Intrinsically Conducting Polymer Composites as Active Masses in Supercapacitors
Abstract
1. Introduction
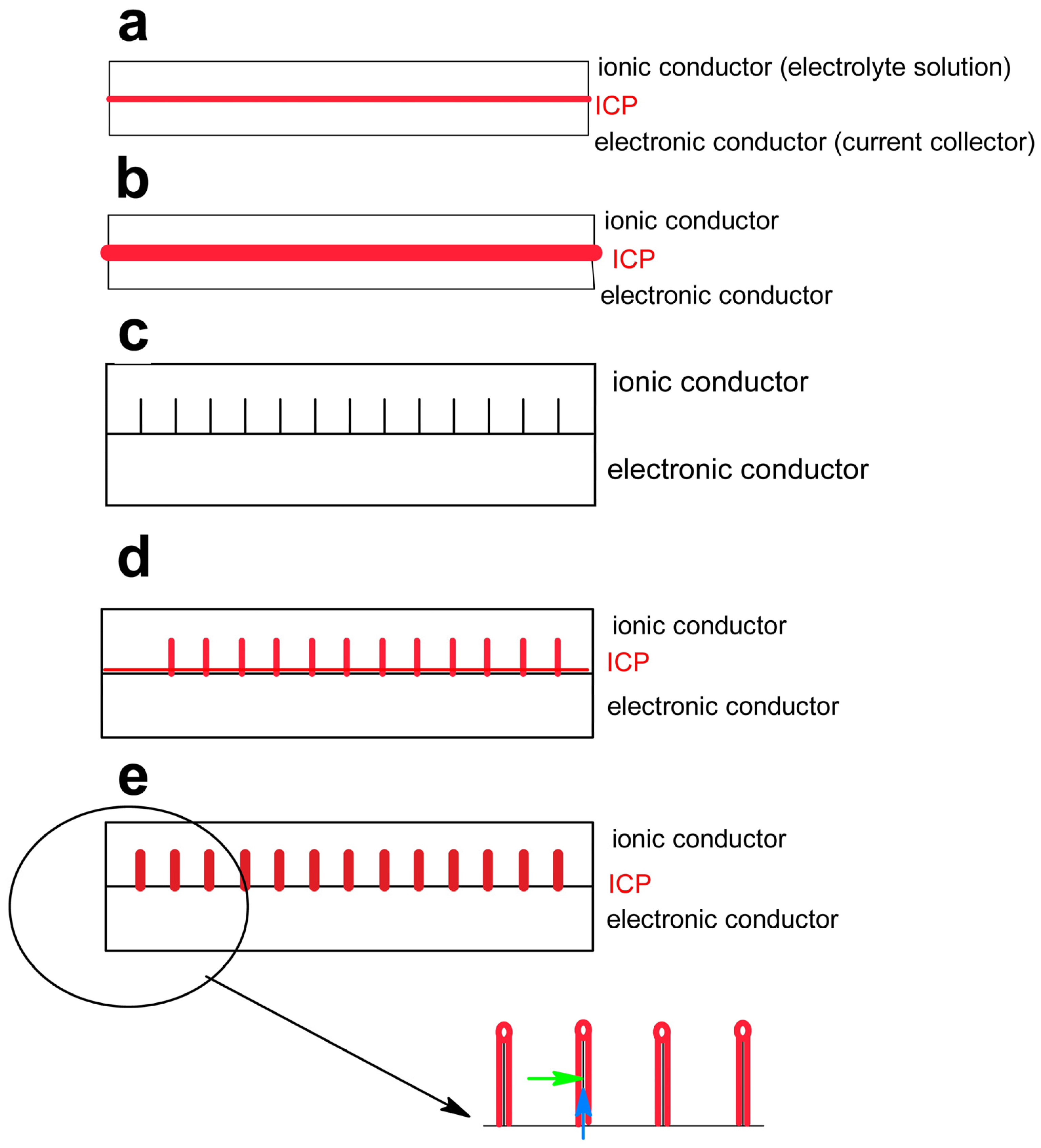
2. Fundamentals
- –
- ICP
- material for charge storage
- mechanical binder
- mechanical stabilizer compensating volume changes
- electronic conductance enhancer
- active mass dissolution inhibitor
- –
- second constituent
- material for charge storage
- template for structuring ICP morphology
- structural/mechanical support for ICP
3. The Materials
4. The Combinations
- Enhanced stability (includes mitigation of volume changes, inhibition of materials dissolution)
- Enhanced conductivity
4.1. ICPs and Carbonaceous Materials
4.2. ICPs and Chalcogenides
4.3. Further Binary Combinations
4.4. Ternary Composites with ICPs
4.5. Further Combinations
4.6. Miscellaneous Observations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sebastian, J.; Samuel, J.M. Recent advances in the applications of substituted polyanilines and their blends and composites. Polym. Bull. 2019, 77, 6641–6669. [Google Scholar]
- Kickelbick, G. (Ed.) Hybrid Materials; WILEY-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Kurc, B.; Pigłowska, M.; Rymaniak, Ł.; Fuć, P. Modern nanocomposites and hybrids as electrode materials used in energy car-riers. Nanomaterials 2021, 11, 538. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar]
- Dahiya, Y.; Hariram, M.; Kumar, M.; Jain, A.; Sarkar, D. Modified transition metal chalcogenides for high performance supercapacitors: Current trends and emerging opportunities. Coord. Chem. Rev. 2022, 451, 214265. [Google Scholar]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: New York, NY, USA, 1999. [Google Scholar]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar]
- Holze, R. Composites of intrinsically conducting polymers with carbonaceous materials for supercapacitors—An update. Univers. J. Electrochem. submitted.
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Source 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Lu, S.; Varanasi, C.V.; Liu, J. Flexible asymmetric supercapacitors with high energy and high power density in aqueous electrolytes. Nanoscale 2013, 5, 1067–1073. [Google Scholar] [CrossRef]
- Xu, L.; Xu, J.; Yang, Y.; Mao, X.; He, X.; Yang, W.; Zhao, Y.; Zhou, Y. A flexible fabric electrode with hierarchical carbon-polymer composite for functional supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 2322–2330. [Google Scholar] [CrossRef]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward flexible polymer and paper-based energy storage devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar]
- Ramachandran, R.; Chen, T.W.; Chen, S.M.; Raja, P.; Fernandez, C.; Rani, S.D.; Gajendran, P.; Raju, G.; Baskar, T. Highly Enhanced Electrochemical Performance of Novel based Electrode Materials for Supercapacitor Applications—An Overview. Int. J. Electrochem. Sci. 2019, 14, 1634–1648. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, X.; Lu, Y.; Su, Y.; Chen, R.; Wu, F. Flexible Electrode Assembled from Different Microstructures. Prog. Chem. 2019, 31, 464–474. [Google Scholar]
- Cheng, M.; Meng, Y.N.; Wei, Z.X. Conducting Polymer Nanostructures and their Derivatives for Flexible Supercapacitors. Isr. J. Chem. 2018, 58, 1299–1314. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for porta-ble/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Chakraborty, S.; Mary, N.L. Review—An Overview on Supercapacitors and Its Applications. J. Electrochem. Soc. 2022, 169, 020552. [Google Scholar] [CrossRef]
- Dhandapani, E.; Thangarasu, S.; Ramesh, S.; Ramesh, K.; Vasudevan, R.; Duraisamy, N. Recent development and prospective of carbonaceous material, conducting polymer and their composite electrode materials for supercapacitor—A review. J. Energy Storage 2022, 52, 104937. [Google Scholar]
- Patel, K.K.; Singhal, T.; Pandey, V.; Sumangala, T.P.; Sreekanth, M.S. Evolution and recent developments of high performance electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103366. [Google Scholar]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Elkodous, M.A.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S. Advanced materials and technologies for supercapacitors used in energy conversion and storage: A review. Environ. Chem. Lett. 2021, 19, 375–439. [Google Scholar]
- Bigdeloo, M.; Kowsari, E.; Ehsani, A.; Chinnappan, A.; Ramakrishna, S.; Ali Akbari, R. Review on innovative sustainable nanomaterials to enhance the performance of supercapacitors. J. Energy Storage 2021, 37, 102474. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar]
- Li, Z.; Xu, K.; Pan, Y. Recent development of Supercapacitor Electrode Based on Carbon Materials. Nanotechnol. Rev. 2019, 8, 35–49. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capac-itance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Bhardwaj, P.; Grace, A.N.; Stephan, A.M. Role of Polymers in Enhancing the Performance of Electrochemical Supercapacitors: A Review. Batter. Supercaps 2021, 4, 571–584. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Abd Sata, S.; Buniran, S. Multiwalled carbon nanotubes based nanocomposites for supercapacitors: A review of electrode materials. Nano 2012, 7, 12300022. [Google Scholar] [CrossRef]
- Dubal, D.P.; Wu, Y.; Holze, R. Supercapacitors as fast storage systems for electric energy. Bunsen-Magazin 2015, 17, 216–227. [Google Scholar]
- Shukla, A.K.; Sampath, S.; Vijayamohanan, K. Electrochemical supercapacitors: Energy storage beyond batteries. Curr. Sci. 2000, 79, 1656–1661. [Google Scholar]
- Dubal, D.P.; Wu, Y.P.; Holze, R. Supercapacitors: From the Leyden jar to electric busses. ChemTexts 2016, 2, 13. [Google Scholar] [CrossRef]
- Yu, A.; Chabot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery—Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Inamuddin; Ahmer, M.F.; Asiri, A.M.; Zaidi, S. Electrochemical Capacitors in: Materials Research Foundations; Materials Research Forum LLC: Millersville, PA, USA, 2018; Volume 26. [Google Scholar]
- Stevic, Z. Supercapacitor Design and Applications; IntechOpen: London, UK, 2016. [Google Scholar]
- Miller, J.M. Ultracapacitor Applications; The Institution of Engineering and Technology: London, UK, 2011. [Google Scholar]
- Shaikh, N.S.; Ubale, S.B.; Mane, V.J.; Shaikh, J.S.; Lokhande, V.C.; Praserthdam, S.; Lokhande, C.D.; Kanjanaboos, P. Novel electrodes for supercapacitor: Conducting polymers, metal oxides, chalcogenides, carbides, nitrides, MXenes, and their com-posites with graphene. J. Alloys Compd. 2022, 893, 161998. [Google Scholar]
- Beguin, F.; Frackowiak, E. Supercapacitors; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Obreja, V.V.N. Supercapacitors specialities—Materials review. AIP Conf. Proc. 2014, 1597, 98–120. [Google Scholar]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar]
- Ge, Y.; Xie, X.; Roscher, J.; Holze, R.; Qu, Q. How to measure and report the capacity of electrochemical double layers, su-percapacitors, and their electrode materials. J. Solid State Electrochem. 2020, 24, 3215–3230. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, Z.; Wu, Y.; Holze, R. On the utilization of supercapacitor electrode materials. Electrochim. Acta 2021, 366, 137390. [Google Scholar]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Conway, B.E.; Gileadi, E. Kinetic Theory of Pseudo-Capacitance and Electrode Reactions at Appreciable Surface Coverage. Trans. Faraday Soc. 1962, 58, 2493–2509. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications, Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar]
- Kulkarni, P.; Nataraj, S.K.; Balakrishna, R.G.; Nagaraju, D.H.; Reddy, M.V. Nanostructured binary and ternary metal sulfides: Synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A 2017, 5, 22040–22094. [Google Scholar]
- Chen, D.; Wang, Q.; Wang, R.; Shen, G. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, C.; An, C.; Xu, Y.; Dong, Y.; Zhang, Q.; Wang, Y.; Jiao, L.; Yuan, H. Facile synthesis of diverse transition metal oxide nanoparticles and electrochemical properties. Inorg. Chem. Front. 2016, 3, 1048–1057. [Google Scholar] [CrossRef]
- Tian, X.D.; Li, X.; Yang, T.; Song, Y.; Liu, Z.J.; Guo, Q.G. Recent Advances on Synthesis and Supercapacitor Application of Binary Metal Oxide. J. Inorg. Mater. 2017, 32, 459–468. [Google Scholar]
- Wang, R.; Wu, J. Structure and basic properties of ternary metal oxides and their prospects for application in supercapacitors. In Metal Oxides in Supercapacitors; Dubal, D.P., Gomez-Romero, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–132. [Google Scholar]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Volkov, A.I.; Dubal, D.P.; Holze, R.; Wu, Y. Mixed metal chalcogenides as active masses for supercapacitor electrodes—A review. Adv. Function. Mater. submitted.
- Holze, R.; Wu, Y.P. Intrinsically conducting polymers in electrochemical energy technology: Trends and progress. Electrochim. Acta 2014, 122, 93–107. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting Polymers for Pseudocapacitive Energy Storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent advancements in synthesis, properties, and applications of conductive polymers for electrochemical energy storage devices: A review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Holze, R. Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes-An Introduction. Polymers 2020, 12, 1835. [Google Scholar] [CrossRef]
- Kondratiev, V.V.; Holze, R. Intrinsically conducting polymers and their combinations with redox-active molecules for re-chargeable battery electrodes: An update. Chem. Pap. 2021, 75, 4981–5007. [Google Scholar] [CrossRef]
- Holze, R. Conjugated Molecules and Polymers in Secondary Batteries: A Perspective. Molecules 2022, 27, 546. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research progress on applications of polyaniline (PANI) for electrochemical energy storage and conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Dev. 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Liu, S.; Li, L.; Zhang, C.; Liu, T. Conducting polymer composites: Material synthesis and applications in elec-trochemical capacitive energy storage. Mater. Chem. Front. 2017, 1, 251–268. [Google Scholar] [CrossRef]
- Chabi, S.; Peng, C.; Hu, D.; Zhu, Y. Ideal Three-Dimensional Electrode Structures for Electrochemical Energy Storage. Adv. Mater. 2014, 26, 2440–2445. [Google Scholar] [CrossRef]
- Liu, T.T.; Shao, G.J.; Ji, M.T.; Ma, Z.P. Research Progress in Nano-Structured MnO2 as Electrode Materials for Supercapacitors. Asian J. Chem. 2013, 25, 7065–7070. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Wang, X.; Gu, W.; Benson, J.; Zhao, E.; Magasinski, A.; Evanoff, K. Nanostructured composites for high energy batteries and supercapacitors. In Proceedings of the 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO), Rome, Italy, 27–30 July 2015; pp. 572–576. [Google Scholar]
- Cherusseri, J.; Sambath Kumar, K.; Choudhary, N.; Nagaiah, N.; Jung, Y.; Roy, T.; Thomas, J. Novel mesoporous electrode materials for symmetric, asymmetric and hybrid supercapacitors. Nanotechnology 2019, 30, 202001. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled synthesis and energy applications of one-dimensional conducting polymer nanostructures: An overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Chae, J.H.; Ng, K.C.; Chen, G.Z. Nanostructured materials for the construction of asymmetrical supercapacitors. Proc. Inst. Mech. Eng. Part A J. Power Energy 2010, 224, 479–503. [Google Scholar] [CrossRef]
- Malinauskas, A.; Malinauskiene, J.; Ramanavicius, A. Conducting polymer-based nanostructurized materials: Electrochemical aspects. Nanotechnology 2005, 16, R51–R62. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Chojak, M.; Cuentas-Gallegos, K.; Asensio, J.A.; Kulesza, P.J.; Casañ-Pastor, N.; Lira-Cantú, M. Hybrid organic-inorganic nanocomposite materials for application in solid state electrochemical supercapacitors. Electrochem. Commun. 2003, 5, 149–153. [Google Scholar] [CrossRef]
- Holze, R. From current peaks to waves and capacitive currents-on the origins of capacitor-like electrode behavior. J. Solid State Electrochem. 2017, 21, 2601–2607. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Lyu, L.; Seong, K.D.; Ko, D.; Choi, J.; Lee, C.; Hwang, T.; Cho, Y.; Jin, X. Recent development of biomass-derived carbons and composites as electrode materials for supercapacitors. Mater. Chem. Front. 2019, 3, 2543–2570. [Google Scholar] [CrossRef]
- Mustaqeem, M.; Naikoo, G.A.; Yarmohammadi, M.; Pedram, M.Z.; Pourfarzad, H.; Dar, R.A.; Taha, S.A.; Hassan, I.U.; Bhat, Y. Rational design of metal oxide based electrode materials for high performance supercapacitors—A review. J. Energy Storage 2022, 55, 105419. [Google Scholar] [CrossRef]
- AL-Refai, H.H.; Ganash, A.A.; Hussein, M.A. Polythiophene and its derivatives -Based nanocomposites in electrochemical sensing: A mini review. Mater. Today Commun. 2021, 26, 101935. [Google Scholar] [CrossRef]
- Kausar, A. Conjugated polymer/graphene oxide nanocomposites—State-of-the-art. J. Compos. Sci. 2021, 5, 292. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Majumder, M. Recent advances on redox active composites of metal-organic framework and conducting polymers as pseudocapacitor electrode material. Renew. Sustain. Energy Rev. 2021, 145, 110854. [Google Scholar] [CrossRef]
- Waltman, R.J.; Diaz, A.F.; Bargon, J. Substituent Effects in the Electropolymerization of Aromatic Heterocyclic Compounds. J. Phys. Chem. 1984, 88, 4343–4346. [Google Scholar] [CrossRef]
- Arjomandi, J.; Nematollahi, D.; Amani, A. Enhanced electrical conductivity of polyindole prepared by electrochemical polymerization of indole in ionic liquids. J. Appl. Polym. Sci. 2014, 131, 40094. [Google Scholar] [CrossRef]
- Mudila, H.; Prasher, P.; Kumar, M.; Kumar, A.; Zaidi, M.G.H.; Kumar, A. Critical analysis of polyindole and its composites in supercapacitor application. Mater. Renew. Sustain. Energy 2019, 8, 9. [Google Scholar] [CrossRef]
- Dai, L. Conjugated and fullerene-containing polymers for electronic and photonic applications: Advanced syntheses and mi-crolithographic fabrications. J. Macromol. Sci. Polym. Rev. 1999, 39, 273–387. [Google Scholar] [CrossRef]
- Pettersson, L.A.; Carlsson, F.; Inganäs, O.; Arwin, H. Spectroscpic ellipsometry Study of the optical properties of doped poly(3,4-ethylenedioxythiophene): An anisotropic metal. Thin Solid Films 1998, 313–314, 356–361. [Google Scholar] [CrossRef]
- Czardybon, A.; Lapkowski, M. Synthesis and electropolymerisation of 3,4-ethylenedoxythiophene functionalized with alkoxy groups. Synth. Met. 2001, 119, 161–162. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Yadav, B.C. Conducting Polymers: Synthesis, Properties and Applications. Int. Adv. Res. J. Sci. Eng. Technol. 2015, 2, 110–124. [Google Scholar]
- Dai, L. Intelligent Macromolecules for Smart Devices; Springer: London, UK, 2004. [Google Scholar]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Holze, R. Metal oxide/conducting polymer hybrids for application in supercapacitors. In Metal Oxides in Supercapacitors; Dubal, D., Gomez-Romero, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; p. 219. [Google Scholar]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef]
- Ouyang, J. Recent Advances of Intrinsically Conductive Polymers. Acta Phys. -Chim. Sin. 2018, 34, 1211–1220. [Google Scholar] [CrossRef]
- Uke, S.K.; Mardikar, S.P.; Kumar, A.; Kumar, Y.; Gupta, M.; Kumar, Y. A review of π-conjugated polymer-based nanocom-posites for metal-ion batteries and supercapacitors. R. Soc. Open Sci. 2021, 8, 210567. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Hu, D.; Chen, G.Z. Theoretical specific capacitance based on charge storage mechanisms of conducting polymers: Comment on ‘Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties’. Chem. Commun. 2011, 47, 4105–4107. [Google Scholar] [CrossRef] [PubMed]
- Kuila, B.K.; Nandan, B.; Böhme, M.; Janke, A.; Stamm, M. Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties. Chem. Commun. 2009, 2009, 5749–5751. [Google Scholar] [CrossRef] [PubMed]
- Down, M.P.; Banks, C.E. 2D materials as the basis of supercapacitor devices. In 2D Nanomaterials for Energy Applications: Graphene and Beyond; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–130. [Google Scholar]
- Holze, R. Porphyrinoids as active masses in electrochemical energy storage. In Smart Materials Applications of Porphyrinoids as Functional Materials; Lang, H., Rüffer, T., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; Volume 38, pp. 79–91. [Google Scholar]
- Zhao, X.; Sánchez, B.M.; Dobson, P.J.; Grant, P.S. The role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 2011, 3, 839–855. [Google Scholar] [CrossRef]
- Sardana, S.; Gupta, A.; Singh, K.; Maan, A.S.; Ohlan, A. Conducting polymer hydrogel based electrode materials for super-capacitor applications. J. Energy Storage 2022, 45, 103510. [Google Scholar] [CrossRef]
- Samtham, M.; Singh, D.; Hareesh, K.; Devan, R.S. Perspectives of conducting polymer nanostructures for high-performance electrochemical capacitors. J. Energy Storage 2022, 51, 104418. [Google Scholar] [CrossRef]
- Liu, M.; Cai, C.; Zhang, Z.; Liu, R. Carbon Nanomaterials Supported Transition Metal Oxides as Supercapacitor Electrodes: A Review. Mater. Rev. 2019, 33, 103–109. [Google Scholar]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their compo-sites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Wei, Z. Conducting Polymer Nanowire Arrays for High Performance Supercapacitors. Small 2014, 10, 14–31. [Google Scholar] [CrossRef]
- Montilla, F.; Cotarelo, M.A.; Morallón, E. Hybrid sol-gel-conducting polymer synthesised by electrochemical insertion: Tailoring the capacitance of polyaniline. J. Mater. Chem. 2009, 19, 305–310. [Google Scholar] [CrossRef]
- Cho, S.I.; Lee, S.B. Fast electrochemistry of conductive polymer nanotubes: Synthesis, mechanism, and application. Acc. Chem. Res. 2008, 41, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, T.; Weng, X.; Zhang, B.; Wang, Z.; He, T. Application of Electrospun Fibers in Supercapacitors. Prog. Chem. 2021, 33, 1159–1174. [Google Scholar]
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.M.; Alothman, Z.A.; Alsheetan, K.M.; AL-Anazy, M.m.; Ragupathy, D. Elec-trospinning technique for production of polyaniline nanocomposites/nanofibres for multi-functional applications: A review. Synthet. Met. 2021, 271, 116609. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, Y.i.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Review of recent progress in electrospinning-derived freestanding and binder-free electrodes for supercapacitors. Coord. Chem. Rev. 2022, 460, 214466. [Google Scholar] [CrossRef]
- Nie, G.; Zhu, Y.; Tiani, D.; Wang, C. Research Progress in the Electrospun Nanofiber. based Supercapacitor Electrode Materials. Chem. J. Chin. Univ. 2018, 39, 1349–1363. [Google Scholar]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun Nanomaterials for Supercapacitor Electrodes: Designed Architectures and Electrochemical Performance. Adv. Energy Mater. 2017, 7, 1601301. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Feleni, U. Electroconductive Green Metal-polyaniline Nanocomposites: Synthesis and Application in Sensors. Electroanalysis 2022, 34. [Google Scholar] [CrossRef]
- Tian, D.; Lu, X.; Li, W.; Li, Y.; Wang, C. Research on Electrospun Nanofiber-Based Binder-Free Electrode Materials for Super-capacitors. Acta Phys.-Chim. Sin. 2020, 36, 1904056. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalagan, T.; Basu, R.N. Nanostructured conducting polymers for energy applications: Towards a sustainable platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, J.S.; Joo, H. Recent Developments of the Solution-Processable and Highly Conductive Polyaniline Composites for Optical and Electrochemical Applications. Polymers 2019, 11, 1965. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.D.; Wang, T.; Smoukov, S.K. Multidimensional performance optimization of conducting polymer-based supercapacitor electrodes. Sustainable Energy Fuels 2017, 1, 1857–1874. [Google Scholar] [CrossRef]
- Amarnath, C.A.; Chang, J.H.; Kim, D.; Mane, R.S.; Han, S.H.; Sohn, D. Electrochemical supercapacitor application of electroless surface polymerization of polyaniline nanostructures. Mater. Chem. Phys. 2009, 113, 14–17. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Balducci, A.; Belanger, D.; Brousse, T.; Long, J.W.; Sugimoto, W. A Guideline for Reporting Performance Metrics with Electrochemical Capacitors: From Electrode Materials to Full Devices. J. Electrochem. Soc. 2017, 164, A1487–A1488. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode mate-rial’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes com-posites. J. Phys. Chem. Sol. 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Wu, F.; Xu, B. Progress on the application of carbon nanotubes in supercapacitors. New Carbon Mater. 2006, 21, 176–184. [Google Scholar]
- Chen, W.X.; Chen, W.L.; Xu, Z.D.; Liu, Z.J.; Tu, J.P.; Zhang, X.B.; Guo, H.T. Characteristics of carbon nanotubes and high-quality composites. Acta Mater. Compos. Sin. 2001, 18, 1–5. [Google Scholar]
- Wu, M.; Snook, G.A.; Gupta, V.; Shaffer, M.; Fray, D.J.; Chen, G.Z. Electrochemical fabrication and capacitance of composite films of carbon nanotubes and polyaniline. J. Mater. Chem. 2005, 15, 2297–2303. [Google Scholar] [CrossRef]
- Liu, F.; Luo, S.; Liu, D.; Chen, W.; Huang, Y.; Dong, L.; Wang, L. Facile Processing of Free-Standing Polyaniline/SWCNT Film as an Integrated Electrode for Flexible Supercapacitor Application. ACS Appl. Mater. Interfaces 2017, 9, 33791–33801. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.G.; Yang, B.C.; Hu, Y.D.; Wang, B.H. Study of supercapacitor based on carbon nanotube-polyaniline nanocomposite. Acta Chim. Sin. 2005, 63, 1127–1130. [Google Scholar]
- Kausar, A. Polymeric nanocomposites reinforced with nanowires: Opening doors to future applications. J. Plastic Film Sheeting 2019, 35, 65–98. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Source 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Chen, Y.; Du, L.; Yang, P.; Sun, P.; Yu, X.; Mai, W. Significantly enhanced robustness and electrochemical performance of flexible carbon nanotube-based supercapacitors by electrodepositing polypyrrole. J. Power Source 2015, 287, 68–74. [Google Scholar] [CrossRef]
- Baibarac, M.; Gómez-Romero, P. Nanocomposites based on conducting polymers and carbon nanotubes: From fancy materials to functional applications. J. Nanosci. Nanotechnol. 2006, 6, 289–302. [Google Scholar] [CrossRef]
- Meer, S.; Kausar, A.; Iqbal, T. Trends in Conducting Polymer and Hybrids of Conducting Polymer/Carbon Nanotube: A Review. Polym. Plast. Technol. Eng. 2016, 55, 1416–1440. [Google Scholar] [CrossRef]
- Zhou, H.; Han, G.; Xiao, Y.; Chang, Y.; Zhai, H.J. A comparative study on long and short carbon nanotubes-incorporated polypyrrole/poly(sodium 4-styrenesulfonate) nanocomposites as high-performance supercapacitor electrodes. Synth. Met. 2015, 209, 405–411. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Gao, L. A promising way to enhance the electrochemical behavior of flexible single-walled carbon nano-tube/polyaniline composite films. J. Phys. Chem. C 2010, 114, 19614–19620. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, X. The study of multiwalled carbon nanotube deposited with conducting polymer for supercapacitor. Electrochim. Acta 2003, 48, 575–580. [Google Scholar] [CrossRef]
- Hughes, M.; Shaffer, M.S.P.; Renouf, A.C.; Singh, C.; Chen, G.Z.; Fray, D.J.; Windle, A.H. Electrochemical capacitance of nanocomposite films formed by coating aligned arrays of carbon nanotubes with polypyrrole. Adv. Mater. 2002, 14, 382–385. [Google Scholar] [CrossRef]
- Hughes, M.; Chen, G.Z.; Shaffer, M.S.P.; Fray, D.J.; Windle, A.H. Electrochemical capacitance of a nanoporous composite of carbon nanotubes and polypyrrole. Chem. Mater. 2002, 14, 1610–1613. [Google Scholar] [CrossRef]
- Bandeira, M.C.E.; Holze, R. Impedance measurements at thin polyaniline films—The influence of film morphology. Microchim. Acta 2006, 156, 125–131. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Zhang, P.; Ma, S. Preparation of Electrode Materials Based on Carbon Cloth via Hydrothermal Method and Their Application in Supercapacitors. Materials 2021, 14, 7148. [Google Scholar] [CrossRef]
- Horng, Y.Y.; Lu, Y.C.; Hsu, Y.K.; Chen, C.C.; Chen, L.C.; Chen, K.H. Flexible supercapacitor based on polyaniline nan-owires/carbon cloth with both high gravimetric and area-normalized capacitance. J. Power Source 2010, 195, 4418–4422. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Liu, X.; Zeng, R.; Li, M.; Huang, Y.; Hu, X. Constructing Hierarchical Tectorum- like α-Fe2O3/PPy Nanoarrays on Carbon Cloth for Solid-State Asymmetric Supercapacitors. Angew. Chem. Int. Ed. 2017, 56, 1105–1110. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Han, Y.; Zhao, Y.T.; Zeng, Y.; Yu, M.H.; Liu, Y.J.; Tang, H.L.; Tong, Y.X.; Lu, X.H. Advanced Ti-Doped Fe2O3@PEDOT Core/Shell Anode for High-Energy Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Bin Tan, Y.; Lee, J.M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Murugan, P.; Nagarajan, R.D.; Shetty, B.H.; Govindasamy, M.; Sundramoorthy, A.K. Recent trends in the applications of thermally expanded graphite for energy storage and sensors—A review. Nanoscale Adv. 2021, 3, 6294–6309. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Valdez, A.; Shaffer, M.S.P.; Boccaccini, A.R. Applications of graphene electrophoretic deposition. A review. J. Phys. Chem. B 2013, 117, 1502–1515. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Synthesis and electrochemical applications of the composites of conducting polymers and chemically converted graphene. Electrochim. Acta 2011, 56, 10737–10743. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Synergetic Effect of Polyaniline and Graphene in Their Composite Supercapacitor Electrodes: Impact of Components and Parameters of Chemical Oxidative Polymerization. Nanomaterials 2022, 12, 2531. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, S.; Tian, X.N.; Zhao, X.S. Layered graphene oxide nanostructures with sandwiched conducting polymers as supercapacitor electrodes. Langmuir 2010, 26, 17624–17628. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Z.; Yang, D.; Lu, H. Hierarchically structured graphene-based supercapacitor electrodes. RSC Adv. 2013, 3, 21183–21191. [Google Scholar] [CrossRef]
- Fan, X.; Phebus, B.D.; Li, L.; Chen, S. Graphene-based composites for supercapacitor electrodes. Sci. Adv. Mater. 2015, 7, 1916–1944. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M. Development of Graphene-Based Polymeric Nanocomposites: A Brief Overview. Polymers 2021, 13, 2978. [Google Scholar] [CrossRef]
- Cai, X.; Sun, K.; Qiu, Y.; Jiao, X. Recent advances in graphene and conductive polymer composites for supercapacitor electrodes: A review. Crystals 2021, 11, 947. [Google Scholar] [CrossRef]
- Shen, F.; Pankratov, D.; Chi, Q. Graphene-conducting polymer nanocomposites for enhancing electrochemical capacitive energy storage. Curr. Opin. Electrochem. 2017, 4, 133–144. [Google Scholar] [CrossRef]
- Shen, C.; Lu, Y. Progress in the research of graphene/conducting polymer composites for the application of supercapacitor electrode materials. Acta Polym. Sin. 2014, 24, 1328–1341. [Google Scholar]
- Ates, M. Graphene and its nanocomposites used as an active materials for supercapacitors. J. Solid State Electrochem. 2016, 20, 1509–1526. [Google Scholar] [CrossRef]
- Fu, S.; Ma, L.; Gan, M.; Wang, S. Recent Advances in Preparation of Three-dimensional Graphene and Relevant Composites and Their Applications in Supercapacitors. Mater. Rev. 2017, 31, 9–15. [Google Scholar]
- Xu, Y.; Shi, G.; Duan, X. Self-Assembled Three-Dimensional Graphene Macrostructures: Synthesis and Applications in Supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Z.; Ren, Y.; Ran, W.; Chen, X.; Wu, J.; Gao, F. Vapor deposition polymerization of aniline on 3D hierarchical porous carbon with enhanced cycling stability as supercapacitor electrode. J. Power Source 2015, 286, 1–9. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Zhang, J.; Wang, Z.; He, Y.; Huang, W. Three-dimensional graphene monolith-based composite: Superiority in properties and applications. Int. Mater. Rev. 2018, 63, 204–225. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.X. Design and construction of three-dimensional graphene/conducting polymer for supercapacitors. Chin. Chem. Lett. 2016, 27, 1437–1444. [Google Scholar] [CrossRef]
- Bashir, S.; Hasan, K.; Hina, M.; Ali Soomro, R.; Mujtaba, M.A.; Ramesh, S.; Ramesh, K.; Duraisamy, N.; Manikam, R. Con-ducting polymer/graphene hydrogel electrodes based aqueous smart Supercapacitors: A review and future prospects. J. Electroanal. Chem. 2021, 898, 115626. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Lei, S.; Song, Y. Graphene-based polyaniline nanocomposites: Preparation, properties and applications. J. Mater. Chem. A 2014, 2, 4491–4509. [Google Scholar] [CrossRef]
- Bao, C.; Han, J.; Cheng, J.; Zhang, R. Electrode Materials Blended with Graphene/Polyaniline for Supercapacitor. Prog. Chem. 2018, 30, 1349–1363. [Google Scholar]
- Chen, Z.X.; Lu, H.B. Overview of graphene/polyaniline composite for high-performance supercapacitor. Gaodeng Xuexiao Huaxue Xuebao/Chem. J. Chin. Univ. 2013, 34, 2020–2033. [Google Scholar]
- Gajendran, P.; Saraswathi, R. Polyaniline-carbon nanotube composites. Pure Appl. Chem. 2008, 80, 2377–2395. [Google Scholar] [CrossRef]
- Lakra, R.; Kumar, R.; Sahoo, P.K.; Thatoi, D.; Soam, A. A mini-review: Graphene based composites for supercapacitor appli-cation. Inorg. Chem. Commun. 2021, 133, 108929. [Google Scholar] [CrossRef]
- Idumah, C.I. A review on polyaniline and graphene nanocomposites for supercapacitors. Polym. Plast. Technol. Mater. 2022, 61, 1871–1907. [Google Scholar]
- Kausar, A. Polythiophene/Graphene Nanocomposite: Top-notch properties and competence. Polym. Plast. Technol. Mater. 2022, 61, 2032–2048. [Google Scholar]
- Li, Q.; Horn, M.; Wang, Y.; MacLeod, J.; Motta, N.; Liu, J. A review of supercapacitors based on graphene and redox-active organic materials. Materials 2019, 12, 703. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, G. Graphene/polymer composites for energy applications. J. Polym. Sci. B 2013, 51, 231–253. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, X.; Li, J.; Lin, S.; Zhu, H. Recent developments in graphene conductive ink: Preparation, printing technology and application. Chin. Sci. Bull. 2017, 62, 3217–3235. [Google Scholar] [CrossRef]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem. Commun. 2014, 50, 6818–6830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Annamalai, K.P.; Liu, L.; Chen, T.; Tao, Y. A mini review over the applications of nano-carbons and their composites in supercapacitors. Rec. Innov. Chem. Eng. 2016, 9, 4–19. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ge, K.K.; Rehman, S.U.; Bi, H. Nanocarbon-based electrode materials applied for supercapacitors. Rare Metals 2022, 41, 3957–3975. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Chee, M.O.L.; Dong, P.; Ye, M.; Shen, J. Recent advances in micro-supercapacitors. Nanoscale 2019, 11, 5807–5821. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Dutta, K.; Kader, M.A.; Nayak, S.K. An overview on the recent developments in polyaniline-based supercapacitors. Polym. Adv. Technol. 2019, 30, 1902–1921. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on carbon/polyaniline hybrids: Design and synthesis for supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xu, K.; Wu, C. Recent progress in supercapacitors based on the advanced carbon electrodes. Nanotechnol. Rev. 2019, 8, 299–314. [Google Scholar] [CrossRef]
- Mei, B.; Qin, Y.; Agbolaghi, S. A review on supramolecules/nanocomposites based on carbonic precursors and dielec-tric/conductive polymers and their applications. Mater. Sci. Eng. B 2021, 269, 115181. [Google Scholar] [CrossRef]
- Liu, Q.; Nayfeh, O.; Nayfeh, M.H.; Yau, S.T. Flexible supercapacitor sheets based on hybrid nanocomposite materials. Nano Energy 2013, 2, 133–137. [Google Scholar] [CrossRef]
- Sardar, A.; Gupta, P.S. Polypyrrole based nanocomposites for supercapacitor applications: A review. AIP Conf. Proc. 2018, 1953, 030020. [Google Scholar]
- Lota, K.; Lota, G.; Sierczynska, A.; Acznik, I. Carbon/polypyrrole composites for electrochemical capacitors. Synth. Met. 2015, 203, 44–48. [Google Scholar] [CrossRef]
- Marriam, I.; Wang, Y.; Tebyetekerwa, M. Polyindole batteries and supercapacitors. Energy Storage Mater. 2020, 33, 336–359. [Google Scholar] [CrossRef]
- Cai, Z.J.; Zhang, Q.; Song, X.Y. Improved electrochemical performance of polyindole/carbon nanotubes composite as electrode material for supercapacitors. Electron. Mater. Lett. 2016, 12, 830–840. [Google Scholar] [CrossRef]
- Kausar, A. Polyaniline and quantum dot-based nanostructures: Developments and perspectives. J. Plast. Film Sheet. 2020, 36, 430–447. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, Y.; Zhang, S.; Fan, Z. Application of carbon-/graphene quantum dots for supercapacitors. Acta Phys. -Chim. Sin. 2019, 36, 1903052. [Google Scholar] [CrossRef]
- Dinari, M.; Momeni, M.M.; Goudarzirad, M. Nanocomposite films of polyaniline/graphene quantum dots and its supercapacitor properties. Surf. Eng. 2016, 32, 535–540. [Google Scholar] [CrossRef]
- Mondal, S.; Rana, U.; Malik, S. Graphene quantum dot-doped polyaniline nanofiber as highperformance supercapacitor electrode materials. Chem. Commun. 2015, 51, 12365–12368. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Liu, D.; Du, P.; Liu, P. Synthesis of flake-shaped nitrogen-doped carbon quantum dot/polyaniline (N-CQD/PANI) nanocomposites via rapid-mixing polymerization and their application as electrode materials in supercapac-itors. Synth. Met. 2017, 231, 120–126. [Google Scholar] [CrossRef]
- Das, H.T.; Barai, P.; Dutta, S.; Das, N.; Das, P.; Roy, M.; Alauddin, M.; Barai, H.R. Polymer Composites with Quantum Dots as Potential Electrode Materials for Supercapacitors Application: A Review. Polymers 2022, 14, 1053. [Google Scholar] [CrossRef]
- Kausar, A. Polymer dots and derived hybrid nanomaterials: A review. J. Plastic Films Sheet. 2021, 37, 510–528. [Google Scholar] [CrossRef]
- Admassie, S.; Ajjan, F.N.; Elfwing, A.; Inganäs, O. Biopolymer hybrid electrodes for scalable electricity storage. Mater. Horiz. 2016, 3, 174–185. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Casado, N.; Rebiś, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganäs, O. High performance PEDOT/lignin bi-opolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Vagin, M.; Rebiś, T.; Aguirre, L.E.; Ouyang, L.; Inganäs, O. Scalable Asymmetric Supercapacitors Based on Hybrid Organic/Biopolymer Electrodes. Adv. Sustain. Syst. 2017, 1, 1700054. [Google Scholar] [CrossRef]
- Hu, Y.; Tong, X.; Zhuo, H.; Zhong, L.; Peng, X. Biomass-Based Porous N-Self-Doped Carbon Framework/Polyaniline Composite with Outstanding Supercapacitance. ACS Sustain. Chem. Eng. 2017, 5, 8663–8674. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Kasaw, E.; Fentahun, B.; Loghin, E.; Luebben, J.F. Banana Peel and Conductive Polymers-Based Flexible Su-percapacitors for Energy Harvesting and Storage. Energies 2022, 15, 2471. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Kwon, K. A review on biomass-derived N-doped carbons as electrocatalysts in electrochemical energy ap-plications. Chem. Eng. J. 2022, 446, 137116. [Google Scholar] [CrossRef]
- Dubal, D.P.; Holze, R. Synthesis, properties, and performance of nanostructured metal oxides for supercapacitors. Pure Appl. Chem. 2014, 86, 611–632. [Google Scholar] [CrossRef]
- Zhao, W.; Rubio, S.J.B.; Dang, Y.; Suib, S.L. Green Electrochemical Energy Storage Devices Based on Sustainable Manganese Dioxides. ACS EST Eng. 2022, 2, 20–42. [Google Scholar] [CrossRef]
- Shin, J.; Seo, J.K.; Yaylian, R.; Huang, A.; Meng, Y.S. A review on mechanistic understanding of MnO2 in aqueous electrolyte for electrical energy storage systems. Int. Mater. Rev. 2020, 65, 356–387. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Zhang, D.; Miao, Z.C.; Zhang, X.L.; Chou, S.L. Research Progress in MnO2-Carbon Based Supercapacitor Elec-trode Materials. Small 2018, 14, 1702883. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Wang, Z.; Yu, J.; Huang, H.; Qiu, J. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives. Nano Energy 2019, 57, 459–472. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Wang, Y.; Walsh, F.C.; Ouyang, J.H.; Jia, D.; Zhou, Y. Materials and fabrication of electrode scaffolds for depo-sition of MnO2 and their true performance in supercapacitors. J. Power Source 2015, 293, 657–674. [Google Scholar] [CrossRef]
- Su, D.; Ma, J.; Huang, M.; Liu, F.; Chen, T.; Liu, C.; Ni, H. Manganese oxides-based composite electrodes for supercapacitors. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012087. [Google Scholar] [CrossRef]
- Wang, J.G.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Bhat, T.S.; Jadhav, S.A.; Beknalkar, S.A.; Patil, S.S.; Patil, P.S. MnO2 core-shell type materials for high-performance superca-pacitors: A short review. Inorg. Chem. Commun. 2022, 141, 109493. [Google Scholar] [CrossRef]
- Tang, X.N.; Zhu, S.K.; Ning, J.; Yang, X.F.; Hu, M.Y.; Shao, J.J. Charge storage mechanisms of manganese dioxide-based su-percapacitors: A review. New Carbon Mater. 2021, 36, 702–710. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition Metal Oxide Electrode Materials for Su-percapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef]
- Balaji, T.E.; Das, H.T.; Maiyalagan, T. Recent Trends in Bimetallic Oxides and Their Composites as Electrode Materials for Supercapacitor Applications. ChemElectroChem 2021, 8, 1723–1746. [Google Scholar] [CrossRef]
- Lokhande, V.C.; Lokhande, A.C.; Lokhande, C.D.; Kim, J.H.; Ji, T. Supercapacitive composite metal oxide electrodes formed with carbon, metal oxides and conducting polymers. J. Alloys Compd. 2016, 682, 381–403. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef]
- Zheng, M.; Xiao, X.; Li, L.; Gu, P.; Dai, X.; Tang, H.; Hu, Q.; Xue, H.; Pang, H. Hierarchically nanostructured transition metal oxides for supercapacitors. Sci. China Mater. 2018, 61, 185–209. [Google Scholar] [CrossRef]
- Kwon, N.H.; Lee, K.G.; Kim, H.K.; Hwang, S.J. MnO2-based nanostructured materials for various energy applications. Mater. Chem. Front. 2021, 5, 3549–3575. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, D.; Zhu, Z.; Wu, Q.; Li, J. Review and prospect of MnO2-based composite materials for supercapacitor electrodes. Ionics 2021, 27, 3699–3714. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Bai, T.; Wang, W.; He, F.; Ye, M. Nickel and cobalt sulfide-based nanostructured materials for electrochemical energy storage devices. Chem. Eng. J. 2021, 409, 127237. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Li, Z.; Du, C.F.; Guo, R. A Review of MnO2 Composites Incorporated with Conductive Materials for Energy Storage. Chem. Rec. 2022, 22, e202200118. [Google Scholar] [CrossRef]
- Yue, T.; Shen, B.; Gao, P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: A review. Renew. Sustain. Energy Rev. 2022, 158, 112131. [Google Scholar]
- Pisal, K.B.; Babar, B.M.; Mujawar, S.H.; Kadam, L.D. Overview of molybdenum disulfide based electrodes for supercapacitors: A short review. J. Energy Storage 2021, 43, 103297. [Google Scholar] [CrossRef]
- Nithya, V.D.; Sabari Arul, N. Progress and development of Fe3O4 electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 10767–10778. [Google Scholar] [CrossRef]
- Yu, S.; Ng, V.M.H.; Wang, F.; Xiao, Z.; Li, C.; Kong, L.B.; Que, W.; Zhou, K. Synthesis and application of iron-based nano-materials as anodes of lithium-ion batteries and supercapacitors. J. Mater. Chem. A 2018, 6, 9332–9367. [Google Scholar] [CrossRef]
- Majumdar, D.; Mandal, M.; Bhattacharya, S.K. V2O5 and its carbon-based nanocomposites for supercapacitor applications. ChemElectroChem 2019, 6, 1623–1648. [Google Scholar] [CrossRef]
- Ates, M.; Fernandez, C. Ruthenium oxide-carbon-based nanofiller-reinforced conducting polymer nanocomposites and their supercapacitor applications. Polym. Bull. 2019, 76, 2601–2619. [Google Scholar] [CrossRef]
- Lee, H.; Cho, M.S.; Kim, I.H.; Nam, J.D.; Lee, Y. RuOx/polypyrrole nanocomposite electrode for electrochemical capacitors. Synth. Met. 2010, 160, 1055–1059. [Google Scholar] [CrossRef]
- Xu, J.M.; Yan, A.L.; Wang, X.C.; Wang, B.Q.; Cheng, J.P. A review of cobalt monoxide and its composites for supercapacitors. Ceram. Int. 2021, 47, 22229–22239. [Google Scholar] [CrossRef]
- Majumdar, D.; Ghosh, S. Recent advancements of copper oxide based nanomaterials for supercapacitor applications. J. Energy Storage 2021, 34, 101995. [Google Scholar] [CrossRef]
- Majumdar, D. Recent progress in copper sulfide based nanomaterials for high energy supercapacitor applications. J. Electroanal. Chem. 2021, 880, 114825. [Google Scholar] [CrossRef]
- Wang, X.; Hu, A.; Meng, C.; Wu, C.; Yang, S.; Hong, X. Recent Advance in Co3O4 and Co3O4-Containing Electrode Materials for High-Performance Supercapacitors. Molecules 2020, 25, 269. [Google Scholar] [CrossRef]
- Wu, X.; Meng, L.; Wang, Q.; Zhang, W.; Wang, Y. A flexible asymmetric fibered-supercapacitor based on unique Co3O4@PPy core-shell nanorod arrays electrode. Chem. Eng. J. 2017, 327, 193–201. [Google Scholar] [CrossRef]
- Arunachalam, S.; Kirubasankar, B.; Pan, D.; Liu, H.; Yan, C.; Guo, Z.; Angaiah, S. Research progress in rare earths and their composites based electrode materials for supercapacitors. Green Energy Environ. 2020, 5, 259–273. [Google Scholar] [CrossRef]
- Ates, A.; Kuzgun, O.; Candan, I. Supercapacitor performances of titanium-polymeric nanocomposites: A review study. Iran. Polym. J. 2022, 31, 31–57. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Savekar, V.A.; Bhat, T.S.; Patil, P.S. Recent advances in metal pyrophosphates for electrochemical supercapacitors: A review. J. Energy Storage 2022, 52, 104986. [Google Scholar] [CrossRef]
- BoopathiRaja, R.; Vadivel, S.; Parthibavarman, M.; Prabhu, S.; Ramesh, R. Effect of polypyrrole incorporated sun flower like Mn2P2O7 with lab waste tissue paper derived activated carbon for asymmetric supercapacitor applications. Surf. Interfaces 2021, 26, 101409. [Google Scholar] [CrossRef]
- Meganathan, K.L.; BoopathiRaja, R.; Parthibavarman, M.; Sharmila, V.; Shkir, M.; Gaikwad, S.A.; Praveenkumar, M. Design and fabrication of Cu2P2O7@Ppy electrode for extraordinary capacitance and long-term stability for ideal asymmetric super-capacitor application. J. Mater. Sci. Mater. Electron. 2021, 32, 24736–24747. [Google Scholar] [CrossRef]
- Díaz, R.; Orcajo, M.G.; Botas, J.A.; Calleja, G.; Palma, J. Co8-MOF-5 as electrode for supercapacitors. Mater. Lett. 2012, 68, 126–128. [Google Scholar] [CrossRef]
- Dedek, I.; Kupka, V.; Jakubec, P.; Sedajova, V.; Jayaramulu, K.; Otyepka, M. Metal-organic framework/conductive polymer hybrid materials for supercapacitors. Appl. Mater. Today 2022, 26, 101387. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Horn, M.; Motta, N.; Hu, M.; Li, Y. Recent advancements in metal organic framework based electrodes for supercapacitors. Sci. China-Mater. 2018, 61, 159–184. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-organic framework composites and their electrochemical applications. J. Mater. Chem. A 2019, 7, 7301–7327. [Google Scholar] [CrossRef]
- Mohanadas, D.; Sulaiman, Y. Recent advances in development of electroactive composite materials for electrochromic and supercapacitor applications. J. Power Source 2022, 523, 231029. [Google Scholar] [CrossRef]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.H.; Deep, A. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Wang, T.; Lei, J.; Wang, Y.; Pang, L.; Pan, F.; Chen, K.J.; Wang, H. Approaches to Enhancing Electrical Conductivity of Pristine Metal-Organic Frameworks for Supercapacitor Applications. Small 2022, 18, 2203307. [Google Scholar] [CrossRef]
- Shinde, S.K.; Kim, D.Y.; Kumar, M.; Murugadoss, G.; Ramesh, S.; Tamboli, A.M.; Yadav, H.M. MOFs-Graphene Composites Synthesis and Application for Electrochemical Supercapacitor: A Review. Polymers 2022, 14, 511. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, R.S.; Sambasivam, S.; Gopi, C.V.V.M.; Alzahmi, S.; Kim, H.J.; de Barros, A.L.F.; Obaidat, I.M. Recent Ad-vancements of Polyaniline/Metal Organic Framework (PANI/MOF) Composite Electrodes for Supercapacitor Applications: A Critical Review. Nanomaterials 2022, 12, 1511. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Ren, L.; Piao, Q.; Zhong, J.; Wang, Y.; Li, H.; Chen, Y.; Wang, B. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef]
- Wang, Z.L.; He, X.J.; Ye, S.H.; Tong, Y.X.; Li, G.R. Design of Polypyrrole/Polyaniline Double-Walled Nanotube Arrays for Electrochemical Energy Storage. ACS Appl. Mater. Interfaces 2014, 6, 642–647. [Google Scholar] [CrossRef]
- Holze, R. Copolymers—A refined way to tailor intrinsically conducting polymers. Electrochim. Acta 2011, 58, 10479–10492. [Google Scholar] [CrossRef]
- Islam, S.; Mia, M.M.; Shah, S.S.; Naher, S.; Shaikh, M.N.; Aziz, M.A.; Ahammad, A.J.S. Recent Advancements in Electro-chemical Deposition of Metal-Based Electrode Materials for Electrochemical Supercapacitors. Chem. Rec. 2022, 22, e202200013. [Google Scholar] [CrossRef]
- Gund, G.S.; Park, J.H.; Harpalsinh, R.; Kota, M.; Shin, J.H.; Kim, T.i.; Gogotsi, Y.; Park, H.S. MXene/Polymer Hybrid Materials for Flexible AC-Filtering Electrochemical Capacitors. Joule 2019, 3, 164–176. [Google Scholar] [CrossRef]
- Thomas, S.A.; Patra, A.; Al-Shehri, B.M.; Selvaraj, M.; Aravind, A.; Rout, C.S. MXene based hybrid materials for supercapac-itors: Recent developments and future perspectives. J. Energy Storage 2022, 55, 105765. [Google Scholar] [CrossRef]
- Wu, W.; Wei, D.; Zhu, J.; Niu, D.; Wang, F.; Wang, L.; Yang, L.; Yang, P.; Wang, C. Enhanced electrochemical performances of organ-like Ti3C2 MXenes/polypyrrole composites as supercapacitors electrode materials. Ceram. Int. 2019, 45, 7328–7337. [Google Scholar] [CrossRef]
- Luo, W.; Ma, Y.; Li, T.; Thabet, H.K.; Hou, C.; Ibrahim, M.M.; El-Bahy, S.M.; Xu, B.B.; Guo, Z. Overview of MXene/conducting polymer composites for supercapacitors. J. Energy Storage 2022, 52, 105008. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Pillai, S.C. MXenes-based nanocomposites for supercapacitor applications. Curr. Opin. Electrochem. 2021, 33, 100710. [Google Scholar] [CrossRef]
- Parajuli, D.; Murali, N.; Devendra, K.C.; Karki, B.; Samatha, K.; Kim, A.A.; Park, M.; Pant, B. Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications. Polymers 2022, 14, 3433. [Google Scholar] [CrossRef]
- Sajjad, M. Recent Advances in SiO2 Based Composite Electrodes for Supercapacitor Applications. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3221–3239. [Google Scholar] [CrossRef]
- Gaboriau, D.; Aradilla, D.; Brachet, M.; Le Bideau, J.; Brousse, T.; Bidan, G.; Gentile, P.; Sadki, S. Silicon nanowires and na-notrees: Elaboration and optimization of new 3D architectures for high performance on-chip supercapacitors. RSC Adv. 2016, 6, 81017–81027. [Google Scholar] [CrossRef]
- Aradilla, D.; Bidan, G.; Gentile, P.; Weathers, P.; Thissandier, F.; Ruiz, V.; Gómez-Romero, P.; Schubert, T.J.S.; Sahin, H. Novel hybrid micro-supercapacitor based on conducting polymer coated silicon nanowires for electrochemical energy storage. RSC Adv. 2014, 4, 26462–26467. [Google Scholar] [CrossRef]
- Aradilla, D.; Gaboriau, D.; Bidan, G.; Gentile, P.; Boniface, M.; Dubal, D.; Gómez-Romero, P.; Wimberg, J.; Schubert, T.J.S. An innovative 3D nanoforest heterostructure made of polypyrrole coated silicon nanotrees for new high performance hybrid micro-supercapacitors. J. Mater. Chem. A 2015, 3, 13978–13985. [Google Scholar] [CrossRef]
- Lé, T.; Bidan, G.; Gentile, P.; Billon, F.; Debiemme-Chouvy, C.; Perrot, H.; Sel, O.; Aradilla, D. Understanding the energy storage mechanisms of poly(3,4-ethylenedioxythiophene)-coated silicon nanowires by electrochemical quartz crystal microbalance. Mater. Lett. 2019, 240, 59–61. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Feleni, U. Catalytic and Energy Storage Applications of Metal/Polyaniline Nanocomposites: A Critical Review. J. Electron. Mater. 2022, 51, 5568–5585. [Google Scholar] [CrossRef]
- Krishnaa, G.P.; Vavaneeth, P.; Ramachandaran, T.; Babu, T.G.S.; Suneesh, P.V. Fabrication of polyaniline-platinum nano-composite based flexible supercapacitor. Mater Today Proc. 2020, 33, 2407–2413. [Google Scholar] [CrossRef]
- Hu, C.C.; Chen, E.; Lin, J.Y. Capacitive and textural characteristics of polyaniline-platinum composite films. Electrochim. Acta 2002, 47, 2741–2749. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, A.K.; Gómez-Romero, P. Triple hybrid materials: A novel concept within the field of organic-inorganic hybrids. J. Power Source 2006, 161, 580–586. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchova, M. Conducting polypyrrole nanotubes: A review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
- Kim, J.; Ju, H.; Inamdar, A.I.; Jo, Y.; Han, J.; Kim, H.; Im, H. Synthesis and enhanced electrochemical supercapacitor properties of Ag-MnO2-polyaniline nanocomposite electrodes. Energy 2014, 70, 473–477. [Google Scholar] [CrossRef]
- Zhao, Z.; Richardson, G.F.; Meng, Q.; Zhu, S.; Kuan, H.C.; Ma, J. PEDOT-based composites as electrode materials for super-capacitors. Nanotechnology 2016, 27, 042001. [Google Scholar] [CrossRef]
- Majumdar, D. Review on Current Progress of MnO2-Based Ternary Nanocomposites for Supercapacitor Applications. ChemElectroChem 2021, 8, 291–336. [Google Scholar] [CrossRef]
- Azman, N.H.N.; Mamat @ Mat Nazir, M.S.; Ngee, L.H.; Sulaiman, Y. Graphene-based ternary composites for supercapacitors. Int. J. Energy Res. 2018, 42, 2104–2116. [Google Scholar] [CrossRef]
- Yang, Y.; Han, C.; Jiang, B.; Iocozzia, J.; He, C.; Shi, D.; Jiang, T.; Lin, Z. Graphene-based materials with tailored nanostructures for energy conversion and storage. Mater. Sci. Eng. R 2016, 102, 1–72. [Google Scholar] [CrossRef]
- Huang, Z.D.; Liang, R.; Zhang, B.; He, Y.B.; Kim, J.K. Evolution of flexible 3D graphene oxide/carbon nanotube/polyaniline composite papers and their supercapacitive performance. Compos. Sci. Technol. 2013, 88, 126–133. [Google Scholar] [CrossRef]
- Das, A.K.; Karan, S.K.; Khatua, B.B. High Energy Density Ternary Composite Electrode Material Based on Polyaniline (PANI), Molybdenum trioxide (MoO3) and Graphene Nanoplatelets (GNP) Prepared by Sono-Chemical Method and Their Synergistic Contributions in Superior Supercapacitive Performance. Electrochim. Acta 2015, 180, 1–15. [Google Scholar] [CrossRef]
- Kausar, A. Polyaniline/graphene nanoplatelet nanocomposite towards high-end features and applications. Mater. Res. Innov. 2022, 26, 249–261. [Google Scholar] [CrossRef]
- Alshahrie, A.; Ansari, M.O. High Performance Supercapacitor Applications and DC Electrical Conductivity Retention on Surfactant Immobilized Macroporous Ternary Polypyrrole/Graphitic-C3N4@Graphene Nanocomposite. Electron. Mater. Lett. 2019, 15, 238–246. [Google Scholar] [CrossRef]
- Akbar, A.R.; Tian, W.; Qadir, M.B.; Khaliq, Z.; Liu, Z.; Tahir, M.; Hu, Y.; Xiong, C.; Yang, Q. A novel ternary composite aerogel for high-performance supercapacitor. Coll. Surf. A 2021, 610, 125644. [Google Scholar] [CrossRef]
- Purty, B.; Choudhary, R.B.; Biswas, A.; Udayabhanu, G. Chemically grown mesoporous f-CNT/-MnO2/PIn nanocomposites as electrode materials for supercapacitor application. Polym. Bull. 2019, 76, 1619–1640. [Google Scholar] [CrossRef]
- Ehsani, A.; Heidari, A.A.; Shiri, H.M. Electrochemical Pseudocapacitors Based on Ternary Nanocomposite of Conductive Polymer/Graphene/Metal Oxide: An Introduction and Review to it in Recent Studies. Chem. Rec. 2019, 19, 908–926. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xia, C.; Du, H.; Wang, W. Enhanced electrochemical performance of polyaniline/carbon/titanium nitride nanowire array for flexible supercapacitor. J. Power Source 2015, 286, 561–570. [Google Scholar] [CrossRef]
- Chakraborty, I.; Chakrabarty, N.; Senapati, A.; Chakraborty, A.K. CuO@NiO/Polyaniline/MWCNT Nanocomposite as High-Performance Electrode for Supercapacitor. J. Phys. Chem. C 2018, 122, 27180–27190. [Google Scholar] [CrossRef]
- Sezali, N.A.A.; Ong, H.L.; Jullok, N.; Villagracia, A.R.; Doong, R.A. A Review on Nanocellulose and Its Application in Supercapacitors. Macromol. Mater. Eng. 2021, 306, 2100556. [Google Scholar] [CrossRef]
- Wasim, M.; Shi, F.; Liu, J.; Khan, M.R.; Farooq, A.; Sanbhal, N.; Alfred, M.; Xin, L.; Yajun, C.; Zhao, X. Extraction of cellulose to progress in cellulosic nanocomposites for their potential applications in supercapacitors and energy storage devices J. Mater. Sci. 2021, 56, 14448–14486. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Kuo, D.W.; Lee, R.H. Flexible and freestanding electrodes based on polypyrrole/carbon nanotube/cellulose composites for supercapacitor application. Celulose 2019, 26, 4495–4513. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.; Chen, B.; Zhang, W.; Sharma, S.; Gu, W.; Deng, Y. Solid-state flexible polyaniline/silver cellulose nanofibrils aerogel supercapacitors. J. Power Source 2014, 246, 283–289. [Google Scholar] [CrossRef]
- Ma, L.N.; Shi, C.; Zhao, N.; Bi, Z.J.; Guo, X.X.; Huang, Y.D. Bacterial Cellulose Based Nano-biomaterials for Energy Storage Applications. J. Inorg. Mater. 2020, 35, 145–157. [Google Scholar]
- Xia, W.; Li, Z.; Xu, Y.; Zhuang, X.; Jia, S.; Zhang, J. Bacterial cellulose based electrode material for supercapacitors. Prog. Chem. 2016, 28, 1682–1688. [Google Scholar]
- Oraon, R.; Adhikari, A.D.; Tiwari, S.K.; Nayak, G.C. Nanoclay Co-Doped CNT/Polyaniline Nanocomposite: A High-Performance Electrode Material for Supercapacitor Applications. ChemistrySelect 2017, 2, 8807–8817. [Google Scholar] [CrossRef]
- Idisi, D.O.; Oke, J.A.; Bello, I.T. Graphene oxide/Au nanoparticles: Synthesis, properties, and application: A mini-review. Int. J. Energy Res. 2021, 45, 19772–19788. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, B.; Peng, J.; Su, W.; Zhang, K.; Hu, X.; Wang, G.; Park, J.H. Engineered Polymeric Carbon Nitride Additive for Energy Storage Materials: A Review. Adv. Funct. Mater. 2021, 31, 2102300. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, S.; Li, L.; Dou, S. Bio-Nanotechnology in High-Performance Supercapacitors. Adv. Energy Mater. 2017, 7, 1700592. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Caban-Huertas, Z.; Wolfart, F.; Vidotti, M.; Holze, R.; Lokhande, C.D.; Gómez-Romero, P. Synthetic approach from polypyrrole nanotubes to nitrogen doped pyrolyzed carbon nanotubes for asymmetric supercapacitors. J. Power Source 2016, 308, 158–165. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Li, J.; Wu, Q. Nanoporous materials derived from metal-organic framework for supercapacitor application. J. Energy Storage 2020, 30, 101525. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Yang, W.; Shen, W.; Xue, H.; Pang, H. MOF-Derived Metal Oxide Composites for Advanced Electrochemical Energy Storage. Small 2018, 14, 1704435. [Google Scholar] [CrossRef]
- Xie, X.C.; Huang, K.J.; Wu, X. Metal-organic framework derived hollow materials for electrochemical energy storage. J. Mater. Chem. A 2018, 6, 6754–6771. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal-Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Sonar, P.; Haick, H. Advanced Materials for Use in Soft Self-Healing Devices. Adv. Mater. 2017, 29, 1604973. [Google Scholar] [CrossRef]
- Kumar, Y.; Gupta, A.; Thakur, A.K.; Uke, S.J.; Khatri, V.; Kumar, A.; Gupta, M.; Kumar, Y. Advancement and current scenario of engineering and design in transparent supercapacitors: Electrodes and electrolyte. J. Nanopart. Res. 2021, 23, 119. [Google Scholar] [CrossRef]
- Eisenberg, O.; Algavi, Y.M.; Weissman, H.; Narevicius, J.; Rybtchinski, B.; Lahav, M.; van der Boom, M.E. Dual Function Metallo-Organic Assemblies for Electrochromic-Hybrid Supercapacitors. Adv. Mater. Interfaces 2020, 7, 2000718. [Google Scholar] [CrossRef]
- Lingappan, N.; Lim, S.; Lee, G.H.; Tung, H.T.; Luan, V.H.; Lee, W. Recent advances on fiber-reinforced multifunctional composites for structural supercapacitors. Funct. Compos. Struct. 2022, 4, 012001. [Google Scholar] [CrossRef]
- Lv, H.; Pan, Q.; Song, Y.; Liu, X.X.; Liu, T. A Review on Nano-/Microstructured Materials Constructed by Electrochemical Technologies for Supercapacitors. Nano-Micro Lett. 2020, 12, 118. [Google Scholar] [CrossRef]
- Kulandaivalu, S.; Sulaiman, Y. Recent Advances in Layer-by-Layer Assembled Conducting Polymer Based Composites for Supercapacitors. Energies 2019, 12, 2107. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, Z.; Yu, J.; Zhao, J.; Wang, G.; Cao, D.; Ding, B.; Li, Y. 3D Printing of Tunable Energy Storage Devices with Both High Areal and Volumetric Energy Densities. Adv. Energy Mater. 2019, 9, 1802578. [Google Scholar] [CrossRef]
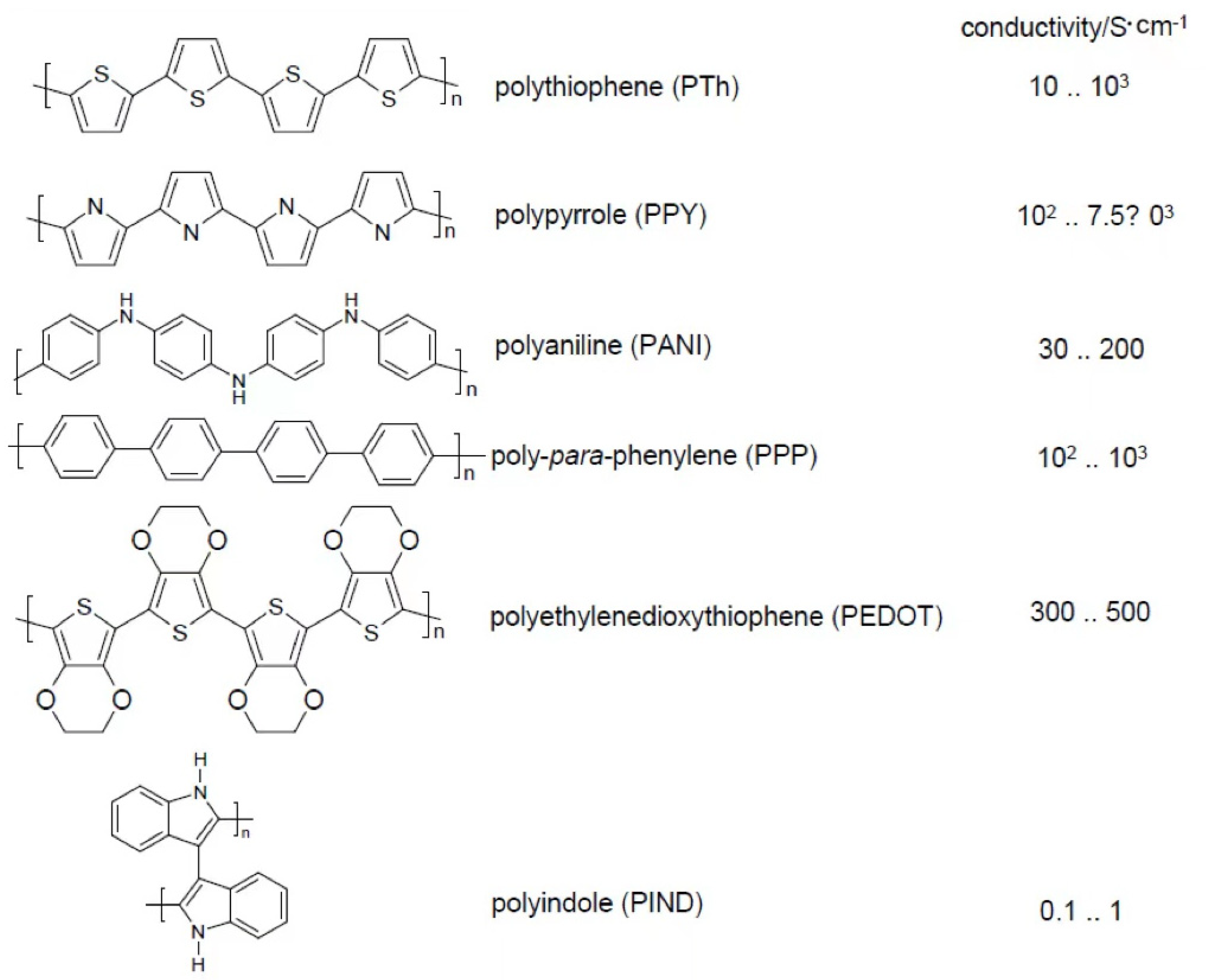
| Material | Molecular Weight of Repeat Unit/g | Oxidation Level */– | Theor. Q # | Measur. Q/F·g−1 |
|---|---|---|---|---|
| PANI | 93 | 0.5 | 750 F·g−1 (ΔE = 0.7 V) | 240 |
| PPy | 67 | 0.33 | 620 F·g−1 (ΔE = 0.8 V) | 530 |
| PTh | 84 | 0.33 | 485 F·g−1 (ΔE = 0.8 V) | – |
| PEDOT | 142 | 0.33 | 210 F·g−1 (ΔE = 1.2 V) | 92 |
| Quinone/HQ | 108 | 2 | 1787 As·g−1 | – |
| Ferrocene | 185 | 1 | 522 As·g−1 | – |
| Li | 6.939 | 1 | 13,904 As·g−1 | – |
| Al | 26.98 | 3 | 10,728 As·g−1 | – |
| PbO2 | 239 | 2 | 807 As·g−1 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ul Hoque, M.I.; Holze, R. Intrinsically Conducting Polymer Composites as Active Masses in Supercapacitors. Polymers 2023, 15, 730. https://doi.org/10.3390/polym15030730
Ul Hoque MI, Holze R. Intrinsically Conducting Polymer Composites as Active Masses in Supercapacitors. Polymers. 2023; 15(3):730. https://doi.org/10.3390/polym15030730
Chicago/Turabian StyleUl Hoque, Md. Ikram, and Rudolf Holze. 2023. "Intrinsically Conducting Polymer Composites as Active Masses in Supercapacitors" Polymers 15, no. 3: 730. https://doi.org/10.3390/polym15030730
APA StyleUl Hoque, M. I., & Holze, R. (2023). Intrinsically Conducting Polymer Composites as Active Masses in Supercapacitors. Polymers, 15(3), 730. https://doi.org/10.3390/polym15030730








