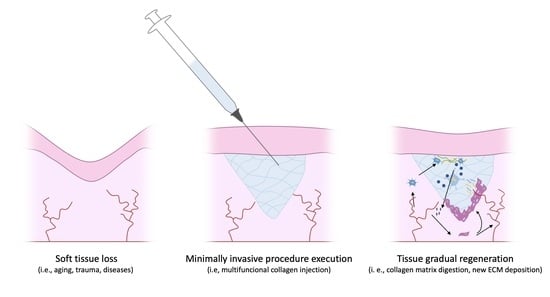
An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications
Abstract
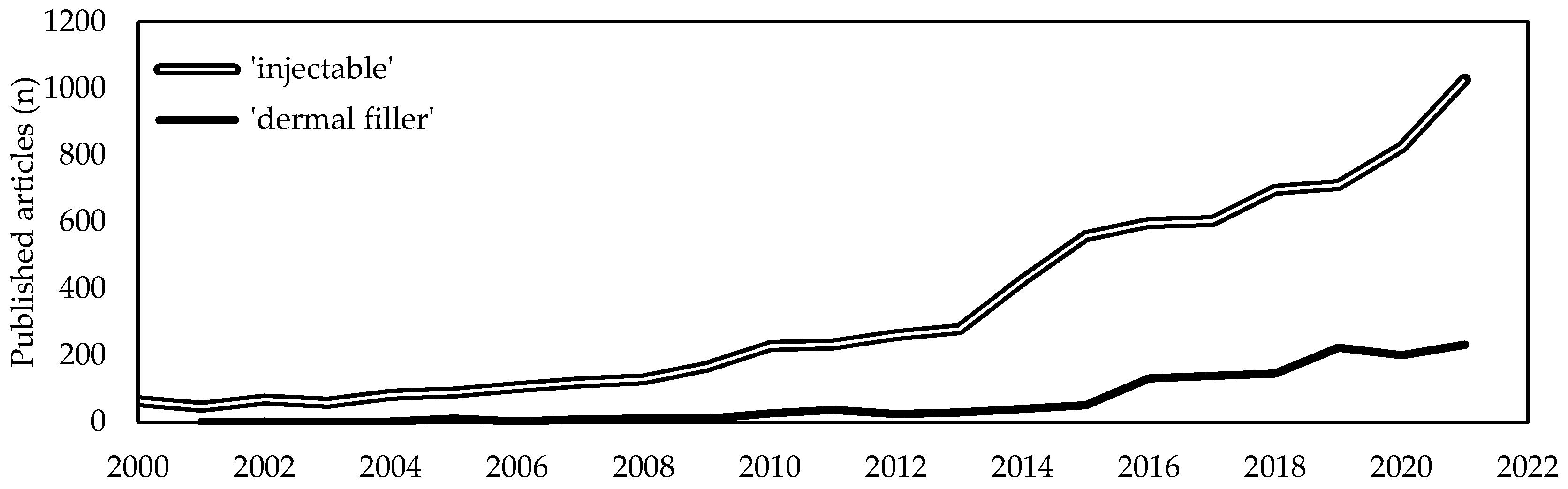
1. Introduction
2. Methodology
3. Collagen as Biomaterial
4. Historical Overview on Collagen-Based Injectable Formulations
5. Collagen-Based Injectable Formulations
| Source | Manufacturer | Product | Additives | Applications | Ref. |
|---|---|---|---|---|---|
| Equine | Euroresearch S.r.l. (Milan, Italy) www.euroresearch.it, accessed on 14 February 2023 | Nithya | – | Integumental | [163] |
| Linerase | – | Integumental | [164,165,166,167,179] | ||
| Nearmedic Italy S.r.l. (Como, Italy) www.salvecoll.com, accessed on 14 February 2023 | Salvecoll-E | – | Integumental | [60] | |
| Bioteck Spa (Arcugnano, Italy) www.bioteck.com, accessed on 14 February 2023 | Biocollagen gel | Type III collagen, bone spongy powder | Musculoskeletal | – | |
| Biocollagen crunch | Type III collagen, bone powder, bone spongy chips | Musculoskeletal | – | ||
| ActivaBone CLX gel | Bone powder, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone Injectable Paste | Demineralized bone matrix, bone powder, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone modulable paste | Demineralized bone matrix, bone powder, bone cortical and spongy granules, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone Crunch | Demineralized bone matrix, bone powder, cortical and spongy chips, exur, Vitamin C | Musculoskeletal | – | ||
| Bovine | Bioteck Spa (Arcugnano, Italy) www.bioteck.com, accessed on 14 February 2023 | CHondroGrid | – | Musculoskeletal | [112] |
| Integra LifeScience Corp. (Princeton, NJ, USA) www.integralife.com, accessed on 14 February 2023 | Integra Flowable Wound Matrix | Glycosaminoglycans | Integumental | [88] | |
| Helitene | – | Soft tissues | [129] | ||
| Rofil Medical International (Breda, The Netherlands) | Resoplast | Lidocaine hydrochloride | Integumental | – | |
| Suneva Medical (San Diego, CA, USA) www.sunevamedical.com, accessed on 14 February 2023 | ArteFill | Polymethylmethacrylate, lidocaine | Integumental | [75,77,78,79,80,81,82,83,84,85] | |
| Datascope Corp., (Montvale, NJ, USA) | VasoSeal | – | Cardiovascular | [128] | |
| BioMimetic Therapeutics, LLC (Franklin, TN, USA) www.biomimetics.com, accessed on 14 February 2023 | Augment | β-tricalcium phosphate, recombinant human platelet-derived growth factor-BB | Musculoskeletal | [97,99,100,101,102,103,104,105,106,107,108,109,110,111] | |
| KOKEN Co., Ltd. (Bunkyo-ku, Tokyo, Japan) www.kokenmpc.co.jp, accessed on 14 February 2023 | Atelocell | Type III collagen | Integumental, gastrointestinal | [86,87,113,114], NCT01060943 | |
| B. Braun (Crissier, Switzerland) www.bbraun.com, accessed on 14 February 2023 | Gelofusine | – | Cardiovascular | [126,127] | |
| Allergan, Inc. (Dublin, Ireland) www.abbvie.it, accessed on 14 February 2023 | Zyplast | Glutaraldehyde | Integumental | [6,76,83,89,90,91,92,95,96,98,116,117,119,180] | |
| Zyderm | – | Integumental | [6,83,89,90,93,94,118,120,180] | ||
| Contigen | glutaraldehyde | Gastrointestinal and genitourinary | [115,121,122,123,124,125] | ||
| Swine | GUNA (Milan, Italy) www.guna.com, accessed on 14 February 2023 | Dental Skin BioRegulation | Vitamin C, magnesium gluconate, pyridozine chlorhydrate, riboflavin, thiamine chlorhydrate | Skin | [181] |
| Dental ATM BioRegulation | Hypericum | Musculoskeletal | [130] | ||
| MD-HIP | Calcium phosphate | Musculoskeletal | [131] | ||
| MD-ISCHIAL | Rhododendron | Musculoskeletal | [132] | ||
| MD-KNEE | Arnica | Musculoskeletal | [133,143,144] | ||
| MD-LUMBAR | Hamemelis | Musculoskeletal | [132,134,135] | ||
| MD-NECK | Silicio | Musculoskeletal | – | ||
| MD-SHOULDERS | Iris | Musculoskeletal | [145,146] | ||
| MD-SMALL JOINTS | Viola | Musculoskeletal | – | ||
| MD-THORACIC | Cimifuga | Musculoskeletal | – | ||
| MD-MATRIX | Citric acid, nicotinamide | Soft tissues | [135,136,160] | ||
| MD-MUSCLE | Hypericum | Musculoskeletal | [130,132,133,134,135,136,137,146,160] | ||
| MD-POLY | Drosera | Musculoskeletal | – | ||
| MD-NEURAL | Citrullus | Musculoskeletal | [132,134,160] | ||
| MD-TISSUE | Ascorbic acid, magnesium gluconate, pyridoxine chlorhydrate, riboflavin, thiamine chlorhydrate | Soft tissues | – | ||
| Joint Biomaterials S.r.l. (Mestre, Italy) www.joint-biomateriali.it, accessed on 14 February 2023 | CartiRegen | Fibrin glue | Musculoskeletal | – | |
| Ubiosis (Gyeonggi-do, Republic of Korea) www.ubiosis.com, accessed on 14 February 2023 | COLTRIX CartiRegen | – | Musculoskeletal | – | |
| COLTRIX TendoRegen | – | Musculoskeletal | – | ||
| Sewon Cellontech Co., Ltd. (Seoul, Republic of Korea) www.swcell.com, accessed on 14 February 2023 | CartiFill | Glucose, CaCl, amino acids, vitamin B, fibrin glue | Musculoskeletal | [138,139], NCT02539030, NCT02519881 | |
| CartiZol | Glucose, CaCl, amino acids, vitamin B | Musculoskeletal | [140], NCT02539095 | ||
| RegenSeal | – | Musculoskeletal | [141] | ||
| TheraFill | – | Integumental | [86,87] | ||
| Sunmax Biotechnology Co., Ltd. (Tainan, Taiwan) www.sunmaxbiotech.com, accessed on 14 February 2023 | Facial Gain | Lidocaine | Integumental | NCT03844529 | |
| Collagen Implant I | – | Integumental | – | ||
| Evolence (Skillman, NJ, USA) | Dermicol-P35 | Ribose | Integumental | [2,147,148,149], NCT00929071, NCT00891774 | |
| Mentor Corp. (Santa Barbara, CA, USA) | Fibrel | – | Integumental | [150,151] | |
| Tissue Science Labs. (Aldershot, UK) | Permacol | – | Gastrointestinal | [153,154,155,156,157,158,159] | |
| EternoGen, LLC (Columbia, MO, USA) | RPC Pure Collagen | Ethylenediamine tetraacetic acid | Integumental | [67] | |
| Aspid S.A. de C.V. (Mexico City, Mexico) www.aspidpharma.com, accessed on 14 February 2023 | Fibroquel | Polyvinylpyrrolidone | Musculoskeletal | [161,162], NCT04517162 | |
| ColBar LifeScience Ltd. (Tel Aviv, Israel) www.ortho-dermatologics.com, accessed on 14 February 2023 | Evolence | Ribose | Integumental | [147,152] | |
| Human | Fascia Biosystem (Beverly Hills, CA, USA) | Fascian | Lidocain | Integumental | [6,168,171] |
| Fibrocell Science (Exton, PA, USA) www.fibrocell.com, accessed on 14 February 2023 | Isolagen therapy | – | Integumental | NCT00655356 | |
| Inamed Corporation (Santa Barbara, CA, USA) www.inamed-cro.com, accessed on 14 February 2023 | Cosmoplast | Glutaraldehyde, lidocaine hydrochloride | Integumental | [6,169] | |
| Cosmoderm | lidocaine hydrochloride | Integumental | [6,169] | ||
| Life Cell Corp. (Branchburg, NJ, USA) | Dermalogen | Type and VI collagen, elastin, fibronectin, chondroitin sulfate, and other proteoglycans | Integumental | [170] | |
| Cymetra | Elascin, glycosaminoglycans, Lidocaine hydrochloride | Integumental | [6,118,172,173,174,175] | ||
| Collagenesis, Inc., (Beverly, MA, USA) | Autologen | Elastin, fibronectin, glycosaminoglycans | Integumental | – | |
| Dermologen | - | Integumental | [173] | ||
| Plant | Vesco Pharmaceutical Co. Ltd. (Bangkok, Thailand) www.vescopharma.com, accessed on 14 February 2023 | Collagen C 1000 | Vitamin C | Integumental | – |
| Silkworm | Monodermà (Milan, Italy) www.monoderma.com | Fillagen | Hyaluronic acid, carboxymethylcellulose | Integumental | [178] |
| n. d. | Taumed (Rome, Italy) www.taumed.it, accessed on 14 February 2023 | Karisma | Hyaluronic acid, carboxymethylcellulose | Integumental | – |
| n. d. | LABO International S.r.l. (Padova, Italy) www.labosuisse.com, accessed on 14 February 2023 | Fillerina con 3D collagen | Hyaluronic acid | Integumental | – |
| n. d. | Hebey Mepha Pharm Group Co., Ltd. (Shandong, Hebei, China) www.mephacn.com, accessed on 14 February 2023 | Collagen Plus | – | Integumental | – |
| n. d. | Pierre Mulot Laboratories (Paris, France) | Neutroskin | Vitamin C | Integumental | – |
| n. d. | Elements Pharmaceuticals (Shijiazhuang, Hebei, China) www.elementspharma.com, accessed on 14 February 2023 | Ele-collagen | Vitamin C, Vitamin B6 | Integumental | – |
| n. d. | Globus Medical (Audubon, PA, USA) www.globusmedical.com, accessed on 14 February 2023 | Kinex Bioactive gel | Bioglass, hyaluronic acid | Musculoskeletal | – |
6. Clinical Efficacy of Collagen-Based Injectable Implants
6.1. Integumental Apparatus
| Collagen Source | Equine | Swine | Bovine | Human | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Product Name | Nithya | Dermicol-P35 | Permacol | Therafill | RPC Pure-Collagen | Sunmax FacialGain | Zyplast | Artecoll | Koken | CosmoDerm | CosmoPlast | Isolagen | ||||||||||||||||||
| Injection specification | Number (n) | 1 | 2 | 2 | 1 | 1–2 | 1–2 | 2 | 2 | 1 | 1 | 1 | 1 | n. d. | 1 | 1 | 2 | 1 | 1 | 3 | 1–3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1–4 | 3 |
| Volume (mL) | 2–5 | 2.2 | 2 | n. d. | n. d. | 0.6 | 1 | 1 | 1 | n. d. | n. d. | 1.5–3.0 | 1 | 2 | 2 | 2.56 | n. d. | 1.5–3.0 | 2 | 1.6 | 1 | n. d. | 1.8 | 2 | 1 | n. d. | n. d. | 1 | n. d. | |
| Amount (mg) | 80 | 77 | 70 | n. d. | n. d. | 21–42 | 70 | 70 | 35 | n. d. | n. d. | 52–105 | 30 | 60 | 60 | 89.6 | n. d. | 53–105 | 70–210 | 56–168 | 35 | n. d. | 54 | 70 | 35 | n. d. | n. d. | n. d. | n. d. | |
| Interval (inj./weeks) | 1 | 0.5/w | 0.5/w | n. d. | 0.5/w | 0.25/w | 0.04/w | 0.25/w | n. d. | n. d. | n. d. | n. d. | 0.5/w | n. d. | n. d. | 1/w | n. d. | n. d. | 0.8/w | 0.5/w | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | 0.3/w | 0.6/w | |
| Observation time (weeks) | 24 | 48 | 48 | 24 | 32 | n. d. | 8 | 1 | 1 | 240 | 48 | 12 | 24 | 48 | 12 | 52 | 48 | 24 | 24 | 12 | 136 | 24 | 48 | 6 | 24 | 6 | 100 | 29 | ||
| Participants (n) | 72 | 148 | 24 | 20 | 172 | 12 | 1 | 1 | 10 | 30 | 1 | 19 | 73 | 57 | 57 | 30 | n. d. | 18 | 439 | 138 | 118 | 153 | 57 | 57 | 3 | n. d. | 1 | 117 | 110 | |
| Adverse events | Severe | 0 | 0 | 149 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | n. d. | 0 | 0 | 1 | n. d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n. d. | 0 | 0 | 0 |
| Non severe | n. d. | 5 | 0 | 16 | 154 | 0 | 0 | 0 | 0 | 38 | 0 | 18 | n. d. | 12 | 1 | 0 | n. d. | 1 | n. d. | 124 | 109 | n. d. | 18 | 5 | 1 | n. d. | 1 | 42 | 32 | |
| Allergic reaction | n. d. | 1 | 2 | |||||||||||||||||||||||||||
| Pain | 9 | 1 | 58 | 58 | 4 | |||||||||||||||||||||||||
| Infection | 12 | 9 | 1 | 8 | ||||||||||||||||||||||||||
| Papule/erythema | v | 80 | 17 | 14 | 7 | 24 | 391 | 75 | 8 | 1 | 1 | 1 | 4/31 | 14 | ||||||||||||||||
| Nodule/lumpiness | 1 | 16 | 369 | 15 | 52 | 1 | 7 | |||||||||||||||||||||||
| Bruise | v | 27 | 1 | 1 | 241 | 66 | 51 | 20 | 1 | 5 | ||||||||||||||||||||
| Edema/swelling | 42 | 22 | 2 | 1 | 24 | 378 | 101 | 61 | 6 | 3 | 1 | 5 | ||||||||||||||||||
| Hemorrhage | 3 | 10 | ||||||||||||||||||||||||||||
| Itching | 24 | ## | 24 | 1 | 1 | 9 | ||||||||||||||||||||||||
| Induration/tenderness | 3 | 36 | 1 | 391 | 88 | 14 | 2 | 1 | ||||||||||||||||||||||
| Discoloration | 1 | 12 | 1 | 149 | 31 | 2 | ||||||||||||||||||||||||
| References | [163] | [149] | [208] | [148] | NCT00891774 | [209] | [207] | [210] | NCT00929071 | [2], NCT00911872 | [147] | [95] | NCT01060943 | [86] | [87] | [67] | NCT03844529 | [95] | [96] | [76] | NCT00876265 | [77] | [86] | [87] | [217] | NCT01212809 | [217] | [205,206], NCT00444210, NCT00444353 | NCT00655356 | |
6.2. Musculoskeletal Apparatus
6.3. Urogenital System
6.4. Gastrointestinal Apparatus
6.5. Others
7. Adverse Reactions to Collagen-Based Injectable Implants
| Application | Product | Disease | Injection Specification | Observation Time (Weeks) | Participants (n) | Adverse Events | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Number (n) | Volume (mL) | Inj./Time (w) | Severe | Mild | ||||||
| Musculoskeletal apparatus | Augment | Non fused foot and ankle | 1 | 3–6 | 1 | 36 | 14 | 0 | 36 | [108] |
| 1 | 6–9 | 1 | 12 | 7 | 0 | 0 | [110] | |||
| 1 | 1–9 | 1 | 52 | 26 | 0 | 0 | [109] NCT00583375 | |||
| 1 | n. d. | 1 | 52 | 132 | 75 | 27 | [101], NCT01305356 | |||
| Arthritis | 1 | 1–9 | 1 | 52 | 394 | 136 | n. d. | [102], NCT00583375 | ||
| Cartifill | Knee cartilage | 1 | 1 | 1 | 96 | 52 | 0 | 5 | [139], NCT02539030 | |
| Cartilage lesion | 1 | 3 | 1 | 6 | 1 | 0 | 0 | [138] | ||
| CartiZol | Osteoarthritis | 1 | 3 | 1 | 24 | 101 | 0 | 7 | [140] | |
| Chondromalacia, osteoarthritis | 1 | n. d. | 1 | n. d. | n. d. | n. d. | n. d. | NCT02539095 | ||
| ChondroGrid | Osteoarthritis | 3 | 2 | 0.5/w (2 w), 0.25/w (1 w) | 24 | 70 | 0 | 3 | [142] | |
| 3 | 6 | 32 | 20 | 0 | 0 | [112] | ||||
| Fibroquel | Osteoarthritis | 3 | 1.5 | 1/w | 24 | n. d. | n. d. | n. d. | NCT04019782 | |
| 5 | 2 | 1/w | 24 | 10 | 0 | n. d. | [182] | |||
| Linerase | Gingival recession | 3 | 14 | 1.5/w | n. d. | 18 | 0 | 0 | [167] | |
| MD-Hip | Osteoarthritis | 1 | 2 | 1 | 96 | 24 | 0 | 1 | [131] | |
| MD-Knee, MD-Muscle | Osteoarthritis | 10 | n. d. | 2/w (2 w), 1/w (6 we) | 12 | 30 | 0 | 0 | [133] | |
| MD-Lumbar, MD-Muscle, MD-Neural | Lumbar spine pain | 5 | 20 | 2/w (2 w), 1/w (1 w) | 6 | 73 | 0 | 0 | [134] | |
| MD-Knee, MD-Matrix | Sprained knee | 6 | n. d. | 2/w | 3 | 10 | 0 | 0 | [143] | |
| MD-Muscle or MD-Matrix | Piriformis syndrome | 1–3 | n. d. | n. d. | n. d. | 28 | 0 | 0 | [136] | |
| MD-Lumbar, MD-Ischial | Chronic pain due to arthrosis, myalgia | 1 | 1 | 1 | 10 | 71 | 0 | 0 | [132] | |
| MD-Lumbar, MD-Matrix | Back pain | 10 | n. d. | 2/w (2 w), 1/w (6 w) | 8 | 1 | 0 | 0 | [135] | |
| MD-Lumbar, MD-Muscle, MD-Matrix | Lumbar joint block | 9 | n. d. | 2/w (2 w), 1/w (5 w) | 7 | 1 | 0 | 0 | [135] | |
| MD-Muscle, MD-Neural | Muscle pain | 1 | 1 | 1 | 10 | 53 | 0 | 0 | [132] | |
| MD Shoulder | Calcific supraspinatus tendinitis | 4 | n. d. | 1/w | 6 | 10 | 0 | 0 | [145] | |
| MD-Shoulder, MD-Muscle | Shoulders periarthritis | 10 | n. d. | 2/w (2 w), 1/w (6 w) | 8 | 22 | 0 | 0 | [146] | |
| MD Muscle | Myofascial pain | 2 | 2 | 1/w | 2 | 18 | 0 | 9 | [130], NCT03323567 | |
| RegenSeal | Plantar fasciitis | n. d. | n. d. | n. d. | n | n. d. | n. d. | n. d. | NCT02539082 | |
| Rotator cuff tears | 1 | 1 | 1 | 48 | 62 | 0 | 0 | [141] | ||
| n. d. | Rotator cuff tears | 4 | 8 | 1/w | 72 | 1 | 0 | 0 | [183] | |
| n. d. | Osteoarthritis | 1 | 2 | 1 | 24 | n. d. | n. d. | n. d. | NCT04998188 | |
| Gastro-intestinal apparatus | Atelocell | Vocal folds paralysis | 1 | 0.5–1.3 | 1 | 12 | 155 | 0 | 0 | [114] |
| 1 | 0.5–1.3 | 1 | 12 | 40 | 0 | 0 | [113] | |||
| Cymetra | Vocal folds paralysis | 1 | 1 | 1 | 4 | 8 | 0 | 0 | [203] | |
| 1 | n. d. | 1 | 2 | 1 | 1 | 0 | [173] | |||
| Dermologen | Laryngoplasy | 2 | n. d. | 0.7/w | 4 | 1 | 1 | 0 | [173] | |
| Permacol | Fecal incontinence | 1 | 1.5 | 1 | 6 | 28 | 0 | 8 | [204], NCT01528995 | |
| n. d. | n. d. | n. d. | 48 | 14 | 0 | 0 | [155] | |||
| Anal fistula | 1 | n. d. | 1 | 36 | 11 | 0 | 0 | [157] | ||
| 1 | n. d. | 1 | 48 | 28 | 0 | 7 | [156] | |||
| 1 | n. d. | 1 | 12 | 1 | 0 | 0 | [153] | |||
| Rectovaginal fistula | 1 | n. d. | 1 | 8 | 1 | 0 | 0 | [154] | ||
| Salvecoll-E | Anorectal fistula | 1 | 2 | 1 | 48 | 70 | 0 | 0 | [60] | |
| Zyderm | Laryngeal paralysis | 1 | 0.2–0.5 | 1 | 24 | 7 | 0 | 1 | [118] | |
| Zyplast | Laryngoplasy | 1 | n. d. | 1 | 24 | 100 | 0 | 0 | [116] | |
| urinary system | Contigen | Sphincter incontinence | 1–5 | 2–4 | 0.05/w | 84 | 63 | 0 | 0 | [188] |
| Urethra hypermobility | 1–4 | 14 | 1 | 172 | 58 | 0 | 0 | [187] | ||
| Neurogenic bladder dysfunction | 1–4 | n. d. | n. d. | 192 | 20 | 0 | 0 | [190] | ||
| Linerase | Lichen sclerosus | 6 | 27 | 2/week (2 w), 1/8 w | 8 | 1 | 0 | 1 | [165] | |
| n. d. | Retrograde ejaculation | 2 | 6 | 1/year | 96 | 1 | 0 | 0 | [198] | |
| n. d. | Bilateral vesicoureteral reflux | 1 | 2.5 | 1 | 144 | 1 | 1 | 0 | [223] | |
| n. d. | Stress urinary incontinence | 1–2 | n. d. | 0.25/w | 36 | 40 | 0 | 1 | [185] | |
| n. d. | Premature ovarian failure | 1 | n. d. | 1 | 12 | 8 | 0 | 0 | [212], NCT02644447 | |
| n. d. | Erectile disfunction | n. d. | n. d. | n. d. | n. d. | n. d. | n. d | n. d. | NCT02745808 | |
| Circulatory system | Gelofusine | Blood volume | 1 | n. d. | 1 | n. d. | n. d. | n. d. | n. d. | NCT02808325 |
| Fluid retention | 1 | 500 | 1 | n. d. | n. d. | n. d. | n. d. | NCT04637308 | ||
| Severe sepsis | 1 | 500 | 1 | 13 | 608 | n. d. | n. d. | [127], NCT02715466 | ||
| Abdominal surgery | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. | NCT01515397 | ||
| Blood volume | 1 | 10 mg/k | 1 | n. d. | 5 | 0 | 0 | [230], NCT02631356 | ||
| Blood volume | 1 | 1 L | 1 | 4 h | 12 | 0 | 0 | [214], NCT00868062 | ||
| others | Fibroquel | COVID-19 due hyperinflammatory syndrome | 10 | 15 | 14/w (3 days), 12/w (4 days) | 12 | 45 | 0 | 33 | [162], NCT04517162 |
| 7 | 10.5 | 7/w | 1 | 35 | 0 | 0 | [161], NCT04517162 | |||
| Flowable Wound Matrix | Hand scar due to severe burns | 1 | 3–6 | 1 | 24 | 8 | 0 | 0 | [88] | |
| Helitene | Organ protection during ablation | 1 | 10–30 | 1 | 12 | 3 | 0 | 0 | [128] | |
| Linerase | Vitiligo | 6 | 27 | 0.5/w | 12 | 5 | 0 | 0 | [164] | |
| MD Neural, MD Matrix, MD Muscle mix | Facial nerve palsy | 16 | 13 | 2/w | 8 | 21 | 0 | 8 | [160], NCT04353908 | |
| n. d. | Decompensated cirrhosis | n. d. | n. d. | n. d. | n | n. d. | n. d. | n. d. | NCT02786017 | |
| n. d. | Brain injury | 1 | n. d. | 1 | n. d. | n. d. | n. d. | n. d. | NCT02767817 | |
| n. d. | Ischemic cardiomyopathy | 1 | n. d. | 1 | 48 | 50 | 1, heart failure | 0 | [226], NCT02635464 | |
8. Regulation
9. Concluding Remarks
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Go, B.C.; Frost, A.S.; Friedman, O. Using Injectable Fillers for Midface Rejuvenation. Plast. Aesthetic Res. 2021, 8, 39. [Google Scholar] [CrossRef]
- Solish, N.J. Assessment of Recovery Time for the Collagen Products Dermicol-P35 27G and 30G. J. Am. Acad. Dermatol. 2010, 62, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Øvrebø, Ø.; Perale, G.; Wojciechowski, J.P.; Echalier, C.; Jeffers, J.R.T.; Stevens, M.M.; Haugen, H.J.; Rossi, F. Design and Clinical Application of Injectable Hydrogels for Musculoskeletal Therapy. Bioeng. Transl. Med. 2022, 7, e10295. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable Biomaterials for Regenerating Complex Craniofacial Tissues. Adv. Mater. 2009, 21, 3368–3393. [Google Scholar] [CrossRef] [PubMed]
- Béduer, A.; Genta, M.; Kunz, N.; Verheyen, C.; Martins, M.; Brefie-Guth, J.; Braschler, T. Design of an Elastic Porous Injectable Biomaterial for Tissue Regeneration and Volume Retention. Acta Biomater. 2022, 142, 73–84. [Google Scholar] [CrossRef]
- Eppley, B.L.; Dadvand, B. Injectable Soft-Tissue Fillers: Clinical Overview. Plast. Reconstr. Surg. 2006, 118, 98–106. [Google Scholar] [CrossRef]
- Buck, D.W.; Alam, M.; Kim, J.Y.S. Injectable Fillers for Facial Rejuvenation: A Review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 11–18. [Google Scholar] [CrossRef]
- Requena, L.; Requena, C.; Christensen, L.; Zimmermann, U.S.; Kutzner, H.; Cerroni, L. Adverse Reactions to Injectable Soft Tissue Fillers. J. Am. Acad. Dermatol. 2011, 64, 1–34. [Google Scholar] [CrossRef]
- Luebberding, S.; Alexiades-Armenakas, M. Safety of Dermal Fillers. J. Drugs Dermatol. 2012, 11, 1053–1058. [Google Scholar]
- Cheng, L.; Sun, X.; Tang, M.; Jin, R.; Cui, W.; Zhang, Y.-G. An Update Review on Recent Skin Fillers. Plast. Aesthetic Res. 2016, 3, 92–99. [Google Scholar] [CrossRef]
- Ginat, D.T.; Schatz, C.J. Imaging Features of Midface Injectable Fillers and Associated Complications. Am. J. Neuroradiol. 2013, 34, 1488–1495. [Google Scholar] [CrossRef]
- Lemperle, G. Injectable Dermal Fillers—Resorbable or Permanent? In Aesthetic Surgery of the Facial Mosaic; Springer: Berlin/Heidelberg, Germany, 2007; pp. 650–664. [Google Scholar]
- Oranges, C.M.; Brucato, D.; Schaefer, D.J.; Kalbermatten, D.F.; Harder, Y. Complications of Nonpermanent Facial Fillers: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, C.; Wei, Z.; Bai, Y.; Bhaduri, S.B.; Webster, T.J.; Bian, L.; Yang, L. Injectable Biomaterials for Translational Medicine. Mater. Today 2019, 28, 81–97. [Google Scholar] [CrossRef]
- Attenello, N.H.; Maas, C.S. Injectable Fillers: Review of Material and Properties. Facial Plast. Surg. 2015, 31, 29–34. [Google Scholar] [CrossRef]
- Cockerham, K.; Hsu, V. Collagen-Based Dermal Fillers: Past, Present, Future. Facial Plast. Surg. 2009, 25, 106–113. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Avila Rodriguez, M.I.; Rodriguez Barroso, G.L.; Sanchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef]
- Sandri, M.; Tampieri, A.; Salvatore, L.; Sannino, A.; Ghiron, J.H.L.; Condorelli, G. Collagen Based Scaffold for Biomedical Applications. J. Biotechnol. 2010, 150, 29. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Kim, G.K. Collagen. In High Yield Orthopaedics; Elsevier: Amsterdam, The Netherlands, 2010; pp. 107–109. [Google Scholar]
- Goldberga, I.; Li, R.; Duer, M.J. Collagen Structure–Function Relationships from Solid-State NMR Spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Owczarzy, A.; Kurasinski, R.; Kulig, K.; Rogoz, W.; Szkudlarek, A.; Maciazek-Juczyk, M. Collagen—Stucture, Properties and Applications. Eng. Biomater. 2020, 156, 17–23. [Google Scholar]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef]
- Shoulder, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E.; Brückner, P. Collagens, Suprastructures, and Collagen Fibril Assembly. In The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–115. [Google Scholar]
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An Insight on Type I Collagen from Horse Tendon for the Manufacture of Implantable Devices. Int. J. Biol. Macromol. 2020, 154, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Ignat’eva, N.Y.; Danilov, N.A.; Averkiev, S.V.; Obrezkova, M.V.; Lunin, V.V.; Sobol, E.N. Determination of Hydroxyproline in Tissues and the Evaluation of the Collagen Content of the Tissues. J. Anal. Chem. 2007, 62, 51–57. [Google Scholar] [CrossRef]
- Bou-Gharios, G.; Abraham, D.; de Crombrugghe, B. Type I Collagen Structure, Synthesis, and Regulation. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 295–337. [Google Scholar]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen Fibril Formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.J.; Andriotis, O.G.; Nedelkovski, V.; Frank, M.; Katsamenis, O.L.; Thurner, P.J. Bone Micro- and Nanomechanics. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 22–44. [Google Scholar]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; de Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Campa, L.; Natali, M.L.; Salvatore, L.; Madaghiele, M.; de Caro, L.; et al. Investigations of Processing–Induced Structural Changes in Horse Type-I Collagen at Sub and Supramolecular Levels. Front. Bioeng. Biotechnol. 2019, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K. Structure, Biosynthesis and Gene Regulation of Collagens in Cartilage and Bone. In Dynamics of Bone and Cartilage Metabolism; Elsevier: Amsterdam, The Netherlands, 2006; pp. 3–40. [Google Scholar]
- Exposito, J.-Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of Collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef]
- Chu, M.-L.; de Wet, W.; Bernard, M.; Ding, J.-F.; Morabito, M.; Myers, J.; Williams, C.; Ramirez, F. Human Proα1(I) Collagen Gene Structure Reveals Evolutionary Conservation of a Pattern of Introns and Exons. Nature 1984, 310, 337–340. [Google Scholar] [CrossRef]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The Triple Helix of Collagens—An Ancient Protein Structure That Enabled Animal Multicellularity and Tissue Evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.-Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The Fibrillar Collagen Family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef]
- Madaghiele, M.; Salvatore, L.; Sannino, A. Tailoring the Pore Structure of Foam Scaffolds for Nerve Regeneration; Woodhead Publishing Limited: Sawston, UK, 2014; ISBN 9780857096968. [Google Scholar]
- Salvatore, L.; Madaghiele, M.; Parisi, C.; Gatti, F.; Sannino, A. Crosslinking of Micropatterned Collagen-Based Nerve Guides to Modulate the Expected Half-Life. J. Biomed. Mater. Res. A 2014, 102, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Calò, E.; Salvatore, L.; Bonfrate, V.; Pedone, D.; Frigione, M.; Sannino, A. Assessment of Collagen Crosslinking and Denaturation for the Design of Regenerative Scaffolds. J. Biomed. Mater. Res. A 2016, 104, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Calò, E.; Bonfrate, V.; Pedone, D.; Gallo, N.; Natali, M.L.; Sannino, A.; Madaghiele, M. Exploring the Effects of the Crosslink Density on the Physicochemical Properties of Collagen-Based Scaffolds. Polym. Test. 2021, 93, 106966. [Google Scholar] [CrossRef]
- Parisi, C.; Salvatore, L.; Veschini, L.; Serra, M.P.; Hobbs, C.; Madaghiele, M.; Sannino, A.; di Silvio, L. Biomimetic Gradient Scaffold of Collagen–Hydroxyapatite for Osteochondral Regeneration. J. Tissue Eng. 2020, 11, 2041731419896068. [Google Scholar] [CrossRef]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of Processing on Structural, Mechanical and Biological Properties of Collagen-Based Substrates for Regenerative Medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Curci, C.; Picerno, A.; Gallone, A.; Vulpi, M.; Vitarelli, A.; Ditonno, P.; Cascione, M.; Sallustio, F.; et al. Analysis of the Physico-Chemical, Mechanical and Biological Properties of Crosslinked Type-I Collagen from Horse Tendon: Towards the Development of Ideal Scaffolding Material for Urethral Regeneration. Materials 2021, 14, 7648. [Google Scholar] [CrossRef]
- Yañez-Mó, M.; Barreiro, O.; Gonzalo, P.; Batista, A.; Megías, D.; Genís, L.; Sachs, N.; Sala-Valdés, M.; Alonso, M.A.; Montoya, M.C.; et al. MT1-MMP Collagenolytic Activity Is Regulated through Association with Tetraspanin CD151 in Primary Endothelial Cells. Blood 2008, 112, 3217–3226. [Google Scholar] [CrossRef]
- Kwansa, A.L.; de Vita, R.; Freeman, J.W. Mechanical Recruitment of N- and C-Crosslinks in Collagen Type I. Matrix Biol. 2014, 34, 161–169. [Google Scholar] [CrossRef]
- Adhikari, A.S.; Chai, J.; Dunn, A.R. Mechanical Load Induces a 100-Fold Increase in the Rate of Collagen Proteolysis by MMP-1. J. Am. Chem. Soc. 2011, 133, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.S.; Glassey, E.; Dunn, A.R. Conformational Dynamics Accompanying the Proteolytic Degradation of Trimeric Collagen I by Collagenases. J. Am. Chem. Soc. 2012, 134, 13259–13265. [Google Scholar] [CrossRef] [PubMed]
- Maternini, M.; Guttadauro, A.; Mascagni, D.; Milito, G.; Stuto, A.; Renzi, A.; Ripamonti, L.; Bottini, C.; Nudo, R.; del Re, L.; et al. Non Cross-Linked Equine Collagen (Salvecoll-E Gel) for Treatment of Complex Ano-Rectal Fistula. Asian J. Surg. 2019, 43, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Sandor, M.; Xu, H.; Connor, J.; Lombardi, J.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host Response to Implanted Porcine-Derived Biologic Materials in a Primate Model of Abdominal Wall Repair. Tissue Eng. Part A 2008, 14, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Bohn, G.; Liden, B.; Schultz, G.; Yang, Q.; Gibson, D.J. Ovine-Based Collagen Matrix Dressing: Next-Generation Collagen Dressing for Wound Care. Adv. Wound Care 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Neuber, F. Fettransplantation bericht uber die verhandlungen der deutscht gesellsch chir. Zentralbl. Chir. 1893, 22, 66. [Google Scholar]
- Kontis, T.; Rivkin, A. The History of Injectable Facial Fillers. Facial Plast. Surg. 2009, 25, 067–072. [Google Scholar] [CrossRef]
- Cespedes, R.D. Collagen Injection or Artificial Sphincter for Postprostatectomy Incontinence: Collagen. Urology 2000, 55, 5–7. [Google Scholar] [CrossRef]
- Cho, K.-H.; Uthaman, S.; Park, I.-K.; Cho, C.-S. Injectable Biomaterials in Plastic and Reconstructive Surgery: A Review of the Current Status. Tissue Eng. Regen. Med. 2018, 15, 559–574. [Google Scholar] [CrossRef]
- Inglefield, C.; Samuelson, E.U.; Landau, M.; DeVore, D. Bio-Dermal Restoration With Rapidly Polymerizing Collagen: A Multicenter Clinical Study. Aesthetic Surg. J. 2018, 38, 1131–1138. [Google Scholar] [CrossRef]
- Alnojeidi, H.; Kilani, R.T.; Ghahary, A. Evaluating the Biocompatibility of an Injectable Wound Matrix in a Murine Model. Gels 2022, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Camilleri-Brennan, J. Anal Injectables and Implantables for Faecal Incontinence. In Fecal Incontinence—Causes, Management and Outcome; InTech: Vienna, Austria, 2014. [Google Scholar]
- Thomas, K.; Engler, A.J.; Meyer, G.A. Extracellular Matrix Regulation in the Muscle Satellite Cell Niche. Connect. Tissue Res. 2015, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and Immunogenicity of Collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Ellingsworth, L.R.; de Lustro, F.; Brennan, J.E.; Sawamura, S.; Mc Pherson, J. The Human Immune Response to Reconstituted Bovine Collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar] [CrossRef]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a Bovine Collagen Implant: Clinical and Immunologic Study in 705 Patients. J. Am. Acad. Dermatol. 1989, 21, 1203–1208. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular Matrix-Based Biomaterial Scaffolds and the Host Response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef]
- Lemperle, G.; Morhenn, V.; Charrier, U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Aesthetic Plast. Surg. 2003, 27, 354–366. [Google Scholar] [CrossRef]
- Narins, R.S.; Brandt, F.; Leyden, J.; Lorenc, Z.P.; Rubin, M.; Smith, S. A Randomized, Double-Blind, Multicenter Comparison of the Efficacy and Tolerability of Restylane versus Zyplast for the Correction of Nasolabial Folds. Dermatol. Surg. 2003, 29, 588–595. [Google Scholar] [CrossRef]
- Solomon, P.; Sklar, M.; Zener, R. Facial Soft Tissue Augmentation with Artecoll®: A Review of Eight Years of Clinical Experience in 153 Patients. Can. J. Plast. Surg. 2012, 20, 28–32. [Google Scholar] [CrossRef]
- Lemperle, G.; Romani, J.J.; Busso, M. Soft Tissue Augmentation With Artecoll: 10-Year History, Indications, Techniques, and Complications. Dermatol. Surg. 2003, 29, 573–587. [Google Scholar] [CrossRef]
- Cohen, S.R.; Holmes, R.E. Artecoll: A Long-Lasting Injectable Wrinkle Filler Material: Report of a Controlled, Randomized, Multicenter Clinical Trial of 251 Subjects. Plast. Reconstr. Surg. 2004, 114, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Haneke, E. Polymethyl Methacrylate Microspheres in Collagen. Semin. Cutan. Med. Surg. 2004, 23, 227–232. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, H.W.; Lee, M.W.; Choi, J.H.; Moon, K.C.; Koh, J.K. Artecoll Granuloma: A Rare Adverse Reaction Induced by Microimplant in the Treatment of Neck Wrinkles. Dermatol. Surg. 2004, 30, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Rullan, P.P. Soft Tissue Augmentation Using Artecoll: A Personal Experience. Facial Plast. Surg. 2004, 20, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Thaler, M.P.; Ubogy, Z.I. Artecoll: The Arizona Experience and Lessons Learned. Dermatol. Surg. 2005, 31, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.; Ng, C.L.; Kerzner, J.; Rival, R. Facial Soft Tissue Augmentation with Bellafill: A Review of 4 Years of Clinical Experience in 212 Patients. Plast. Surg. 2021, 29, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.R.; Berner, C.F.; Busso, M.; Gleason, M.C.; Hamilton, D.; Holmes, R.E.; Romano, J.J.; Rullan, P.P.; Thaler, M.P.; Ubogy, Z.; et al. ArteFill: A Long-Lasting Injectable Wrinkle Filler Material—Summary of the U.S. Food and Drug Administration Trials and a Progress Report on 4- to 5-Year Outcomes. Plast. Reconstr. Surg. 2006, 118, 64–76. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, Y.J.; Rhie, J.W.; Suh, D.S.; Oh, D.Y.; Lee, J.H.; Kim, Y.J.; Kim, S.M.; Jun, Y.J. Comparative Study of the Effectiveness and Safety of Porcine and Bovine Atelocollagen in Asian Nasolabial Fold Correction. J. Plast. Surg. Hand Surg. 2015, 49, 147–152. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, Y.S.; Kim, S.M.; Kim, Y.J.; Rhie, J.W.; Jun, Y.J. Efficacy and Safety of Porcine Collagen Filler for Nasolabial Fold Correction in Asians: A Prospective Multicenter, 12 Months Follow-up Study. J. Korean Med. Sci. 2014, 29, S217–S221. [Google Scholar] [CrossRef] [PubMed]
- Hirche, C.; Senghaas, A.; Fischer, S.; Hollenbeck, S.T.; Kremer, T.; Kneser, U. Novel Use of a Flowable Collagen-Glycosaminoglycan Matrix (IntegraTM Flowable Wound Matrix) Combined with Percutaneous Cannula Scar Tissue Release in Treatment of Post-Burn Malfunction of the Hand—A Preliminary 6 Month Follow-Up. Burns 2016, 42, e1–e7. [Google Scholar] [CrossRef]
- Kligman, A.M.; Armstrong, R.C. Histologic Respose to Intradermal Zyderm and Zyplast (Glutaraldehyde Cross-Linked) Collagen in Humans. J. Dermatol. Surg. Oncol. 1986, 12, 351–357. [Google Scholar] [CrossRef]
- Stegman, S.J.; Chu, S.; Bensch, K.; Armstrong, R. A Light and Electron Microscopic Evaluation of Zyderm Collagen and Zyplast Implants in Aging Human Facial Skin: A Pilot Study. Arch. Dermatol. 1987, 123, 1644–1649. [Google Scholar] [CrossRef]
- Elson, M.L. Clinical Assessment of Zyplast Implant: A Year of Experience for Soft Tissue Contour Correction. J. Am. Acad. Dermatol. 1988, 18, 707–713. [Google Scholar] [CrossRef]
- Matti, B.A.; Nicolle, F.v. Clinical Use of Zyplast in Correction of Age- and Disease-Related Contour Deficiencies of the Face. Aesthetic Plast. Surg. 1990, 14, 227–234. [Google Scholar] [CrossRef]
- Cooperman, L.S.; Mackinnon, V.; Bechler, G.; Pharriss, B.B. Injectable Collagen: A Six-Year Clinical Investigation. Aesthetic Plast. Surg. 1985, 9, 145–151. [Google Scholar] [CrossRef]
- Castrow, F.F.; Krull, E.A. Injectable Collagen Implant—Update. J. Am. Acad. Dermatol. 1983, 9, 889–893. [Google Scholar] [CrossRef]
- Downie, J.; Mao, Z.; Rachel Lo, T.W.; Barry, S.; Bock, M.; Siebert, J.P.; Bowman, A.; Ayoub, A. A Double-Blind, Clinical Evaluation of Facial Augmentation Treatments: A Comparison of PRI 1, PRI 2, Zyplast® and Perlane®. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1636–1643. [Google Scholar] [CrossRef]
- Baumann, L.S.; Shamban, A.T.; Lupo, M.P.; Monheit, G.D.; Thomas, J.A.; Murphy, D.K.; Walker, P.S. Comparison of Smooth-Gel Hyaluronic Acid Dermal Fillers with Cross-Linked Bovine Collagen: A Multicenter, Double-Masked, Randomized, within-Subject Study. Dermatol. Surg. 2007, 33, 128–135. [Google Scholar] [CrossRef]
- Nevins, M.; Giannobile, W.V.; McGuire, M.K.; Kao, R.T.; Mellonig, J.T.; Hinrichs, J.E.; McAllister, B.S.; Murphy, K.S.; McClain, P.K.; Nevins, M.L.; et al. Platelet-Derived Growth Factor Stimulates Bone Fill and Rate of Attachment Level Gain: Results of a Large Multicenter Randomized Controlled Trial. J. Periodontol. 2005, 76, 2205–2215. [Google Scholar] [CrossRef]
- Nevins, A.; Crespi, P. A Clinical Study Using the Collagen Gel Zyplast in Endodontic Treatment. J. Endod. 1998, 24, 610–613. [Google Scholar] [CrossRef]
- Gadomski, B.C.; Labus, K.M.; Puttlitz, C.M.; McGilvray, K.C.; Regan, D.P.; Nelson, B.; Seim, H.B.; Easley, J.T. Evaluation of Lumbar Spinal Fusion Utilizing Recombinant Human Platelet Derived Growth Factor-B Chain Homodimer (RhPDGF-BB) Combined with a Bovine Collagen/β-Tricalcium Phosphate (β-TCP) Matrix in an Ovine Model. JOR Spine 2021, 4, e1166. [Google Scholar] [CrossRef]
- Scott, R.T.; McAlister, J.E.; Rigby, R.B. Allograft Bone: What Is the Role of Platelet-Derived Growth Factor in Hindfoot and Ankle Fusions. Clin. Podiatr. Med. Surg. 2018, 35, 37–52. [Google Scholar] [CrossRef]
- Daniels, T.R.; Anderson, J.; Swords, M.P.; Maislin, G.; Donahue, R.; Pinsker, E.; Quiton, J.D. Recombinant Human Platelet–Derived Growth Factor BB in Combination with a Beta-Tricalcium Phosphate (RhPDGF-BB/β-TCP)-Collagen Matrix as an Alternative to Autograft. Foot Ankle Int. 2019, 40, 1068–1078. [Google Scholar] [CrossRef]
- DiGiovanni, C.W.; Lin, S.S.; Baumhauer, J.F.; Daniels, T.; Younger, A.; Glazebrook, M.; Anderson, J.; Anderson, R.; Evangelista, P.; Lynch, S.E.; et al. Recombinant Human Platelet-Derived Growth Factor-BB and Beta-Tricalcium Phosphate (RhPDGF-BB/β-TCP): An Alternative to Autogenous Bone Graft. J. Bone Jt. Surg. 2013, 95, 1184–1192. [Google Scholar] [CrossRef]
- Daniels, T.; DiGiovanni, C.; Lau, J.T.C.; Wing, K.; Alastair, Y. Prospective Clinical Pilot Trial in a Single Cohort Group of RhPDGF in Foot Arthrodeses. Foot Ankle Int. 2010, 31, 473–479. [Google Scholar] [CrossRef]
- Abidi, N.A.; Younger, A.; Digiovanni, C.W. Role of Platelet-Derived Growth Factor in Hindfoot Fusion. Tech. Foot Ankle Surg. 2012, 11, 34–38. [Google Scholar]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant Human Platelet-Derived Growth Factor: Biology and Clinical Applications. J. Bone Jt. Surg. 2008, 90, 48–54. [Google Scholar] [CrossRef]
- DiGiovanni, C.W.; Petricek, J.M. The Evolution of RhPDGF-BB in Musculoskeletal Repair and Its Role in Foot and Ankle Fusion Surgery. Foot Ankle Clin. 2010, 15, 621–640. [Google Scholar] [CrossRef]
- Digiovanni, C.W.; Lin, S.; Pinzur, M. Recombinant Human PDGF-BB in Foot and Ankle Fusion. Expert Rev. Med. Devices 2012, 9, 111–122. [Google Scholar] [CrossRef]
- DiGiovanni, C.W.; Baumhauer, J.; Lin, S.S.; Berberian, W.S.; Flemister, A.S.; Enna, M.J.; Evangelista, P.; Newman, J. Prospective, Randomized, Multi-Center Feasibility Trial of RhPDGF-BB versus Autologous Bone Graft in a Foot and Ankle Fusion Model. Foot Ankle Int. 2011, 32, 344–354. [Google Scholar] [CrossRef]
- DiGiovanni, C.W.; Lin, S.S.; Daniels, T.R.; Glazebrook, M.; Evangelista, P.; Donahue, R.; Beasley, W.; Baumhauer, J.F. The Importance of Sufficient Graft Material in Achieving Foot or Ankle Fusion. J. Bone Jt. Surg. Am. Vol. 2016, 98, 1260–1267. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Daniels, T.; Roach, S.; Beasley, W.; Snel, L.B. Effect of Implantation of Augment® Bone Graft on Serum Concentrations of Platelet-Derived Growth Factors: A Pharmacokinetic Study. Clin. Drug Investig. 2013, 33, 143–149. [Google Scholar] [CrossRef]
- Perrien, D.S.; Young, C.S.; Alvarez-Urena, P.P.; Dean, D.D.; Lynch, S.E.; Hollinger, J.O. Percutaneous Injection of Augment Injectable Bone Graft (RhPDGF-BB and β-Tricalcium Phosphate [β-TCP]/Bovine Type i Collagen Matrix) Increases Vertebral Bone Mineral Density in Geriatric Female Baboons. Spine J. 2013, 13, 580–586. [Google Scholar] [CrossRef]
- de Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; de Girolamo, L.; Volpi, P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional in Vitro Investigation and a Pilot Retrospective Clinical Study. J. Clin. Med. 2019, 8, 975. [Google Scholar] [CrossRef]
- Kimura, M.; Nito, T.; Imagawa, H.; Tayama, N.; Chan, R.W. Collagen Injection as a Supplement to Arytenoid Adduction for Vocal Fold Paralysis. Ann. Otol. Rhinol. Laryngol. 2008, 117, 430–436. [Google Scholar] [CrossRef]
- Kimura, M.; Nito, T.; Sakakibara, K.I.; Tayama, N.; Niimi, S. Clinical Experience with Collagen Injection of the Vocal Fold: A Study of 155 Patients. Auris Nasus Larynx 2008, 35, 67–75. [Google Scholar] [CrossRef]
- Stojkovic, S.G.; Lim, M.; Burke, D.; Finan, P.J.; Sagar, P.M. Intra-Anal Collagen Injection for the Treatment of Faecal Incontinence. Br. J. Surg. 2006, 93, 1514–1518. [Google Scholar] [CrossRef]
- Jamal, N.; Mundi, J.; Chhetri, D.K. Higher Risk of Superficial Injection during Injection Laryngoplasty in Women. Am. J. Otolaryngol. 2014, 35, 159–163. [Google Scholar] [CrossRef]
- Ford, C.N.; Bless, D.M.; Loftus, J.M. Role of Injectable Collagen in the Treatment of Glottic Insufficiency: A Study of 119 Patients. Ann. Otol. Rhinol. Laryngol. 1992, 101, 237–247. [Google Scholar] [CrossRef]
- Hoffman, H.; McCabe, D.; McCulloch, T.; Jin, S.M.; Karnell, M. Laryngeal Collagen Injection as an Adjunct to Medialization Laryngoplasty. Laryngoscope 2002, 112, 1407–1413. [Google Scholar] [CrossRef]
- Luu, Q.; Tsai, V.; Mangunta, V.; Berke, G.S.; Chhetri, D.K. Safety of Percutaneous Injection of Bovine Dermal Crosslinked Collagen for Glottic Insufficiency. Otolaryngol. Head Neck Surg. 2007, 136, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Kamer, F.M.; Churukian, M.M. Clinical Use of Injectable Collagen. Arch. Otolaryngol. 1984, 110, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Kawabe, K.; Kageyama, S.; Koiso, K.; Akaza, H.; Kakizoe, T.; Koshiba, K.; Yokoyama, E.; Aso, Y. Injection of Glutaraldehyde Cross-Linked Collagen for Urinary Incontinence: Two-Year Efficacy by Self-Assessment. Int. J. Urol. 1996, 3, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Elsergany, R.; Elgamasy, A.N.; Ghoniem, G.M. Transurethral Collagen Injection for Female Stress Incontinence. Int. Urogynecol. J. 1998, 9, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, R.D.; Leng, W.W.; McGuire, E.J. Collagen Injection Therapy for Postprostatectomy Incontinence. Urology 1999, 54, 597–602. [Google Scholar] [CrossRef]
- Corcos, J.; Fournier, C. Periurethral Collagen Injection for the Treatment of Female Stress Urinary Incontinence: 4-Year Follow-up Results. Urology 1999, 54, 815–818. [Google Scholar] [CrossRef]
- Gorton, E.; Stanton, S.; Monga, A.; Wiskind, A.K.; Lentz, G.M.; Bland, D.R. Periurethral Collagen Injection: A Long-Term Follow-up Study. BJU Int. 1999, 84, 966–971. [Google Scholar] [CrossRef]
- Vandenbulcke, L.; Lapage, K.G.; Vanderstraeten, K.V.; de Somer, F.M.; de Hert, S.G.; Moerman, A.T. Microvascular Reactivity Monitored with Near-Infrared Spectroscopy Is Impaired after Induction of Anaesthesia in Cardiac Surgery Patients. Eur. J. Anaesthesiol. 2017, 34, 688–694. [Google Scholar] [CrossRef]
- Marx, G.; Zacharowski, K.; Ichai, C.; Asehnoune, K.; Černý, V.; Dembinski, R.; Ferrer Roca, R.; Fries, D.; Molnar, Z.; Rosenberger, P.; et al. Efficacy and Safety of Early Target-Controlled Plasma Volume Replacement with a Balanced Gelatine Solution versus a Balanced Electrolyte Solution in Patients with Severe Sepsis/Septic Shock: Study Protocol, Design, and Rationale of a Prospective, Randomized, Controlled, Double-Blind, Multicentric, International Clinical Trial: GENIUS—Gelatine Use in ICU and Sepsis. Trials 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Hamraoui, K.; Ernst, S.M.P.G.; van Dessel, P.F.H.M.; Kelder, J.C.; ten Berg, J.M.; Suttorp, J.; Jaarsma, W.; Plokker, H.W. Efficacy and Safety of Percutaneous Treatment of Iatrogenic Femoral Artery Pseudoaneurysm by Biodegradable Collagen Injection. J. Am. Coll. Cardiol. 2002, 39, 1297–1304. [Google Scholar] [CrossRef]
- Majdalany, B.S.; Willatt, J.; Beecham Chick, J.F.; Srinivasa, R.N.; Saad, W.A. Fibrillar Collagen Injection for Organ Protection during Thermal Ablation of Hepatic Malignancies. Diagn. Interv. Radiol. 2017, 23, 381–384. [Google Scholar] [CrossRef]
- Nitecka-Buchta, A.; Walczynska-Dragon, K.; Batko-Kapustecka, J.; Wieckiewicz, M. Comparison between Collagen and Lidocaine Intramuscular Injections in Terms of Their Efficiency in Decreasing Myofascial Pain within Masseter Muscles: A Randomized, Single-Blind Controlled Trial. Pain Res. Manag. 2018, 2018, 8261090. [Google Scholar] [CrossRef] [PubMed]
- Giovannangeli, F.; Bizzi, E.; Massafra, U.; Vacca, F.; Tormenta, S.; Migliore, A. Intra-Articular Administration of MD-HIP in 24 Patients Affected by Symptomatic Hip Osteoarthritis—A 24-Month Cohort Study. Physiol. Regul. Med. 2016, 31–32. [Google Scholar]
- Guitart Vela, J.; Folch Ibanez, J. Collagen MDs for Chronic Pain. Efficacy and Tolerability in Chronic Treatment in 124 Patients. Physiol. Regul. Med. 2016, 9–12. [Google Scholar]
- Reshkova, V.; Rashkov, R.; Nestorova, R. Efficacy and Safety Evaluation of GUNA Collagen MDs Injections in Knee Osteoarthritis—A Case Series of 30 Patients. Physiol. Regul. Med. 2016, 27–29. [Google Scholar]
- Pavelka, K.; Jarosova, H.; Milani, L.; Prochazka, Z.; Kostiuk, P.; Kotlarova, L.; Meroni, A.M.; Slíva, J. Efficacy and Tolerability of Injectable Collagen-Containing Products in Comparison to Trimecaine in Patients with Acute Lumbar Spine Pain (Study FUTURE-MD-Back Pain). Physiol. Res. 2019, 68, s65–s74. [Google Scholar] [CrossRef]
- Massulo, C. Injectable GUNA Collagen Medical Devices in Functional Recovery from Sport. Physiol. Regul. Med. 2016, 3–7. [Google Scholar]
- Staňa, J. 3 Years in Luhačovice Spa with Collagen Medical Devices Injections in the Treatment of Piriformis Syndrome. Physiol. Regul. Med. 2016, 19–20. [Google Scholar]
- Alfieri, N. MD-Muscle in the Management of Myofascial Pain Syndrome. Physiol. Regul. Med. 2016, 23–24. [Google Scholar] [CrossRef]
- Heng, C.H.Y.; Snow, M.; Dave, L.Y.H. Single-Stage Arthroscopic Cartilage Repair With Injectable Scaffold and BMAC. Arthrosc. Tech. 2021, 10, e751–e756. [Google Scholar] [CrossRef]
- Kim, M.S.; Chun, C.H.; Wang, J.H.; Kim, J.G.; Kang, S.B.; Yoo, J.D.; Chon, J.G.; Kim, M.K.; Moon, C.W.; Chang, C.B.; et al. Microfractures Versus a Porcine-Derived Collagen-Augmented Chondrogenesis Technique for Treating Knee Cartilage Defects: A Multicenter Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Oh, K.J.; Moon, Y.W.; In, Y.; Lee, H.J.; Kwon, S.Y. Intra-Articular Injection of Type I Atelocollagen to Alleviate Knee Pain: A Double-Blind, Randomized Controlled Trial. Cartilage 2021, 13, 342S–350S. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.J.; Lee, H.J.; Kim, B.K.; Kim, Y.S. Atelocollagen Injection Improves Tendon Integrity in Partial-Thickness Rotator Cuff Tears: A Prospective Comparative Study. Orthop. J. Sports Med. 2020, 8, 2325967120904012. [Google Scholar] [CrossRef] [PubMed]
- Volpi, P.; Zini, R.; Erschbaumer, F.; Beggio, M.; Busilacchi, A.; Carimati, G. Effectiveness of a Novel Hydrolyzed Collagen Formulation in Treating Patients with Symptomatic Knee Osteoarthritis: A Multicentric Retrospective Clinical Study. Int. Orthop. 2021, 45, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mariconti, P. Usefulness of GUNA Collagen Medical Devices in the Treatment of Knee Pain. Physiol. Regul. Med. 2016, 39–40. [Google Scholar]
- Martin Martin, L.S.; Massafra, U.; Bizzi, E.; Migliore, A. A Double Blind Randomized Active-Controlled Clinical Trial on the Intra-Articular Use of Md-Knee versus Sodium Hyaluronate in Patients with Knee Osteoarthritis (“Joint”). BMC Musculoskelet. Disord. 2016, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Uroz, N.Z. Collagen Medical Device Infiltrations in Shoulder Pathologies. Calcific Supraspinatus Tendinitis. Physiol. Regul. Med. 2016, 15–17. [Google Scholar]
- Nestorova, R.; Rashkov, R.; Petranova, T. Clinical and Sonographic Assessment of the Effectiveness of GUNA Collagen MDs Injections in Patients with Partial Thickness Tear of the Rotator Cuff. Physiol. Regul. Med. 2016, 35–37. [Google Scholar]
- Wolkow, N.; Jakobiec, F.A.; Dryja, T.P.; Lefebvre, D.R. Mild Complications or Unusual Persistence of Porcine Collagen and Hyaluronic Acid Gel Following Periocular Filler Injections. Ophthalmic Plast. Reconstr. Surg. 2018, 34, e143–e146. [Google Scholar] [CrossRef]
- Braun, M.; Braun, S. Nodule Formation Following Lip Augmentation Using Porcine Collagen-Derived Filler. J. Drugs Dermatol. 2008, 7, 579–581. [Google Scholar]
- Narins, R.S.; Brandt, F.S.; Lorenc, Z.P.; Maas, C.S.; Monheit, G.D.; Smith, S.R. Twelve-Month Persistency of a Novel Ribose-Cross-Linked Collagen Dermal Filler. Dermatol. Surg. 2008, 34, 31–39. [Google Scholar] [CrossRef]
- Pollack, S.v. Silicone, Fibrel, and Collagen Implantation for Facial Lines and Wrinkles. J. Dermatol. Surg. Oncol. 1990, 16, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Mittelman, H. Fibrel: A Dermal Implant Comparison With Collagen Implants. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 1379. [Google Scholar] [CrossRef]
- Denton, A.B.; Shoman, N. Porcine Collagen: Evolence. In Office-Based Cosmetic Procedures and Techniques; Cambridge University Press: Cambridge, UK, 2010; pp. 68–70. [Google Scholar] [CrossRef]
- Samalavicius, N.E.; Kavaliauskas, P.; Dulskas, A. PermacolTM Collagen Paste Injection for the Treatment of Complex Anal Fistula—A Video Vignette. Color. Dis. 2020, 22, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Pescatori, M. PermacolTM Collagen Paste for Treating a Rectovaginal Fistula Following Anterior Rectal Prolapsectomy. Tech. Coloproctol. 2017, 21, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Harran, N.; Herold, J.; Bentley, A.; Bebington, B.D. Efficacy of Porcine Dermal Collagen (PermacolTM) Injection for Passive Faecal Incontinence in a Dedicated Colorectal Unit at the Wits Donald Gordon Medical Centre. S. Afr. J. Surg. 2017, 55, 10–13. [Google Scholar] [CrossRef]
- Giordano, P.; Sileri, P.; Buntzen, S.; Stuto, A.; Nunoo-Mensah, J.; Lenisa, L.; Singh, B.; Thorlacius-Ussing, O.; Griffiths, B.; Ziyaie, D. A Prospective Multicentre Observational Study of PermacolTM Collagen Paste for Anorectal Fistula: Preliminary Results. Color. Dis. 2016, 18, 286–294. [Google Scholar] [CrossRef]
- Sileri, P.; Franceschilli, L.; del Vecchio Blanco, G.; Stolfi, V.M.; Angelucci, G.P.; Gaspari, A.L. Porcine Dermal Collagen Matrix Injection May Enhance Flap Repair Surgery for Complex Anal Fistula. Int. J. Color. Dis. 2011, 26, 345–349. [Google Scholar] [CrossRef]
- Milito, G.; Cadeddu, F. Conservative Treatment for Anal Fistula: Collagen Matrix Injection. J. Am. Coll. Surg. 2009, 209, 542–543. [Google Scholar] [CrossRef]
- Harper, C. Permacol: Clinical Experience with a New Biomaterial. Hosp. Med. 2001, 62, 90–95. [Google Scholar] [CrossRef]
- Micarelli, A.; Viziano, A.; Granito, I.; Antonuccio, G.; Felicioni, A.; Loberti, M.; Carlino, P.; Micarelli, R.X.; Alessandrini, M. Combination of In-Situ Collagen Injection and Rehabilitative Treatment in Long-Lasting Facial Nerve Palsy: A Pilot Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 366–375. [Google Scholar] [CrossRef]
- del Carpio-Orantes, L.; García-Méndez, S.; Sánchez-Díaz, J.S.; Aguilar-Silva, A.; Contreras-Sánchez, E.R.; Hernández, S.N.H. Use of Fibroquel® (Polymerized Type I Collagen) in Patients with Hypoxemic Inflammatory Pneumonia Secondary to COVID-19 in Veracruz, Mexico. J. Anesth. Crit. Care 2021, 13, 69–73. [Google Scholar] [CrossRef]
- Méndez-Flores, S.; Priego-Ranero, Á.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; Ochoa-Hein, E.; Perez-Ortiz, A.; Rendón-Macías, M.E.; Rojas-Castañeda, E.; Urbina-Terán, S.; et al. Effect of Polymerised Type I Collagen on Hyperinflammation of Adult Outpatients with Symptomatic COVID-19. Clin. Transl. Med. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Sparavigna, A.; Tateo, A.; Inselvini, E.; Tocchio, M.; Orlandini, M.C.; Botali, G. Anti-Age Activity and Tolerance Evaluation of Collagen Micro-Injection Treatment Associated to Topical Application of a Cosmetic Formulation (Investigator-Initiated Multicentre Trial). J. Clin. Exp. Dermatol. Res. 2017, 8, 1000391. [Google Scholar] [CrossRef]
- Gkouvi, A.; Nicolaidou, E.; Corbo, A.; Selvaggi, G.; Tsimpidakis, A.; Mastraftsi, S.; Gregoriou, S. Heterologous Type i Collagen as an Add-on Therapy to Narrowband Ultraviolet b for the Treatment of Vitiligo: A Pilot Study. J. Clin. Aesthetic Dermatol. 2021, 14, 31–34. [Google Scholar]
- Gkouvi, A.; Corbo, A.; Gregoriou, S. Treatment of Male Genital Lichen Sclerosus with Heterologous Type I Collagen. Clin. Exp. Dermatol. 2020, 45, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Klewin-Steinböck, S.; Nowak-Terpi3owska, A.; Adamski, Z.; Grocholewicz, K.; Wyganowska-Swiatkowska, M. Effect of Injectable Equine Collagen Type I on Metabolic Activity and Apoptosis of Gingival Fibroblasts. Adv. Dermatol. Allergol. 2021, XXXVIII, 440–445. [Google Scholar] [CrossRef]
- Wyganowska-Swiatkowska, M.; Duda-Sobczak, A.; Corbo, A.; Matthews-Brzozowska, T. Atelocollagen Application in Human Periodontal Tissue Treatment—A Pilot Study. Life 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Burres, S. Soft-Tissue Augmentation with Fascian. Clin. Plast. Surg. 2001, 28, 101–110. [Google Scholar] [CrossRef]
- Bauman, L. CosmoDerm/CosmoPlast (Human Bioengineered Collagen) for the Aging Face. Facial Plast. Surg. 2004, 20, 125–128. [Google Scholar] [CrossRef]
- Fagien, S.; Elson, M.L. Facial Soft-Tissue Augmentation with Allogeneic Human Tissue Collagen Matrix (Dermalogen and Dermaplant). Clin. Plast. Surg. 2001, 28, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Burres, S. Fascian. Facial Plast. Surg. 2004, 20, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Maloney, B.P.; Murphy, B.A.; Cole, H.P. Cymetra. Facial Plast. Surg. 2004, 20, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.D.; Sataloff, R.T. Complications of Collagen Injection of the Vocal Fold: Report of Several Unusual Cases and Review of the Literature. J. Voice 2004, 18, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.M.; Lee, J.H.; Robinson, R.A.; Hoffman, H.T. Migration of Cymetra After Vocal Fold Injection for Laryngeal Paralysis. Laryngoscope 2007, 117, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, A.N.; Meleca, R.J.; Dworkin, J.P.; Stachler, R.J. Cymetra Injection for Unilateral Vocal Fold Paralysis. Ann. Otol. Rhinol. Laryngol. 2003, 112, 927–934. [Google Scholar] [CrossRef]
- Douglas, R.S.; Donsoff, I.; Cook, T.; Shorr, N. Collagen Fillers in Facial Aesthetic Surgery. Facial Plast. Surg. 2004, 20, 117–123. [Google Scholar] [CrossRef]
- Homicz, M.R.; Watson, D. Review of Injectable Materials for Soft Tissue Augmentation. Facial Plast. Surg. 2004, 20, 21–29. [Google Scholar] [CrossRef]
- Rinaldi, F.; Pinto, D.; Trink, A.; Giuliani, G.; Sparavigna, A. In Vitro and in Vivo Evaluation on the Safety and Efficacy of a Brand-New Intracutaneous Filler with A1-R-Collagen. Clin. Cosmet. Investig. Dermatol. 2021, 14, 501–512. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Giusti, I.; Dolo, V.; Guerrini, L.; Cifone, M.G.; Giuliani, M.; Cinque, B. Type I Collagen Suspension Induces Neocollagenesis and Myodifferentiation in Fibroblasts in Vitro. Biomed. Res. Int. 2020, 2020, 6093974. [Google Scholar] [CrossRef]
- Keefe, J.; Wauk, L.; Chu, S.; DeLustro, F. Clinical Use of Injectable Bovine Collan: A Decade of OExperience. Clin. Mater. 1992, 9, 155–162. [Google Scholar] [CrossRef]
- Randelli, F.; Menon, A.; Via, A.G.; Mazzoleni, M.; Sciancalepore, F.; Brioschi, M.; Gagliano, N. Effect of a Collagen-Based Compound on Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2018, 7, 246. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Lima, G.; Llorente, L.; Nuñez-Álvarez, C.; Ruiz-Ordaz, B.H.; Echevarría-Zuno, S.; Hernández-Cuevas, V. Polymerized-Type I Collagen Downregulates Inflammation and Improves Clinical Outcomes in Patients with Symptomatic Knee Osteoarthritis Following Arthroscopic Lavage: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Sci. World J. 2012, 2012, 342854. [Google Scholar] [CrossRef]
- Corrado, B.; Bonini, I.; Alessio Chirico, V.; Rosano, N.; Gisonni, P. Use of Injectable Collagen in Partial-Thickness Tears of the Supraspinatus Tendon: A Case Report. Oxf. Med. Case Rep. 2020, 2020, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, Y.S.; You, M.-W.; Kim, J.S.; Young, K.W. Sonoelastography in the Evaluation of Plantar Fasciitis Treatment 3-Month Follow-Up After Collagen Injection. Ultrasound Q. 2016, 32, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.B.; Oliveira, E.; Castro, R.A.; Sartori, M.G.; Baracat, E.C.; Lima, G.R.; Girao, M.J. Clinical and Urodynamic Evaluation in Women with Stress Urinary Incontinence Treated by Periurethral Collagen Injection. Int. Braz. J. Urol. 2007, 33, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Oremus, M.; Tarride, J.-E. An Economic Evaluation of Surgery versus Collagen Injection for the Treatment of Female Stress Urinary Incontinence. Can. J. Urol. 2010, 17, 5087–5093. [Google Scholar]
- Winters, J.C.; Chiverton, A.; Scarpero, H.M.; Prats, L.J. Collagen injection therapy in elderly women: Long-term results and patient satisfaction. Urology 2000, 55, 856–860. [Google Scholar] [CrossRef]
- Groutz, A.; Blaivas, J.G.; Kesler, S.S.; Weiss, J.P.; Chaikin, D.C. Female urology outcome results of transurethral collagen injection for female stress incontinence: Assessment by urinary incontinence score. J. Urol. 2000, 164, 2006–2009. [Google Scholar] [CrossRef]
- Bomalaski, M.D.; Bloom, D.A.; McGuire, E.J.; Panzl, A. Glutaraldehyde cross-linked collagen in the treatment of urinary incontinence in children. J. Urol. 1996, 155, 699–702. [Google Scholar] [CrossRef]
- Kassouf, W.; Capolicchio, G.; Berardinucci, G.; Corcos, J. Collagen injection for treatment of urinary incontinence in children. J. Urol. 2001, 165, 1666–1668. [Google Scholar] [CrossRef] [PubMed]
- Faerber, G.J.; Richardson, T.D. Long-Term Results of Transurethral Collagen Injection in Men with Intrinsic Sphincter Deficiency; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 1997; Volume 11. [Google Scholar]
- Faerber, G.J.; Belville, W.D.; Ohl, D.A.; Plata, A. Comparison of Transurethral versus Periurethral Collagen Injection in Women with Intrinsic Sphincter Deficiency. Tech. Urol. 1998, 4, 124–127. [Google Scholar] [PubMed]
- Richardson, T.D.; Kennelly, M.J.; Faerber, G.J. Endoscopic injection of clutaraldehyde cross-linked collagen for the treatment of intrinsic sphincter deficiency in women. Urology 1995, 43, 378–381. [Google Scholar] [CrossRef]
- Smith, D.N.; Appell, R.A.; Rackley, R.R.; Winters, J.C. Collagen Injection Therapy for Post-Prostatectomy Incontinence. J. Urol. 1998, 160, 364–367. [Google Scholar] [CrossRef]
- Klutke, C.G.; Tiemann, D.D.; Nadler, R.B.; Andriole, G.L. Antegrade Collagen Injection for Stress Incontinence after Radical Prostatectomy: Technique and Early Results; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 1996; Volume 10. [Google Scholar]
- Appell, R.A. Collagen Injection Therapy for Urinary Incontinence. Urol. Clin. N. Am. 1994, 21, 177–182. [Google Scholar] [CrossRef]
- Appell, R.A.; Vasavada, S.P.; Rackley, R.R.; Winters, J.C. Percutaneous antegrade collagen injection therapy for urinary incontinence following radical prostatectomy. Urology 1996, 48, 769–772. [Google Scholar] [CrossRef]
- Nagai, A.; Nasu, Y.; Watanabe, M.; Tsugawa, M.; Iguchi, H.; Kumon, H. Analysis of Retrograde Ejaculation Using Color Doppler Ultrasonography before and after Transurethral Collagen Injection. Int. J. Impot. Res. 2004, 16, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.R.; Sataloff, R.T.; Gould, W.J. The Treatment of Vocal Fold Paralysis with Injectable Collagen: Clinical Concerns. J. Voice 1987, 1, 119–121. [Google Scholar] [CrossRef]
- Remacle, M.; Lawson, G. Results with Collagen Injection into the Vocal Folds for Medialization. Curr. Opin. Otolaryngol. Head Neck Surg 2007, 15, 148–152. [Google Scholar] [CrossRef]
- Remacle, M.; Hamoir, M.; Marbaix, E. Gax-Collagen Injection to Correct Aspiration Problems after Subtotal Laryngectomy. Laryngoscope 1990, 100, 662–669. [Google Scholar]
- Ford, C.N.; Bless, D.M. Selected Problems Treated by Vocal Fold Injection of Collagen. Am. J. Otolaryngol. 1993, 14, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.S.; Casiano, R.R.; McClinton, M.E.; Xue, J.W. Early Results of Transcutaneous Injection Laryngoplasty with Micronized Acellular Dermis Versus Type-I Thyroplasty for Glottic Incompetence Dysphonia Due to Unilateral Vocal Fold Paralysis. J. Voice 2003, 17, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Rydningen, M.; Dehli, T.; Wilsgaard, T.; Rydning, A.; Kumle, M.; Lindsetmo, R.O.; Norderval, S. Sacral Neuromodulation Compared with Injection of Bulking Agents for Faecal Incontinence Following Obstetric Anal Sphincter Injury—A Randomized Controlled Trial. Color. Dis. 2017, 19, O134–O144. [Google Scholar] [CrossRef]
- Brown, S.A.; Rohrich, R.J.; Baumann, L.; Brandt, F.S.; Fagien, S.; Glazer, S.; Kenkel, J.M.; Lowe, N.J.; Monheit, G.D.; Narins, R.S.; et al. Subject Global Evaluation and Subject Satisfaction Using Injectable Poly-l-Lactic Acid versus Human Collagen for the Correction of Nasolabial Fold Wrinkles. Plast. Reconstr. Surg. 2011, 127, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Narins, R.S.; Baumann, L.; Brandt, F.S.; Fagien, S.; Glazer, S.; Lowe, N.J.; Monheit, G.D.; Rendon, M.I.; Rohrich, R.J.; Werschler, W.P. A Randomized Study of the Efficacy and Safety of Injectable Poly-L-Lactic Acid versus Human-Based Collagen Implant in the Treatment of Nasolabial Fold Wrinkles. J. Am. Acad. Dermatol. 2010, 62, 448–462. [Google Scholar] [CrossRef]
- Draelos, Z. Case Study of Dermicol-P35 Used in Patient with Past Hypersensitivity to Crosslinked Bovine Collagen Dermal Filler. Dermatol. Surg. 2010, 36, 825–827. [Google Scholar] [CrossRef]
- Narins, R.S.; Brandt, F.S.; Lorenc, Z.P.; Maas, C.S.; Monheit, G.D.; Smith, S.R.; Mcintyre, S. A Randomized, Multicenter Study of the Safety and Efficacy of Dermicol-P35 and Non-Animal-Stabilized Hyaluronic Acid Gel for the Correction of Nasolabial Folds. Dermatol. Surg. 2007, 33, S213–S221. [Google Scholar] [CrossRef]
- Cassuto, D. The Use of Dermicol-P35 Dermal Filler for Nonsurgical Rhinoplasty. Aesthetic Surg. J. 2009, 29, S22–S24. [Google Scholar] [CrossRef]
- Smith, K.C. Repair of Acne Scars With Dermicol-P35. Aesthetic Surg. J. 2009, 29, S16–S18. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K. The Effect of GUNA-MDs in the Therapy Resistant Facial Paresis. In Proceedings of the International Congress of PRM, Low doses therapies, Prague, Czech Republic, 9 November 2013. Trials and Case Reports. [Google Scholar]
- Ding, L.; Yan, G.; Wang, B.; Xu, L.; Gu, Y.; Ru, T.; Cui, X.; Lei, L.; Liu, J.; Sheng, X.; et al. Transplantation of UC-MSCs on Collagen Scaffold Activates Follicles in Dormant Ovaries of POF Patients with Long History of Infertility. Sci. China Life Sci. 2018, 61, 1554–1565. [Google Scholar] [CrossRef]
- Bechara, C.F.; Annambhotla, S.; Lin, P.H. Access Site Management with Vascular Closure Devices for Percutaneous Transarterial Procedures. J. Vasc. Surg. 2010, 52, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Dharmavaram, S.; Wearn, C.S.; Dube, M.G.; Lobo, D.N. Effects of an Intraoperative Infusion of 4 Succinylated Gelatine (Gelofusine®) and 6 Hydroxyethyl Starch (Voluven®) on Blood Volume. Br. J. Anaesth. 2012, 109, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking Older Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.; Kaye, R.L.; Klein, A.; Stegman, S. Injectable Collagen: A Clinical Overview. Cutis 1983, 31, 543–546. [Google Scholar] [PubMed]
- Nijhawan, R.I.; Cohen, J.I.; Kaufman, J. Persistent Erythema After Human Collagen Filler Injections. Cosmet. Dermatol. 2008, 21, 90–94. [Google Scholar]
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The Effect of Osteoarthritis Definition on Prevalence and Incidence Estimates: A Systematic Review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Meimandi-Parizi, A.; Oryan, A.; Moshiri, A. Role of Tissue Engineered Collagen Based Tridimensional Implant on the Healing Response of the Experimentally Induced Large Achilles Tendon Defect Model in Rabbits: A Long Term Study with High Clinical Relevance. J. Biomed. Sci. 2013, 20, 28. [Google Scholar] [CrossRef]
- Suh, D.-S.; Lee, J.-K.; Yoo, J.-C.; Woo, S.-H.; Kim, G.-R.; Kim, J.-W.; Choi, N.-Y.; Kim, Y.; Song, H.-S. Atelocollagen Enhances the Healing of Rotator Cuff Tendon in Rabbit Model. Am. J. Sport. Med. 2017, 45, 2019–2027. [Google Scholar] [CrossRef]
- Kirlum, H.J.; Stehr, M.; Dietz, H.G. Late Obstruction after Subureteral Collagen Injection. Eur. J. Pediatr. Surg. 2006, 16, 133–134. [Google Scholar] [CrossRef]
- Hartl, D.M.; Riquet, M.; Hans, S.; Laccourreye, O.; Vaissière, J.; Brasnu, D.F. Objective Voice Analysis after Autologous Fat Injection for Unilateral Vocal Fold Paralysis. Ann. Otol. Rhinol. Laryngol. 2001, 110, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Mori, K.; Tanaka, S.; Fujita, M. Vocal Function in Patients with Unilateral Vocal Fold Paralysis Before and After Silicone Injection. Acta. Otolaryngol. 1995, 115, 553–559. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Q.; Zhao, Y.; Zhang, H.; Wang, B.; Pan, J.; Li, J.; Yu, H.; Wang, L.; Dai, J.; et al. Effect of Intramyocardial Grafting Collagen Scaffold With Mesenchymal Stromal Cells in Patients With Chronic Ischemic Heart Disease. JAMA Netw. Open 2020, 3, e2016236. [Google Scholar] [CrossRef] [PubMed]
- Swanson, N.A.; Stoner, J.G.; Siegle, R.J.; Solomon, A.R. Treatment Site Reactions to Zyderm Collagen Implantation. J. Dermatol. Surg. Oncol. 1983, 9, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Elson, M.L. The Role of Skin Testing in the Use of Collagen Injectable Materials. J. Dermatol. Surg. Oncol. 1989, 15, 301–303. [Google Scholar] [CrossRef]
- Aragona, F.; D’Urso, L.; Marcolongo, R. Immunologic Aspects of Bovine Injectable Collagen in Humans. Eur. Urol. 1998, 33, 129–133. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Zhou, X.; Yang, X.; Lu, D.; Kang, W.; Feng, X. Effects of Intraoperative Gelatin on Blood Viscosity and Oxygenation Balance. J. PeriAnesthesia Nurs. 2019, 34, 1274–1281. [Google Scholar] [CrossRef]
- Lucey, P.; Goldberg, D. Complications of Collagen Fillers. Facial Plast. Surg. 2014, 30, 615–622. [Google Scholar] [CrossRef]
- Cooperman, L.; Michaeli, D.; Alto, P.; Francisco, S. The Immunogenicity of Injectable Collagen. II. A Retrospective Review of Seventy-Two Tested and Treated Patients. J. Am. Acad. Dermatol. 1984, 10, 647–651. [Google Scholar] [CrossRef]
- Lemperle, G.; Rullan, P.P.; Gauthier-Hazan, N. Avoiding and Treating Dermal Filler Complications. Plast. Reconstr. Surg. 2006, 118, 92S–107S. [Google Scholar] [CrossRef] [PubMed]
- Vanderveen, E.E.; McCoy, J.P.; Schade, W.; Kapur, J.J.; Hamilton, T.; Ragsdale, C.; Grekin, R.C.; Swanson, N.A. The Association of HLA and Immune Responses to Bovine Collagen Implants. Arch. Dermatol. 1986, 122, 650–654. [Google Scholar] [CrossRef]
- Klein, A.W. In Favor of Double Testing. Dermatol. Surg. 1989, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K. What Do Anti-Collagen Antibodies Mean? Ann. Rheum. Dis. 1990, 49, 62–65. [Google Scholar] [CrossRef]
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of Telopeptides on the Structural and Physical Properties of Polymeric and Monomeric Acid-Soluble Type I Collagen. Mater. Sci. Eng. C 2017, 77, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C.; Timpl, R.; Wolff, I. Immunogenicity and Specificity of Collagen. V. Demonstration of Three Different Antigenic Determinants on Calf Collagen. Immunology 1968, 15, 135–144. [Google Scholar]
- Leonard, M.P.; Decter, A.; Mix, L.W.; Johnson, H.W.; Coleman, G.U. Endoscopic Treatment of Vesicoureteral Reflux with Collagen: Preliminary Report and Cost Analysis. J. Urol. 1996, 155, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Elliott, D.S.; Barrett, D.M. Postprostatectomy Urinary Incontinence: A Comparison of the Cost of Conservative Versus Surgical Management. Urology 1998, 51, 715–720. [Google Scholar] [CrossRef]

| Animal Source | Extraction Tissue | Company |
|---|---|---|
| Equine | Tendon | Euroresearch S.r.l. (Milan, Italy) www.euroresearch.it, accessed on 14 February 2023 |
| Tendon | Opocrin Spa (Formigine, Italy) www.opocrin.it, accessed on 14 February 2023 | |
| Tendon | Typeone Biomaterials S.r.l. (Calimera, Italy) www.typeone.it, accessed on 14 February 2023 | |
| Bovine | Corium, tendon, membranes | Bovine collagen products (Branchburg, NJ, USA) www.bovinecollagenproducts.com, accessed on 14 February 2023 |
| Corium, tendon | Collagen solution (Eden Prairie, MN, USA) www.collagensolutions.com, accessed on 14 February 2023 | |
| n. d. | Royal DSM (Heerlen, The Netherlands) www.dsm.com, accessed on 14 February 2023 | |
| Tendon | Integra LifeScience Corp. (Princeton, NJ, USA) www.integralife.com, accessed on 14 February 2023 | |
| Dermis | Koken Co., Ltd. (Tokyo, Japan), www.kokenmpc.co.jp, accessed on 14 February 2023 | |
| Dermis | Devro Plc (Moodiesburn, UK) www.devro.com, accessed on 14 February 2023 | |
| Tendon | Getinge (Göteborg, Sweden) www.getinge.com, accessed on 14 February 2023 | |
| Dermis | Symatese (Chaponost, France) www.symatese.com, accessed on 14 February 2023 | |
| Hide | Advanced Biomatrix (Carlsbad, CA, USA) www.advancedbiomatrix.com, accessed on 14 February 2023 | |
| Swine | Skin | Ubiosis (Gyeonggi-do, Republic of Korea) www.ubiosis.com, accessed on 14 February 2023 |
| Skin | Botiss Biomaterials GmbH (Zossen, Germany) www.botiss-dental.com, accessed on 14 February 2023 | |
| Jellyfish | n. d. | Jellagen (Cardiff, UK) www.jellagen.co.uk, accessed on 14 February 2023 |
| Plant | Leaves | CollPlant (Rehovot, Israel) www.collplant.com, accessed on 14 February 2023 |
| Formulation | Study Aim | Status | Outcomes | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Injectable Collagen ScaffoldTM HUC-MSCs | Improvement of erectile function in men with diabetes | Unknown | n. d. | NCT02745808 |
| Injectable Collagen ScaffoldTM HUC-MSCs | Improvement of liver function in cases of decompensated cirrhosis | Unknown | n. d. | NCT02786017 |
| Injectable Collagen ScaffoldTM MCSs | Improvement of functional brain recovery in cases of brain injury | Unknown | n. d. | NCT02767817 |
| Gelofusine | Fluid retention prevention in patients with breast cancer | Completed | n. d. | NCT04637308 |
| Gelofusine | Improvement of blood volume in patients scheduled for abdominal or pelvic surgery | Completed | n. d. | NCT02808325 |
| Gelofusine | Improvement of blood volume for intravascular volume compensation during surgery | Completed | n. d. | NCT01515397 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, L.; Natali, M.L.; Brunetti, C.; Sannino, A.; Gallo, N. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers 2023, 15, 1020. https://doi.org/10.3390/polym15041020
Salvatore L, Natali ML, Brunetti C, Sannino A, Gallo N. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers. 2023; 15(4):1020. https://doi.org/10.3390/polym15041020
Chicago/Turabian StyleSalvatore, Luca, Maria Lucia Natali, Chiara Brunetti, Alessandro Sannino, and Nunzia Gallo. 2023. "An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications" Polymers 15, no. 4: 1020. https://doi.org/10.3390/polym15041020
APA StyleSalvatore, L., Natali, M. L., Brunetti, C., Sannino, A., & Gallo, N. (2023). An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers, 15(4), 1020. https://doi.org/10.3390/polym15041020