Polymer Membranes of Zeolitic Imidazole Framework-8 with Sodium Alginate Synthesized from ZIF-8 and Their Application in Light Gas Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystallization of ZIF-8
2.2. Mixed Membrane Preparation
2.3. Characterizations
2.4. Permeability Measurements
- -
- P is the permeability of the gas through the membrane (barrer);
- -
- (1 Barrer = 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1);
- -
- V is the permeate volume (cm3);
- -
- l is the thickness of the membrane layer (cm);
- -
- A is the effective area of the membrane (cm2);
- -
- Pf is the feed pressure (cmHg);
- -
- P0 is the pressure at the standard state (76 cmHg);
- -
- T is the absolute operating temperature (K);
- -
- T0 is the temperature at the standard state (273.15 K);
- -
- (dp/dt)ss is the steady-state pressure increase in the permeate side (cmHg s-1) under the feed pressure;
- -
- (dp/dt)leak is the pressure increase in the permeate side under vacuum (leakage pressure increase).
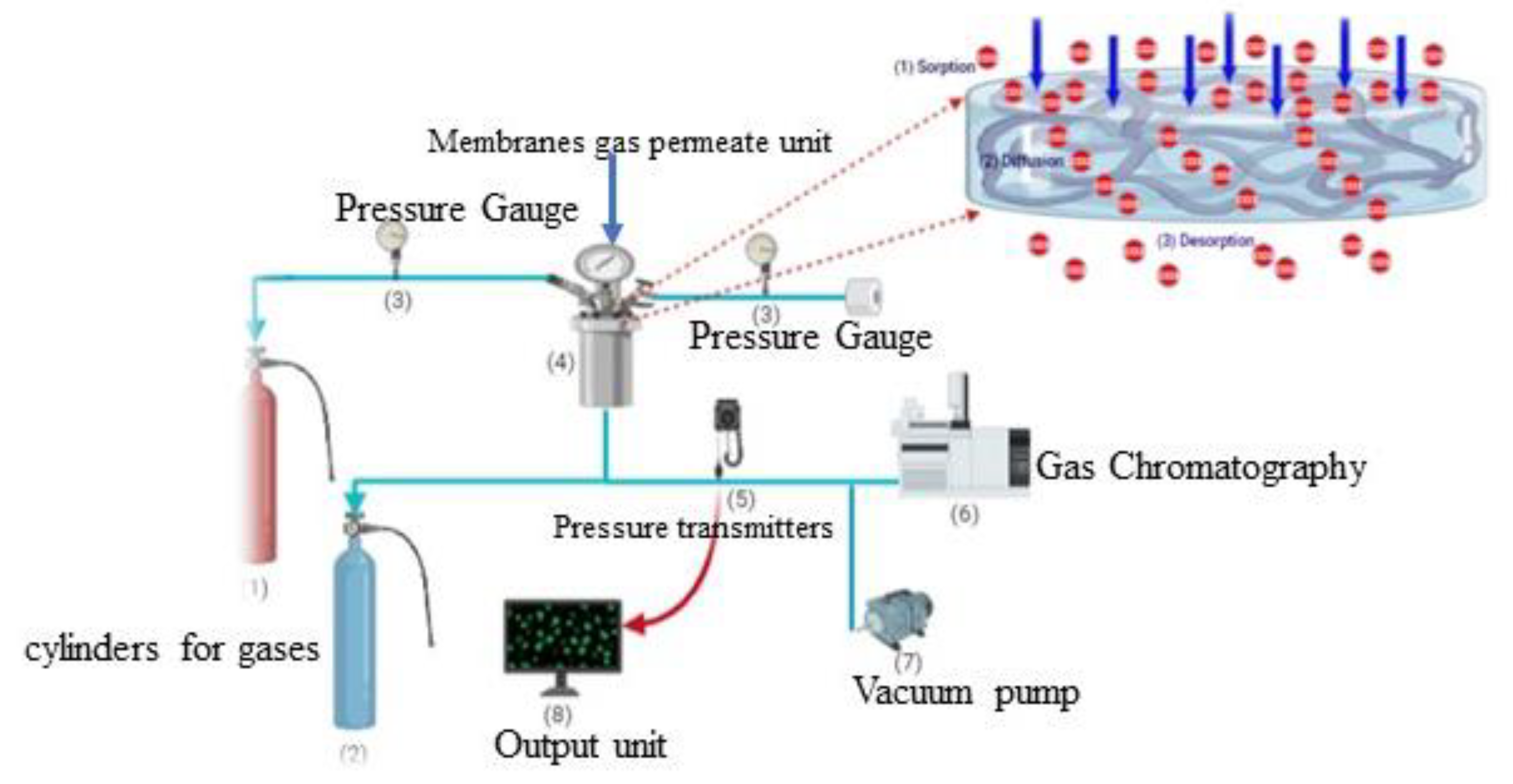
3. Results and Discussion
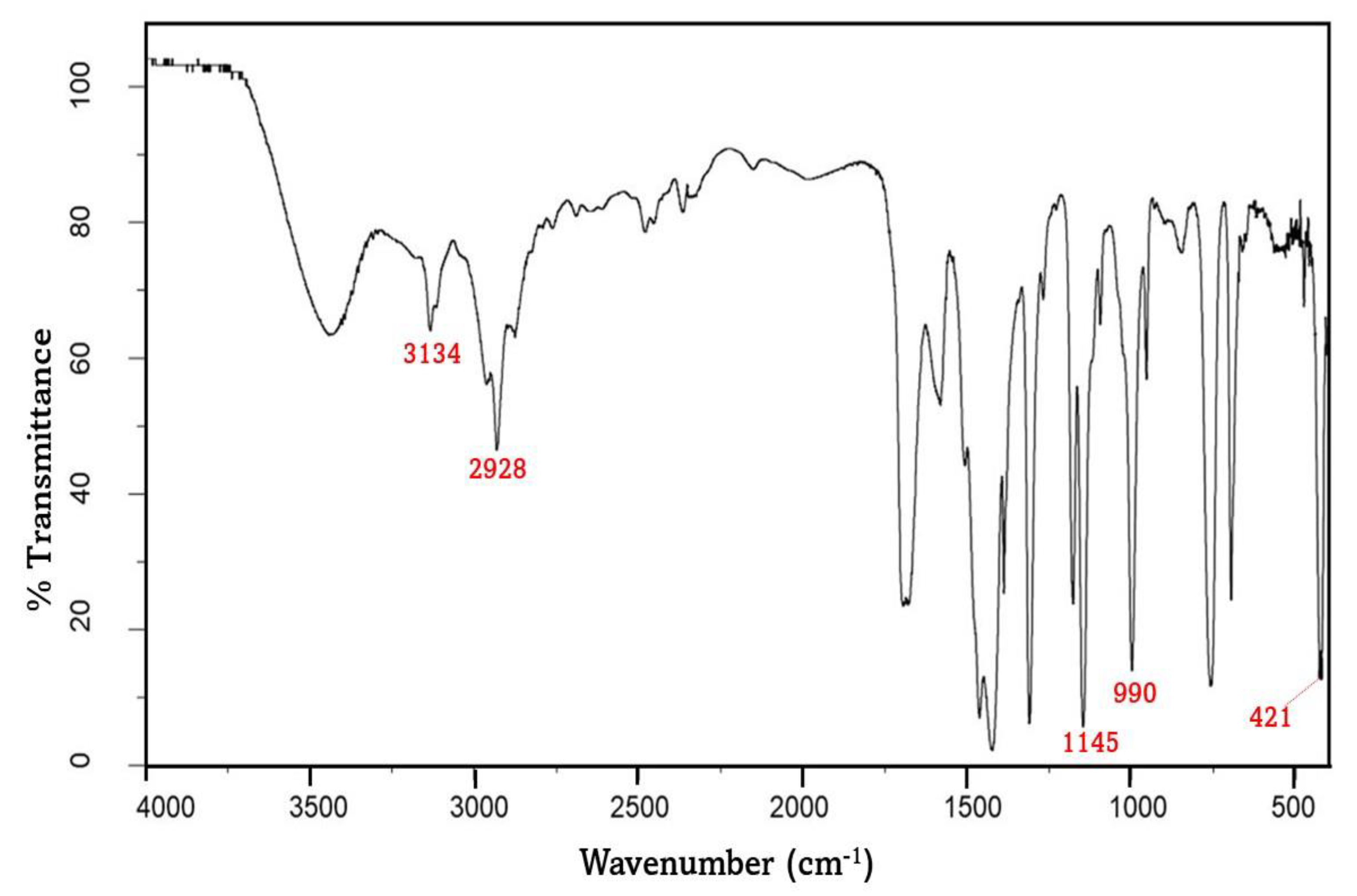
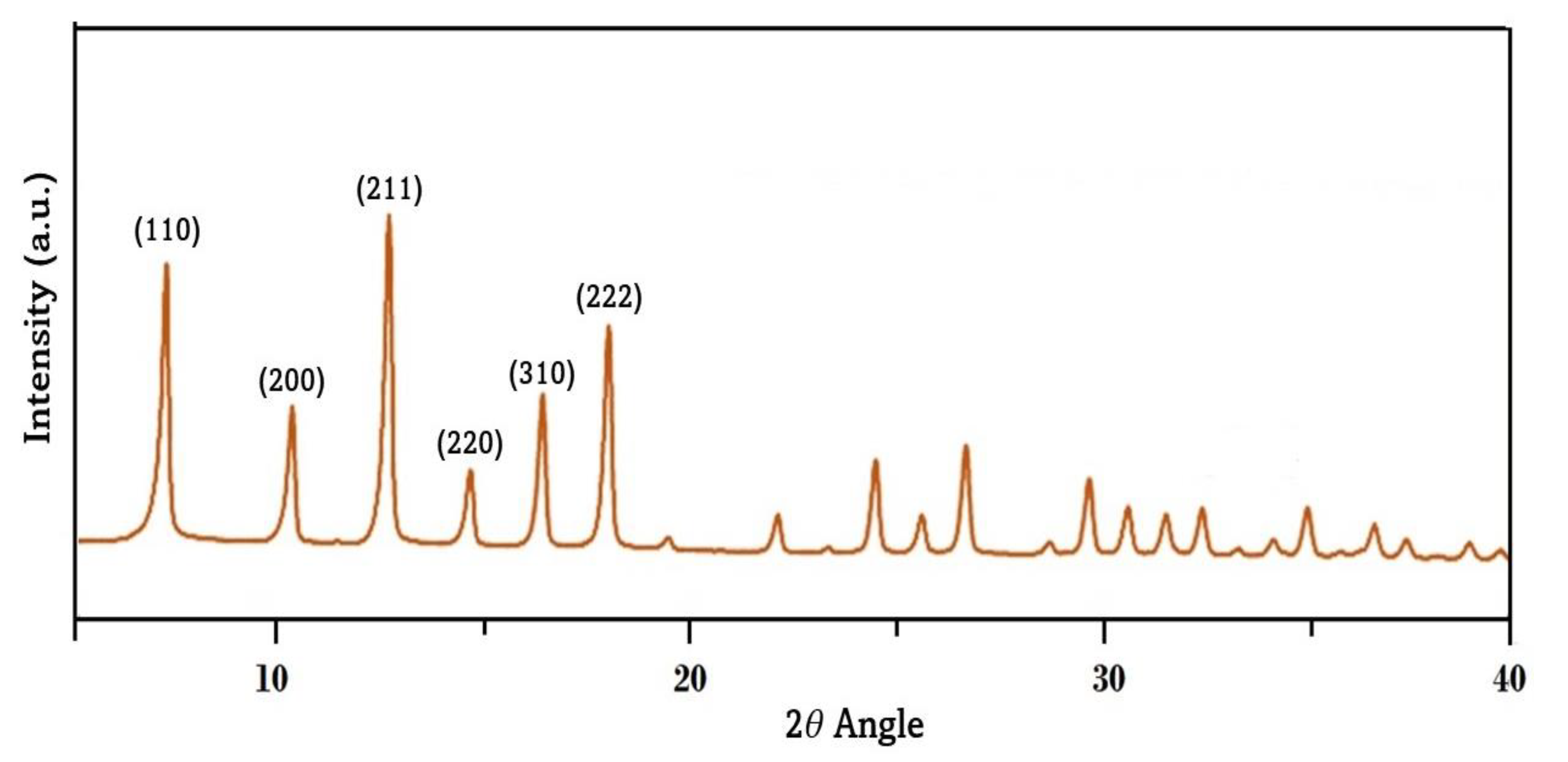
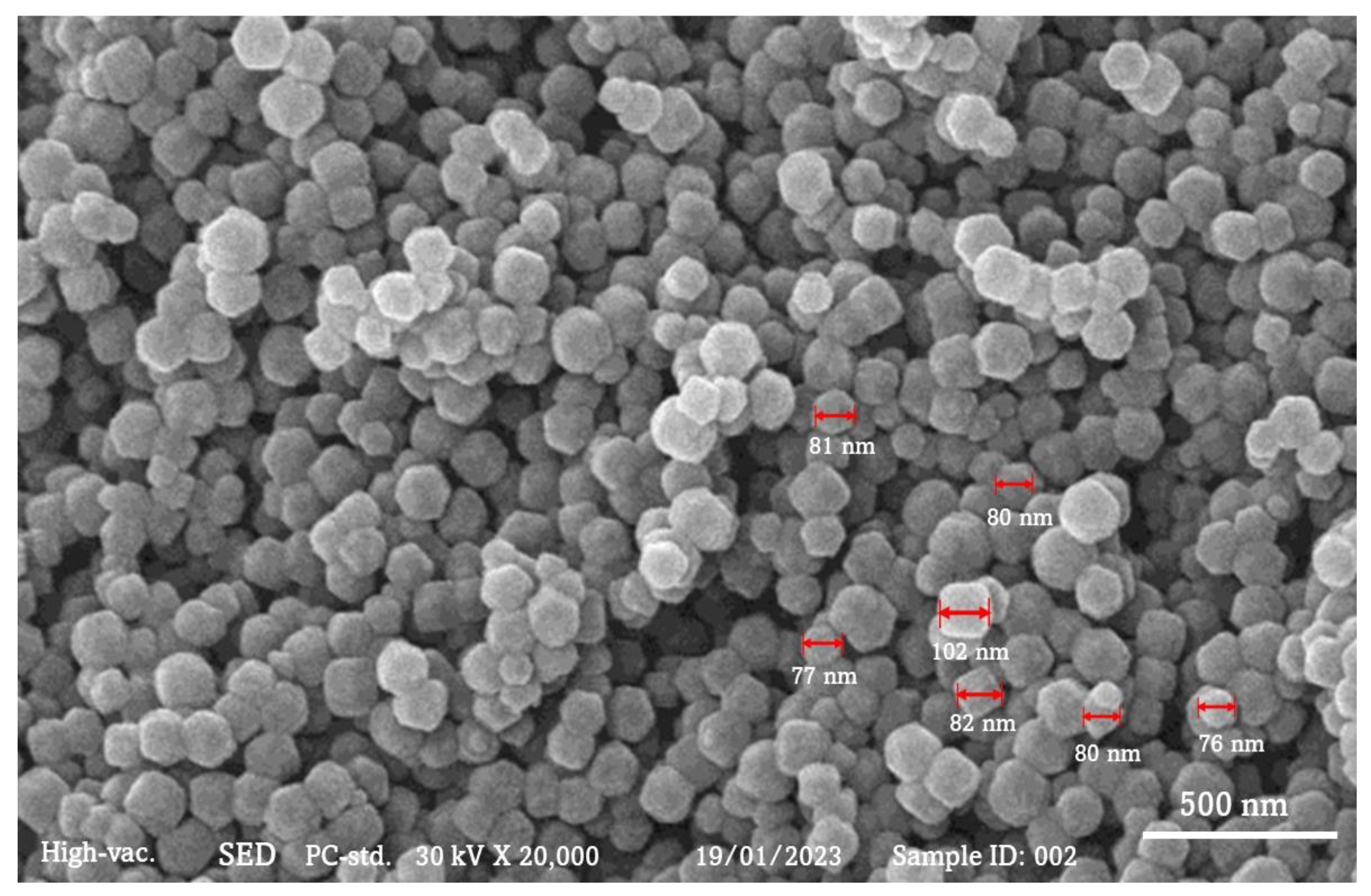
3.1. Characterization of ZIF-8
3.2. XRD
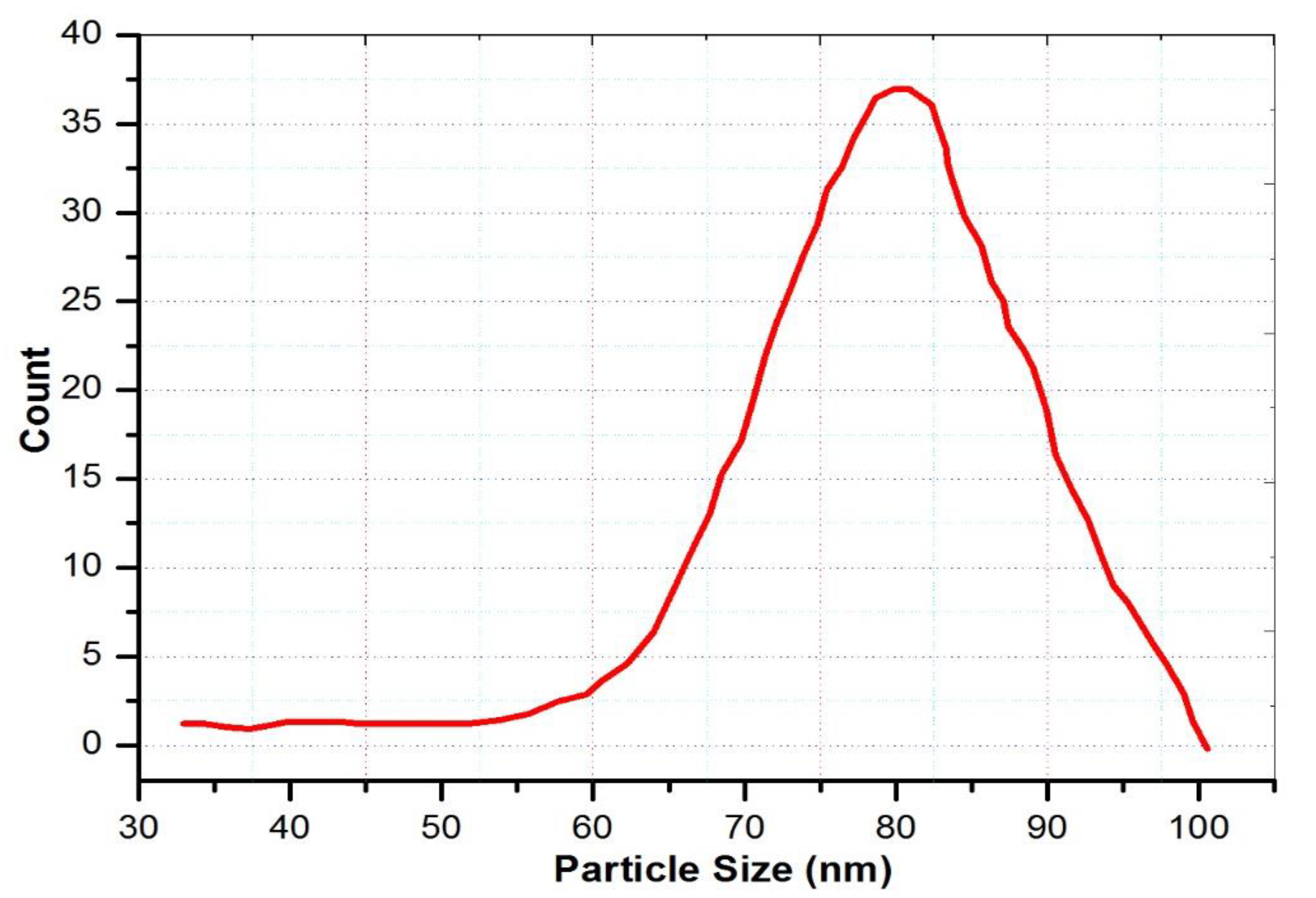
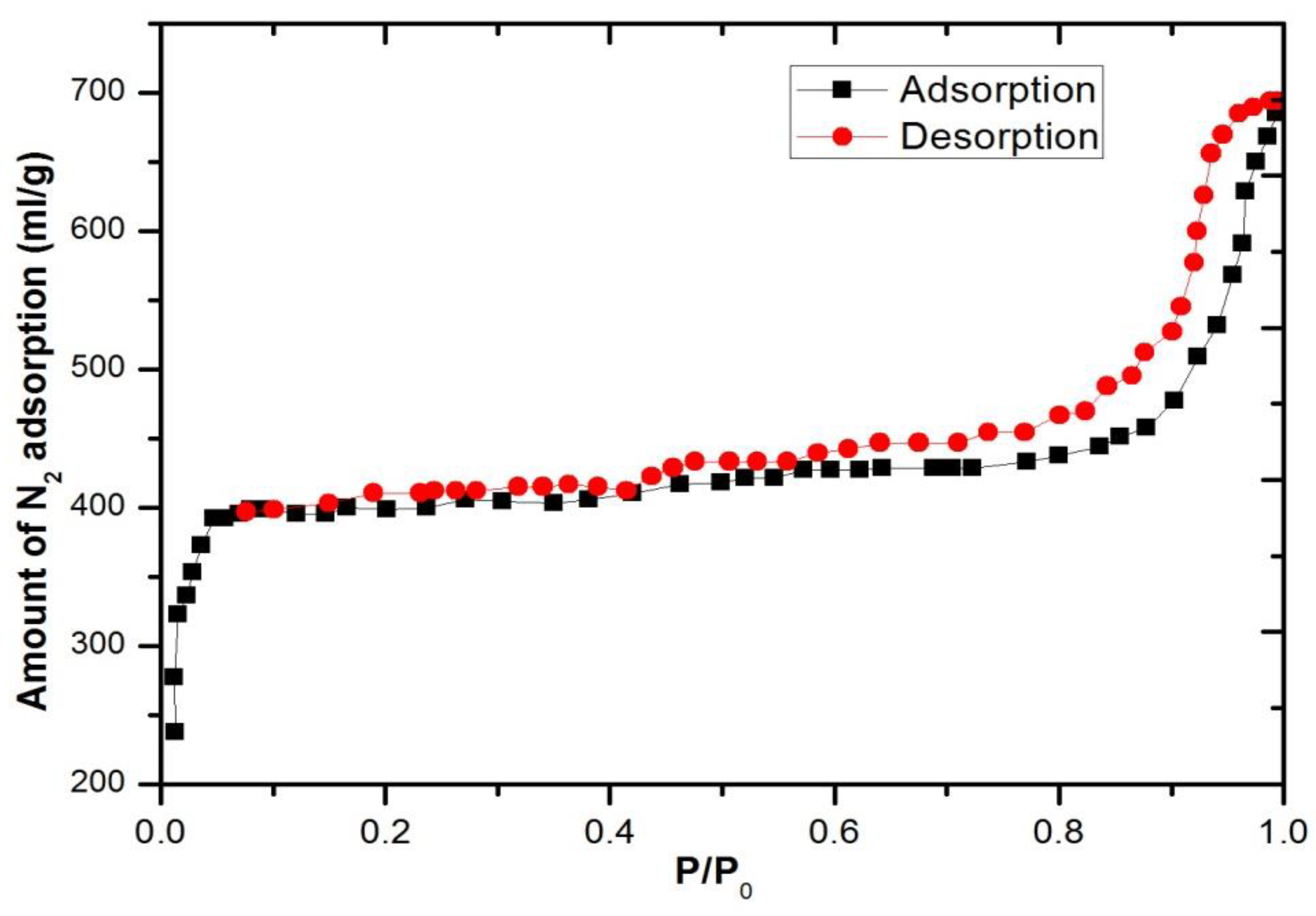
3.3. SEM and Adsorption Analysis
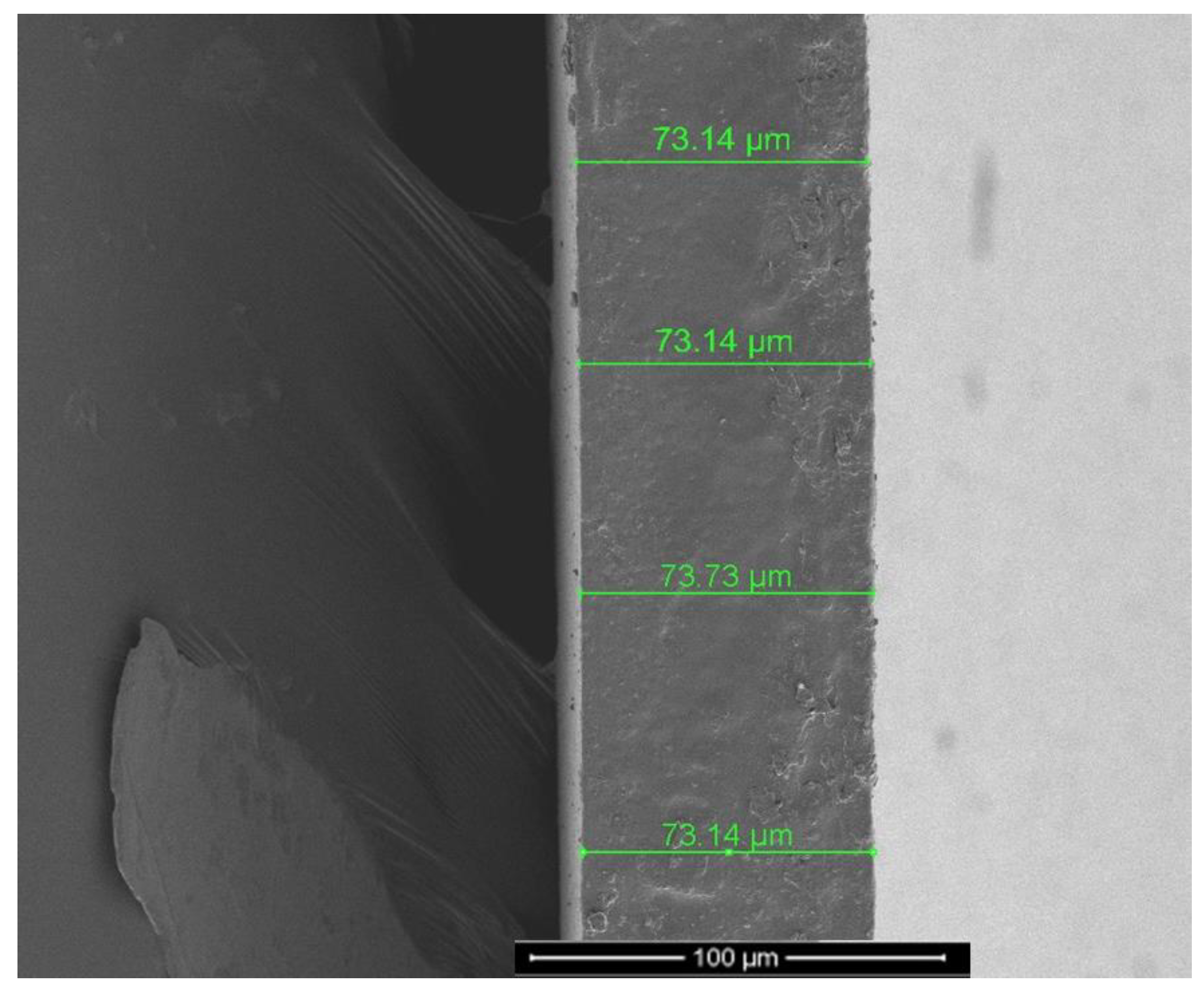
3.4. Characterization of Membranes
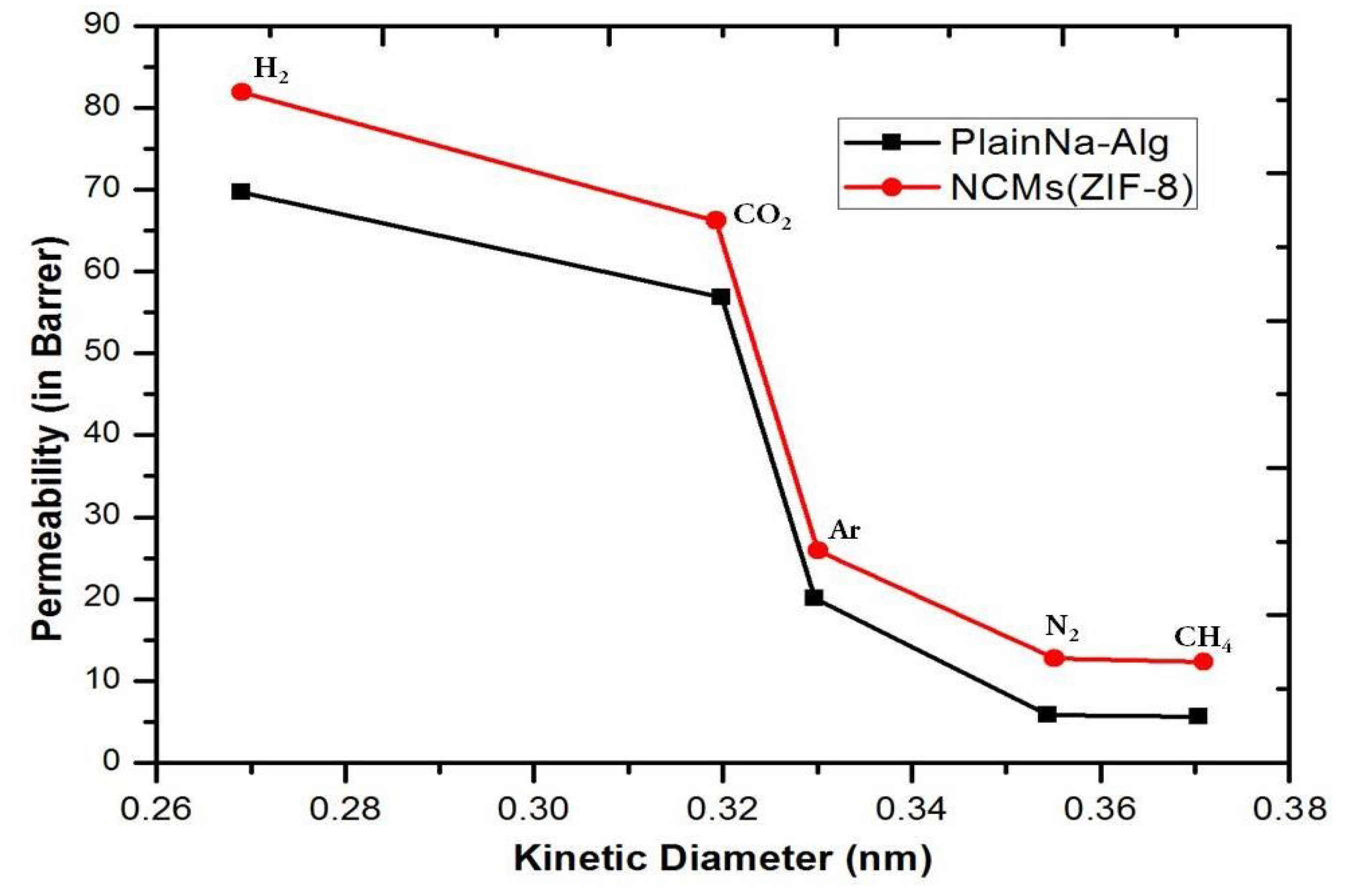
3.5. Single Gas Permeation through Membranes
3.6. Comparison Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Lu, G.; Millar, G.J. Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: State of the art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Menegazzo, F.; Pizzolitto, C.; Ghedini, E.; Di Michele, A.; Cruciani, G.; Signoretto, M. Development of La Doped Ni/CeO2 for CH4/CO2 Reforming. J. Carbon Res. 2018, 4, 60. [Google Scholar] [CrossRef]
- Razmara, N.; Kirch, A.; Meneghini, J.R.; Miranda, C.R. Efficient CH4/CO2 Gas Mixture Separation through Nanoporous Graphene Membrane Designs. Energies 2021, 14, 2488. [Google Scholar] [CrossRef]
- Zhang, Z.; Fuoco, A.; He, G. Membranes for Gas Separation. Membranes 2021, 11, 755. [Google Scholar] [CrossRef]
- Samarasinghe, S.A.S.C.; Chuah, C.Y.; Karahan, H.E.; Sethunga, G.S.M.D.P.; Bae, T.-H. Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles. Membranes 2020, 10, 75. [Google Scholar] [CrossRef]
- Mazumder, A.; Kim, J.M.; Hunter, B.; Beckingham, B.S. Controlling Fractional Free Volume, Transport, and Co-Transport of Alcohols and Carboxylate Salts in PEGDA Membranes. Membranes 2023, 13, 17. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Effect of condensable impurity in CO2/CH4 gas feeds on performance of mixed matrix membranes using carbon molecular sieves. J. Membr. Sci. 2003, 221, 233–239. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed-matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef]
- Aroon, M.; Ismail, A.; Matsuura, T.; Montazer-Rahmati, M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Khdhayyer, M.R.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C.; Attfield, M.P.; Budd, P.M. Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2017, 173, 304–313. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17007. [Google Scholar] [CrossRef]
- Fuoco, A.; Khdhayyer, M.R.; Attfield, M.P.; Esposito, E.; Jansen, J.C.; Budd, P.M. Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation. Membranes 2017, 7, 7. [Google Scholar] [CrossRef]
- Patil, M.B.; Amshumali, M.K. Sweetening of Natural Gas Through Hollow Silica Nanoparticles Embedded Hydroxyethyl Cellulose Membrane. Mater. Sci. Res. India 2018, 15, 256–262. [Google Scholar] [CrossRef]
- Patil, M.; Mathad, S.N.; Patil, A.Y.; Arshad, M.N.; Alorfi, H.S.; Puttegowda, M.; Asiri, A.M.; Khan, A.; Azum, N. Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly (vinyl alcohol)-g-starch-methacrylate and Their Evaluation for Gas Transport Properties. Polymers 2022, 14, 350. [Google Scholar] [CrossRef]
- Smith, S.J.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-synthetic Ti exchanged UiO-66 metal-organic frameworks that deliver exceptional gas permeability in mixed matrix membranes. Sci. Rep. 2015, 5, 7823. [Google Scholar] [CrossRef]
- Anjum, M.W.; Vermoortele, F.; Khan, A.L.; Bueken, B.; de Vos, D.E.; Vankelecom, I.F. Modulated UiO-66-based mixed-matrix membranes for CO2 separation. ACS Appl. Mater. Interfaces 2015, 7, 25193–25201. [Google Scholar] [CrossRef]
- Askari, M.; Chung, T.S. Natural gas purification and olefin/paraffin separation using thermal cross-linkable co-polyimide/ZIF-8 mixed matrix membranes. J. Membr. Sci. 2013, 444, 173–183. [Google Scholar] [CrossRef]
- Wijenayake, S.N.; Panapitiya, N.P.; Versteeg, S.H.; Nguyen, C.N.; Goel, S.; Balkus, K.J.; Jr Musselman, I.H.; Ferraris, J.P. Surface cross-linking of ZIF-8/polyimide mixed matrix membranes (MMMs) for gas separation. Ind. Eng. Chem. Res. 2013, 52, 6991–7001. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Phan, P.T.; Hong, J.; Tran, N.; Le, T.H. The Properties of Microwave-Assisted Synthesis of Metal–Organic Frameworks and Their Applications. Nanomaterials 2023, 13, 352. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Casco, M.E.; Cheng, Y.Q.; Daemen, L.L.; Fairen-Jimenez, D.; Ramos-Ferna’ndez, E.V.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Gate-opening effect in ZIF-8: The first experimental proof using inelastic neutron scattering. Chem. Commun. 2016, 52, 3639. [Google Scholar] [CrossRef]
- Zornoz, B.; Telle, C.; Corona, J.; Gasco, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Nijem, N.; Wu, H.; Canepa, P.; Marti, A.; Balku KJJr Thonhauser, T.; Li, J.; Chabal, Y.J. Tuning the gate opening pressure of metal–organic frameworks (MOFs) for the selective separation of hydrocarbons. J. Am. Chem. Soc. 2012, 134, 15201–15204. [Google Scholar] [CrossRef]
- Patil, M.B.; Patil, S.A.; Veerapur, R.S.; Aminabhavi, T.M. Novel Poly (vinyl alcohol)-tetraethoxysilane hybrid matrix membranes as oxygen barriers. J. Appl. Polym. Sci. 2007, 104, 273–278. [Google Scholar] [CrossRef]
- Patil, M.B.; Rajamani, S.B.; Gani, R.S. Novel Poly (vinyl alcohol)-g-Acrylamide Graft Membranes as Oxygen Barriers and their Application in Fruit and Vegetable Packaging. Acta Chem. Iasi 2020, 28, 162–174. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal–organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef]
- Patil, M.B.; Rajamani, S.B.; Mathad, S.N.; Patil, A.Y.; Hussain, M.A.; Alorfii, H.S.; Khan, A.; Asiri, A.M.; Khan, I.; Puttegowda, M. Microwave-Assisted Synthesis of Poly (Acrylamide-co-2-Hydroxyethyl Methacrylate)/Chitosan Semi-IPN ZnO Nanocomposite Membranes for Food Packaging Applications. J. Mater. Res. Technol. 2022, 20, 3537–3548. [Google Scholar] [CrossRef]
- Huang, D.; Xin, Q.; Ni, Y.; Shuai, Y.; Wang, S.; Li, Y.; Ye, H.; Lin, L.; Ding, X.; Zhang, Y. Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation. RSC Adv. 2018, 8, 6099. [Google Scholar] [CrossRef]



| Sample | BET Surface Area (m2/g) | Mesopore Volume (cm3/g) | Micropore Volume (cm3/g) | Net Pore Volume (cm3/g) |
|---|---|---|---|---|
| ZIF-8 | 1632.3 | 0.521 | 0.569 | 1.09 |
| Membrane | Operating Pressure (kg/cm2) | Temperature (°C) | PN2 (Barrer) | PCO2 (Barrer) | Reference |
|---|---|---|---|---|---|
| Hollow silica nanoparticles embedded hydroxyethyl cellulose membrane | 4 | 30 | 10.10 | 71.30 | 17 |
| Poly(vinyl alcohol)-g-starch methacrylate | 4 | 30 | 0.674 | 10.474 | 18 |
| ZIF-8/graphene oxide-15 | 4 | 30 | 0.31 | 14.50 | 35 |
| Na-Alg-ZIF-8 (15 wt.%) | 4 | 30 | 11.3 | 66.4 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.P.; Patil, M.B.; Rathod, L.P.; Vader, S.G.; Raizada, P.; Singh, P.; Alotaibi, M.M.; Ansari, M.O.; Khan, A.; Azum, N.; et al. Polymer Membranes of Zeolitic Imidazole Framework-8 with Sodium Alginate Synthesized from ZIF-8 and Their Application in Light Gas Separation. Polymers 2023, 15, 1011. https://doi.org/10.3390/polym15041011
Khan AAP, Patil MB, Rathod LP, Vader SG, Raizada P, Singh P, Alotaibi MM, Ansari MO, Khan A, Azum N, et al. Polymer Membranes of Zeolitic Imidazole Framework-8 with Sodium Alginate Synthesized from ZIF-8 and Their Application in Light Gas Separation. Polymers. 2023; 15(4):1011. https://doi.org/10.3390/polym15041011
Chicago/Turabian StyleKhan, Aftab Aslam Parwaz, Mallikarjunagouda B. Patil, Laxmibai P. Rathod, Shivalila G. Vader, Pankaj Raizada, Pardeep Singh, Maha M. Alotaibi, Mohammad Omaish Ansari, Anish Khan, Naved Azum, and et al. 2023. "Polymer Membranes of Zeolitic Imidazole Framework-8 with Sodium Alginate Synthesized from ZIF-8 and Their Application in Light Gas Separation" Polymers 15, no. 4: 1011. https://doi.org/10.3390/polym15041011
APA StyleKhan, A. A. P., Patil, M. B., Rathod, L. P., Vader, S. G., Raizada, P., Singh, P., Alotaibi, M. M., Ansari, M. O., Khan, A., Azum, N., Rub, M. A., Arshad, M. N., & Asiri, A. M. (2023). Polymer Membranes of Zeolitic Imidazole Framework-8 with Sodium Alginate Synthesized from ZIF-8 and Their Application in Light Gas Separation. Polymers, 15(4), 1011. https://doi.org/10.3390/polym15041011














