Biological Characteristics of Polyurethane-Based Bone-Replacement Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reginster, J.Y.; Burler, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, A.R.; Schroeder, G.D.; Kepler, C.K.; Oner, F.C.; Vialle, L.R.; Kandziora, F.; Koerner, J.D.; Kurd, M.F.; Reinhold, M.; Schnake, K.J.; et al. The surgical algorithm for the AOSpine thoracolumbar spine injury classification system. Eur. Spine J. 2016, 25, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Curtis, E.M.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.K.; Kanna, R.M. Management of thoracolumbar fractures in adults: Current algorithm. Int. J. Spine 2019, 4, 10–19. [Google Scholar]
- Jensen, R.K.; Jensen, T.S.; Koes, B.; Hartvigsen, J. Prevalence of lumbar spinal stenosis in general and clinical populations: A systematic review and meta-analysis. Eur. Spine J. 2020, 29, 2143–2163. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, S.; Chen, G.; Zheng, W.; Peng, J.; Huang, X.; Chen, L. Insight into the roles of melatonin in bone tissue and bone-related diseases (Review). Int. J. Mol. Med. 2021, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Le Meng Bao, C.; Teo, E.Y.; Chong, M.S.; Liu, Y.; Choolani, M.; Chan, J.K. Advances in Bone Tissue Engineering. In Regenerative Medicine and Tissue Engineering; IntechOpen: London, UK, 2013; pp. 599–614. [Google Scholar] [CrossRef]
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. [Google Scholar] [CrossRef]
- Szendrői, M.; Antal, I.; Szendrői, A.; Lazáry, Á.; Varga, P.P. Diagnostic algorithm, prognostic factors and surgical treatment of metastatic cancer diseases of the long bones and spine. EFORT Open Rev. 2017, 2, 372–381. [Google Scholar] [CrossRef]
- Anderson, S.R.; Pak, K.Y.; Vincent, A.G.; Ong, A.; Ducic, Y. Reconstruction of the Mandibular Condyle. Facial Plast. Surg. 2021, 37, 728–734. [Google Scholar] [CrossRef]
- Fretwurst, T.; Gad, L.M.; Nelson, K.; Schmelzeisen, R. Dentoalveolar reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 316–322. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Gorbulev, S.; Guehring, T.; Schulz, A.P.; Schupfner, R.; Raschke, M.; Huber-Wagner, S.; Rommens, P.M. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J. Bone Jt. Surg. 2019, 102, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Kampleitner, C.; Brennan, M.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Pelletier, M.H.; Christou, C.; He, J.; Vizesi, F.; Boden, S.D. The in vivo response to a novel Ti coating compared with polyether ether ketone: Evaluation of the periphery and inner surfaces of an implant. Spine J. 2018, 18, 1231–1240. [Google Scholar] [CrossRef]
- Smit, T.H.; Müller, R.; Van Dijk, M.; Wuisman, P.I.J.M. Changes in Bone Architecture During Spinal Fusion: Three Years Follow-up and the Role of Cage Stiffness. Spine 2003, 28, 1802–1808. [Google Scholar] [CrossRef]
- Kashii, M.; Kitaguchi, K.; Makino, T.; Kaito, T. Comparison in the same intervertebral space between titanium-coated and uncoated PEEK cages in lumbar interbody fusion surgery. J. Orthop. Sci. 2020, 25, 565–570. [Google Scholar] [CrossRef]
- Arts, M.P.; Wolfs, J.F.; Corbin, T.P. The CASCADE trial: Effectiveness of ceramic versus PEEK cages for anterior cervical discectomy with interbody fusion; protocol of a blinded randomized controlled trial. BMC Musculoskelet. Disord. 2013, 14, 244. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Nemoto, O.; Asazuma, T.; Yato, Y.; Imabayashi, H.; Yasuoka, H.; Fujikawa, A. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation. Eur. Spine J. 2014, 23, 2150–2155. [Google Scholar] [CrossRef]
- Kuo, M.; Tsai, C.; Huang, J.; Chen, M. PEEK composites reinforced by nano-sized SiO2 and Al2O3 particulates. Mater. Chem. Phys. 2005, 90, 185–195. [Google Scholar] [CrossRef]
- Jiang, N.; Huang, J.; Edwards, L.J.; Liu, B.; Zhang, Y.; Beal, C.D.; Evavold, B.D.; Zhu, C. Two-Stage Cooperative T Cell Receptor-Peptide Major Histocompatibility Complex-CD8 Trimolecular Interactions Amplify Antigen Discrimination. Immunity 2011, 34, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Chavanne, A.; Spitaler, R.; Kropik, K.; Aigner, N.; Ogon, M.; Redl, H. Assessment of different screw augmentation techniques and screw designs in osteoporotic spines. Eur. Spine J. 2008, 17, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Shea, T.M.; Laun, J.; Gonzalez-Blohm, S.A.; Doulgeris, J.J.; Lee, W.E., 3rd; Aghayev, K.; Vrionis, F.D. Designs and Techniques That Improve the Pullout Strength of Pedicle Screws in Osteoporotic Vertebrae: Current Status. BioMed Res. Int. 2014, 2014, 748393. [Google Scholar] [CrossRef]
- Liu, D.; Sheng, J.; Wu, H.-H.; Kang, X.; Xie, Q.-Y.; Luo, Y.; Zhou, J.-J.; Zheng, W. Biomechanical study of injectable hollow pedicle screws for PMMA augmentation in severely osteoporotic lumbar vertebrae: Effect of PMMA distribution and volume on screw stability. J. Neurosurgery: Spine 2018, 29, 639–646. [Google Scholar] [CrossRef]
- Lai, P.-L.; Tai, C.-L.; Chen, L.-H.; Nien, N.-Y. Cement leakage causes potential thermal injury in vertebroplasty. BMC Musculoskelet. Disord. 2011, 12, 116. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Kepler, C.K.; Savage, K.E.; Eachus, B.; Albert, T.J.; Vaccaro, A.R. Neurological deficit due to cement extravasation following a vertebral augmentation procedure. J. Neurosurg. Spine 2013, 19, 61–70. [Google Scholar] [CrossRef]
- Lazáry, Á.; Speer, G.; Varga, P.P.; Balla, B.; Bácsi, K.; Kósa, J.P.; Nagy, Z.; Takács, I.; Lakatos, P. Effect of vertebroplasty filler materials on viability and gene expression of human nucleus pulposus cells. J. Orthop. Res. 2008, 26, 601–607. [Google Scholar] [CrossRef]
- Donaldson, A.J.; Thomson, H.E.; Harper, N.J.; Kenny, N.W. Bone cement implantation syndrome. Br. J. Anaesth. 2009, 102, 12–22. [Google Scholar] [CrossRef]
- Filip, N.; Radu, I.; Veliceasa, B.; Filip, C.; Pertea, M.; Clim, A.; Pinzariu, A.C.; Drochioi, I.C.; Hilitanu, R.L.; Serban, I.L. Biomaterials in Orthopedic Devices: Current Issues and Future Perspectives. Coatings 2022, 12, 1544. [Google Scholar] [CrossRef]
- Szczepańczyk, P.; Szlachta, M.; Złocista-Szewczyk, N.; Chłopek, J.; Pielichowska, K. Recent Developments in Polyurethane-Based Materials for Bone Tissue Engineering. Polymers 2021, 13, 946. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.; Al-Qaddo, H.; Gosau, M.; Smeets, R.; Hartjen, P.; Friedrich, R.E.; Nada, O.A.; Vollkommer, T.; Rashad, A. Cytocompatibility of Bone Substitute Materials and Membranes. In Vivo 2021, 35, 2035–2040. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Pröhl, A.; Batinic, M.; Alkildani, S.; Hahn, M.; Radenkovic, M.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Bone Healing Capacity of a Novel Bone Grafting Material Combined with Hyaluronic Acid. Int. J. Mol. Sci. 2021, 22, 4818. [Google Scholar] [CrossRef] [PubMed]
- Meenapriya, M.; Ashok, V.; Kiran, K.; Dhanraj, G. Biocompatibility of Polylactic Acid as a Bone Substitute: An In Vitro Study. Biosci. Biotechnol. Res. Commun. 2020, 13, 239–243. [Google Scholar] [CrossRef]
- Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Kakiuchi, Y.; Nakano, M.; Suzuki, S.; Handa, K.; Saito, M. Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy. Cells 2021, 10, 2687. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; Qin, K.H.; Hu, D.A.; Wang, E.J.; Li, A.J.; et al. Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. Part B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Yudin, V.; Kovylin, R.; Baten’Kin, M.; Kulikova, T.; Aleynik, D.Y.; Egorikhina, M.; Rubtsova, Y.P.; Charykova, I.; Mlyavykh, S.; Chesnokov, S.; et al. Visible-light induced synthesis of biocompatible porous polymers from oligocarbonatedimethacrylate (OCM-2) in the presence of dialkyl phthalates. Polymer 2020, 192, 122302. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Iizuka, T.; Safi, A.-F. The impact of the size of bone substitute granules on macrophage and osteoblast behaviors in vitro. Clin. Oral Investig. 2021, 25, 4949–4958. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 5304860. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Yetter, A.B. Correlation of visual in vitro cytotoxicity ratings of biomaterials with quantitative in vitro cell viability measurements. Cell Biol. Toxicol. 2007, 24, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, J.N.; Uspenskij, I.V.; Slinjakov, A.J.; Novikov, A.E. Filler Material. Patent RU2518753C1, 6 October 2014. [Google Scholar]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Gopal, B. Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Appl. Surf. Sci. 2014, 303, 277–281. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Hung, S.C.; Hsu, S.H. The effect of elastic biodegradable polyurethane electrospunnanofibers on the differentiation of mesenchymal stem cells. Colloids Surf. 2014, 122, 414–422. [Google Scholar] [CrossRef]
- Yang, W.; Both, S.K.; Zuo, Y.; Birgani, Z.T.; Habibovic, P.; Li, Y.; Jansen, J.A.; Yang, F. Biological evaluation of porous aliphatic polyurethane/hydroxyapatite composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2014, 103, 2251–2259. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Beckman, E.J.; Piesco, N.P.; Agarwal, S. A new peptide-based polymer: Synthesis, biodegradation and potential to support cell growth in vivo. Biomaterials 2000, 21, 1247–1258. [Google Scholar] [CrossRef]
- Gogolewski, S.; Gorna, K. Biodegradable polyurethane cancellous bone graft substitutes in the treatment of iliac crest defects. J. Biomed. Mater. Res. 2007, 80, 94–111. [Google Scholar] [CrossRef]
- Das, B.; Chattopadhyay, P.; Maji, S.; Upadhyay, A.; Das Purkayastha, M.; Mohanta, C.L.; Maity, T.K.; Karak, N. Bio-functionalized MWCNT/hyperbranched polyurethane bionanocomposite for bone regeneration. Biomed. Mater. 2015, 10, 025011. [Google Scholar] [CrossRef]
- Li, B.; Yoshii, T.; Hafeman, A.E.; Nyman, J.S.; Wenke, J.C.; Guelcher, S.A. The effects of rhBMP-2 released from biodegradable polyurethane/microsphere composite scaffolds on new bone formation in rat femora. Biomaterials 2009, 30, 6768–6779. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; An, Y.K.; Kang, Q.K.; Hartsock, L.A.; Gogolewski, S.; Gorna, K. Osteogenesis of osteoblast seeded polyurethane–hydroxyapatite scaffolds in nude mice. Macromol. Symp. 2007, 253, 94–97. [Google Scholar] [CrossRef]
- Marzec, M.; Kucińska-Lipka, J.; Kalaszczyńska, I.; Janik, H. Development of polyurethanes for bone repair. Mater. Sci. Eng. C 2017, 80, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Bierer, D.W. Bismuth subsalicylate: History, chemistry, and safety. Rev. Infect. Dis. 1990, 12, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.J. The use of bismuth in gastroenterology. The ACG Committee on FDA-related Matters. Am. J. Gastroenterol. 1991, 86, 16–25. [Google Scholar] [PubMed]
- Sun, H. Biological Chemistry of Arsenic, Antimony and Bismuth; John Wiley & Sons: Hoboken, NJ, USA, 2011; 400p, ISBN 978-0-470-71390-7. [Google Scholar]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Malfertheiner, P.; Giguère, M.; Santana, J.; Khan, M.; Moayyedi, P. Adverse events with bismuth salts for Helicobacter pylori eradication: Systematic review and meta-analysis. World J. Gastroenterol. 2008, 14, 7361–7370. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, D.; Griffith, D.M. The prevalence of metal-based drugs as therapeutic or diagnostic agents: Beyond platinum. Dalton Trans. 2012, 41, 13239–13257. [Google Scholar] [CrossRef]
- Roth, A.K.; Boon-Ceelen, K.; Smelt, H.; van Rietbergen, B.; Willems, P.C.; van Rhijn, L.W.; Arts, J.J. Radiopaque UHMWPE sublaminar cables for spinal deformity correction: Preclinical mechanical and radiopacifier leaching assessment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 771–779. [Google Scholar] [CrossRef]
- Sox, T.E.; Olson, C.A. Binding and killing of bacteria by bismuth subsalicylate. Antimicrob. Agents Chemother. 1989, 33, 2075–2082. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Yang, M.; Li, Y.; Zhang, L.; Xie, S. One dodecahedral bismuth(III) complex derived from 2-acetylpyridine N(4)-pyridylthiosemicarbazone: Synthesis, crystal structure and biological evaluation. Dalton Trans. 2012, 41, 12882–12887. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-X.; Zhang, L.-Z.; Yang, M.; Niu, J.-Y.; Zhou, J. Synthesis, crystal structures, in vitro biological evaluation of zinc(II) and bismuth(III) complexes of 2-acetylpyrazine N(4)-phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2012, 22, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tai, Y.; Li, M.; Ma, P.; Zhao, J.; Niu, J. Main group bismuth(III), gallium(III) and diorganotin(IV) complexes de-rived from bis(2-acetylpyrazine)thiocarbonohydrazone: Synthesis, crystal structures and biological evaluation. Dalton Trans. 2014, 43, 5182–5189. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Park, S.; Kim, J.; Han, A.; Ahn, S.; Min, S.K.; Jae, H.J.; Chung, J.W.; Lee, J.H.; Jung, H.D.; et al. Enhanced endothelial cell activity induced by incorporation of nano-thick tantalum layer in artificial vascular grafts. Appl. Surf. Sci. 2020, 508, 144801. [Google Scholar] [CrossRef]
- Jang, T.S.; Lee, J.H.; Kim, S.; Park, C.; Song, J.; Jae, H.J.; Kim, H.E.; Chung, J.W.; Jung, H.D. Ta ion implanted nano-ridge-platform for enhanced vascular responses. Biomaterials 2019, 223, 119461. [Google Scholar] [CrossRef]
- Mendonca, A.; Rahman, M.S.; Alhalawani, A.; Rodriguez, O.; Gallant, R.C.; Ni, H.; Clarkin, O.M.; Towler, M.R. The effect of tantalum incorporation on the physical and chemical properties of ternary silicon-calcium-phosphorous mesoporous bi-oactive glasses. J. Biomed. Mater. Res. 2019, 107, 2229–2237. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The physicochemical/biological properties of porous tantalum and the potential sur-face modification techniques to improve its clinical application in dental implantology. Mater. Sci. 2015, 49, 323–329. [Google Scholar]
- Mas-Moruno, C.; Garrido, B.; Rodriguez, D.; Ruperez, E.; Gil, F.J. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J. Mater. Sci. 2015, 26, 109. [Google Scholar] [CrossRef]
- Jin, Y.; Ma, X.; Zhang, S.; Meng, H.; Xu, M.; Yang, X.; Xu, W.; Tian, J. A tantalum oxide-based core/shell nanoparticle for triple-modality image-guided chemo-thermal synergetic therapy of esophageal carcinoma. Cancer Lett. 2017, 397, 61–71. [Google Scholar] [CrossRef]
- Macheras, G.A.; Lepetsos, P.; Leonidou, A.O.; Anastasopoulos, P.; Galanakos, S.P.; Poultsides, L.A. Survivorship of a Porous Tantalum Monoblock Acetabular Component in Primary Hip Arthroplasty With a Mean Follow-Up of 18 Years. J. Arthroplast. 2017, 32, 3680–3684. [Google Scholar] [CrossRef]
- Patel, M.S.; McCormick, J.R.; Ghasem, A.; Huntley, S.R.; Gjolaj, J.P. Tantalum: The next biomaterial in spine surgery? J. Spine Surg. 2020, 6, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Bilandžić, M.D.; Roos, C.; Braun, A.; Jansen, P. Development of a radiopaque dental glass for endodontic laser applications. J. Mater. Res. Technol. 2020, 9, 13994–14001. [Google Scholar] [CrossRef]
- Huo, W.T.; Zhao, L.Z.; Yu, S.; Yu, Z.T.; Zhang, P.X.; Zhang, Y.S. Significantly enhanced osteoblast response to nano-grained pure tantalum. Sci. Rep. 2017, 7, 40868. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wei, L.; Song, B.; Chen, L.; Liu, J.; Deng, B.; Pan, X.; Shao, L. Involvement of autophagy in tantalum nanoparticle-induced osteoblast proliferation. Int. J. Nanomed. 2017, ume 12, 4323–4333. [Google Scholar] [CrossRef]
- An, R.; Fan, P.P.; Zhou, M.J.; Wang, Y.; Goel, S.; Zhou, X.F.; Li, W.; Wang, J.T. Nanolamellar tantalum interfaces in the osteoblast adhesion. Langmuir 2019, 35, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Haddouti, E.M.; Beckert, H.; Biehl, R.; Pariyar, S.; Rüwald, J.M.; Li, X.; Jaenisch, M.; Burger, C.; Wirtz, D.C.; et al. Investigation of cytotoxicity, oxidative stress, and inflammatory responses of tantalum nanoparticles in THP-1-Derived macrophages. Mediat. Inflamm. 2020, 2020, 3824593. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, S.; Wu, H.; Mei, S.; Wang, D.; Pan, Y.; Wang, D.; Zhao, J.; Wei, J. Preparation, characterization, in vitro bioactivity and rBMSCs responses to tantalum pentoxide/polyimide biocomposites for dental and orthopedic implants. Compos. Part B Eng. 2019, 177, 107433. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, P.; Xing, H.; Zhang, R.; Lingling, E.; Liu, H. Cytotoxicity, Oxidative Stress, and Autophagy Effects of Tantalum Nanoparticles on MC3T3-E1 Mouse Osteoblasts. J. Nanosci. Nanotechnol. 2020, 20, 1417–1424. [Google Scholar] [CrossRef]
- Fidan, S.; Muhaffel, F.; Riool, M.; Cempura, G.; de Boer, L.; Zaat, S.; Filemonowicz, A.C.; Cimenoglu, H. Fabrication of oxide layer on zirconium by micro-arc oxidation: Structural and antimicrobial characteristics. Mater. Sci. Eng. C 2017, 71, 565–569. [Google Scholar] [CrossRef]
- Pilz, M.; Staats, K.; Tobudic, S.; Assadian, O.; Presterl, E.; Windhager, R.; Holinka, J. Zirconium Nitride Coating Reduced Staphylococcus epidermidis Biofilm Formation on Orthopaedic Implant Surfaces: An In Vitro Study. Clin. Orthop. Relat. Res. 2019, 477, 461–466. [Google Scholar] [CrossRef]
- Aktuğ, S.L.; Durdu, S.; Yalçın, E.; Çavuşoğlu, K.; Usta, M. In vitro properties of bioceramic coatings produced on zirconium by plasma electrolytic oxidation. Surf. Coat. Technol. 2017, 324, 129–139. [Google Scholar] [CrossRef]
- Korniienko, V.; Oleshko, O.; Husak, Y.; Deineka, V.; Holubnycha, V.; Mishchenko, O.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; Pshenychnyi, R.; Leśniak-Ziółkowska, K.; et al. Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles. Materials 2020, 13, 3913. [Google Scholar] [CrossRef] [PubMed]
- Oleshko, O.; Deineka, V.V.; Husak, Y.; Korniienko, V.; Mishchenko, O.; Holubnycha, V.; Pisarek, M.; Michalska, J.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; et al. Ag Nanoparticle-Decorated Oxide Coatings Formed via Plasma Electrolytic Oxidation on ZrNb Alloy. Materials 2019, 12, 3742. [Google Scholar] [CrossRef]
- Lu, S.-F.; Lou, B.-S.; Yang, Y.-C.; Wu, P.-S.; Chung, R.-J.; Lee, J.-W. Effects of duty cycle and electrolyte concentration on the microstructure and biocompatibility of plasma electrolytic oxidation treatment on zirconium metal. Thin Solid Films 2015, 596, 87–93. [Google Scholar] [CrossRef]
- Sedelnikova, M.; Komarova, E.; Sharkeev, Y.; Tolkacheva, T.; Khlusov, I.; Litvinova, L.; Yurova, K.; Shupletsova, V. Comparative investigations of structure and properties of micro-arc wollastonite-calcium phosphate coatings on titanium and zirconium-niobium alloy. Bioact. Mater. 2017, 2, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, K.; Bai, C.; Li, X.; Dang, X.; Zhang, C. The influence of UV irradiation on the biological properties of MAO-formed ZrO2. Colloids Surf. B Biointerfaces 2012, 89, 40–47. [Google Scholar] [CrossRef]
- Bertoldi, S.; Fare, S.; Denegri, M.; Rossi, D.; Haugen, H.J.; Parolini, O.; Tanzi, M.C. Ability of polyurethane foams to support placenta-derived cell adhesion and osteogenic differentiation: Preliminary results. J. Mater. Sci. Mater. Med. 2009, 21, 1005–1011. [Google Scholar] [CrossRef]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbühl, R.; Bohner, M.; Richter, W. Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef]
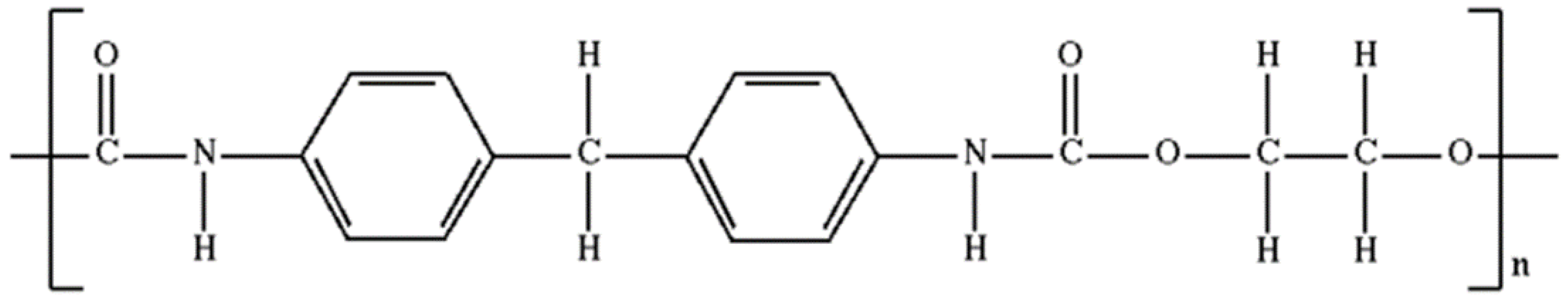



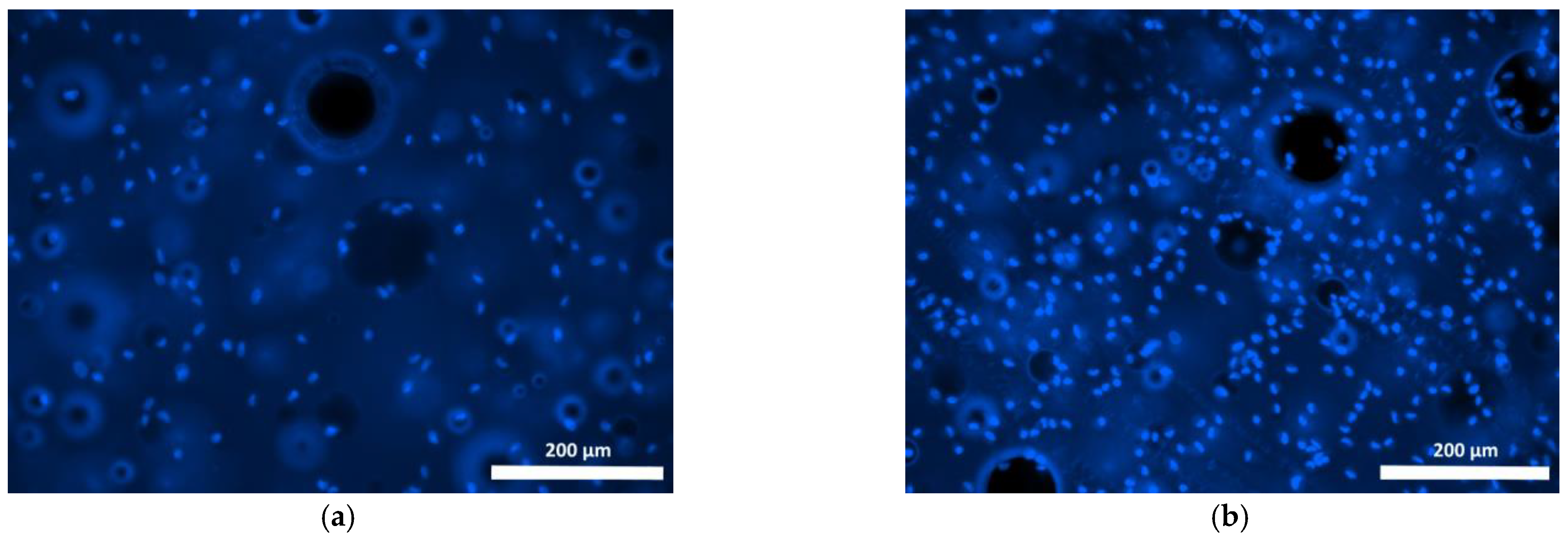
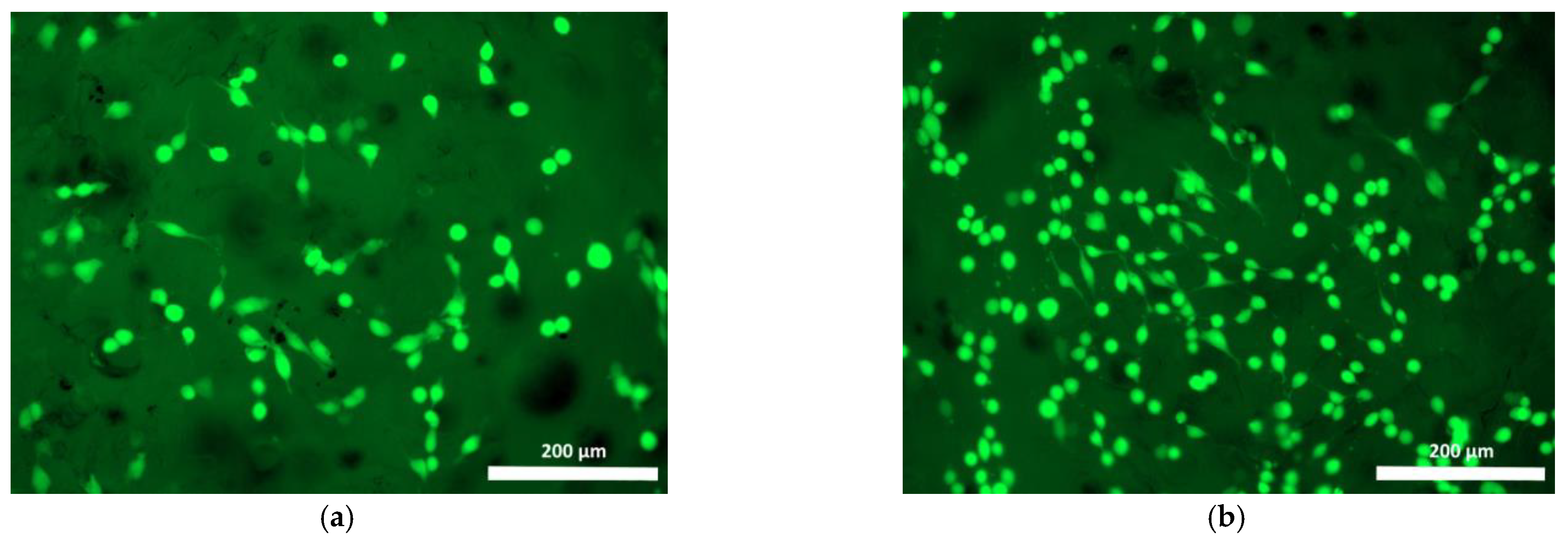

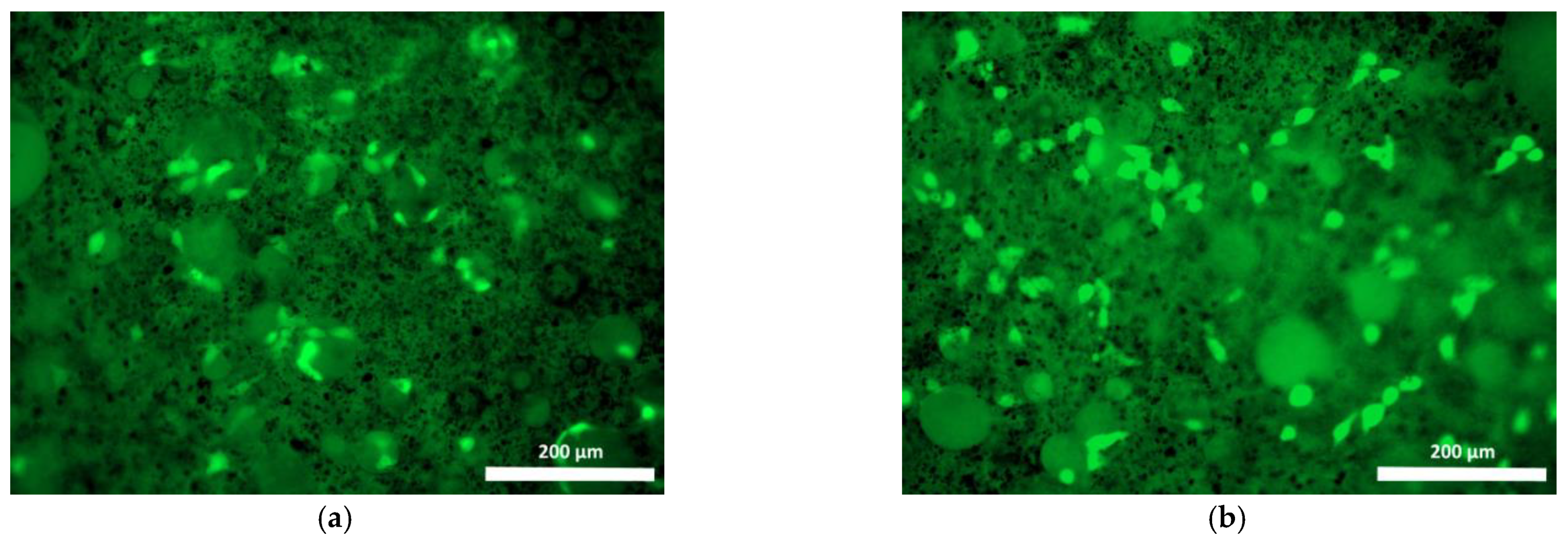
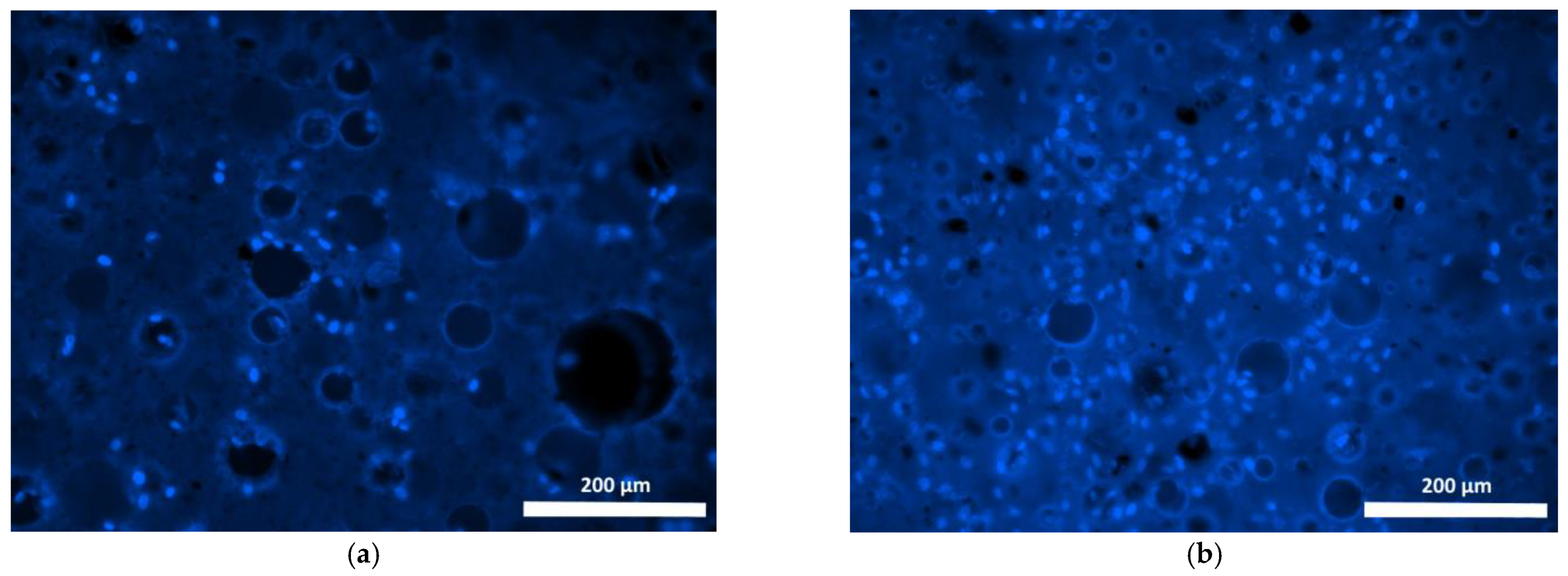
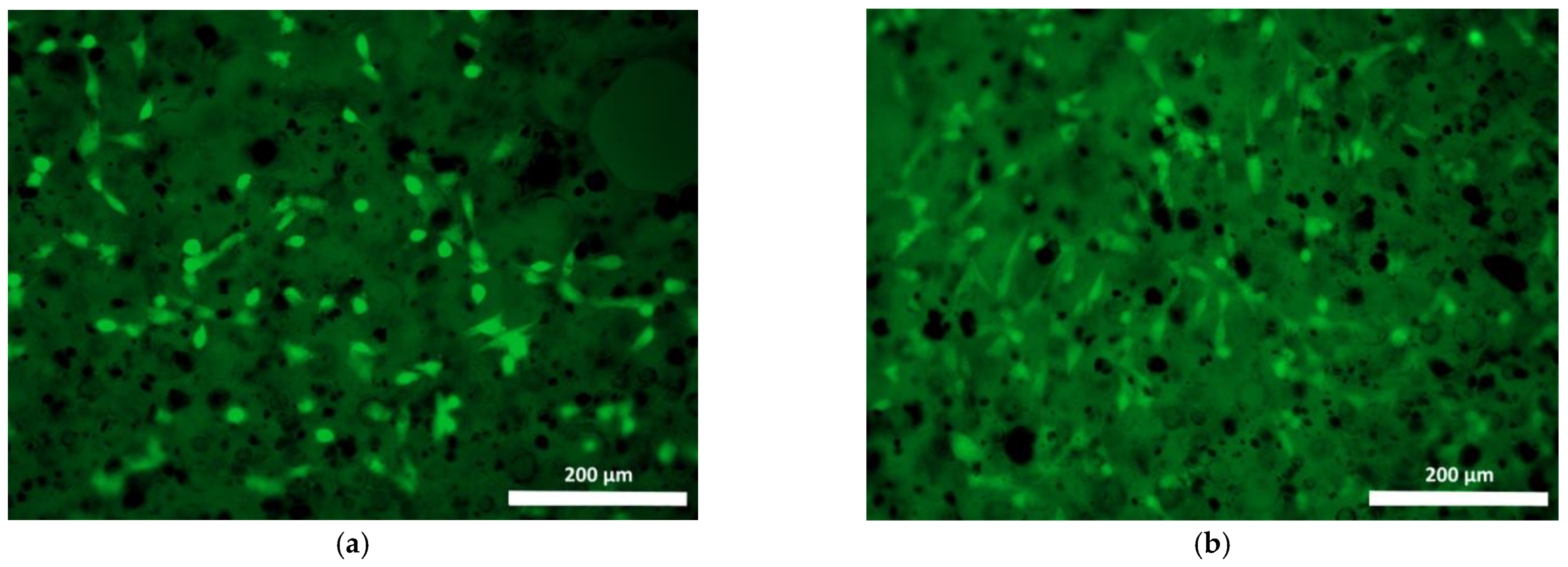
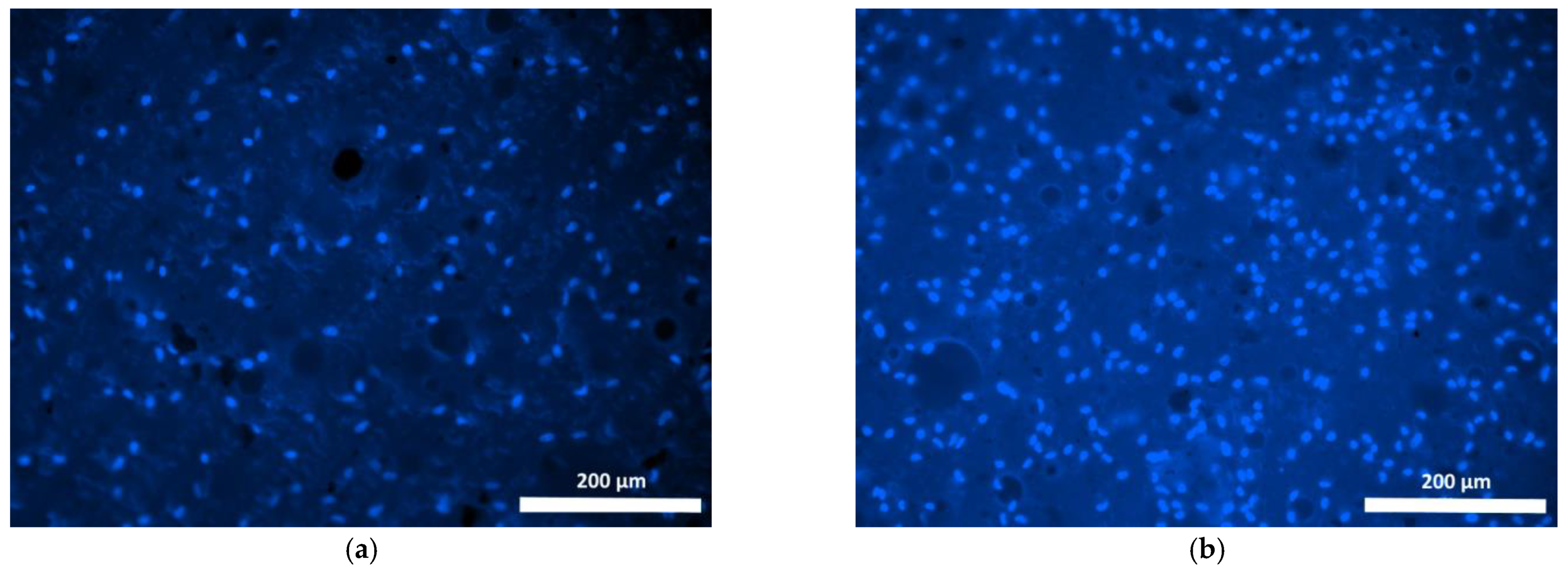
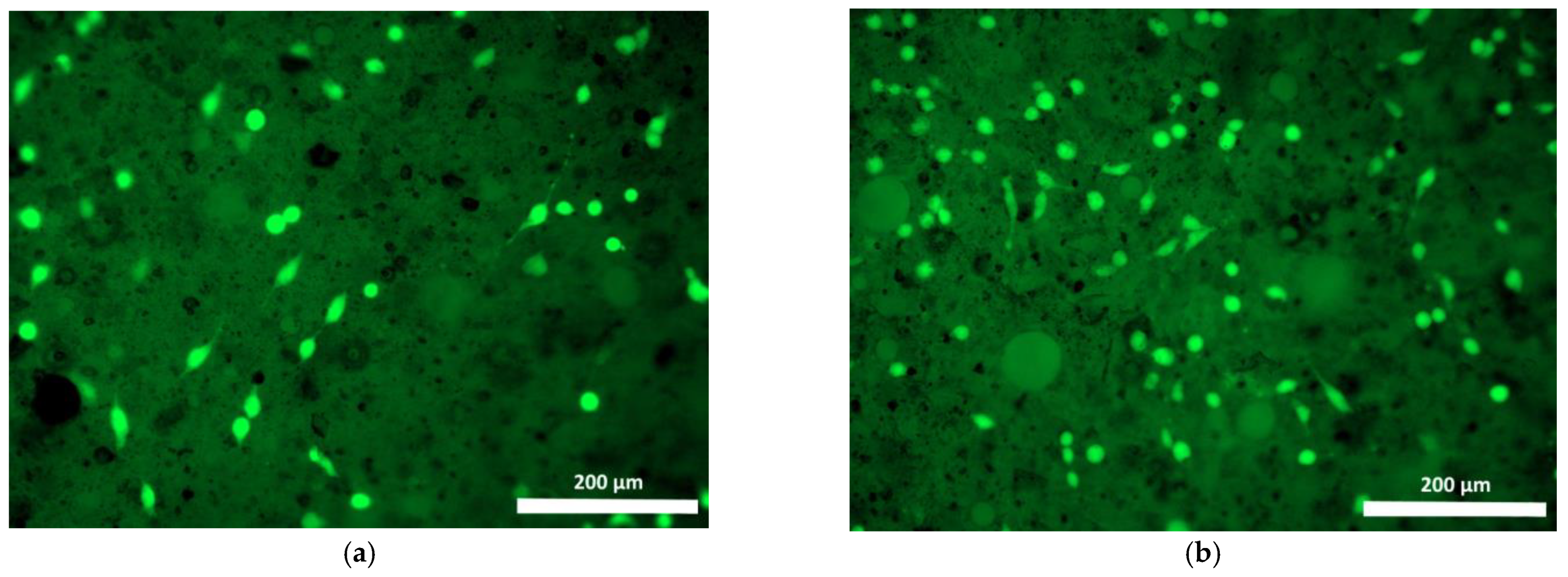
| Specimen | Specimen Composition |
|---|---|
| Composition 1 | Basic composition:
|
| Composition 2 | Basic composition + 15% bismuth oxide |
| Composition 3 | Basic composition + 15% tantalum pentoxide |
| Composition 4 | Basic composition + 15% zirconium oxide |
| Series | Parameter | Specimen Denomination | |||
|---|---|---|---|---|---|
| Composition 1 | Composition 2 | Composition 3 | Composition 4 | ||
| Control (n = 8) | OD | 0.451 ± 0.021 | 0.438 ± 0.018 | 0.382 ± 0.012 | 0.433 ± 0.007 |
| RGR, % | 100 | 100 | 100 | 100 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | |
| Extract (n = 8) | OD | 0.414 ± 0.016 | 0.294 ± 0.018 | 0.350 ± 0.022 | 0.403 ± 0.012 |
| RGR, % | 92 | 67 | 91 | 93 | |
| Cytotoxicity level | 1 | 2 | 1 | 1 | |
| Extract 1:1 (n = 8) | OD | 0.471 ± 0.015 | 0.274 ± 0.010 | 0.279 ± 0.012 | 0.393 ± 0.011 |
| RGR, % | 104 | 63 | 73 | 91 | |
| Cytotoxicity level | 0 | 2 | 2 | 1 | |
| Extract 1:2 (n = 8) | OD | 0.410 ± 0.02 | 0.298 ± 0.009 | 0.282 ± 0.009 | 0.403 ± 0.008 |
| RGR, % | 91 | 68 | 74 | 93 | |
| Cytotoxicity level | 1 | 2 | 2 | 1 | |
| Extract 1:4 (n = 8) | OD | 0.452 ± 0.008 | 0.255 ± 0.009 | 0.314 ± 0.012 | 0.374± 0.008 |
| RGR, % | 100 | 58 | 82 | 86 | |
| Cytotoxicity level | 0 | 2 | 1 | 1 | |
| Extract 1:8 (n = 8) | OD | 0.372 ± 0.017 | 0.308 ± 0.008 | 0.295 ± 0.011 | 0.392 ± 0.021 |
| RGR, % | 82 | 70 | 77 | 91 | |
| Cytotoxicity level | 1 | 2 | 1 | 1 | |
| Series | Parameter | Specimen Denomination | |||
|---|---|---|---|---|---|
| Composition 1 | Composition 2 | Composition 3 | Composition 4 | ||
| Control (n = 8) | OD | 0.329 ± 0.008 | 0.467 ± 0.021 | 0.330 ± 0.010 | 0.339 ± 0.010 |
| RGR, % | 100 | 100 | 100 | 100 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | |
| Extract (n = 8) | OD | 0.352 ± 0.008 | 0.330 ± 0.021 | 0.126 ± 0.004 | 0.302 ± 0.006 |
| RGR, % | 107 | 71 | 38 | 89 | |
| Cytotoxicity level | 0 | 2 | 3 | 1 | |
| Extract 1:1 (n = 8) | OD | 0.318 ± 0.020 | 0.525 ± 0.031 | 0.391 ± 0.011 | 0.404 ± 0.008 |
| RGR, % | 97 | 112 | 118 | 119 | |
| Cytotoxicity level | 1 | 0 | 0 | 0 | |
| Extract 1:2 (n = 8) | OD | 0.259 ± 0.006 | 0.491 ± 0.035 | 0.441 ± 0.019 | 0.447 ± 0.007 |
| RGR, % | 78 | 105 | 134 | 132 | |
| Cytotoxicity level | 1 | 0 | 0 | 0 | |
| Extract 1:4 (n = 8) | OD | 0.262 ± 0.007 | 0.302 ± 0.008 | 0.362 ± 0.015 | 0.475 ± 0.021 |
| RGR, % | 80 | 65 | 110 | 140 | |
| Cytotoxicity level | 1 | 2 | 0 | 0 | |
| Extract 1:8 (n = 8) | OD | 0.287 ± 0.022 | 0.292 ± 0.022 | 0.416± 0.011 | 0.379 ± 0.049 |
| RGR, % | 87 | 63 | 126 | 112 | |
| Cytotoxicity level | 1 | 2 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorikhina, M.N.; Bokov, A.E.; Charykova, I.N.; Rubtsova, Y.P.; Linkova, D.D.; Kobyakova, I.I.; Farafontova, E.A.; Kalinina, S.Y.; Kolmogorov, Y.N.; Aleynik, D.Y. Biological Characteristics of Polyurethane-Based Bone-Replacement Materials. Polymers 2023, 15, 831. https://doi.org/10.3390/polym15040831
Egorikhina MN, Bokov AE, Charykova IN, Rubtsova YP, Linkova DD, Kobyakova II, Farafontova EA, Kalinina SY, Kolmogorov YN, Aleynik DY. Biological Characteristics of Polyurethane-Based Bone-Replacement Materials. Polymers. 2023; 15(4):831. https://doi.org/10.3390/polym15040831
Chicago/Turabian StyleEgorikhina, Marfa N., Andrey E. Bokov, Irina N. Charykova, Yulia P. Rubtsova, Daria D. Linkova, Irina I. Kobyakova, Ekaterina A. Farafontova, Svetlana Ya. Kalinina, Yuri N. Kolmogorov, and Diana Ya. Aleynik. 2023. "Biological Characteristics of Polyurethane-Based Bone-Replacement Materials" Polymers 15, no. 4: 831. https://doi.org/10.3390/polym15040831
APA StyleEgorikhina, M. N., Bokov, A. E., Charykova, I. N., Rubtsova, Y. P., Linkova, D. D., Kobyakova, I. I., Farafontova, E. A., Kalinina, S. Y., Kolmogorov, Y. N., & Aleynik, D. Y. (2023). Biological Characteristics of Polyurethane-Based Bone-Replacement Materials. Polymers, 15(4), 831. https://doi.org/10.3390/polym15040831









