.; Yin, G.; Liao, X.; Yang, Y.; Huang, Z.; Gu, J.; Yao, Y.; Chen, X.; Gao, H. Preparation, characterization and in vitro release properties of morphine-loaded PLLA-PEG-PLLA microparticles via solution enhanced dispersion by supercritical fluids. J. Mater. Sci. Mater. Med. 2013, 24, 1693–1705. [Google Scholar] [CrossRef]
- Jin, H.; Li, S.; Hu, D.; Zhao, Y. Preparation of PLA-PEG nanoparticles by the solution enhanced dispersion with enhanced mass transfer using ultrasound in supercritical CO2. Powder Technol. 2012, 227, 17–23. [Google Scholar] [CrossRef]
- Della Porta, G.; Falco, N.; Giordano, E.; Reverchon, E. PLGA microspheres by Supercritical Emulsion Extraction: A study on insulin release in myoblast culture. J. Biomater. Sci. Polym. Ed. 2013, 24, 1831–1847. [Google Scholar] [CrossRef]
- Della Porta, G.; Campardelli, R.; Reverchon, E. Monodisperse biopolymer nanoparticles by Continuous Supercritical Emulsion Extraction. J. Supercrit. Fluids 2013, 76, 67–73. [Google Scholar] [CrossRef]
- Sheth, P.; Sandhu, H.; Singhal, D.; Malick, W.; Shah, N.; Kislalioglu, M.S. Nanoparticles in the pharmaceutical industry and the use of supercritical fluid technologies for nanoparticle production. Curr. Drug Deliv. 2012, 9, 269–284. [Google Scholar] [CrossRef]
- Gangapurwala, G.; Vollrath, A.; Luis, A.D.S.; Schubert, U.S. PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation? Pharmaceutics 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Lee, C.W.; Bibi, G.; Jung, Y.; Kim, S.H. Supercritical fluid technology parameters affecting size and behavior of stereocomplex polylactide particles and their composites. Polym. Eng. Sci. 2017, 58, 1193–1200. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.; Pereyra, C.; de la Ossa, E.M. Supercritical CO2 precipitation of poly(l-lactic acid) in a wide range of miscibility. J. Supercrit. Fluids 2013, 81, 236–244. [Google Scholar] [CrossRef]
- Bakhbakhi, Y.; Asif, M.; Chafidz, A.; Ajbar, A. Formation of biodegradable polymeric fine particles by supercritical antisolvent precipitation process. Polym. Eng. Sci. 2013, 53, 564–570. [Google Scholar] [CrossRef]
- Montes, A.; Kin, N.; Gordillo, M.; Pereyra, C.; de la Ossa, E.M. Polymer–naproxen precipitation by supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2014, 89, 58–67. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Zahran, F.; Martín, D.; Cabañas, A.; Pando, C. Preparation of 5-fluorouracil microparticles and 5-fluorouracil/poly(l-lactide) composites by a supercritical CO2 antisolvent process. J. Supercrit. Fluids 2019, 143, 64–71. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Heal. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef]
- Tabernero, A.; Martin del Valle, E.M.; Galán, M.A. Supercritical fluids for pharmaceutical particle engineering: Methods, basic fundamentals and modelling. Chem. Eng. Process. Process. Intensif. 2012, 60, 9–25. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Li, H.; Ma, G. Recent research and development of PLGA/PLA microspheres/nanoparticles: A review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2019, 13, 14–27. [Google Scholar] [CrossRef]
- Spenlehauer, G.; Vert, M.; Benoit, J.; Boddaert, A. In vitro and In vivo degradation of poly(D,L lactide/glycolide) type microspheres made by solvent evaporation method. Biomaterials 1989, 10, 557–563. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Vega-González, A.; Subra-Paternault, P.; López-Periago, A.M.; García-González, C.A.; Domingo, C. Supercritical CO2 antisolvent precipitation of polymer networks of l-PLA, PMMA and PMMA/PCL blends for biomedical applications. Eur. Polym. J. 2008, 44, 1081–1094. [Google Scholar] [CrossRef]
- Yoda, S.; Sato, K.; Oyama, H.T. Impregnation of paclitaxel into poly(dl-lactic acid) using high pressure mixture of ethanol and carbon dioxide. RSC Adv. 2011, 1, 156–162. [Google Scholar] [CrossRef]
- Machado, N.D.; Cejudo-Bastante, C.; Goñi, M.L.; Gañán, N.A.; Casas-Cardoso, L.; Mantell-Serrano, C. Screening of the Supercritical Impregnation of Olea europaea Leaves Extract into Filaments of Thermoplastic Polyurethane (TPU) and Polylactic Acid (PLA) Intended for Biomedical Applications. Antioxidants 2022, 11, 1170. [Google Scholar] [CrossRef]
- Ardestani, N.S.; Rojas, A.; Esfandiari, N.; Galotto, M.J.; Babhadiashar, A.; Sajadian, S.A. Supercritical Fluid Extraction from Zataria multiflora Boiss and Impregnation of Bioactive Compounds in PLA for the Development of Materials with Antibacterial Properties. Processes 2022, 10, 1787. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, I.; Maia-Obi, L.; Champeau, M. Aspirin-Loaded Polymeric Films for Drug Delivery Systems: Comparison between Soaking and Supercritical CO2 Impregnation. Pharmaceutics 2021, 13, 824. [Google Scholar] [CrossRef]
- Champeau, M.; Coutinho, I.T.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Tuning the release profile of ketoprofen from poly(l-lactic acid) suture using supercritical CO2 impregnation process. J. Drug Deliv. Sci. Technol. 2020, 55, 101468. [Google Scholar] [CrossRef]
- Sugiura, K.; Ogawa, S.; Tabata, I.; Hori, T. Impregnation of Tranilast to the Poly(lactic acid) Fiber with Supercritical Carbon Dioxide and the Release Behavior of Tranilast. Sen′i Gakkaishi 2005, 61, 159–165. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Jawaid, M.; Boonruang, K.; Chinsirikul, W.; Hararak, B.; Kerddonfag, N.; Chonhenchob, V.; Kenawy, E.-R. Antifungal Poly(lactic acid) Films Containing Thymol and Carvone. MATEC Web Conf. 2016, 67, 6107. [Google Scholar] [CrossRef]
- Coutinho, I.T.; Champeau, M. Synergistic effects in the simultaneous supercritical CO2 impregnation of two compounds into poly(L-lactic acid) and polyethylene. J. Supercrit. Fluids 2020, 166, 105019. [Google Scholar] [CrossRef]
- Rosales, J.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; de la Ossa, E.M. Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Naranjo, L.V.; Cejudo-Bastante, C.; Casas, L.; Mantell, C.; De La Ossa, E.J.M. In Vitro Study of the Release of Drugs Impregnated by Supercritical Technology in Polylactic Acid for Biomedical Applications. Chem. Eng. Trans. 2021, 86, 1063–1068. [Google Scholar] [CrossRef]
- Naranjo, L.V.; Bastante, C.C.; Cardoso, L.C.; Serrano, C.M.; Fernández, E.M.d.l.O. Supercritical Impregnation of Ketoprofen into Polylactic Acid for Biomedical Application: Analysis and Modeling of the Release Kinetic. Polymers 2021, 13, 1982. [Google Scholar] [CrossRef]
- Roß, N.; Frerich, S.C. High pressure differential scanning calorimetry of poly(lactic acid) in presence of CO2 up to 100 MPa. Thermochim. Acta 2021, 706, 179076. [Google Scholar] [CrossRef]
- Huang, E.; Liao, X.; Zhao, C.; Park, C.B.; Yang, Q.; Li, G. Effect of Unexpected CO2’s Phase Transition on the High-Pressure Differential Scanning Calorimetry Performance of Various Polymers. ACS Sustain. Chem. Eng. 2016, 4, 1810–1818. [Google Scholar] [CrossRef]
- Miranda-Villa, P.P.; Gañán, N.A.; Martini, R.E.; Goñi, M.L. Supercritical CO2-assisted impregnation of polylactic acid films with R-carvone: Effect of processing on loading, mass transfer kinetics, and final properties. J. CO2 Util. 2022, 61, 102029. [Google Scholar] [CrossRef]
- Milovanović, S.L.; Kuska, R.M.; Škorić-Lučić, M.L.; Kalagasidis-Krušić, M.T.; Frerich, S.; Žižović, I.T.; Ivanović, J.Z. Swelling kinetics and impregnation of PLA with thymol under supercritical CO2 conditions. Tehnika 2016, 71, 16–20. [Google Scholar] [CrossRef]
- Grosso, P.; Cejudo, C.; Sánchez-Gomar, I.; Durán-Ruiz, M.C.; Moreno-Luna, R.; Casas, L.; Pereyra, C.; Mantell, C. Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures. Polymers 2022, 14, 2706. [Google Scholar] [CrossRef]
- Lukic, I.; Pajnik, J.; Nisavic, J.; Tadic, V.; Vági, E.; Szekely, E.; Zizovic, I. Application of the Integrated Supercritical Fluid Extraction–Impregnation Process (SFE-SSI) for Development of Materials with Antiviral Properties. Processes 2022, 10, 680. [Google Scholar] [CrossRef]
- Lukic, I.; Pajnik, J.; Tadic, V.; Milovanovic, S. Supercritical CO2-assisted processes for development of added-value materials: Optimization of starch aerogels preparation and hemp seed extracts impregnation. J. CO2 Util. 2022, 61, 102036. [Google Scholar] [CrossRef]
- Ivanovic, J.; Rezwan, K.; Kroll, S. Supercritical CO2 deposition and foaming process for fabrication of biopolyester-ZnO bone scaffolds. J. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Bozbağ, S.E.; Erkey, C. Supercritical deposition: Current status and perspectives for the preparation of supported metal nanostructures. J. Supercrit. Fluids 2015, 96, 298–312. [Google Scholar] [CrossRef]
- Erkey, C. Preparation of metallic supported nanoparticles and films using supercritical fluid deposition. J. Supercrit. Fluids 2009, 47, 517–522. [Google Scholar] [CrossRef]
- Türk, M.; Erkey, C. Synthesis of supported nanoparticles in supercritical fluids by supercritical fluid reactive deposition: Current state, further perspectives and needs. J. Supercrit. Fluids 2018, 134, 176–183. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-Printed, Dual Crosslinked and Sterile Aerogel Scaffolds for Bone Tissue Engineering. Polymers 2022, 14, 1211. [Google Scholar] [CrossRef]
- Thiele, M.; Kutlu, Y.; Dobbelstein, H.; Petermann, M.; Esen, C.; Ostendorf, A. Direct generation of 3D structures by laser polymer deposition. J. Laser Appl. 2021, 33, 022002. [Google Scholar] [CrossRef]
- Maga, D.; Hiebel, M.; Thonemann, N. Life cycle assessment of recycling options for polylactic acid. Resour. Conserv. Recycl. 2019, 149, 86–96. [Google Scholar] [CrossRef]


| Company | Industrial Name | Intended Processing Methods | Applications |
|---|---|---|---|
| NatureWorks (Plymouth, MN, USA) | Ingeo® |
|
|
| TotalEnergies Corbion (Gorinchem, The Netherlands) | Luminy® |
|
|
| Evonik Industries (Essen, Germany) | Resomer® |
|
|
| ThyssenKrupp (Essen, Germany) | PLAneo® |
|
|
| Technique | Product | Products’ Main Properties |
|---|---|---|
| Drying | Aerogels Scaffolds |
|
| Foaming | Foams |
|
| Particle generation | Microparticles Nanoparticles |
|
| Impregnation | Variety of forms |
|
| PLA | Producer | Solvent | Method | P (MPa) | T (°C) | Particle Size | Application | Lit |
|---|---|---|---|---|---|---|---|---|
| PLLA, PDLA | Synthetized | DME, chloroform | Static PGSS | 10 | 60–100 | d = 1.1–11.6 µm | Filler in composites | [111] |
| PLA | Sigma-Aldrich | Ethanol, acetone, DCM | RESS | 27.5 | 35 | d = 2–3 µm | Drug delivery | [99] |
| PLLA | Sigma-Aldrich | DCM, TCM, methanol, acetone | RESS, SAS | 15–30 10–25 | 45–100 35 | dRESS = n.i.a dSAS = 1–104 µm | Drug delivery | [112] |
| PLLA | Sigma-Aldrich | DCM | SAS | n.i.a. | 35–50 | d = 0.6–47.3 µm | Biomedical applications | [113] |
| PLLA | Purac | DCM | SAS | 8–16 | 40 | d = 5–25 µm | Veterinary applications | [101] |
| PLLA | Sigma-Aldrich | DCM | SAS | 10–20 | 40 | d = 0.08–1.43 µm | Drug delivery | [114] |
| PLA | Synthetized | DCM | SEDS-EM | 7.9–9.8 | 24–38 | d < 400–1000 nm | Controlled release | [106] |
| PLA | Evonik | Ethylacetate | SEE-C | 8 | 38 | d = 203 nm–0.9 µm | Bactericidal nanocomposites | [42] |
| PLA | Boehringer | Acetone | SEE-C | 8 | 38 | d = 212–233 nm | Controlled release | [108] |
| PLLA | Sigma-Aldrich | DMSO+DCM | SAS | 18 | 50 | d < 1 µm | Drug delivery | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanovic, S.; Lukic, I.; Horvat, G.; Novak, Z.; Frerich, S.; Petermann, M.; García-González, C.A. Green Processing of Neat Poly(lactic acid) Using Carbon Dioxide under Elevated Pressure for Preparation of Advanced Materials: A Review (2012–2022). Polymers 2023, 15, 860. https://doi.org/10.3390/polym15040860
Milovanovic S, Lukic I, Horvat G, Novak Z, Frerich S, Petermann M, García-González CA. Green Processing of Neat Poly(lactic acid) Using Carbon Dioxide under Elevated Pressure for Preparation of Advanced Materials: A Review (2012–2022). Polymers. 2023; 15(4):860. https://doi.org/10.3390/polym15040860
Chicago/Turabian StyleMilovanovic, Stoja, Ivana Lukic, Gabrijela Horvat, Zoran Novak, Sulamith Frerich, Marcus Petermann, and Carlos A. García-González. 2023. "Green Processing of Neat Poly(lactic acid) Using Carbon Dioxide under Elevated Pressure for Preparation of Advanced Materials: A Review (2012–2022)" Polymers 15, no. 4: 860. https://doi.org/10.3390/polym15040860
APA StyleMilovanovic, S., Lukic, I., Horvat, G., Novak, Z., Frerich, S., Petermann, M., & García-González, C. A. (2023). Green Processing of Neat Poly(lactic acid) Using Carbon Dioxide under Elevated Pressure for Preparation of Advanced Materials: A Review (2012–2022). Polymers, 15(4), 860. https://doi.org/10.3390/polym15040860