Polylactide—Meso-Substituted Arylporphyrin Composites: Structure, Properties and Antibacterial Activity
Abstract
1. Introduction
- Physically fixed porphyrins;
- Chemically bound porphyrins.
2. Materials and Methods
2.1. Sample Preparation
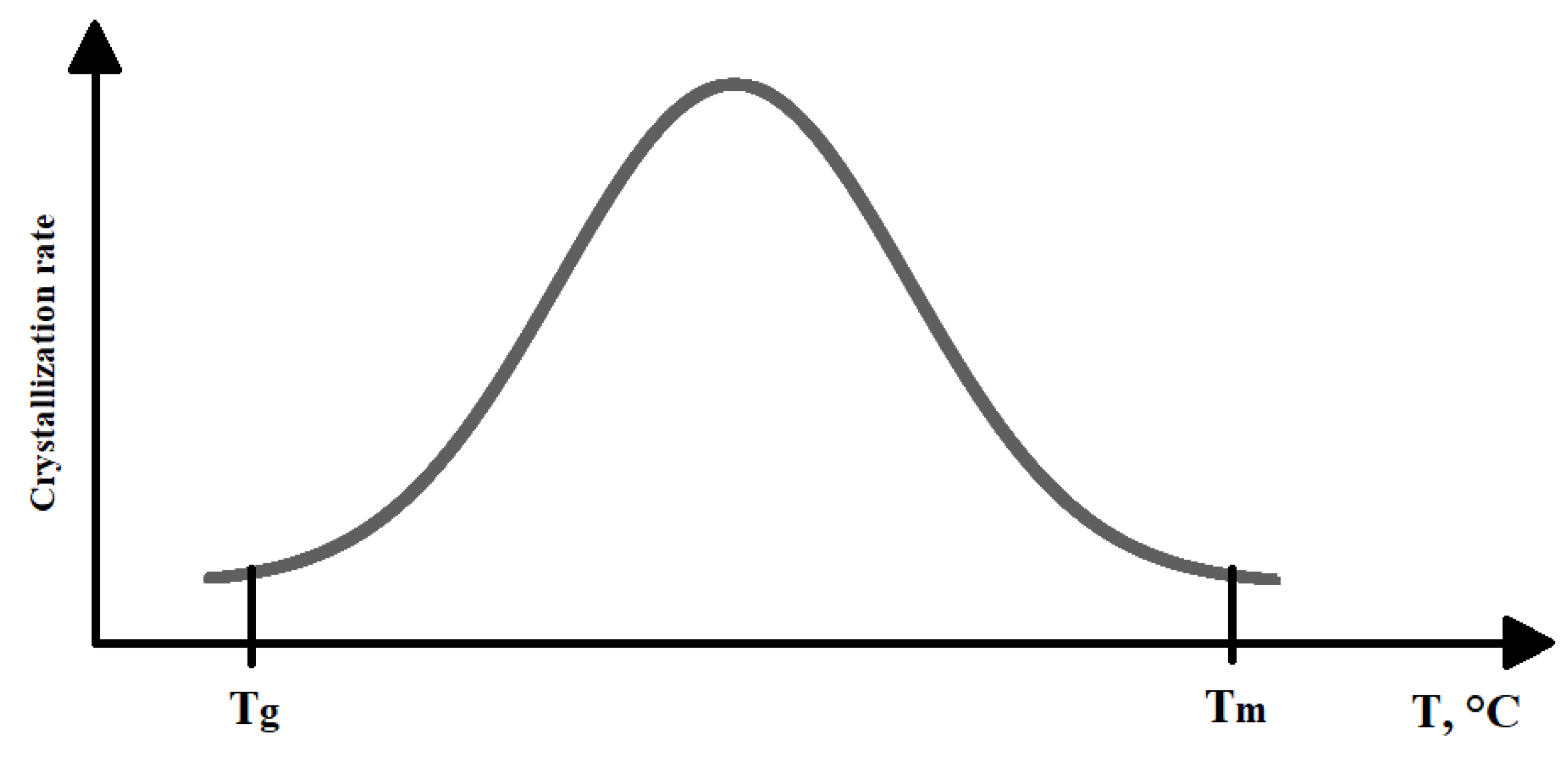
2.2. Analysis of Crystallization
2.3. Morphology of the Sample
2.4. X-ray Analysis
2.5. UV-vis Spectroscopyy
2.6. Ultrasonic Tests
2.7. Bacteriological Test
2.8. Statistical Processing
3. Results and Discussion
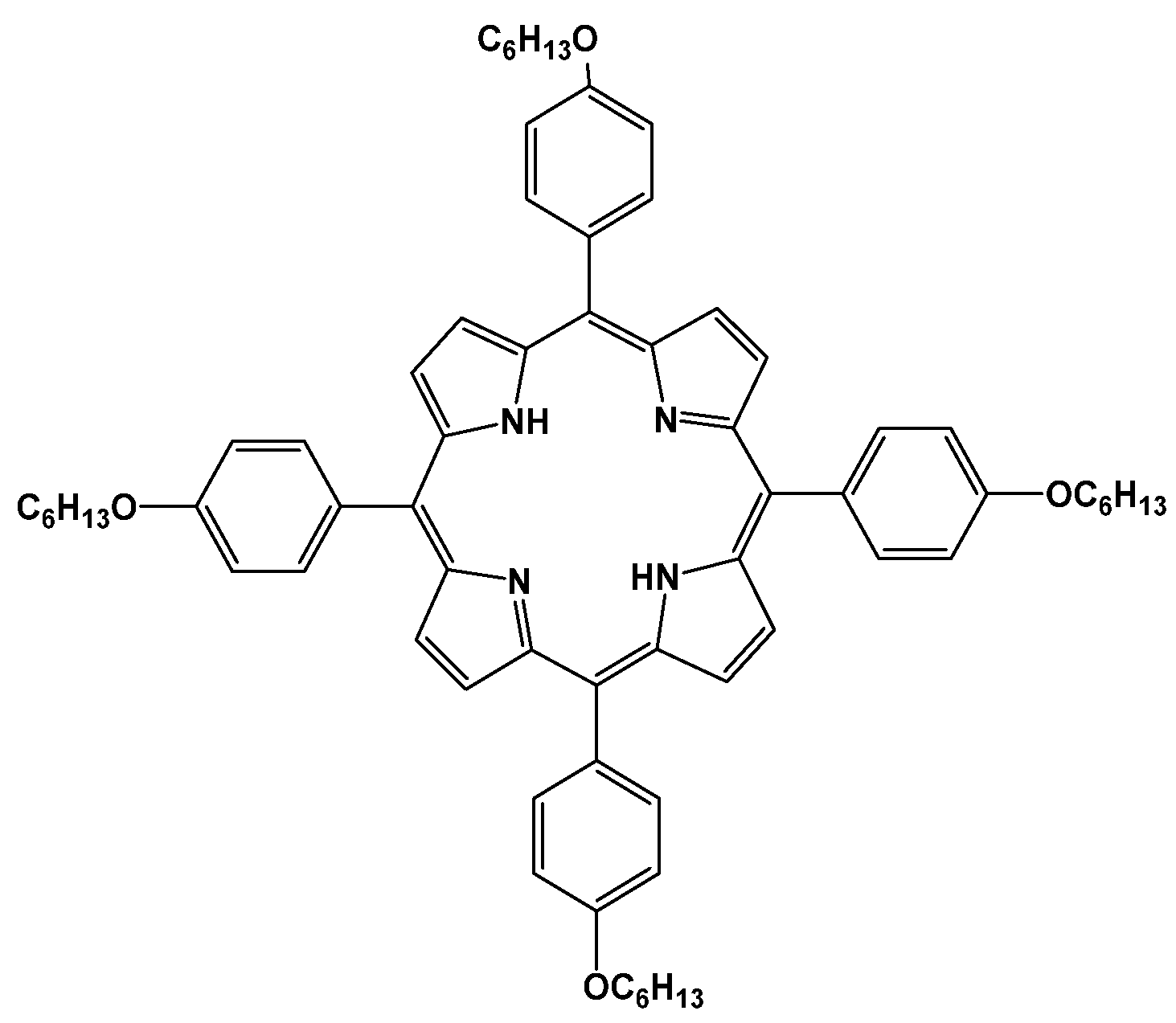
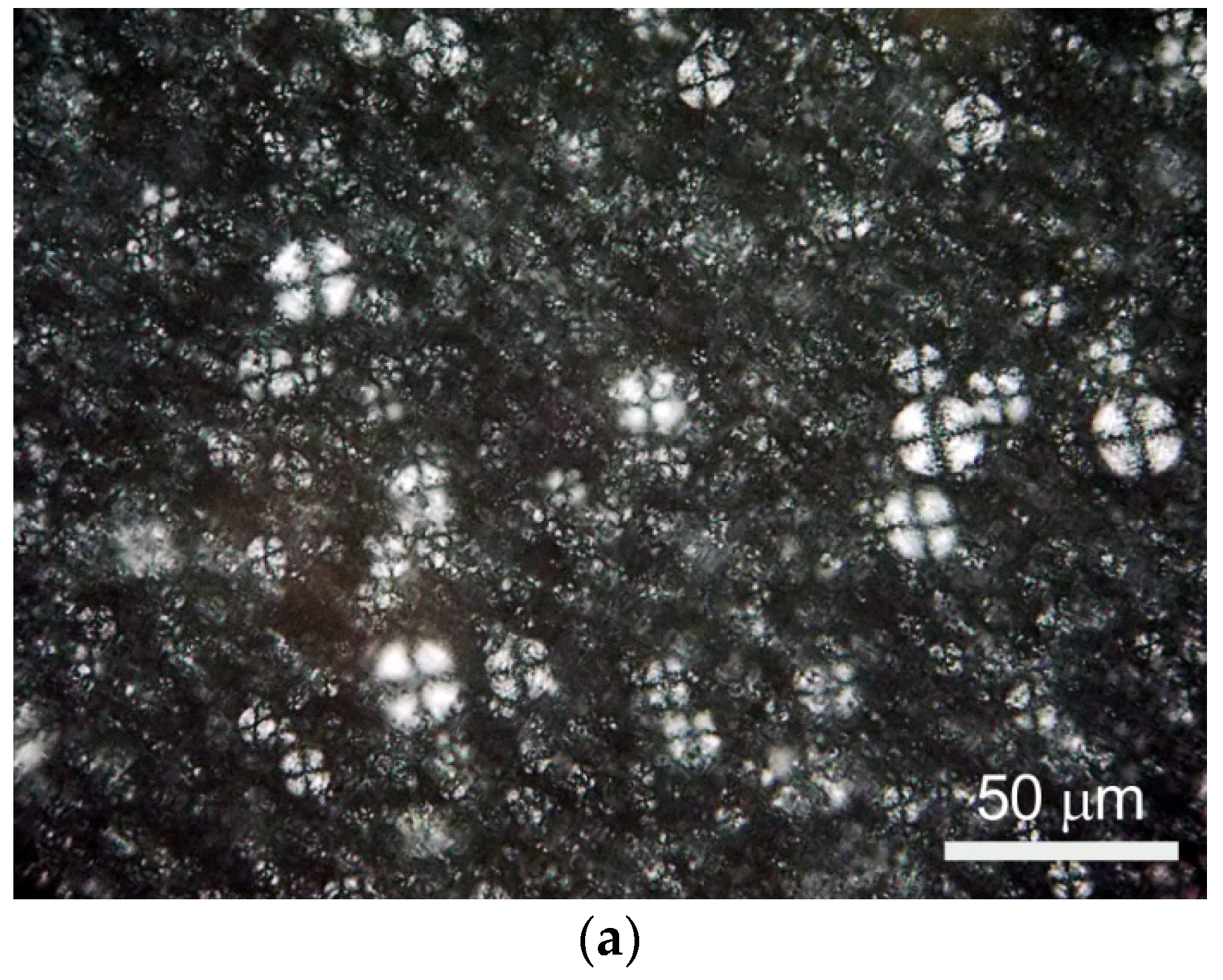
3.1. Morphology
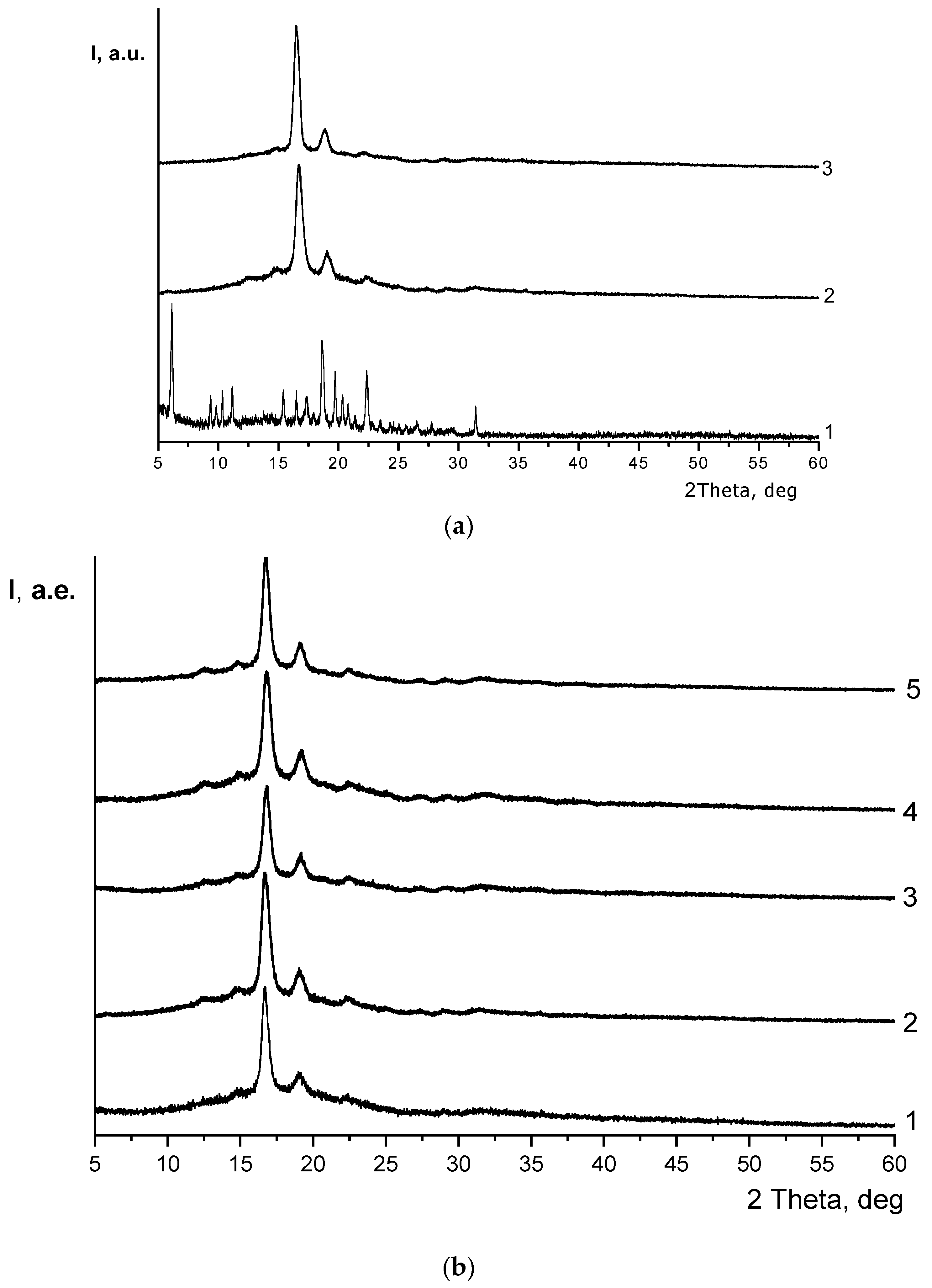
3.2. X-ray Diffraction
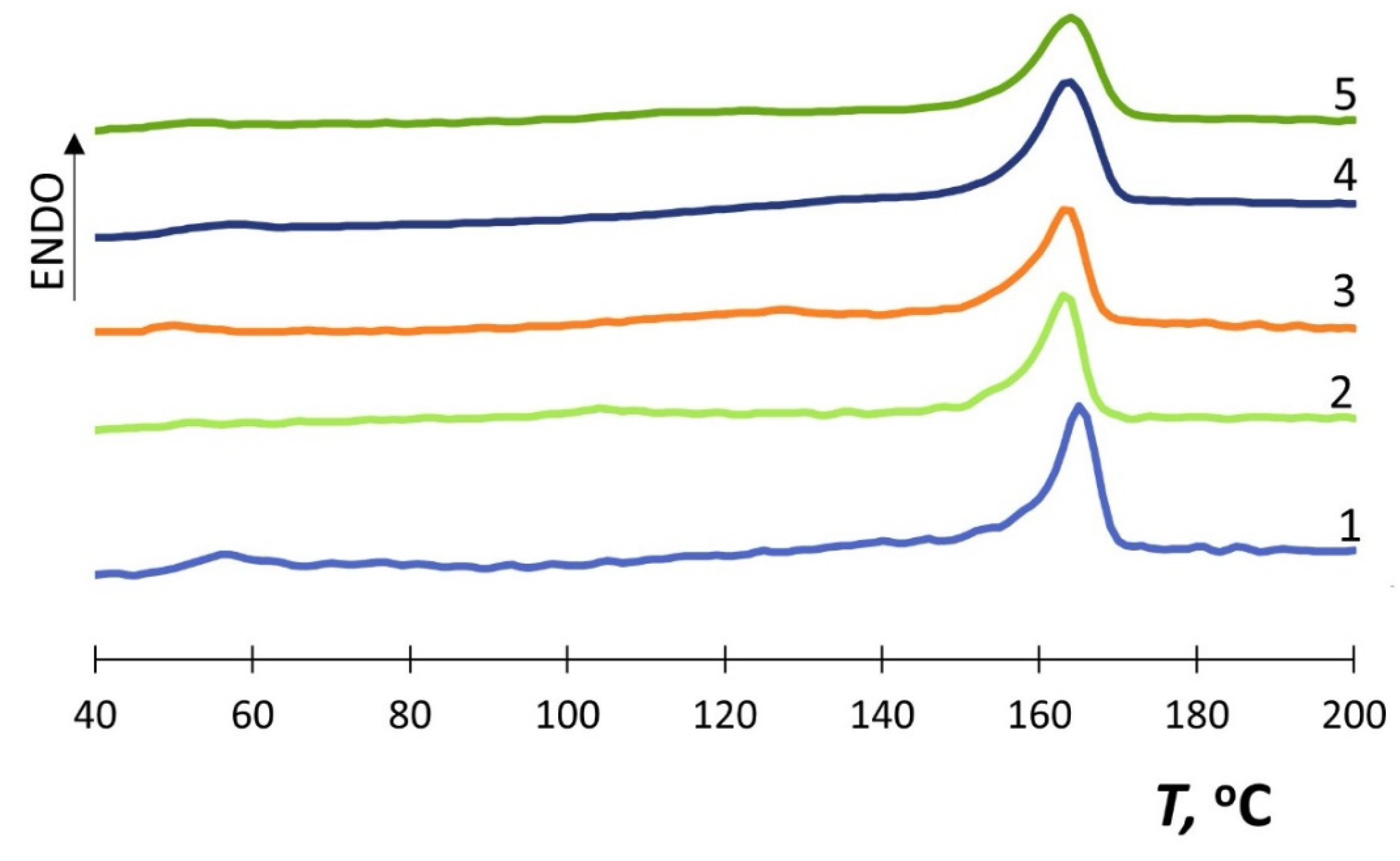
3.3. Differential Scanning Calorimetry (DSC)
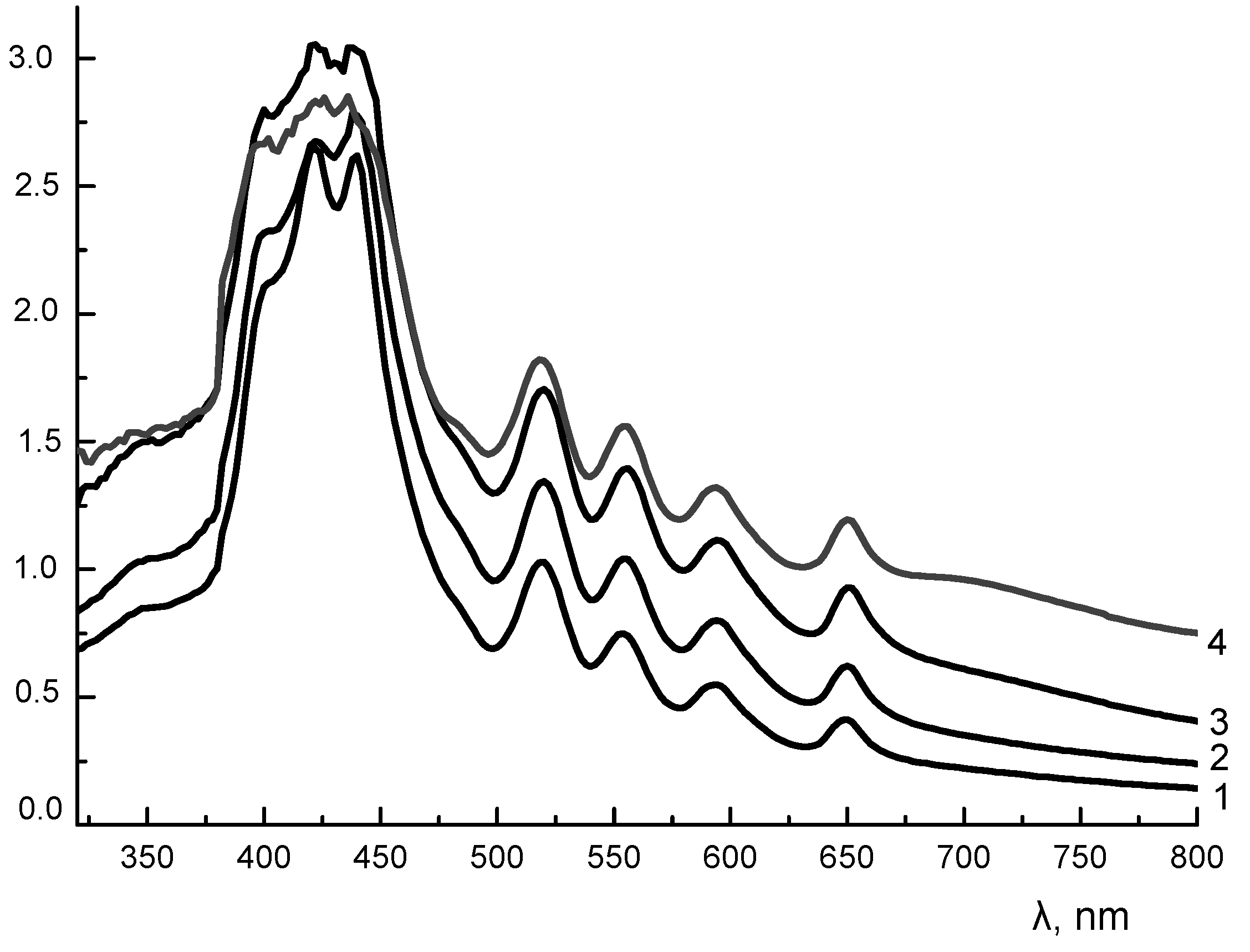
3.4. UV-vis Spectra
3.5. Ultrasonic Measurements
3.6. Biological Tests
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef]
- Han, R.; Kim, S.; Janda, K.J.; Fleischer, E.B. Structural study of fluoro-tetraphenylporphyrins relating to large variability in solubilities of the para-F-TPP, ortho-F-TPP and meta-F-TPP. J. Porphyr. Phthalocyanines 2018, 22, 355–358. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-based compounds and their applications in materials and medicine. Dyes Pigments 2021, 188, 109136. [Google Scholar] [CrossRef]
- Mironov, A.F.; Zhdanova, K.A.; Bragina, N.A. Nanosized vehicles for delivery of photosensitizers in photodynamic diagnosis and therapy of cancer. Russ. Chem. Rev. 2018, 87, 859–881. [Google Scholar] [CrossRef]
- Koifman, O.I.; Ageeva, T.A. Porphyrin Polymers: Synthesis, Properties, and Application. Polym. Sci. Ser. C 2004, 46, 49–72. [Google Scholar]
- Ageeva, T.A.; Titov, V.A.; Vershinina, I.A.; Gornukhina, O.V.; Shikova, T.G.; Golubchikov, O.A. Application of Porphyrins for Modification of Polymer Materials by Plasma Chemical Methods. J. Porphyr. Phthalocyan. 2004, 8, 588–598. [Google Scholar]
- Tertyshnaya, Y.; Lobanov, A.; Karpova, S.; Pantyukhov, P. Composites based on polylactide and manganese (III) tetraphenylporphyrin. Influence of concentration on the structure and properties. J. Mol. Liq. 2020, 302, 112176. [Google Scholar] [CrossRef]
- Karpova, S.G.; Ol’Khov, A.A.; Zhul’Kina, A.L.; Popov, A.A.; Iordanskii, A.L. Nonwoven Materials Based on Electrospun Ultrathin Fibers of Poly(3-hydroxybutyrate) and Complex Tin Chloride–Porphyrin. Polym. Sci. Ser. A 2021, 63, 369–381. [Google Scholar] [CrossRef]
- Ol’Khov, A.A.; Tyubaeva, P.M.; Zernova, Y.N.; Kurnosov, A.S.; Karpova, S.G.; Iordanskii, A.L. Structure and Properties of Biopolymeric Fibrous Materials Based on Polyhydroxybutyrate–Metalloporphyrin Complexes. Russ. J. Gen. Chem. 2021, 91, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Kuruppuarachchi, M.; Savoie, H.; Lowry, A.; Alonso, C.; Boyle, R.W. Polyacrylamide Nanoparticles as a Delivery System in Photodynamic Therapy. Mol. Pharm. 2011, 8, 920–931. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Co-ord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Dai, X.-H.; Jin, H.; Cai, M.-H.; Wang, H.; Zhou, Z.-P.; Pan, J.-M.; Wang, X.-H.; Yan, Y.-S.; Liu, D.-M.; Sun, L. Fabrication of thermosensitive, star-shaped poly(L-lactide)-block-poly(N-isopropylacrylamide) copolymers with porphyrin core for photodynamic therapy. React. Funct. Polym. 2015, 89, 9–17. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Lobanov, A.V.; Khvatov, A.V. Morphology and Antibacterial Properties of Composites Based on Polylactide and Manganese(III) Complex with Tetraphenylporphyrin. Russ. J. Phys. Chem. B 2020, 14, 1022–1027. [Google Scholar] [CrossRef]
- Moghnie, S.; Tovmasyan, A.; Craik, J.; Batinic-Haberle, I.; Benov, L. Cationic amphiphilic Zn-porphyrin with high antifungal photodynamic potency. Photochem. Photobiol. Sci. 2017, 16, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Basso, G.; Cargnelutti, J.F.; Oliveira, A.L.; Acunha, T.V.; Weiblen, R.; Flores, E.F.; Iglesias, B.A. Photodynamic inactivation of selected bovine viruses by isomeric cationic tetra-platinated porphyrins. J. Porphyr. Phthalocyanines 2019, 23, 1041–1046. [Google Scholar] [CrossRef]
- Dai, X.-H.; Wang, Z.-M.; Gao, L.-Y.; Pan, J.-M.; Wang, X.-H.; Yan, Y.-S.; Liu, D.-M. Star-shaped poly(l-lactide)-b-poly(ethylene glycol) with porphyrin core: Synthesis, self-assembly, drug-release behavior and singlet oxygen research. N. J. Chem. 2014, 38, 3569–3578. [Google Scholar] [CrossRef]
- Chen, R.-J.; Chen, P.-C.; Prasannan, A.; Vinayagam, J.; Huang, C.-C.; Chou, P.-Y.; Weng, C.-C.; Tsai, H.C.; Lin, S.-Y. Formation of gold decorated porphyrin nanoparticles and evaluation of their photothermal and photodynamic activity. Mater. Sci. Eng. C 2016, 63, 678–685. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Khvatov, A.V.; Lobanov, A.V. Morphological features of composites prepared from polylactide and iron(III)−tetraphenylporphyrin complex. Russ. J. Phys. Chem. B 2017, 11, 828–832. [Google Scholar] [CrossRef]
- Pechnikova, N.L.; Alopina, E.V.; Kuznetsov, O.Y.; Ageeva, T.A.; Koifman, O.I. Synthesis and Study of Antibacterial Activity of Bromine Derivatives of Porhyrin Polymers and Their Zinc Complexes. ChemChemTech 2017, 60, 52–59. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. In Biomedical Science, Engineering and Technology; Ghista, D.N., Ed.; IntechOpen: London, UK, 2012; pp. 247–282. [Google Scholar]
- Auras, R.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 345–381. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Karpova, S.G.; Podzorova, M.V.; Khvatov, A.V.; Moskovskiy, M.N. Thermal Properties and Dynamic Characteristics of Electrospun Polylactide/Natural Rubber Fibers during Disintegration in Soil. Polymers 2022, 14, 1058. [Google Scholar] [CrossRef] [PubMed]
- Kurtycz, P.; Karwowska, E.; Ciach, T.; Olszyna, A.; Kunicki, A. Biodegradable polylactide (PLA) fiber mats containing Al2O3-Ag nanopowder prepared by electrospinning technique—Antibacterial properties. Fibers Polym. 2013, 14, 1248–1254. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Tertyshnaya, Y.V.; Zakharov, M.S.; Zhdanova, K.A.; Bragina, N.A. The Spectral Characteristics and Morphology of a Composite Material Based on Polylactide and Alkoxy-Substituted meso-Arylporphyrins. Polym. Sci. Ser. B 2021, 63, 905–914. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Morokov, E.S.; Demina, V.A.; Sedush, N.G.; Kalinin, K.T.; Khramtsova, E.A.; Dmitryakov, P.V.; Bakirov, A.V.; Grigoriev, T.E.; Levin, V.M.; Chvalun, S.N.; et al. Noninvasive high-frequency acoustic microscopy for 3D visualization of microstructure and estimation of elastic properties during hydrolytic degradation of lactide and ε-caprolactone polymers. Acta Biomater. 2020, 109, 61–72. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Brinke, G.T.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(L-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Kalb, B.; Pennings, A. General crystallization behaviour of poly(l-lactic acid). Polymer 1980, 21, 607–612. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Iura, K.; Ono, Y.; Dan, Y.; Takahashi, K. Crystallization behavior of poly(l-lactic acid). Polymer 2006, 47, 7554–7563. [Google Scholar] [CrossRef]
- Ohtani, Y.; Okumura, K.; Kawaguchi, A. Crystallization Behavior of Amorphous Poly(l-Lactide). J. Macromol. Sci. Part B 2003, 42, 875–888. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jimenez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Briassoulis, D.; Athanasoulia, I.-G.; Tserotas, P. PHB/PLA plasticized by olive oil and carvacrol solvent-cast films with optimised ductility and physical ageing stability. Polym. Degrad. Stab. 2022, 200, 109958. [Google Scholar] [CrossRef]
- Karpova, S.G.; Chumakova, N.A.; Lobanov, A.V.; Olkhov, A.A.; Vetcher, A.A.; Iordanskii, A.L. Evaluation and Characterization of Ultrathin Poly(3-hydroxybutyrate) Fibers Loaded with Tetraphenylporphyrin and Its Complexes with Fe(III) and Sn(IV). Polymers 2022, 14, 610. [Google Scholar] [CrossRef] [PubMed]
- Berezin, B.D.; Enikolopyan, N.S. Porfiriny: Struktura, Svoistva, Sintez [Porphyrins: Structure, Properties, Synthesis]; Enikolopyan, N.S., Ed.; Nauka: Moscow, Russia, 1985. (In Russian) [Google Scholar]
- Williamson, C.E.; Corwin, A.H. Dyes as biologic probes, color and dye microenvironments. J. Colloid Interface Sci. 1972, 38, 567–576. [Google Scholar] [CrossRef]
- Shulevich, Y.V.; Zakharova, J.A.; Motyakin, M.V.; Dukhanina, E.G.; Ionova, I.S.; Navrotskii, A.V.; Novakov, I.A. Surfactant Micelles as a Possible Template for Radical Polymerization: Evaluation Using ESR Spectroscopy. Russ. Chem. Bull. 2022, 71, 593–1603. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Wei, Y.; Duan, M.; Chen, X.; Hu, Y. Transformation of H-Aggregates and J-Dimers of Water-Soluble Tetrakis (4-carboxyphenyl) Porphyrin in Polyion Complex Micelles. Polymers 2018, 10, 494. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, R.; Li, J.; Li, Y.; An, Y.; Shi, L. J- and H-Aggregates of 5,10,15,20-Tetrakis-(4-sulfonatophenyl)-porphyrin and Interconversion in PEG-b-P4VP Micelles. Biomacromolecules 2008, 9, 2601–2608. [Google Scholar] [CrossRef]
- Karayianni, M.; Koufi, D.; Pispas, S. Development of Double Hydrophilic Block Copolymer/ Porphyrin Polyion Complex Micelles towards Photofunctional Nanoparticles. Polymers 2022, 14, 5186. [Google Scholar] [CrossRef]
- Kazakevich, G.S.; Rudskoy, A.I. Continuum Mechanics; SPb GPU: Saint Petersburg, Russia, 2003. [Google Scholar]
- Dutikova, Y.V.; Ol’shevskaya, V.A.; Shtil, A.A.; Kaluzhny, D.N. Beyond photodynamic therapy: Porphyrins and their derivatives as antitumor DNA-ligands. Rus. Biotherap. J. 2011, 10, 47–53, ISSN 1726-9792. [Google Scholar]
- Mu, W.-Y.; Chen, C.-H.; Chen, Q.-Y. Bacterium-Sculpted Porphyrin–Protein–Iron Sulfide Clusters for Distinction and Inhibition of Staphylococcus aureus. Langmuir 2022, 38, 10385–10391. [Google Scholar] [CrossRef]
- Wesseling, C.M.J.; Martin, N.I. Synergy by Perturbing the Gram-Negative Outer Membrane: Opening the Door for Gram-Positive Specific Antibiotics. ACS Infect. Dis. 2022, 8, 1731–1757. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.J.; Sheffield, J.B.; Gh, M.S.; Wu, Y.; Spahr, C.; Gonella, G.; Xu, B.; Dai, H.-L. Gram’s Stain Does Not Cross the Bacterial Cytoplasmic Membrane. ACS Chem. Biol. 2015, 10, 1711–1717. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-H.; Wang, Z.-M.; Liu, W.; Dong, C.-M.; Pan, J.-M.; Yuan, S.-S.; Yan, Y.-S.; Liu, D.-M.; Sun, L. Biomimetic star-shaped porphyrin-cored poly(l-lactide)-b-glycopolymer block copolymers for targeted photodynamic therapy. Colloid Polym. Sci. 2014, 292, 2111–2122. [Google Scholar] [CrossRef]



| ТРР4ОС6, wt.% | Thermophysical Characteristics | |||
|---|---|---|---|---|
| Tg, °C (Δ ± 0.5 °C) | Tm, °C (Δ ± 0.5 °C) | Tcc, °C (Δ ± 0.5%) | χc, % (Δ ± 1%) | |
| 0 | 57.5 | 165 | - | 37 |
| 0.2 | (53) | 163 | - | 33 |
| 0.3 | (53) | 163 | - | 33 |
| 0.4 | (53) | 163 | - | 34 |
| 0.5 | (52) | 164 | - | 34 |
| TPP4OC6, wt.% | CL, km/s | CT, km/s | ρ, g/cm3 | G, GPa | K, GPa | E, GPa | μ |
|---|---|---|---|---|---|---|---|
| 0 | 2.28 | 1.15 | 1.26 | 1.7 | 4.3 | 4.4 | 0.33 |
| 0.2 | 2.28 | 1.13 | 1.24 | 1.6 | 4.3 | 4.2 | 0.34 |
| 0.3 | 2.28 | 1.12 | 1.24 | 1.6 | 4.4 | 4.2 | 0.34 |
| 0.4 | 2.28 | 1.12 | 1.24 | 1.6 | 4.4 | 4.2 | 0.34 |
| 0.5 | 2.28 | 1.12 | 1.23 | 1.5 | 4.3 | 4.1 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tertyshnaya, Y.V.; Lobanov, A.V.; Morokov, E.S.; Buzanov, G.A.; Abushakhmanova, Z.R. Polylactide—Meso-Substituted Arylporphyrin Composites: Structure, Properties and Antibacterial Activity. Polymers 2023, 15, 1027. https://doi.org/10.3390/polym15041027
Tertyshnaya YV, Lobanov AV, Morokov ES, Buzanov GA, Abushakhmanova ZR. Polylactide—Meso-Substituted Arylporphyrin Composites: Structure, Properties and Antibacterial Activity. Polymers. 2023; 15(4):1027. https://doi.org/10.3390/polym15041027
Chicago/Turabian StyleTertyshnaya, Yulia V., Anton V. Lobanov, Egor S. Morokov, Grigorii A. Buzanov, and Zubarzhat R. Abushakhmanova. 2023. "Polylactide—Meso-Substituted Arylporphyrin Composites: Structure, Properties and Antibacterial Activity" Polymers 15, no. 4: 1027. https://doi.org/10.3390/polym15041027
APA StyleTertyshnaya, Y. V., Lobanov, A. V., Morokov, E. S., Buzanov, G. A., & Abushakhmanova, Z. R. (2023). Polylactide—Meso-Substituted Arylporphyrin Composites: Structure, Properties and Antibacterial Activity. Polymers, 15(4), 1027. https://doi.org/10.3390/polym15041027








