Hybrid Hydrogels for Neomycin Delivery: Synergistic Effects of Natural/Synthetic Polymers and Proteins
Abstract
1. Introduction
2. Materials and Methods
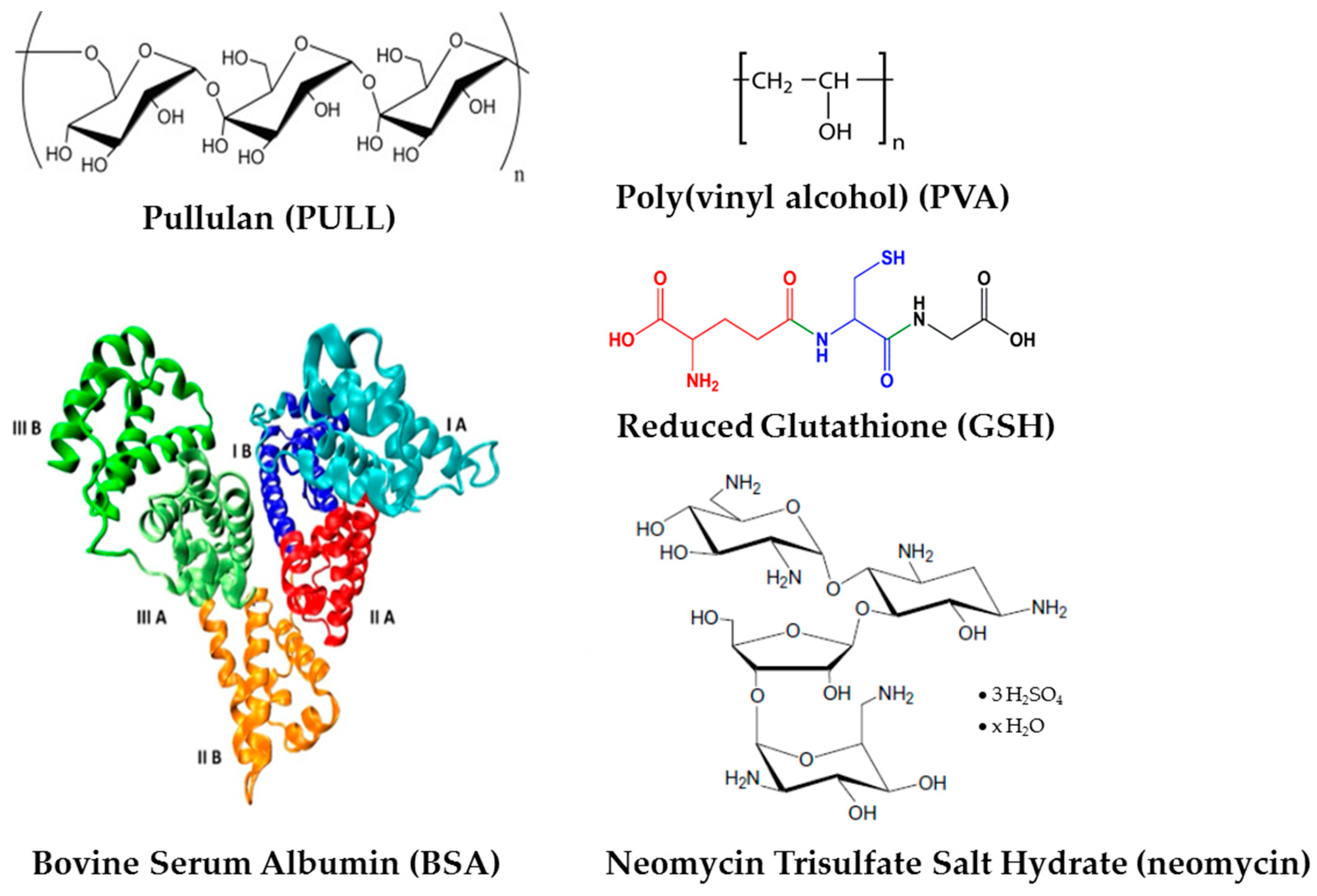
2.1. Materials
2.2. Hydrogel Preparation
2.3. Scanning Electron Microscopy Studies
2.4. Fourier Transform Infrared Spectroscopy
2.5. Rheological Investigation
2.6. Swelling Behavior
2.7. Delivery
3. Results
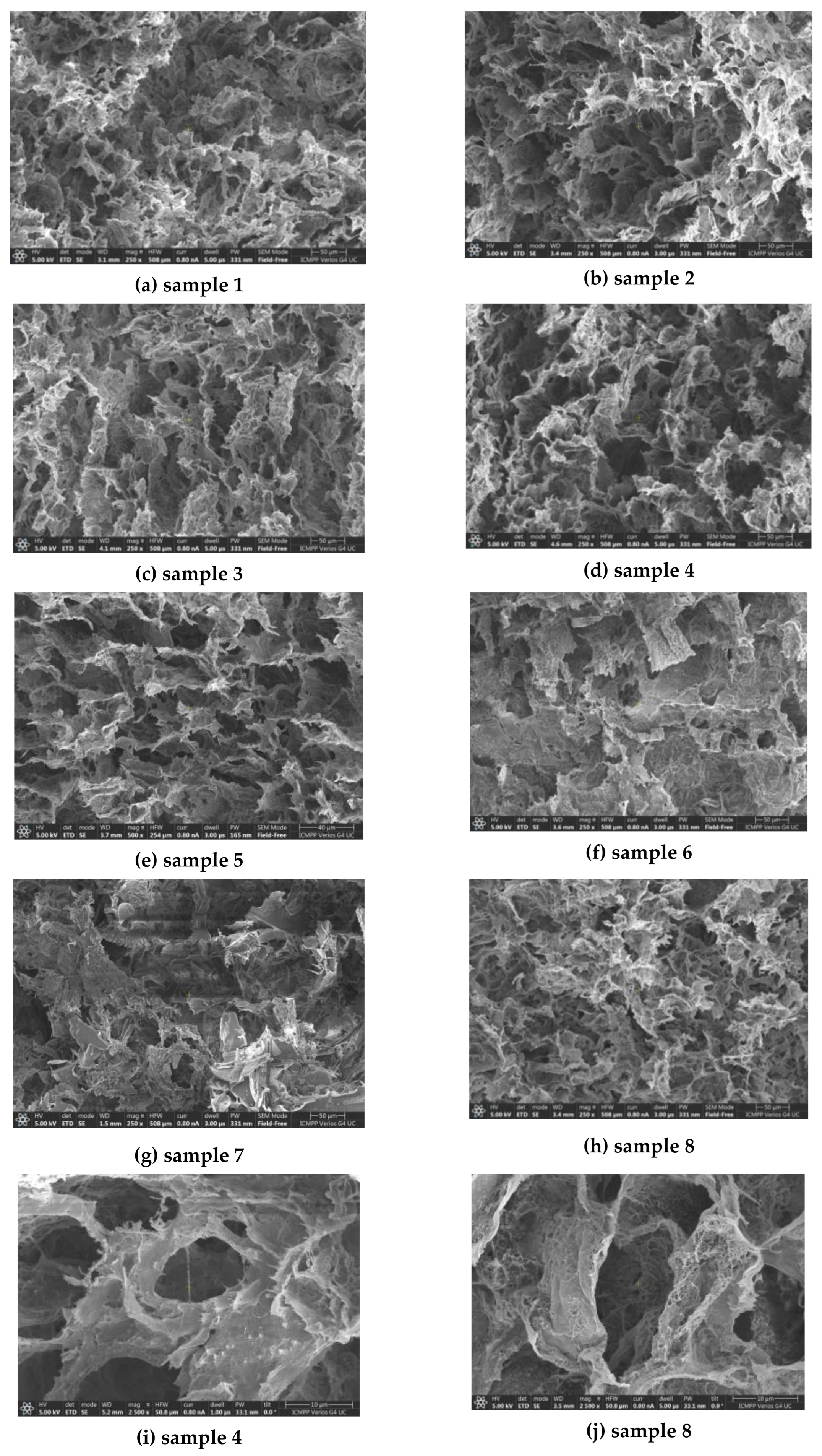
3.1. Morphology of the Hydrogels
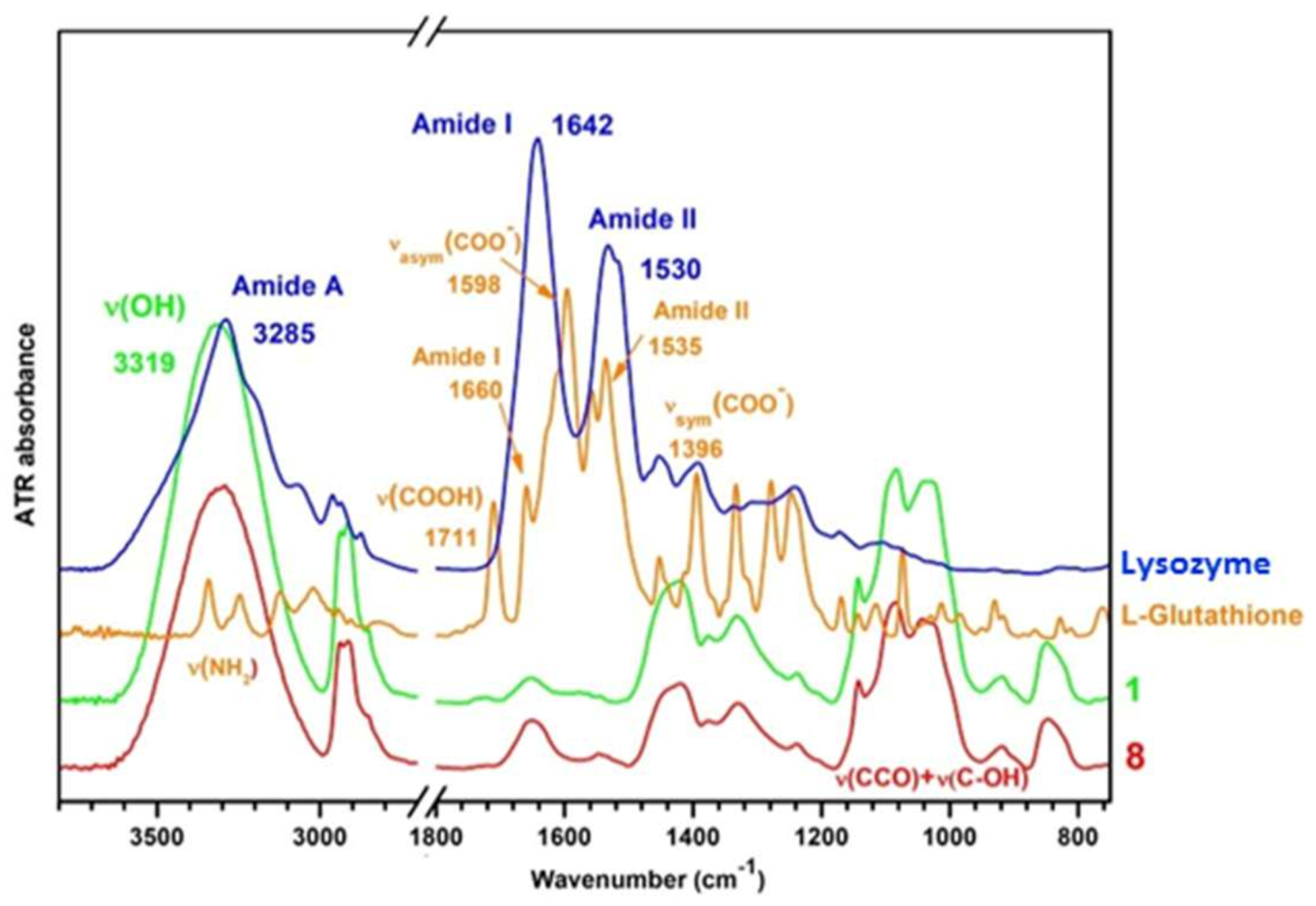
3.2. Infrared Spectroscopy of Hydrogels
3.2.1. Interactions in the Polymer Matrix in Absence and Presence of Proteins
3.2.2. Conformational Changes of BSA in Presence of PULL/PVA Mixtures
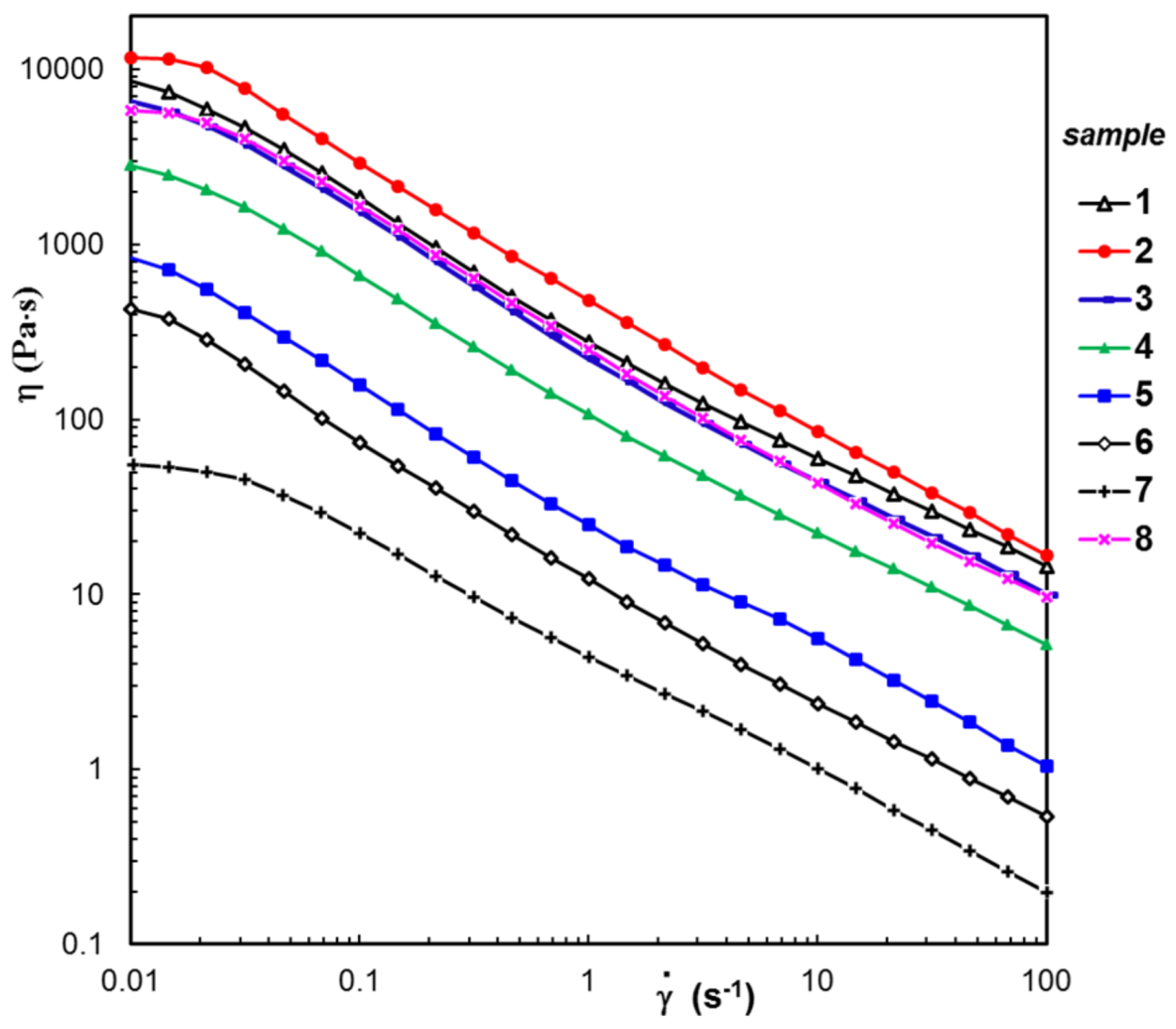
3.3. Rheological Behavior
3.4. Swelling
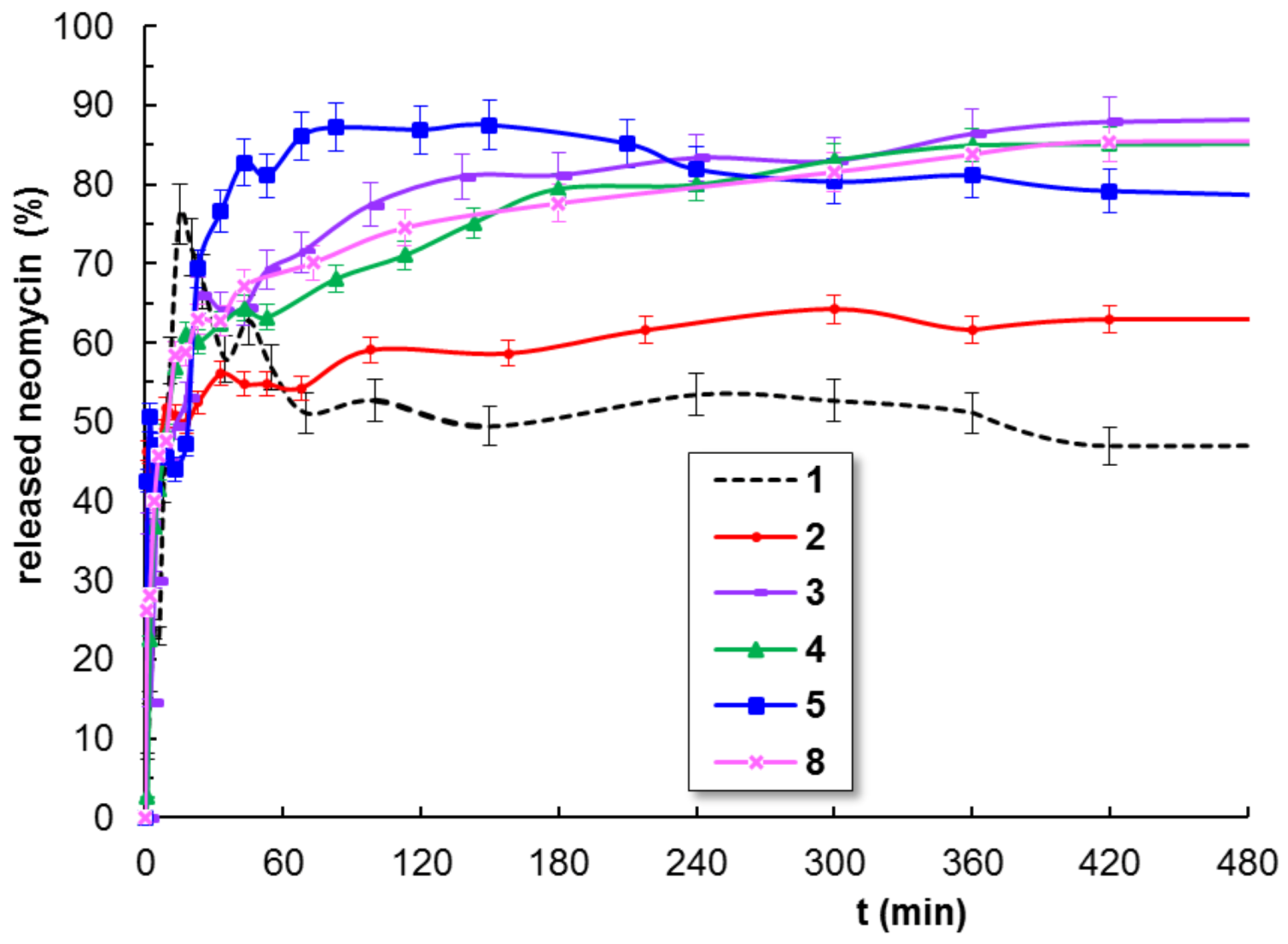
3.5. Neomicin Delivery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Ye, B.; Wu, B.; Su, Y.; Sun, T.; Guo, X. Recent advances in the application of natural and synthetic polymer-based scaffolds in musculoskeletal regeneration. Polymers 2022, 14, 4566. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Júnior, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Niem, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-cross-linked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic hydrogels to promote wound healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, D.W.; Kim, D.S.; Kim, J.O.; Yong, C.S.; Cho, K.H.; Youn, Y.S.; Jin, S.G.; Choi, H.-G. Novel neomycin sulfate-loaded hydrogel dressing with enhanced physical dressing properties and wound curing effect. Drug Deliv. 2016, 23, 2806–2812. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Rusu, D. In-situ gelation of aqueous solutions of entangled poly(vinyl alcohol). Soft Matter 2013, 9, 1244–1253. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Teodorescu, M. Rheological investigation of poly(vinyl alcohol)/poly(N-vinyl pyrrolidone) mixtures in aqueous solution and hydrogel state. J. Polym. Res. 2016, 23, 142. [Google Scholar] [CrossRef]
- Joshi, N.; Suman, K.; Joshi, Y.M. Rheological behavior of aqueous poly(vinyl alcohol) solution during a freeze–thaw gelation process. Macromolecules 2020, 53, 3452–3463. [Google Scholar] [CrossRef]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S.; Albulescu, R. Polyurethane/poly(vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- Coşkun, G.; Karaca, E.; Ozyurtlu, M.; Özbek, S.; Yermezler, A.; Çavuşoğlu, I. Histological evaluation of wound healing performance of electrospun poly(vinylalcohol)/sodium alginate as wound dressing in vivo. Biomed. Mater. Eng. 2014, 24, 1527–1536. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, S.-J.; Oh, D.H.; Ku, S.K.; Li, D.X.; Yong, C.S.; Choi, H.-G. Wound healing evaluation of sodium fucidate-loaded polyvinylalcohol/sodium carboxymethylcellulose-based wound dressing. Arch. Pharm. Res. 2010, 33, 1083–1089. [Google Scholar] [CrossRef]
- Lin, S.-P.; Lo, K.-Y.; Tseng, T.-N.; Liu, J.-M.; Shih, T.-Y.; Cheng, K.-C. Evaluation of PVA/dextran/chitosan hydrogel for wound dressing. Cell. Polym. 2019, 38, 15–30. [Google Scholar] [CrossRef]
- Shamloo, A.; Sarmadi, M.; Aghababaie, Z.; Vossoughi, M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int. J. Pharm. 2018, 537, 278–289. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.M.; Morariu, S.; Plugariu, I.A.; Gradinaru, R. Tailoring the properties of PVA/HPC/BSA hydrogels for wound dressing applications. React. Funct. Polym. 2022, 170, 105094. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Bibire, L.E.; Carja, G. Chitosan/poly(vinyl alcohol)/LDH biocomposites with pH-sensitive properties. Int. J. Polym. Mat. Polym. Biomat. 2015, 64, 628–636. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical hydrogels of oxidized polysaccharides and poly(vinyl alcohol) for wound dressing applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef] [PubMed]
- Teekamp, N.; Tian, Y.; Visser, J.C.; Olinga, P.; Frijlink, H.W.; Woerdenbag, H.J.; Hinrichs, W.L.J. Addition of pullulan to trehalose glasses improves the stability of β-galactosidase at high moisture conditions. Carbohydr. Polym. 2017, 176, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, K.; Lazurko, C.; Muñoz, M.; McTiernan, C.D.; Alarcon, E.I. Peptide-based functional biomaterials for soft-tissue repair. Front. Bioeng. Biotechnol. 2019, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, H.; Yang, Y.; Wang, H.; Li, X.; Wu, X. Stretchable and biocompatible bovine serum albumin fibrous films supported silver for accelerated bacteria-infected wound healing. Chem. Eng. J. 2021, 417, 129145. [Google Scholar] [CrossRef]
- Bercea, M. Self-healing behavior of polymer/protein hybrid hydrogels. Polymers 2022, 14, 130. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Xiong, L.; Zhang, X.; Zheng, Y. Conductance changes in Bovine Serum Albumin caused by drug-binding triggered structural transitions. Materials 2019, 12, 1022. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human disease. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef]
- Sokolowski, K.; Pham, H.M.; Wenzler, E.; Gemeinhart, R.A. Glutathione-conjugated hydrogels: Flexible vehicles for personalized treatment of bacterial infection. Pharm. Res. 2021, 38, 1247–1261. [Google Scholar] [CrossRef]
- Bercea, M. Bioinspired hydrogels as platforms for life-science applications: Challenges and opportunities. Polymers 2022, 14, 2365. [Google Scholar] [CrossRef]
- Chakravarthi, S.; Bulleid, N.J. Glutathione is required to regulate the formation of native disulfide bonds within proteins entering the secretory pathway. J. Biol. Chem. 2004, 279, 39872–39879. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Altoé, L.S.; Alves, R.S.; Sarandy, M.M.; Morais-Santos, M.; Novaes, R.D.; Gonçalves, R.V. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. PLoS ONE 2019, 14, e0223511. [Google Scholar] [CrossRef]
- Hosny, K.M.; Naveen, N.R.; Kurakula, M.; Sindi, A.M.; Sabei, F.Y.; Fatease, A.A.; Jali, A.M.; Alharbi, W.S.; Mushtaq, R.Y.; Felemban, M.; et al. Design and development of neomycin sulfate gel loaded with solid lipid nanoparticles for buccal mucosal wound healing. Gels 2022, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Mohamed, G.G.; Ismail, S.H.; Mousa, M.R.; Elbanna, H.A.; Osman, A.S. Evaluation of the wound healing effect of neomycin-silver nano-composite gel in rats. Int. J. Immunopathol. Pharmacol. 2022, 36, 1–12. [Google Scholar] [CrossRef]
- dos Santos, W.G.; Buck, G.A. Simultaneous stable expression of neomycin phosphotransferase and green fluorescence protein genes in Trypanosoma cruzi. J. Parasitol. 2000, 86, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Ayliffe, G.A.J. Stability of neomycin resistance in Staphylococcus aureus. J. Clin. Path. 1970, 23, 19–23. [Google Scholar] [CrossRef]
- Simone, R.M.; Popino, R.P. The stability of neomycin in several pharmaceutical preparations. J. Am. Pharm. Ass. 1955, 44, 275–280. [Google Scholar] [CrossRef]
- Zafalon, A.T.; Dos Santos, V.J.; Lugão, A.B.; Rangari, V.; Temesgen, S.; Parra, D.F. Stability of the neomycin antibiotic in irradiated polymeric biomaterials. Eur. J. Biomed. Pharm. Sci. 2018, 5, 49–57. [Google Scholar]
- Stypulkowska, K.; Blazewicz, A.; Fijalek, K.; Warowna-Grzeskiewicz, M.; Srebrzynska, K. Determination of neomycin and related substances in pharmaceutical preparations by reversed-phase high performance liquid chromatography with mass spectrometry and charged aerossol detection. J. Pharm. Biomed. Anal. 2013, 76, 207–214. [Google Scholar] [CrossRef]
- Wan, Y.C.; Liu, Y.; Liu, C.; Ma, H.; Yu, H.; Kang, J.; Gao, C.; Wu, Z.; Zheng, D.; Lu, B. Rapid determination of neomycin in biological samples using fluorescent sensor based on quantum dots with doubly selective binding sites. J. Pharm. Biomed. Anal. 2018, 154, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yu, F.; Lu, J.; Zhang, M.; Wang, H.; Xu, D.; Lu, L. In vivo effects of neomycin sulfate on non-specific immunity, oxidative damage and replication of cyprinid herpesvirus 2 in crucian carp (Carassius auratus gibelio). Aquac. Fish. 2019, 4, 67–73. [Google Scholar] [CrossRef]
- Neomycin for dogs and cats. Available online: https://www.wedgewoodpharmacy.com/medications/neomycin (accessed on 18 January 2023).
- Napoli, S.; Carbone, G.M.; Catapano, C.V.; Shaw, N.; Arya, D.P. Neomycin improves cationic lipid-mediated transfection of DNA in human cells. Bioorg. Med. Chem. Lett. 2005, 15, 3467–3469. [Google Scholar] [CrossRef] [PubMed]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Petty, M.M.; Gautam, S.P. Formulation and characterization of neomycin sulfate loaded ethosomes for transdermal delivery. J. Pharm. Drug Res. 2021, 4, 462–469. [Google Scholar]
- Carvajal-Aldaz, D.G.; Guice, J.L.; Page, R.C.; Raggio, A.M.; Martin, R.J.; Husseneder, C.; Durham, H.A.; Geaghan, J.; Janes, M.; Gauthier, T.; et al. Simultaneous delivery of antibiotics neomycin and ampicillin in drinking water inhibits fermentation of resistant starch in rats. Mol. Nutr. Food Res. 2017, 61, 1600609. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 2006, 27, 5440–5451. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Ambalangodage, C.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biophacodyn. 1978, 6, 165–175. [Google Scholar] [CrossRef]
- Tüting, L.; Ye, W.X.; Settanni, G.; Schmid, F.; Wolf, B.A.; Ahijado-Guzman, R.; Sönnichsen, C. Potassium triggers a reversible specific stiffness transition of polyethylene glycol. J. Phys. Chem. C 2017, 121, 22396–22402. [Google Scholar] [CrossRef]
- Bercea, M.; Wolf, B.A. Intrinsic viscosities of polymer blends: Sensitive probes of specific interactions between the counterions of polyelectrolytes and uncharged macromolecules. Macromolecules 2018, 51, 7483–7490. [Google Scholar] [CrossRef]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Panahi-Azar, V.; Sajedi, S. Interaction of glutathione with bovine serum albumin: Spectroscopy and molecular docking. Food Chem. 2016, 202, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Shingel, K.I. Determination of structural peculiarities of dextran, pullulan and -irradiated pullulan by Fourier-transform IR spectroscopy. Carbohydr. Res. 2002, 337, 1445–1451. [Google Scholar] [CrossRef]
- Boulkanz, L.; Balcar, N.; Baron, M.-H. FT-IR analysis for structural characterization of albumin adsorbed on the reversed-phase support RP-C6. Appl. Spectrosc. 1995, 49, 1737–1746. [Google Scholar] [CrossRef]
- Murayama, K.; Tomida, M. Heat-induced secondary structure and conformation change of bovine serum albumin investigated by Fourier Transform Infrared Spectroscopy. Biochemistry 2004, 43, 11526–11532. [Google Scholar] [CrossRef]
- Güler, G.; Vorob’ev, M.M.; Vogel, V.; Mäntele, W. Proteolytically-induced changes of secondary structural protein conformation of bovine serum albumin monitored by Fourier transform infrared (FT-IR) and UV-circular dichroism spectroscopy. Spectrochim. Acta A 2016, 161, 8–18. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gumy, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable device. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Peppas, N.A.; Simmons, R.E.P. Mechanistic analysis of protein delivery from porous poly(vinyl alcohol) systems. J. Drug Del. Sci. Tech. 2004, 14, 285–289. [Google Scholar] [CrossRef]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Nitanan, T.; Akkaramongkolporn, P.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Neomycin-loaded poly(styrene sulfonic acid-co-maleic acid) (PSSAMA)/polyvinyl alcohol (PVA) ion exchange nanofibers for wound dressing materials. Int. J. Pharm. 2013, 448, 71–78. [Google Scholar] [CrossRef]
- Adams, E.; Schepers, R.; Roets, E.; Hoogmartens, J. Determination of neomycin sulfate by liquid chromatography with pulsed electrochemical detection. J. Chromatogr. A 1996, 741, 233–240. [Google Scholar] [CrossRef]
- Clarot, I.; Regazzeti, A.; Auzeil, N.; Laadani, F.; Citton, M.; Netter, P.; Nicolas, A. Analysis of neomycin sulfate and framycetin sulfate by high-performance liquid chromatography using evaporative light scattering detection. J. Chromatogr. A 2005, 1087, 236–244. [Google Scholar] [CrossRef]
- Jabeen, S.; Islam, A.; Ghaffar, A.; Gull, N.; Hameed, A.; Bashir, A.; Jamil, T.; Hussain, T. Development of a novel pH sensitive silane crosslinked injectable hydrogel for controlled release of neomycin sulfate. Int. J. Biol. Macromol. 2017, 97, 218–227. [Google Scholar] [CrossRef]
- Neomycin sulfate 25 mg/mL oral solution. Available online: https://www.uspharmacist.com/article/neomycin-sulfate-25-mgml-oral-solution (accessed on 14 January 2023).
- Singh, M.; Jonnalagadda, S. Design and characterization of 3D printed, neomycin-eluting poly-L-lactide mats for wound-healing applications. J. Mater. Sci. Mater. Med. 2021, 32, 44. [Google Scholar] [CrossRef]



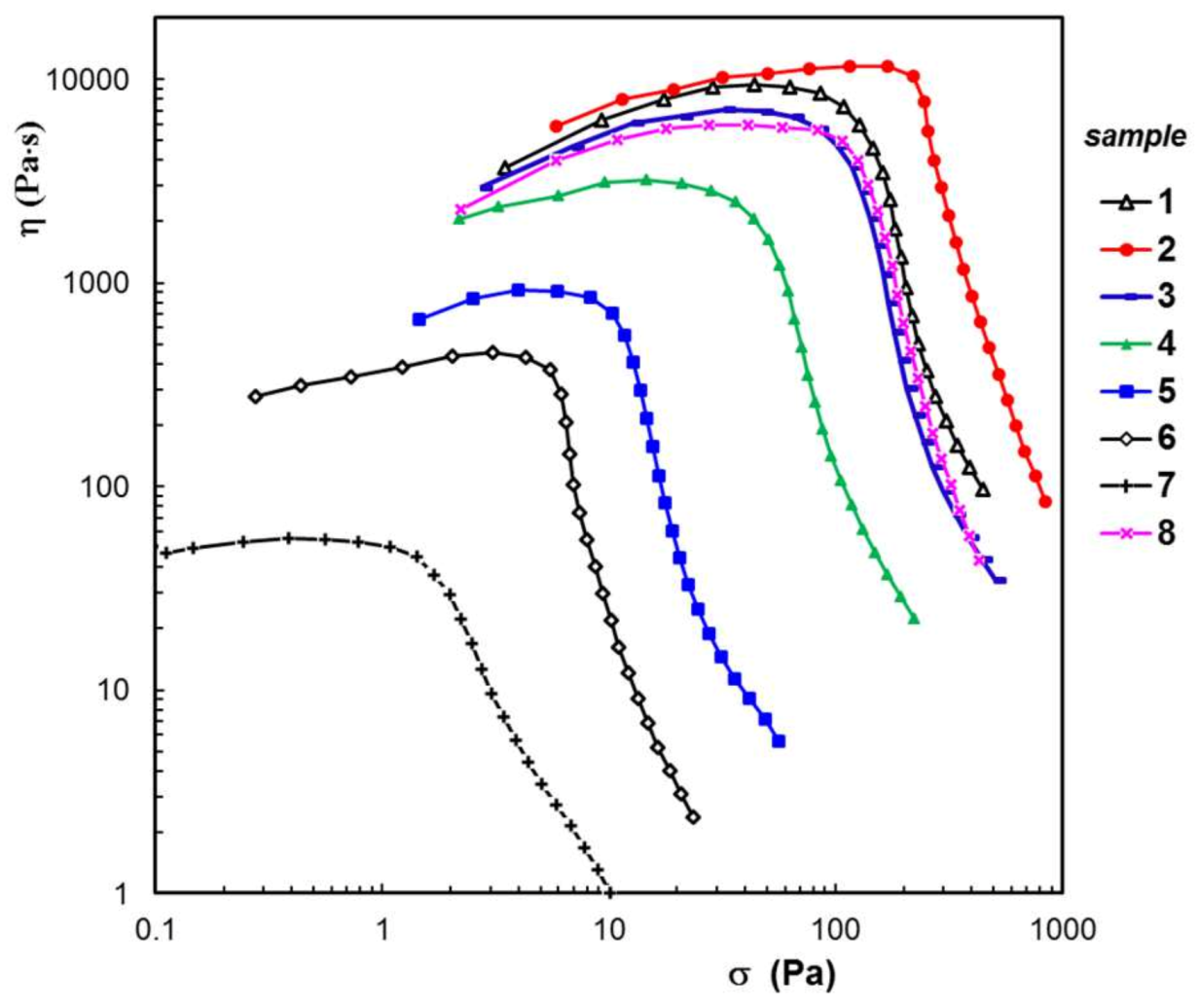
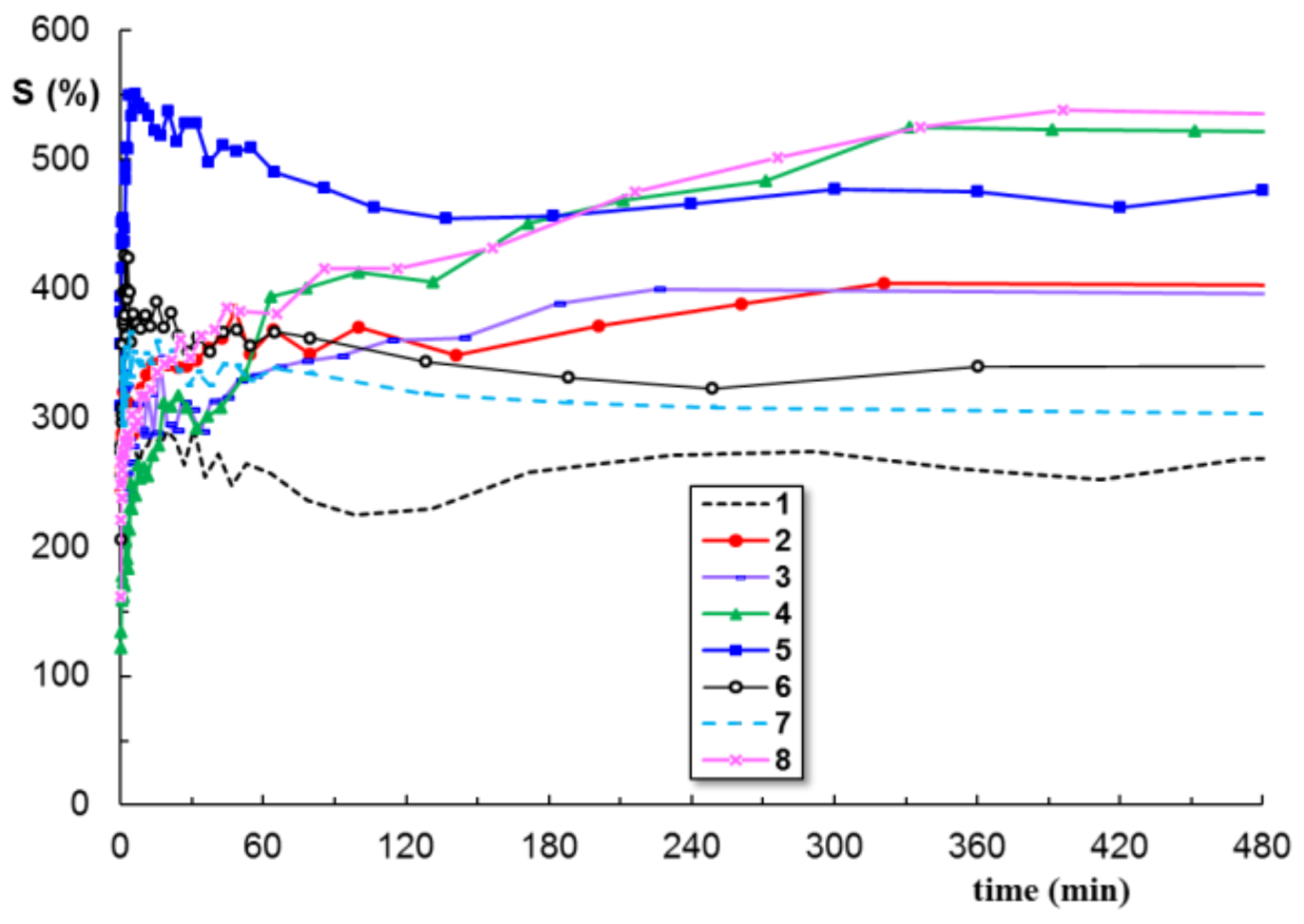
| Sample Composition a | SEM | Rheology b | Swelling in PBS b | Disintegration c | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Code | wBSA (wt. %) | Average Pore Size (μm) | G′ (Pa) | ηo (Pa⋅s) | σo (Pa) | Seq (%) | R2 | Mass Loss (%) | |
| 1 | 0 | 21.1 ± 2.6 | 2230 | 8540 | 63 | 224 | 0.0207 | 0.994 | 0.88 |
| 2 | 5 | 18.6 ± 3.3 | 2650 | 11,500 | 170 | 356 | 0.0437 | 0.981 | 1.24 |
| 3 | 10 | 21.8 ± 3.1 | 1726 | 6560 | 41 | 364 | 0.0148 | 0.983 | 3.35 |
| 4 | 30 | 18.9 ± 1.9 | 799 | 2830 | 21 | 468 | 0.1205 | 0.969 | 5.78 |
| 5 | 50 | 15.7 ± 2.2 | 174 | 844 | 8 | 457 | 0.0608 | 0.936 | 8.65 |
| 6 | 70 | 16.2 ± 1.8 | 78.6 | 428 | 4 | 349 | 0.0459 | 0.877 | 36.14 |
| 7 | 90 | 24.5 ± 1.7 | 22.6 | 54.7 | 1 | 256 | 0.0311 | 0.843 | 59.77 |
| 8 | 0 | 20.3 ± 1.1 | 1340 | 5910 | 79 | 533 | 0.0866 | 0.964 | 2.42 |
| Sample Code | Korsmeyer-Peppas Equation (5) | First Order Equation (6) | Higuchi Equation (7) | Peppas-Sahlin Equation (8) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | AIC | k11 (min−1) | AIC | kh (min−1/2) | AIC | k1 (min−m) | k2 (min−2m) | m | AIC | |
| 1 | 0.4664 | -44.9337 | 0.1428 | -40.9695 | 0.2799 | -40.9695 | 0.2619 | -0.0214 | 0.3717 | -32.7543 |
| 2 | 0.2562 | -76.0326 | 0.0562 | -24.0109 | 0.0401 | -38.8007 | 0.3675 | -0.0379 | 0.3235 | -62.6196 |
| 3 | 0.2543 | -74.3310 | 0.0424 | -26.2339 | 0.0603 | -12.8497 | 0.2763 | -0.0215 | 0.3454 | -63.7953 |
| 4 | 0.1986 | -75.9132 | 0.0516 | -30.0502 | 0.0837 | -15.9917 | 0.2640 | -0.0197 | 0.3928 | -69.7078 |
| 5 | 0.2631 | -80.7675 | 0.0498 | -16.4281 | 0.0993 | -17.6851 | 0.2598 | -0.0166 | 0.4120 | -74.5820 |
| 8 | 0.2220 | -82.4251 | 0.0479 | -35.2077 | 0.1111 | -25.8943 | 0.3802 | -0.0363 | 0.2823 | -73.8514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bercea, M.; Plugariu, I.-A.; Gradinaru, L.M.; Avadanei, M.; Doroftei, F.; Gradinaru, V.R. Hybrid Hydrogels for Neomycin Delivery: Synergistic Effects of Natural/Synthetic Polymers and Proteins. Polymers 2023, 15, 630. https://doi.org/10.3390/polym15030630
Bercea M, Plugariu I-A, Gradinaru LM, Avadanei M, Doroftei F, Gradinaru VR. Hybrid Hydrogels for Neomycin Delivery: Synergistic Effects of Natural/Synthetic Polymers and Proteins. Polymers. 2023; 15(3):630. https://doi.org/10.3390/polym15030630
Chicago/Turabian StyleBercea, Maria, Ioana-Alexandra Plugariu, Luiza Madalina Gradinaru, Mihaela Avadanei, Florica Doroftei, and Vasile Robert Gradinaru. 2023. "Hybrid Hydrogels for Neomycin Delivery: Synergistic Effects of Natural/Synthetic Polymers and Proteins" Polymers 15, no. 3: 630. https://doi.org/10.3390/polym15030630
APA StyleBercea, M., Plugariu, I.-A., Gradinaru, L. M., Avadanei, M., Doroftei, F., & Gradinaru, V. R. (2023). Hybrid Hydrogels for Neomycin Delivery: Synergistic Effects of Natural/Synthetic Polymers and Proteins. Polymers, 15(3), 630. https://doi.org/10.3390/polym15030630








