Manuka Honey/2-Hydroxyethyl Methacrylate/Gelatin Hybrid Hydrogel Scaffolds for Potential Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
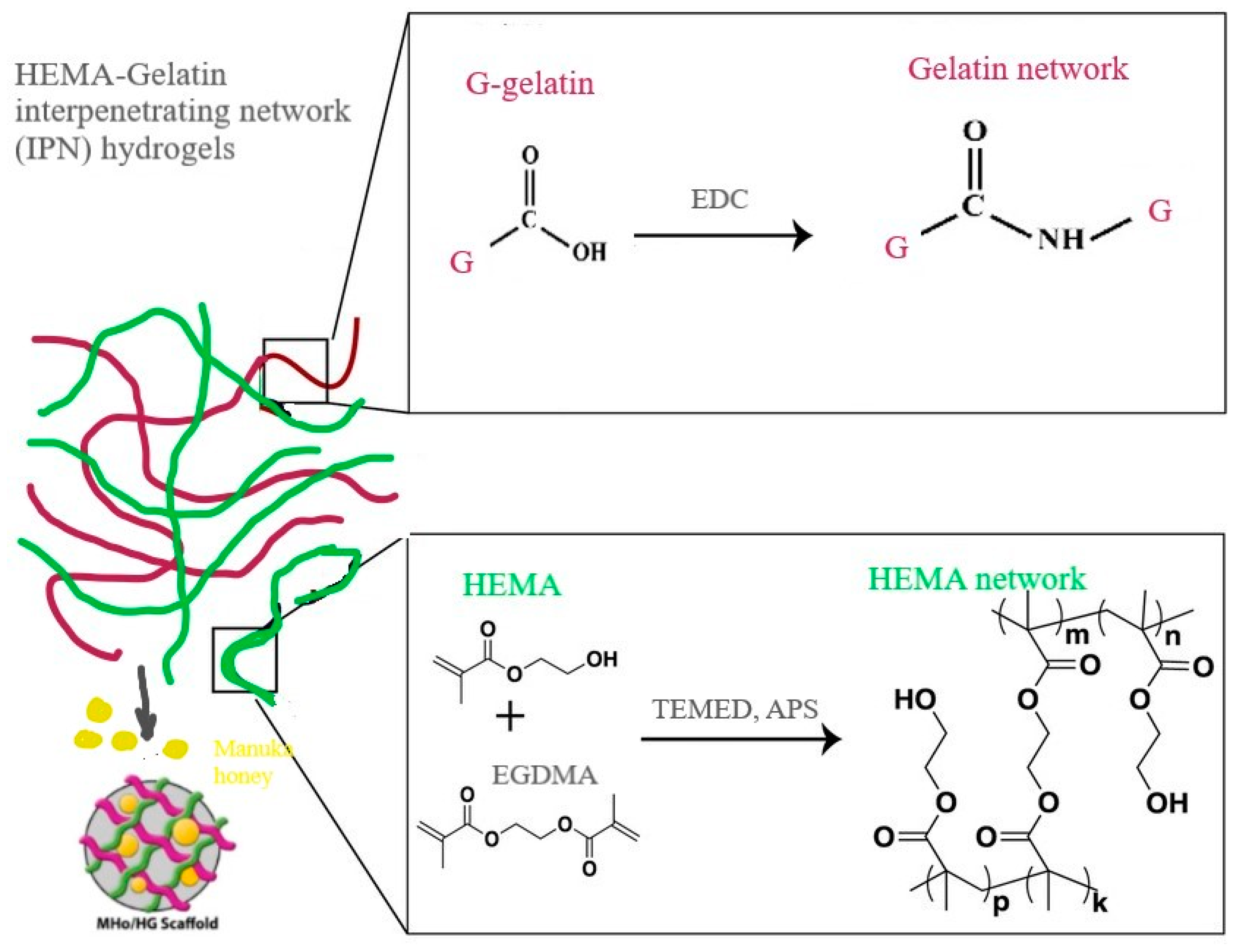
2.2. Hydrogel Scaffold Syntheses
2.3. Hybrid Hydrogel Scaffold Characterization
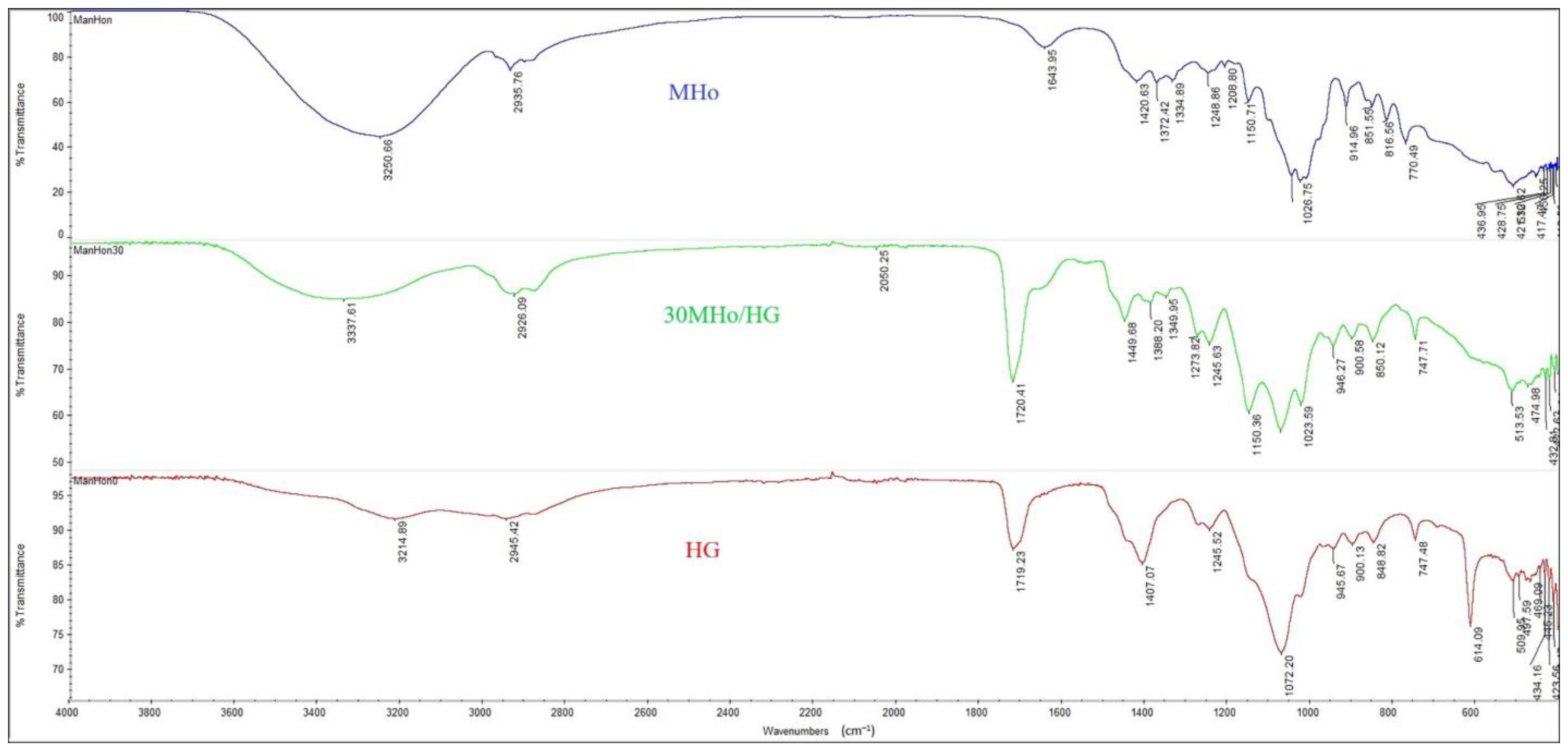
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
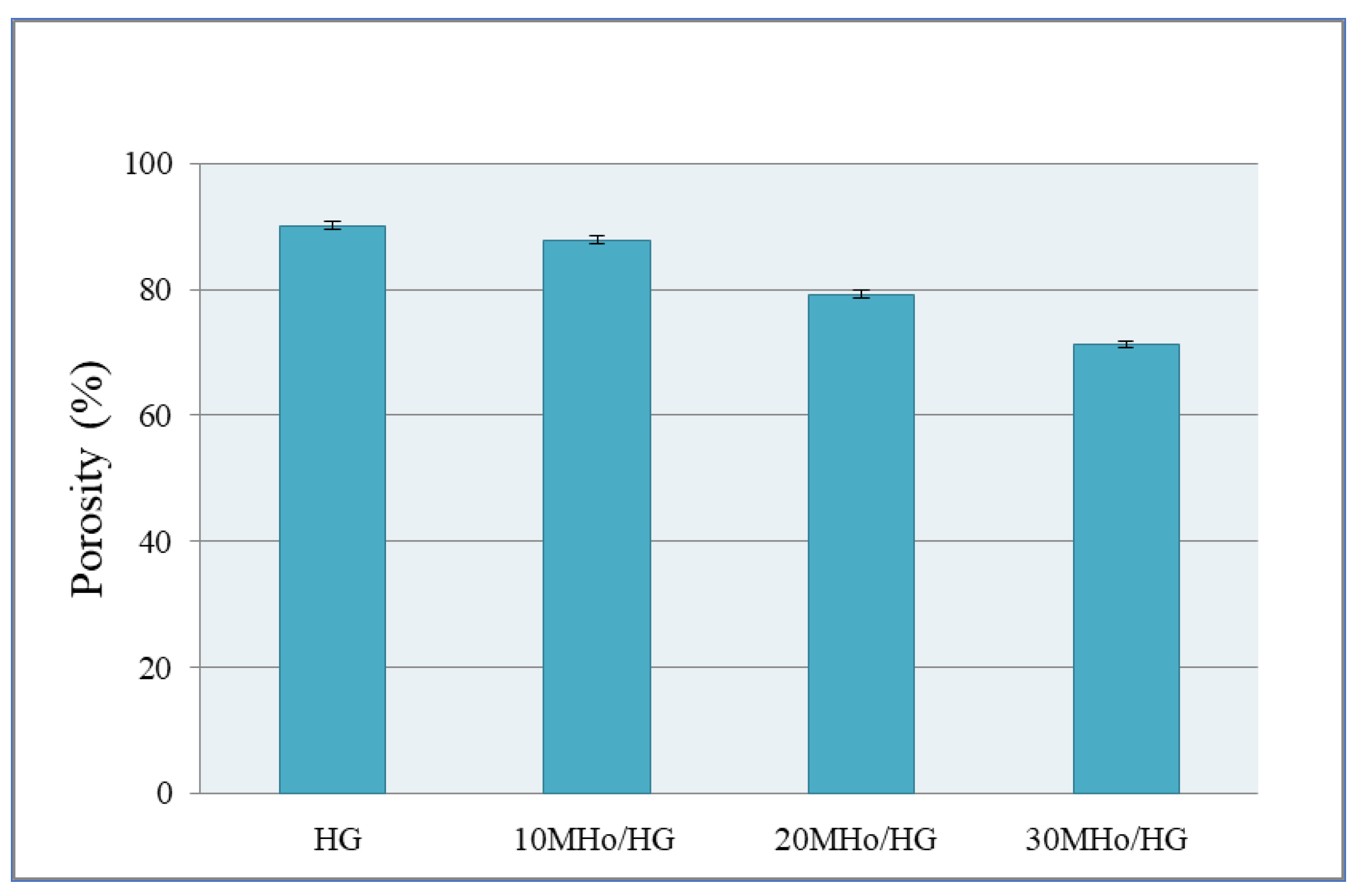
2.3.2. Porosity Measurements
2.3.3. In Vitro pH- and Temperature-Dependent Swelling Studies
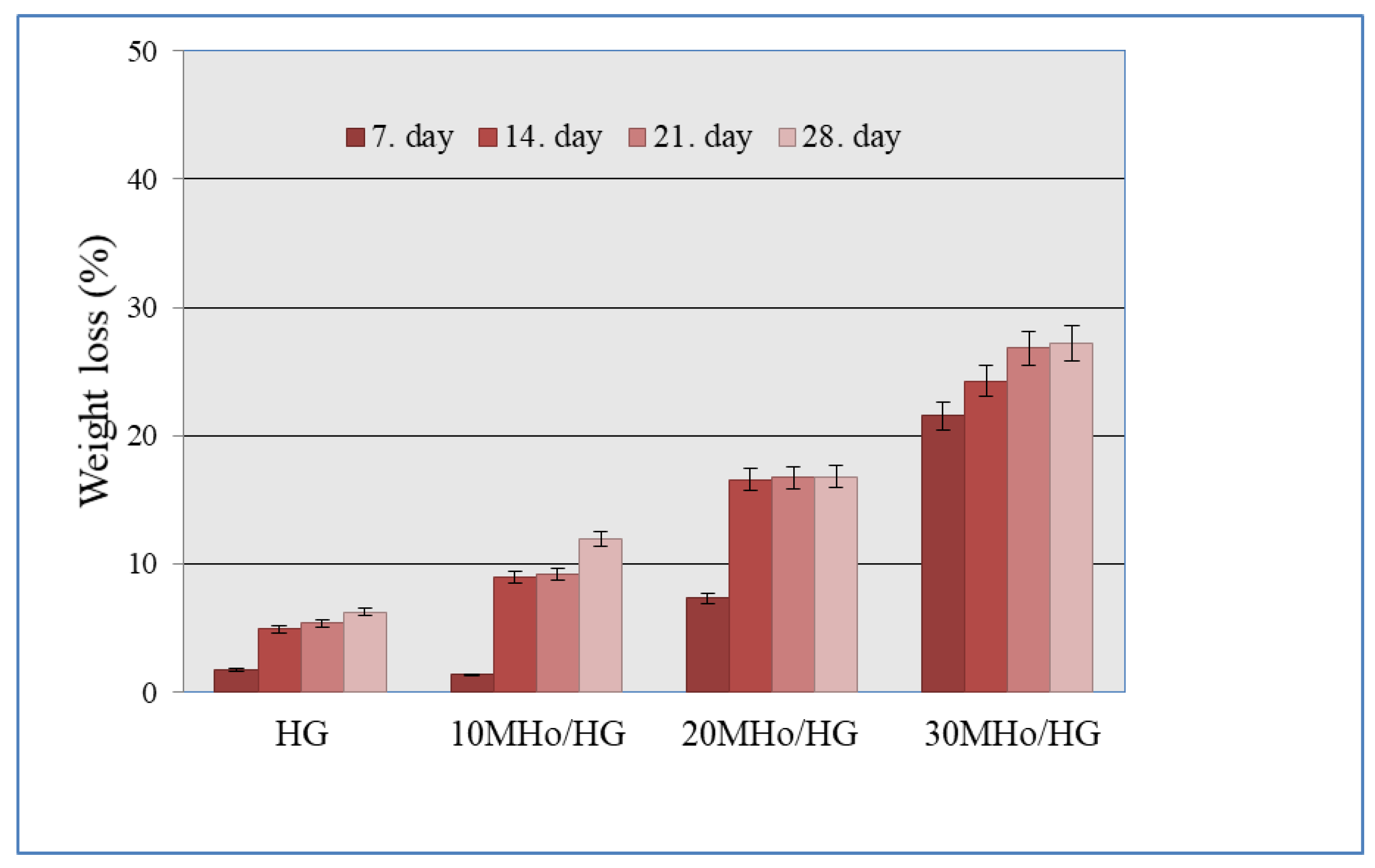
2.3.4. In Vitro Degradation Study
2.4. In Vitro Biocompatibility Assay
3. Results and Discussion
3.1. Structural Features of MHo/HG Hybrid Hydrogel Scaffolds
3.2. Porosity of MHo/HG Hybrid Hydrogel Scaffolds
3.3. Swelling Features of MHo/HG Hybrid Hydrogel Scaffolds
3.4. In Vitro Degradation Behavior of MHo/HG Hybrid Hydrogel Scaffolds
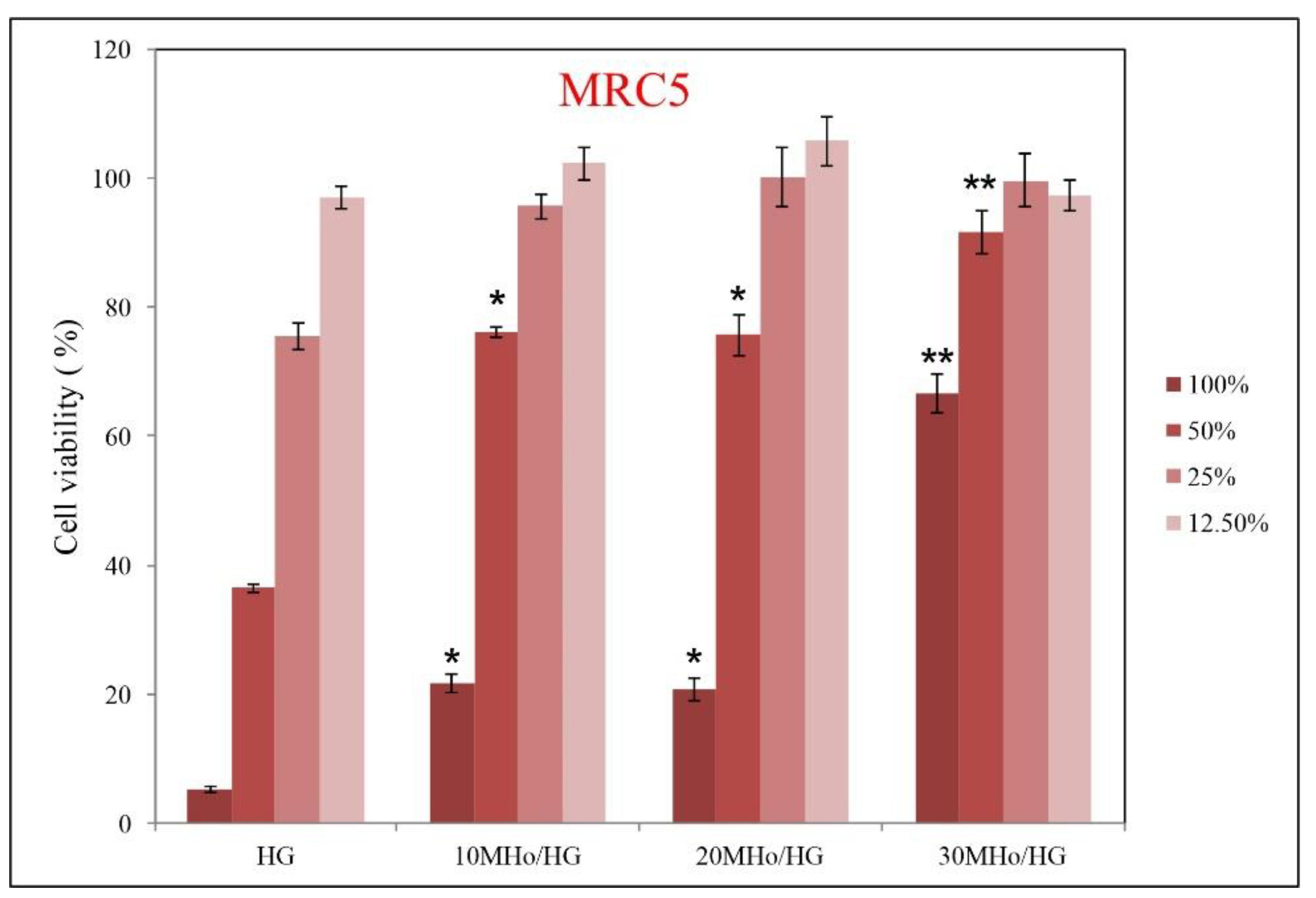
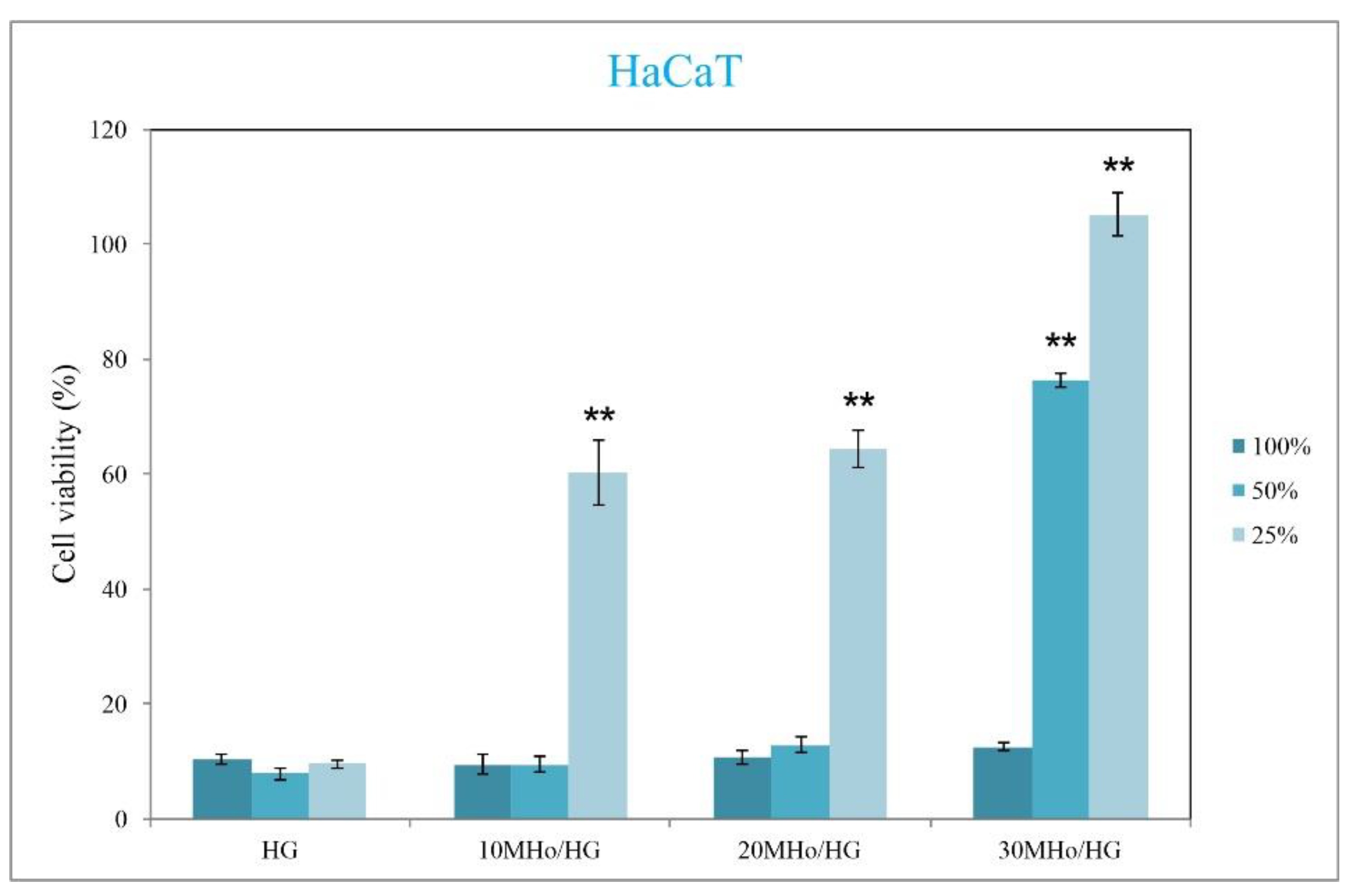
3.5. Biocompatibility Assays of MHo/HG Hybrid Hydrogel Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An Advanced Antimicrobial and Wound Healing Biomaterial for Tissue Engineering Applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef] [PubMed]
- Angioi, R.; Morrin, A.; White, B. The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques. Appl. Sci. 2021, 11, 5192. [Google Scholar] [CrossRef]
- Speer, S.L.; Schreyack, G.E.; Bowlin, G.L. Manuka Honey: A Tissue Engineering Essential Ingredient. J. Sci. Eng. 2015, 6, 1–3. [Google Scholar] [CrossRef]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.K.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-Dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid. Med. Cell Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef]
- Frydman, G.H.; Olaleye, D.; Annamalai, D.; Layne, K.; Yang, I.; Kaafarani, H.M.A.; Fox, J.G. Manuka honey microneedles for enhanced wound healing and the prevention and/or treatment of Methicillin-resistant Staphylococcus aureus (MRSA) surgical site infection. Sci. Rep. 2020, 10, 13229. [Google Scholar] [CrossRef]
- Jenkins, R.; Roberts, A.E.L.; Brown, H.L. On the antibacterial effects of manuka honey: Mechanistic insights. Res. Rep. Biol. 2015, 6, 215–224. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A Preliminary Study on the Potential of Manuka Honey and Platelet-Rich Plasma inWound Healing. Int. J. Biomater. 2012, 2012, 313781. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Gounari, P.; Skourtis, A.; Panagos, J.; Kazazis, C. Honey and its Anti-Inflammatory, Anti-Bacterial and Anti-Oxidant Properties. Gen. Med. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- McLoone, P.; Tabys, D.; Fyfe, L. Honey Combination Therapies for Skin and Wound Infections: A Systematic Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2020, 13, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Tomblin, V.; Ferguson, L.R.; Han, D.Y.; Murray, P.; Schlothauer, R. Potential pathway of anti-inflammatory effect by New Zealand honeys. Int. J. Gen. Med. 2014, 7, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Bogner, S.J.; Ronning-Arnesen, G.; Janowiak, B.E.; Sell, S.A. Investigatingmanuka honey antibacterial properties when incorporated into cryogel, hydrogel, and electrospun tissue engineering scaffolds. Gels 2019, 5, 21. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Cengiz, I.F.; Silva-Correia, J.; Sousa, R.A.; Oliveira, J.M.; Reis, R.L. “Smart” hydrogels in tissue engineering and regenerative medicine applications. In Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine, 2nd ed.; Gilson Khang, G., Ed.; Jenny Stanford Publishing Pte. Ltd.: Singapore, 2017; pp. 333–364. [Google Scholar]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 32–33. [Google Scholar] [CrossRef]
- Bettinger, C.; Borenstein, J.; Langer, R. Microfabrication techniques in scaffold development. In Nanotechnology and Regenerative Engineering, 2nd ed.; Laurencin, C.T., Nair, L.S., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 103–142. [Google Scholar]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Khandan, A.; Jazayeri, H.; Fahmy, M.D.; Razavi, M. Hydrogels: Types, structure, properties, and applications. In Frontiers in Biomaterials-Biomaterials for Tissue Engineering; Bentham Science: Sharjah, United Arab Emirates, 2017; Volume 4, pp. 143–169. [Google Scholar]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremiao, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Kasinski, A.; Zielinska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels–synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Johnson, N.; Wang, Y. Drug delivery systems for wound healing. Curr. Pharm. Biotechnol. 2015, 16, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayan, R.; Maji, S.; Behera, S.; Kulanthaivel, S.; Maiti, T.K.; Banerjee, I.; Pal, K.; Giri, S. Gelatin/carboxymethyl chitosan based scaffolds for dermal tissue engineering applications. Int. J. Biol. Macromol. 2016, 93, 1499–1506. [Google Scholar] [CrossRef]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control Release 2010, 142, 149–159. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Lasch, K.; Weidinger, J.; Boccaccini, A.R. Manuka honey and zein coatings impart bioactive glass bone tissue scaffolds antibacterial properties and superior mechanical properties. Front. Mater. 2021, 7, 610889. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Honey, wound repair and regenerative medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Tshai, K.Y.; Nordin, N.; Prasad, V. Gelatin based scaffolds for tissue engineering—A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Shevchenko, R.V.; Eeman, M.; Rowshanravan, B.; Allan, L.U.; Savina, I.N.; Illsley, M.; Salmon, M.; James, S.L.; Mikhalovsky, S.V.; James, S.E. The in vitro characterization of a gelatin scaffold, prepared by cryogelation and assessed in vivo as a dermal replacement in wound repair. Acta Biomater. 2014, 10, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.V.; Jungvid, H.; Galaev, I.Y. Gelatin-fibrinogen cryogel dermal matrices for wound repair: Preparation, optimisation and in vitro study. Biomaterials 2010, 31, 67–76. [Google Scholar] [CrossRef]
- Allan, I.U.; Tolhurst, B.A.; Shevchenko, R.V.; Dainiak, M.B.; Illsley, M.; Ivanov, A.; Jungvid, H.; Galaev, I.Y.; James, S.L.; Mikhalovsky, S.V.; et al. An in vitro evaluation of fibrinogen and gelatin containing cryogels as dermal regeneration scaffolds. Biomater. Sci. 2016, 4, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhossein, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutic. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Horák, D. Application of poly(2-hydroxyethyl methacrylate) in medicine. In Polymers and Composites: Synthesis, Properties, and Applications, Polymer Yearbook; Pethrick, R.A., Zaikov, G.E., Horák, D., Eds.; Nova Science Publishers: New York, NY, USA, 2007; Volume 21, pp. 1–33. [Google Scholar]
- Kopeček, J. Hydrogels from soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. A 2009, 47, 5929–5946. [Google Scholar] [CrossRef]
- Park, S.; Nam, S.H.; Koh, W.-G. Preparation of collagen-immobilized poly(ethylene glycol)/poly(2-hydroxyethyl methacrylate) interpenetrating network hydrogels for potential application of artificial cornea. J. Appl. Polym. Sci. 2012, 123, 637–645. [Google Scholar] [CrossRef]
- Hidzir, N.M.; Radzali, N.A.M.; Rahman, I.A.; Shamsudin, S.A. Gamma irradiation-induced grafting of 2-hydroxyethyl methacrylate (HEMA) onto ePTFE for implant applications. Nucl. Eng. Technol. 2020, 52, 2320–2327. [Google Scholar] [CrossRef]
- Babić Radić, M.M.; Filipović, V.V.; Vuković, J.S.; Vukomanović, M.; Rubert, M.; Hofmann, S.; Müller, R.; Tomić, S.L. Bioactive interpenetrating hydrogel networks based on 2-hydroxyethyl methacrylate and gelatin intertwined with alginate and dopped with apatite as scaffolding biomaterials. Polymers 2022, 14, 3112. [Google Scholar] [CrossRef] [PubMed]
- Filipović, V.V.; Babić Radić, M.M.; Vuković, J.S.; Vukomanović, M.; Rubert, M.; Hofmann, S.; Müller, R.; Tomić, S.L. Biodegradable hydrogel scaffolds based on 2-hydroxyethyl methacrylate, gelatin, poly(β-amino esters), and hydroxyapatite. Polymers 2022, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Tomić, S.L.; Nikodinović-Runić, J.; Vukomanović, M.; Babić, M.M.; Vuković, J.S. Novel hydrogel scaffolds based on alginate, gelatin, 2-hydroxyethyl methacrylate, and hydroxyapatite. Polymers 2021, 13, 932. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.F.; Dias, D.R.C.; Bastos, G.N.T.; Jardini, A.L.; Benatti, A.C.B.; Dias, C.G.B.T.; Maciel Filho, R. pHEMA hydrogels: Synthesis, kinetics and in vitro tests. J. Therm. Anal. Calorim. 2016, 125, 361–368. [Google Scholar] [CrossRef]
- Dobić, S.N.; Filipović, J.M.; Tomić, S.L. Synthesis and characterization of poly(2-hydroxyethyl methacrylate/itaconic acid)/poly(ethyleneglycol dimethacrylate) hydrogels. Chem. Eng. J. 2012, 179, 372–380. [Google Scholar] [CrossRef]
- Filipović, V.V.; Bozić Nedeljković, B.D.; Vukomanović, M.; Tomić, S.L. Biocompatible and degradable scaffolds based on 2-hydroxyethyl methacrylate, gelatin and poly(beta amino ester) crosslinkers. Polym. Test. 2018, 68, 270–278. [Google Scholar] [CrossRef]
- Bell, C.L.; Peppas, N.A. Measurement of swelling force in ionic polymer networks. III. Swelling force of interpolymer complexes. J. Control Release 1995, 37, 77–280. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymer. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić, M.M.; Antić, K.M.; Vuković, J.S.; Malešić, N.B.; Filipović, J.M. pH-sensitive hydrogels based on (meth)acrylates and itaconic acid. Macromol. Res. 2014, 22, 1203–1213. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhao, Y.; Ding, J.; Lin, S. Investigation on complex coacervation between fish skin gelatin from cold-water fish and gum arabic: Phase behavior, thermodynamic, and structural properties. Food Res. Int. 2018, 107, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Svečnjak, L.; Biliškov, N.; Bubalo, D.; Barišić, D. Application of infrared spectroscopy in honey analysis. Agric. Conspec. Sci. 2011, 76, 191–195. [Google Scholar]
- Arslan, A.; Simsek, M.; Aldemir, S.D.; Kazaroglu, N.M.; Gumusderelioglu, M. Honey-based PET or PET/chitosan fibrous wound dressings: Effect of honey on electrospinning process. J. Biomater. Sci. Polym. Ed. 2014, 25, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Head, J.; Kinyanjui, J.; Talbott, M. FTIR-ATR Characterization of Commercial Honey Samples and Their Adulteration with Sugar Syrups Using Chemometric Analysis; Shimadzu Scientific Instruments: Columbia, MD, USA, 2015. [Google Scholar]
- Sabri, N.F.M.; See, H.H. Classification of honey using Fourier transform infrared spectroscopy and chemometrics. EProceedings Chem. 2016, 1, 22–26. [Google Scholar]
- Ebrahimi, M. Porosity parameters in biomaterial science: Definition, impact, and challenges in tissue engineering. Front. Mater. Sci. 2021, 15, 352–373. [Google Scholar] [CrossRef]
- Rodrıguez-Rodrıguez, R.; Garcıa-Carvajal, Z.; Jimenez-Palomar, I.; Jimenez-Avalos, J.; Espinosa-Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2019, 136, 47149–47168. [Google Scholar] [CrossRef]
- Bakravi, A.; Ahamadian, Y.; Hashemi, H.; Namazi, H. Synthesis of gelatin-based biodegradable hydrogel nanocomposite and their application as drug delivery agent. Adv. Polym. Technol. 2018, 37, 2625–2635. [Google Scholar] [CrossRef]
- Boral, S.; Gupta, A.N.; Bohidar, H.B. Swelling and de-swelling kinetics of gelatin hydrogels in ethanol-water marginal solvent. Int. J. Biol. Macromol. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Tomić, S.L. Synthesis, Structure, and Properties of Hydrogels Based on Vinyl Monomers. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2006. [Google Scholar]
- Zhang, H.; Zhou, L.; Zhang, W. Control of scaffold degradation in tissue engineering: A Review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Biocompatibility and drug delivery systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- Williams, D.F. Biocompatibility. In Tissue Engineering, 1st ed.; Van Blitterswijk, C., De Boer, J., Thomsen, P., Hubbell, J., Cancedda, R., de Bruijn, J.D., Lindahl, A., Sohier, J., Williams, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 255–278. [Google Scholar]
- Tronci, G. Synthesis, Characterization, and Biological Evaluation of Gelatin-Based Scaffolds. Ph.D. Thesis, University of Potsdam, Potsdam, Germany, 2010. [Google Scholar]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.; Kang, I.-K. Biocompatible polymers and their potential biomedical applications: A review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef] [PubMed]
- Nasonova, M.V.; Glushkova, T.V.; Borisov, V.V.; Velikanova, E.A.; Burago, A.Y.; Kudryavtseva, Y.A. Biocompatibility and structural features of biodegradable polymer scaffolds. Bull. Exp. Biol. Med. 2015, 160, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef]
- Yang, L.; Xing, S.; Wang, K.; Yi, H.; Du, B. Paeonol attenuates aging MRC-5 cells and inhibits epithelial-mesenchymal transition of premalignant HaCaT cells induced by aging MRC-5 cell-conditioned medium. Mol. Cell Biochem. 2018, 439, 117–129. [Google Scholar] [CrossRef]
- Grare, M.; Mourer, M.; Fontanay, S.; Regnouf-de-Vains, J.-B.; Finance, C.; Duval, R.E. In vitro activity of para-guanidinoethylcalix[4]arene against susceptible and antibiotic-resistant Gram-negative and Gram-positive bacteria. J. Antimicrob. Chemother. 2007, 60, 575–581. [Google Scholar] [CrossRef]
- Sakač, M.; Jovanov, P.; Marić, A.; Četojević-Simin, D.; Novaković, A.; Plavšić, D.; Škrobot, D.; Kovač, R. Antioxidative, antibacterial and antiproliferative properties of Honey types from the Western Balkans. Antioxidants 2022, 11, 1120. [Google Scholar] [CrossRef]
- Tonks, A.J.; Dudley, E.; Porter, N.G.; Parton, J.; Brazier, J.; Smith, E.L.; Tonks, A.A. 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J. Leukoc. Biol. 2007, 82, 1147–1155. [Google Scholar] [CrossRef]
- White, R. Manuka honey in wound management: Greater than the sum of its parts? J. Wound Care 2016, 25, 539–543. [Google Scholar] [CrossRef]


| Sample | Component 1 | Component 2 | Component 3 | Crosslinker for HEMA | Crosslinker for Gelatin | Initiator/Activator |
|---|---|---|---|---|---|---|
| HG | HEMA | Gelatin | – | EGDMA | EDC | APS/TEMED |
| 10MHo/HG | HEMA | Gelatin | MHo | EGDMA | EDC | APS/TEMED |
| 20MHo/HG | HEMA | Gelatin | MHo | EGDMA | EDC | APS/TEMED |
| 30MHo/HG | HEMA | Gelatin | MHo | EGDMA | EDC | APS/TEMED |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomić, S.L.; Vuković, J.S.; Babić Radić, M.M.; Filipović, V.V.; Živanović, D.P.; Nikolić, M.M.; Nikodinovic-Runic, J. Manuka Honey/2-Hydroxyethyl Methacrylate/Gelatin Hybrid Hydrogel Scaffolds for Potential Tissue Regeneration. Polymers 2023, 15, 589. https://doi.org/10.3390/polym15030589
Tomić SL, Vuković JS, Babić Radić MM, Filipović VV, Živanović DP, Nikolić MM, Nikodinovic-Runic J. Manuka Honey/2-Hydroxyethyl Methacrylate/Gelatin Hybrid Hydrogel Scaffolds for Potential Tissue Regeneration. Polymers. 2023; 15(3):589. https://doi.org/10.3390/polym15030589
Chicago/Turabian StyleTomić, Simonida Lj., Jovana S. Vuković, Marija M. Babić Radić, Vuk. V. Filipović, Dubravka P. Živanović, Miloš M. Nikolić, and Jasmina Nikodinovic-Runic. 2023. "Manuka Honey/2-Hydroxyethyl Methacrylate/Gelatin Hybrid Hydrogel Scaffolds for Potential Tissue Regeneration" Polymers 15, no. 3: 589. https://doi.org/10.3390/polym15030589
APA StyleTomić, S. L., Vuković, J. S., Babić Radić, M. M., Filipović, V. V., Živanović, D. P., Nikolić, M. M., & Nikodinovic-Runic, J. (2023). Manuka Honey/2-Hydroxyethyl Methacrylate/Gelatin Hybrid Hydrogel Scaffolds for Potential Tissue Regeneration. Polymers, 15(3), 589. https://doi.org/10.3390/polym15030589









