Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams
Abstract
:1. Introduction
- It represents a renewable energy resource. When considering the valorization of the natural residues to generate energy, it can be considered practically inexhaustible due to the continuous waste generation in nature.
- The utilization can lead to the reduction of greenhouse gas emissions. The emissions during the burning process are reabsorbed during the formation process of the new biomass. However, the overall CO2 balance depends on the biomass processing strategy.
- It has a low market price. Compared to conventional fuels, biomass recovered energy has the potential to offer a more economical alternative, with a reduction of the costs up to 33%.
- Biomass constitutes an abundant global resource. Almost all regions on the planet can permit the generation of natural biomass waste, which is available for local use. In addition, generally, there is no need for large infrastructure to make biomass available for use.
- In some areas, due to local conditions, the recovery of biomass can be more expensive than in others. This is an important aspect when considering the biomass collection, pretreatment, and storage of different types of biomass.
- It requires large land areas for the processes utilized to obtain energy from biomass, especially for storage, because the residues tend to display a low energy density.
- In some cases, the overutilization of this energy resource can cause damage to ecosystems or fragmentation due to the activities during the biomass residue collection process.
2. Polyols
3. The Mechanism for Liquefaction of Lignocellulosic Biomass in the Presence of Polyols
4. Influence of the Reaction Parameters on the Lignocellulose Solvolysis Process
4.1. Type of Lignocellulose Biomass
4.2. Liquefaction Solvent
4.3. Catalysts, Temperature, and Reaction Time
4.4. Heating Method—Process Intensification Strategies
4.5. Mechanism of Acid-Catalyzed Liquefaction of Cellulose and Lignin in Phenols
4.6. Polycondensation Reaction in Lignocellulosic Liquefaction
4.7. Polyols Obtained by Liquefaction
5. Applications of Products Obtained by Biomass Liquefaction
6. Discussion and Limitations
7. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Kaltschmitt, M.; Themelis, N.J.; Bronicki, L.Y.; Söder, L.; Vega, L.A. Renewable Energy Systems; Springer: New York, NY, USA, 2012. [Google Scholar]
- Basso, T.P.; Basso, T.O.; Basso, L.C. Biotechnological Applications of Biomass; IntechOpen: London, UK, 2021. [Google Scholar]
- Adeleke, A.A.; Odusote, J.K.; Lasode, O.A.; Ikubanni, P.P.; Madhurai, M.; Paswan, D. Evaluation of thermal decomposition characteristics and kinetic parameters of melina wood. Biofuels 2022, 13, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Kheybari, S.; Rezaie, F.M.; Naji, S.A.; Najafi, F. Evaluation of energy production technologies from biomass using analytical hierarchy process: The case of Iran. J. Clean. Prod. 2019, 232, 257–265. [Google Scholar] [CrossRef]
- Ahiduzzaman, M.; Islam, A.K.M.S. Greenhouse gas emission and renewable energy sources for sustainable development in Bangladesh. Renew. Sustain. Energy Rev. 2011, 15, 4659–4666. [Google Scholar] [CrossRef]
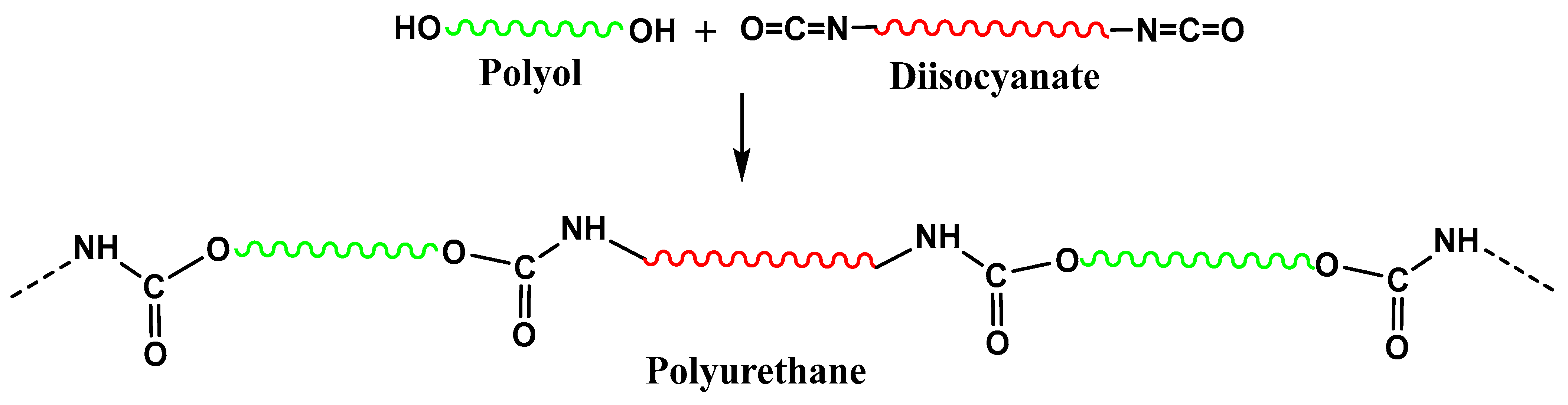
- Hu, S.; Luo, X.; Li, Y. Polyols and Polyurethanes from the Liquefaction of Lignocellulosic Biomass. ChemSusChem 2014, 7, 66–72. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Akiyama, M.; Sato, S.; Takahashi, R.; Inui, K.; Yokota, M. Dehydration–hydrogenation of glycerol into 1,2-propanediol at ambient hydrogen pressure. Appl. Catal. A Gen. 2009, 371, 60–66. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt. Appl. Catal. A Gen. 2010, 378, 11–18. [Google Scholar] [CrossRef]
- Chia, M.; Pagán-Torres, Y.J.; Hibbitts, D.; Tan, Q.; Pham, H.N.; Datye, A.K.; Neurock, M.; Davis, R.J.; Dumesic, J.A. Selective Hydrogenolysis of Polyols and Cyclic Ethers over Bifunctional Surface Sites on Rhodium–Rhenium Catalysts. J. Am. Chem. Soc. 2011, 133, 12675–12689. [Google Scholar] [CrossRef]
- Gandarias, I.; Requies, J.; Arias, P.L.; Armbruster, U.; Martin, A. Liquid-phase glycerol hydrogenolysis by formic acid over Ni–Cu/Al2O3 catalysts. J. Catal. 2012, 290, 79–89. [Google Scholar] [CrossRef]
- Amada, Y.; Koso, S.; Nakagawa, Y.; Tomishige, K. Hydrogenolysis of 1,2-Propanediol for the Production of Biopropanols from Glycerol. ChemSusChem 2010, 3, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Elliott, W.T. Alkyd Resins. In Surface Coatings: Volume 1 Raw Materials and Their Usage; Springer: Dordrecht, The Netherlands, 1993; pp. 76–109. [Google Scholar]
- Timberlake, J.F.; Barksby, N.; Gerkin, R.M.; Lawler, L.F. Polymer Polyol for Improved Chain Extender Compatibility for Use in Elastomers. J. Elastomers Plast. 1989, 21, 92–99. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Malani, R.S.; Malshe, V.C.; Thorat, B.N. Polyols and polyurethanes from renewable sources: Past, present, and future—Part 2: Plant-derived materials. J. Coat. Technol. Res. 2022, 19, 361–375. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Lan, J.C.-W.; Veziroglu, A.; Veziroglu, T.N.; Tomo, T.; Chang, J.-S.; Allakhverdiev, S.I. A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int. J. Hydrog. Energy 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Behrendt, F.; Neubauer, Y.; Oevermann, M.; Wilmes, B.; Zobel, N. Direct Liquefaction of Biomass. Chem. Eng. Technol. 2008, 31, 667–677. [Google Scholar] [CrossRef]
- Evtiouguina, M.; Margarida Barros, A.; Cruz-Pinto, J.J.; Pascoal Neto, C.; Belgacem, N.; Pavier, C.; Gandini, A. The oxypropylation of cork residues: Preliminary results. Bioresour. Technol. 2000, 73, 187–189. [Google Scholar] [CrossRef]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of lignocellulosic materials and its applications in wood adhesives—A review. Ind. Crops Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Lange, J.-P. Lignocellulose Liquefaction to Biocrude: A Tutorial Review. ChemSusChem 2018, 11, 997–1014. [Google Scholar] [CrossRef]
- Kumar, S.; Lange, J.-P.; Van Rossum, G.; Kersten, S.R.A. Liquefaction of Lignocellulose: Process Parameter Study to Minimize Heavy Ends. Ind. Eng. Chem. Res. 2014, 53, 11668–11676. [Google Scholar] [CrossRef]
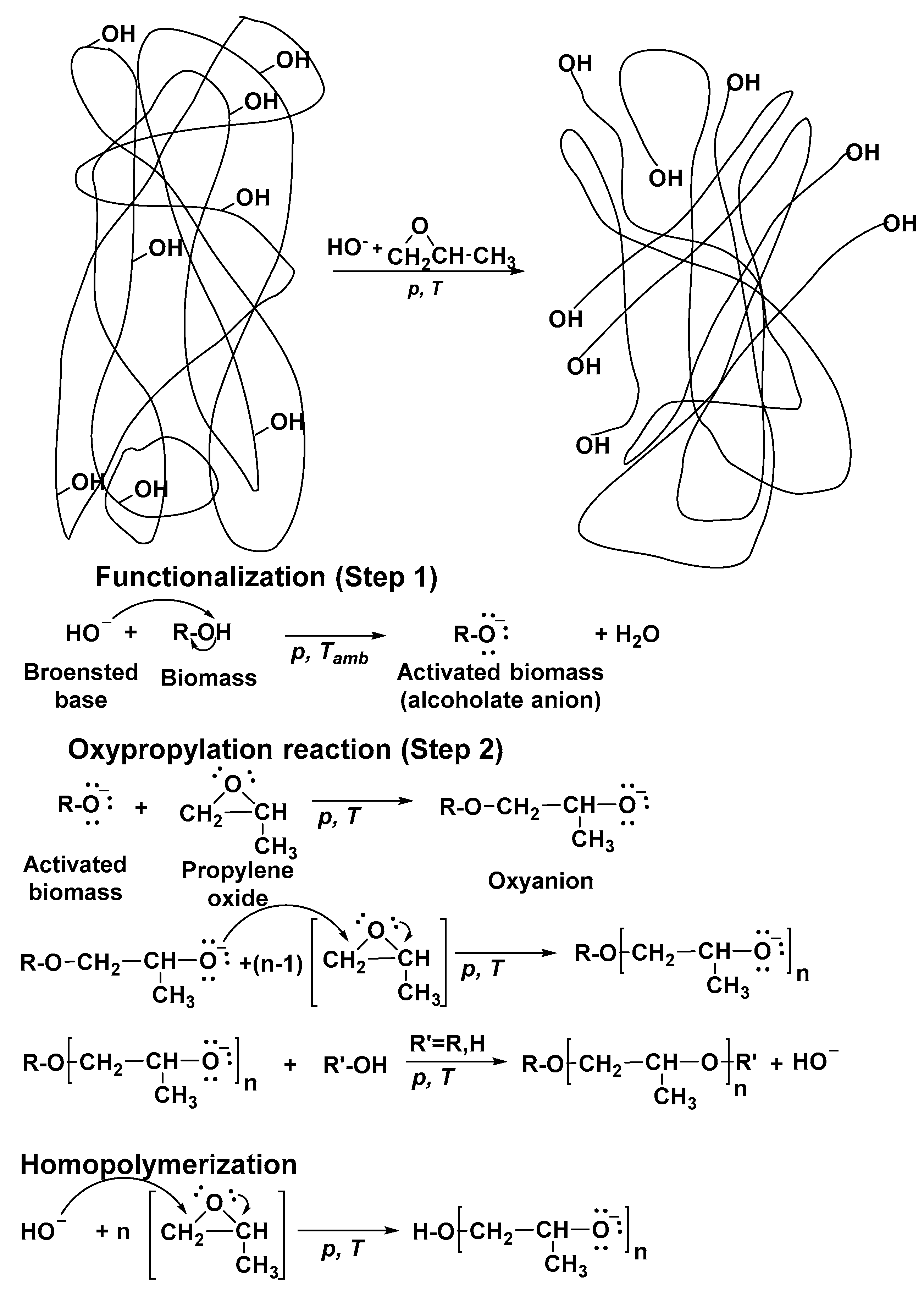
- Aniceto, J.P.S.; Portugal, I.; Silva, C.M. Biomass-Based Polyols through Oxypropylation Reaction. ChemSusChem 2012, 5, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Evtiouguina, M.; Barros-Timmons, A.; Cruz-Pinto, J.J.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Oxypropylation of Cork and the Use of the Ensuing Polyols in Polyurethane Formulations. Biomacromolecules 2002, 3, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lu, Z. Liquefaction of wheat straw and preparation of rigid polyurethane foam from the liquefaction products. J. Appl. Polym. Sci. 2009, 111, 508–516. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoshioka, M.; Shiraishi, N. Liquefaction of corn bran (CB) in the presence of alcohols and preparation of polyurethane foam from its liquefied polyol. J. Appl. Polym. Sci. 2000, 78, 319–325. [Google Scholar] [CrossRef]
- Yu, F.; Le, Z.; Chen, P.; Liu, Y.; Lin, X.; Ruan, R. Atmospheric Pressure Liquefaction of Dried Distillers Grains (DDG) and Making Polyurethane Foams from Liquefied DDG. Appl. Biochem. Biotechnol. 2008, 148, 235–243. [Google Scholar] [CrossRef]
- Hu, S.; Wan, C.; Li, Y. Production and characterization of biopolyols and polyurethane foams from crude glycerol based liquefaction of soybean straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef]
- Yan, Y.; Pang, H.; Yang, X.; Zhang, R.; Liao, B. Preparation and characterization of water-blown polyurethane foams from liquefied cornstalk polyol. J. Appl. Polym. Sci. 2008, 110, 1099–1111. [Google Scholar] [CrossRef]
- Yamada, T.; Ono, H. Characterization of the products resulting from ethylene glycol liquefaction of cellulose. J. Wood Sci. 2001, 47, 458–464. [Google Scholar] [CrossRef]
- Yamada, T.; Aratani, M.; Kubo, S.; Ono, H. Chemical analysis of the product in acid-catalyzed solvolysis of cellulose using polyethylene glycol and ethylene carbonate. J. Wood Sci. 2007, 53, 487–493. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, F.; Luo, C.; Xiong, L.; Chen, X. Liquefaction and characterization of acid hydrolysis residue of corncob in polyhydric alcohols. Ind. Crops Prod. 2012, 39, 47–51. [Google Scholar] [CrossRef]
- Lee, S.-H.; Teramoto, Y.; Shiraishi, N. Biodegradable polyurethane foam from liquefied waste paper and its thermal stability, biodegradability, and genotoxicity. J. Appl. Polym. Sci. 2002, 83, 1482–1489. [Google Scholar] [CrossRef]
- Kobayashi, M.; Asano, T.; Kajiyama, M.; Tomita, B. Analysis on residue formation during wood liquefaction with polyhydric alcohol. J. Wood Sci. 2004, 50, 407–414. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, H.; Shi, J.; Fu, T.; Liao, B. Investigation of liquefied wood residues based on cellulose, hemicellulose, and lignin. J. Appl. Polym. Sci. 2012, 123, 850–856. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Takeda, M.; Doi, S.; Tamura, Y.; Ono, H. Network structures and thermal properties of polyurethane films prepared from liquefied wood. Bioresour. Technol. 2001, 77, 33–40. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Doi, S.; Tamura, Y. Species Effects on Wood-Liquefaction in Polyhydric Alcohols. Holzforschung 1999, 53, 617–622. [Google Scholar] [CrossRef]
- Shi, Y.; Xia, X.; Li, J.; Wang, J.; Zhao, T.; Yang, H.; Jiang, J.; Jiang, X. Solvolysis kinetics of three components of biomass using polyhydric alcohols as solvents. Bioresour. Technol. 2016, 221, 102–110. [Google Scholar] [CrossRef]
- Jo, Y.J.; Ly, H.V.; Kim, J.; Kim, S.-S.; Lee, E. Preparation of biopolyol by liquefaction of palm kernel cake using PEG#400 blended glycerol. J. Ind. Eng. Chem. 2015, 29, 304–313. [Google Scholar] [CrossRef]
- El-barbary, M.H.; Shukry, N. Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind. Crops Prod. 2008, 27, 33–38. [Google Scholar] [CrossRef]
- Gao, L.-L.; Liu, Y.-H.; Lei, H.; Peng, H.; Ruan, R. Preparation of semirigid polyurethane foam with liquefied bamboo residues. J. Appl. Polym. Sci. 2010, 116, 1694–1699. [Google Scholar] [CrossRef]
- You, X.; Chaudhari, M.; Rempe, S.; Pratt, L.R. Dielectric Properties of Ethylene Carbonate and Propylene Carbonate Using Molecular Dynamics Simulations. ECS Trans. 2015, 69, 107. [Google Scholar] [CrossRef]
- Yamada, T.; Ono, H. Rapid liquefaction of lignocellulosic waste by using ethylene carbonate. Bioresour. Technol. 1999, 70, 61–67. [Google Scholar] [CrossRef]
- Wang, T.-P.; Li, D.; Wang, L.-J.; Yin, J.; Chen, X.D.; Mao, Z.-H. Effects of CS/EC ratio on structure and properties of polyurethane foams prepared from untreated liquefied corn stover with PAPI. Chem. Eng. Res. Des. 2008, 86, 416–421. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Q.; Lin, X.; Jiang, K.; Han, D. The Degradation and Repolymerization Analysis on Solvolysis Liquefaction of Corn Stalk. Polymers 2020, 12, 2337. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.-Z. A novel method of utilizing the biomass resource: Rapid liquefaction of wheat straw and preparation of biodegradable polyurethane foam (PUF). J. Chin. Inst. Chem. Eng. 2007, 38, 95–102. [Google Scholar] [CrossRef]
- Maldas, D.; Shiraishi, N. Liquefaction of Wood in the Presence of Polyol Using NaOH as a Catalyst and its Application to Polyurethane Foams. Int. J. Polym. Mater. Polym. Biomater. 1996, 33, 61–71. [Google Scholar] [CrossRef]
- Alma, M.H.; Shiraishi, N. Preparation of polyurethane-like foams from NaOH-catalyzed liquefied wood. Holz als Roh- und Werkstoff 1998, 56, 245–246. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, C.-J.; Kim, S.B. Kinetic study of recycled newspaper liquefaction in polyol solvent. Biotechnol. Bioprocess Eng. 2009, 14, 349. [Google Scholar] [CrossRef]
- Lu, Z.; Fan, L.; Wu, Z.; Zhang, H.; Liao, Y.; Zheng, D.; Wang, S. Efficient liquefaction of woody biomass in polyhydric alcohol with acidic ionic liquid as a green catalyst. Biomass Bioenergy 2015, 81, 154–161. [Google Scholar] [CrossRef]
- Chang, C.; Liu, L.; Li, P.; Xu, G.; Xu, C. Preparation of flame retardant polyurethane foam from crude glycerol based liquefaction of wheat straw. Ind. Crops Prod. 2021, 160, 113098. [Google Scholar] [CrossRef]
- Shao, H.; Zhao, H.; Xie, J.; Qi, J.; Shupe, T.F. Agricultural and Forest Residues towards Renewable Chemicals and Materials Using Microwave Liquefaction. Int. J. Polym. Sci. 2019, 2019, 7231263. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lee, E.Y. Liquefaction of Red Pine Wood, Pinus densiflora, Biomass Using Peg-400-Blended Crude Glycerol for Biopolyol and Biopolyurethane Production. J. Wood Chem. Technol. 2016, 36, 353–364. [Google Scholar] [CrossRef]
- Kim, K.H.; Jo, Y.J.; Lee, C.G.; Lee, E. Solvothermal liquefaction of microalgal Tetraselmis sp. biomass to prepare biopolyols by using PEG#400-blended glycerol. Algal Res. 2015, 12, 539–544. [Google Scholar] [CrossRef]
- Kržan, A.; Žagar, E. Microwave driven wood liquefaction with glycols. Bioresour. Technol. 2009, 100, 3143–3146. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Guo, J.; Chen, L.; Li, D.; Liu, J.; Ye, N. Microwave-assisted direct liquefaction of Ulva prolifera for bio-oil production by acid catalysis. Bioresour. Technol. 2012, 116, 133–139. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiz, Á.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Rana, K.K.; Rana, S. Microwave Reactors: A Brief Review on its Fundamental Aspects and Applications. Open Access Libr. J. 2014, 1, e686. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiz, A.; Prieto, P. CHAPTER 1 Microwave-Assisted Green Organic Synthesis. In Alternative Energy Sources for Green Chemistry; The Royal Society of Chemistry: London, UK, 2016; pp. 1–33. [Google Scholar]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; He, Y.; Wang, F.; Wu, J.; Ci, Z.; Chen, L.; Xu, R.; Yang, M.; Lin, J.; Han, L.; et al. Microwave technology: A novel approach to the transformation of natural metabolites. Chin. Med. 2021, 16, 87. [Google Scholar] [CrossRef]
- Li, Z.; Han, L.; Xiao, W. Influence of Microwave Heating on the Liquefaction Kinetics of Corn Stover in Ethylene Glycol. Bioresources 2013, 8, 3453–3460. [Google Scholar] [CrossRef] [Green Version]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupta, R. Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: Parametric study and products characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Kumar, M.; Olajire Oyedun, A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Lin, H.; Zheng, Y.; Zhao, J.; Pelletier, A.; Li, K. Effects of solvents and catalysts in liquefaction of pinewood sawdust for the production of bio-oils. Biomass Bioenergy 2013, 59, 158–167. [Google Scholar] [CrossRef]
- Minowa, T.; Zhen, F.; Ogi, T. Cellulose decomposition in hot-compressed water with alkali or nickel catalyst. J. Supercrit. Fluids 1998, 13, 253–259. [Google Scholar] [CrossRef]
- Lin, L.; Yao, Y.; Yoshioka, M.; Shiraishi, N. Liquefaction mechanism of cellulose in the presence of phenol under acid catalysis. Carbohydr. Polym. 2004, 57, 123–129. [Google Scholar] [CrossRef]
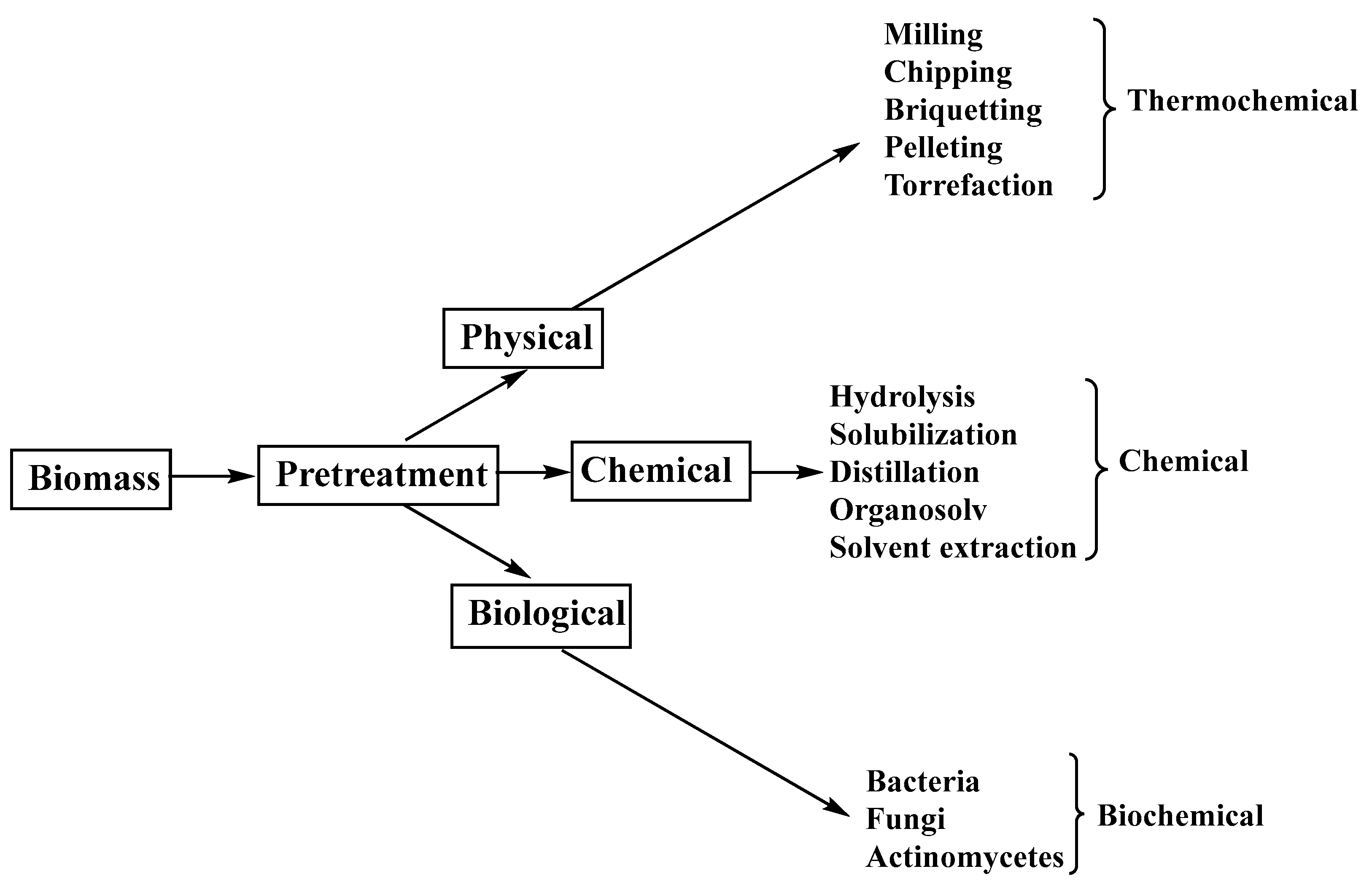
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Jasiukaitytė-Grojzdek, E.; Kunaver, M.; Crestini, C. Lignin Structural Changes During Liquefaction in Acidified Ethylene Glycol. J. Wood Chem. Technol. 2012, 32, 342–360. [Google Scholar] [CrossRef]
- Flores, E.M.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-assisted biomass valorization to industrial interesting products: State-of-the-art, perspectives and challenges. Ultrason. Sonochemistry 2021, 72, 105455. [Google Scholar] [CrossRef] [PubMed]
- Kunaver, M.; Jasiukaitytė, E.; Čuk, N. Ultrasonically assisted liquefaction of lignocellulosic materials. Bioresour. Technol. 2012, 103, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-S.; Kasapis, S.; Huang, D. Molecular weight and crystallinity alteration of cellulose via prolonged ultrasound fragmentation. Food Hydrocoll. 2012, 26, 365–369. [Google Scholar] [CrossRef]
- Mateus, M.M.; Acero, N.F.; Bordado, J.C.; dos Santos, R.G. Sonication as a foremost tool to improve cork liquefaction. Ind. Crops Prod. 2015, 74, 9–13. [Google Scholar] [CrossRef]
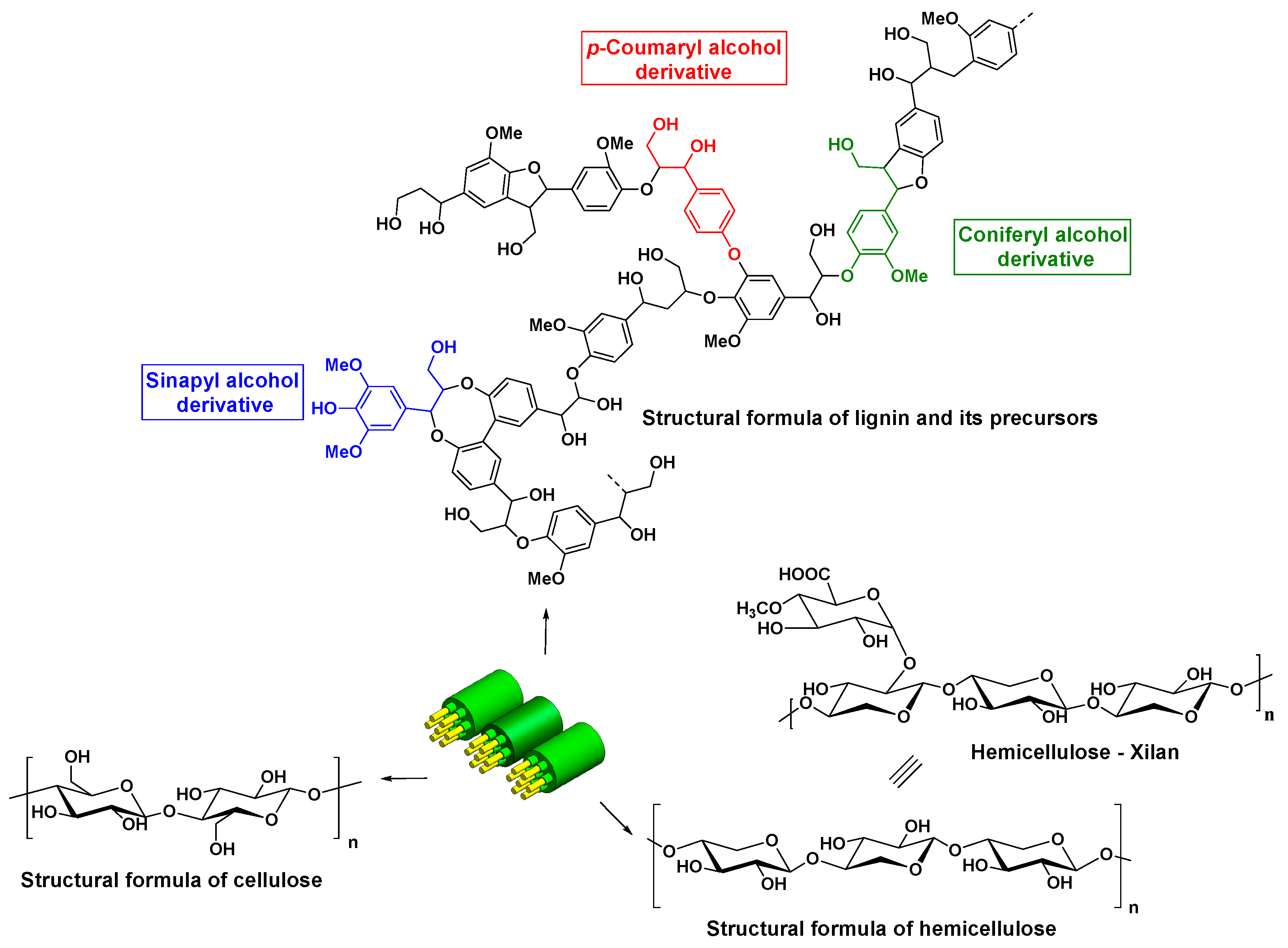
- Niu, M.; Zhao, G.-J.; Alma, M.H. Polycondensation reaction and its mechanism during lignocellulosic liquefaction by an acid catalyst: A review. For. Stud. China 2011, 13, 71–79. [Google Scholar] [CrossRef]
- Balan, V.; Chiaramonti, D.; Kumar, S. Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels Bioprod. Biorefining 2013, 7, 732–759. [Google Scholar] [CrossRef] [Green Version]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications of Lignocellulose-Based Polyurethanes: A Review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Bernard, F.L.; dos Santos, L.M.; Cobalchina, F.W.; Schwab, M.B.; Einloft, S. Polyurethane/poly (Ionic Liquids) Cellulosic Composites and their Evaluation for Separation of CO2 from Natural Gas. Mater. Res. 2020, 22. [Google Scholar] [CrossRef]
- Velvizhi, G.; Goswami, C.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Valorisation of lignocellulosic biomass to value-added products: Paving the pathway towards low-carbon footprint. Fuel 2022, 313, 122678. [Google Scholar] [CrossRef]
- D’Souza, J.; Camargo, R.; Yan, N. Biomass Liquefaction and Alkoxylation: A Review of Structural Characterization Methods for Bio-based Polyols. Polym. Rev. 2017, 57, 668–694. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology: Shewsbury, UK, 2005. [Google Scholar]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane foams from vegetable oil-based polyols: A review. Polym. Bull. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tuohedi, N. Polyurethane Foams and Bio-Polyols from Liquefied Cotton Stalk Agricultural Waste. Sustainability 2020, 12, 4214. [Google Scholar] [CrossRef]
- Ma, Y.; Xiao, Y.; Zhao, Y.; Bei, Y.; Hu, L.; Zhou, Y.; Jia, P. Biomass based polyols and biomass based polyurethane materials as a route towards sustainability. React. Funct. Polym. 2022, 175, 105285. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.T.; Piszczyk, Ł. Biopolyols obtained via crude glycerol-based liquefaction of cellulose: Their structural, rheological and thermal characterization. Cellulose 2016, 23, 2929–2942. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, S.H.; Oh, K.W. Bio-Based Polyurethane Foams with Castor Oil Based Multifunctional Polyols for Improved Compressive Properties. Polymers 2021, 13, 576. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of Bio-Based Polyurethanes: Perspective on Applications and Bio-Degradation. Macromol 2022, 2, 284–314. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Roslan, R.; Jaafar, S.N.S.; Salleh, K.M. Polyols and rigid polyurethane foams derived from liquefied lignocellulosic and cellulosic biomass. Cellulose 2019, 26, 3231–3246. [Google Scholar] [CrossRef]
- Gosz, K.; Tercjak, A.; Olszewski, A.; Haponiuk, J.; Piszczyk, Ł. Bio-Based Polyurethane Networks Derived from Liquefied Sawdust. Materials 2021, 14, 3138. [Google Scholar] [CrossRef]
- Yang, W.; Han, Y.; Zhang, W.; Zhang, D. High-Strength and Low-Cost Biobased Polyurethane Foam Composites Enhanced by Poplar Wood Powder Liquefaction. Polymers 2021, 13, 2999. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Cornelis, F.; Peng, X.-P.; Xie, J.-L.; Qi, J.-Q.; Jiang, Y.-Z.; Xiao, H.; Nie, S.-X. Thermal stability analysis of polyurethane foams made from microwave liquefaction bio-polyols with and without solid residue. BioResources 2018, 13, 3346–3361. [Google Scholar] [CrossRef]
- Zheng, Z.F.; Pan, H.; Huang, Y.B.; Chung, Y.H. Bio-Based Rigid Polyurethane Foam from Liquefied Products of Wood in the Presence of Polyhydric Alcohols. Adv. Mater. Res. 2011, 168–170, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Serrano, L.; Rincón, E.; García, A.; Rodríguez, J.; Briones, R. Bio-Degradable Polyurethane Foams Produced by Liquefied Polyol from Wheat Straw Biomass. Polymers 2020, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; De Hoop, C.F.; Xie, J.; Wu, Q.; Boldor, D.; Qi, J. High bio-content polyurethane (PU) foam made from bio-polyol and cellulose nanocrystals (CNCs) via microwave liquefaction. Mater. Des. 2018, 138, 11–20. [Google Scholar] [CrossRef]
- Yue, D.; Oribayo, O.; Rempel; Garry, L.; Pan, Q. Liquefaction of waste pine wood and its application in the synthesis of a flame retardant polyurethane foam. RSC Adv. 2017, 7, 30334–30344. [Google Scholar] [CrossRef]

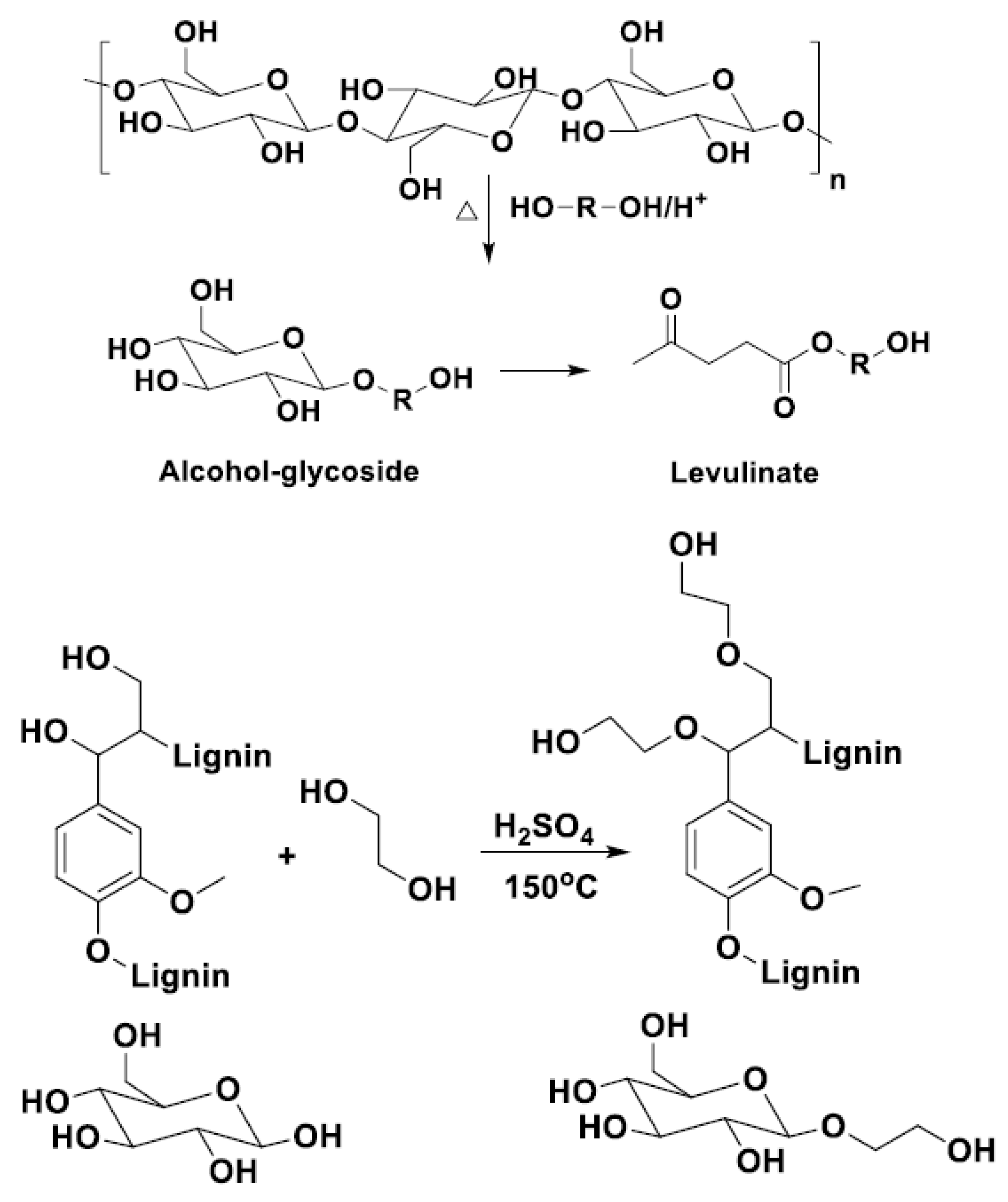
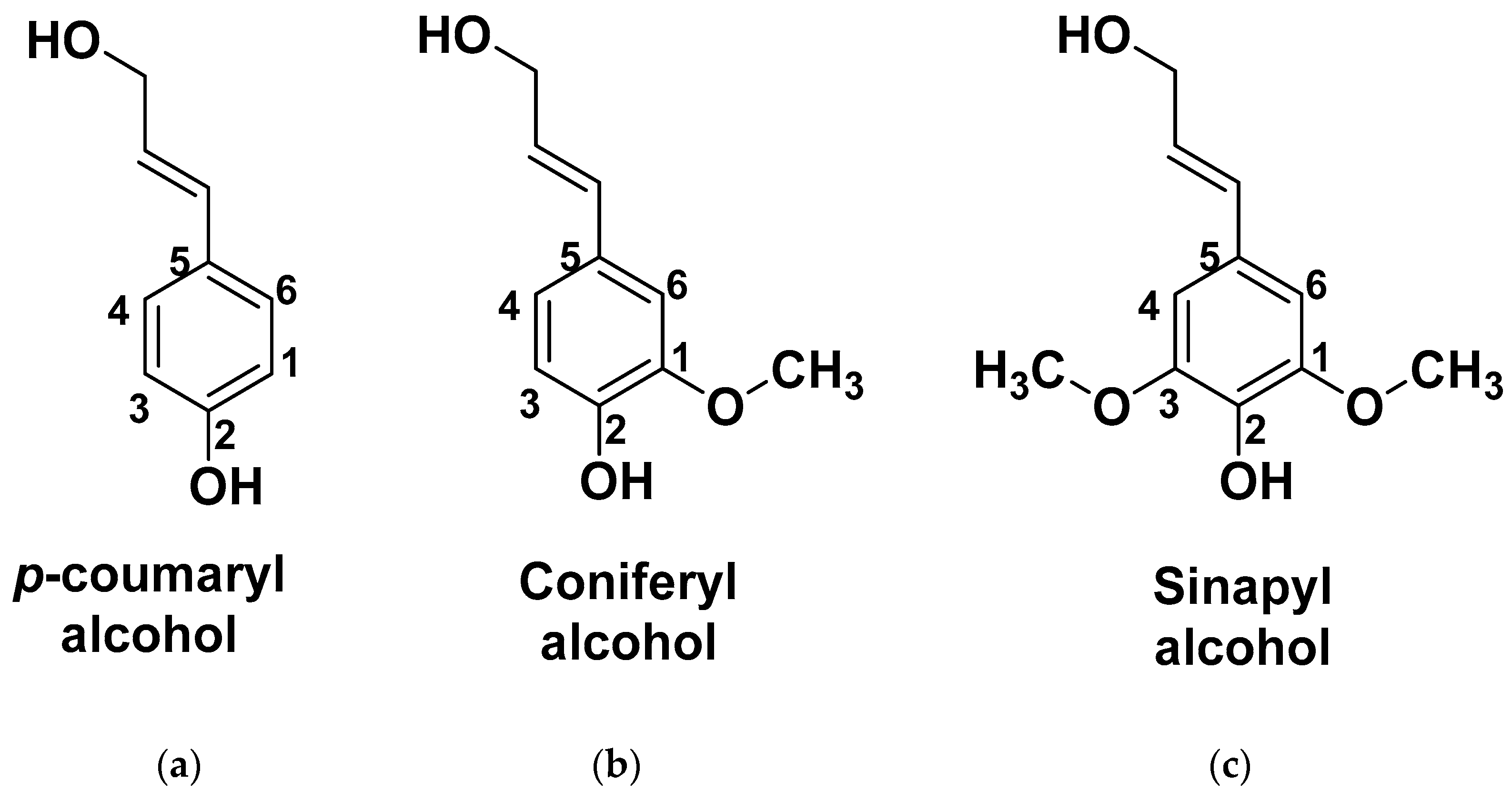
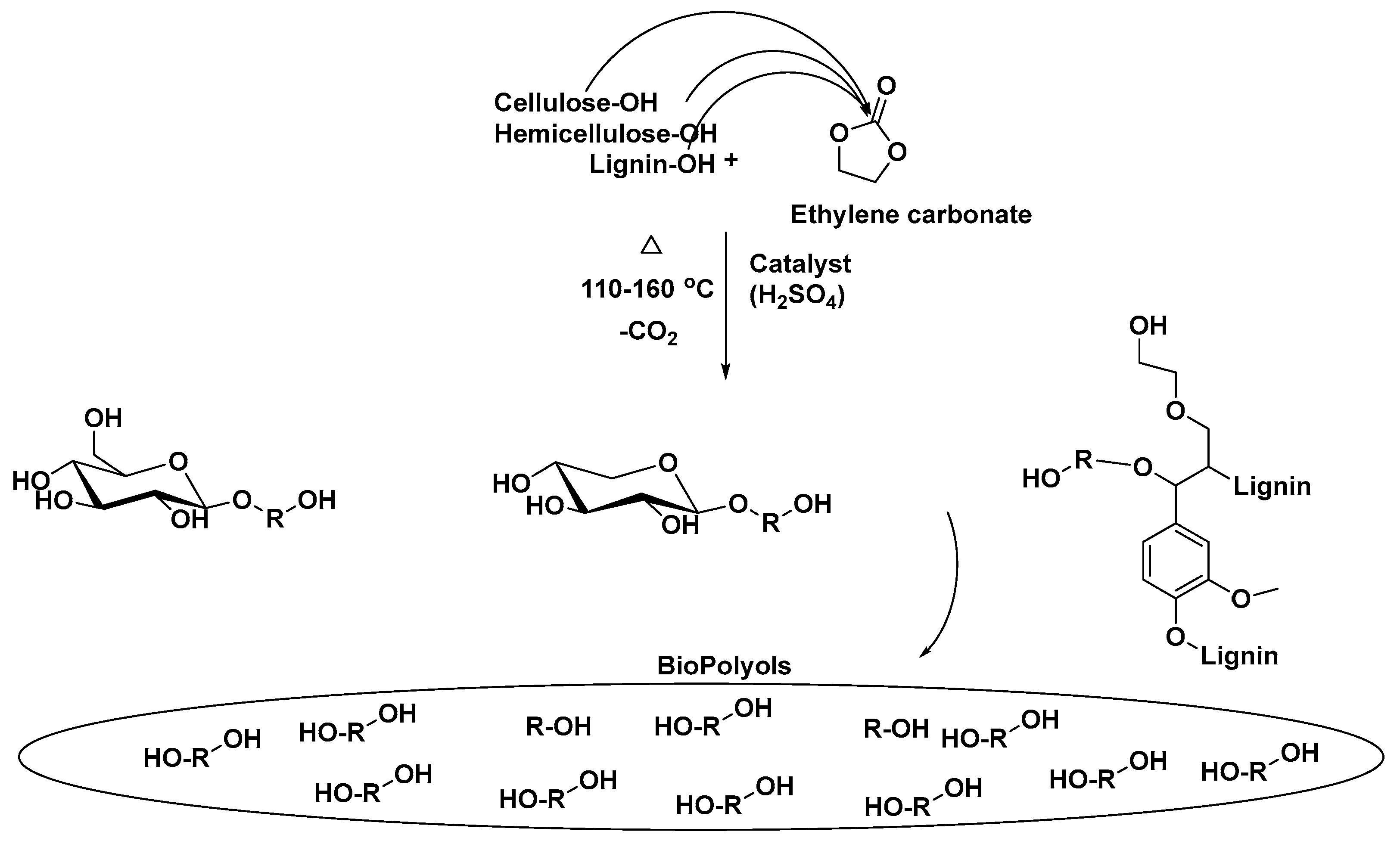
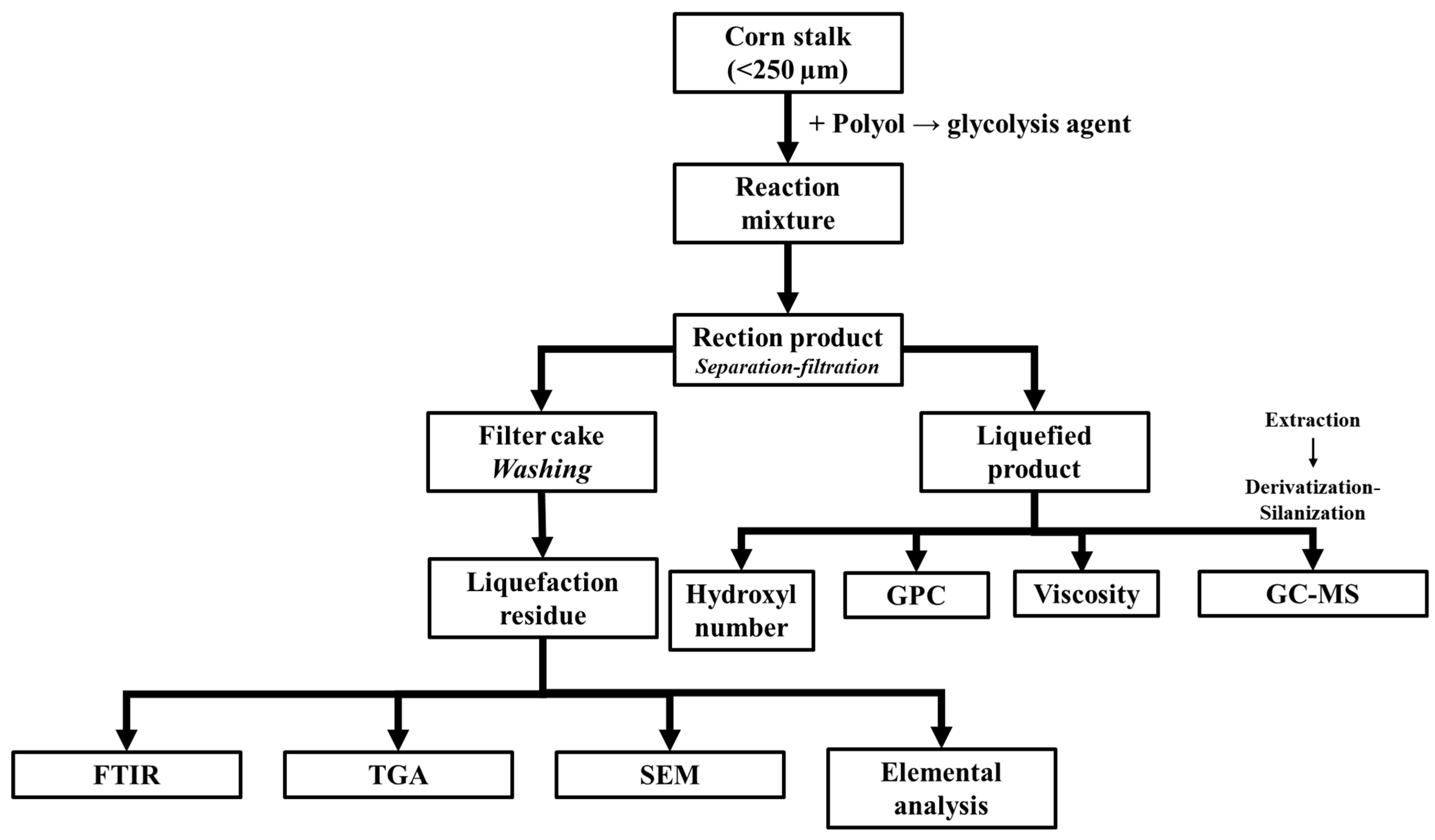

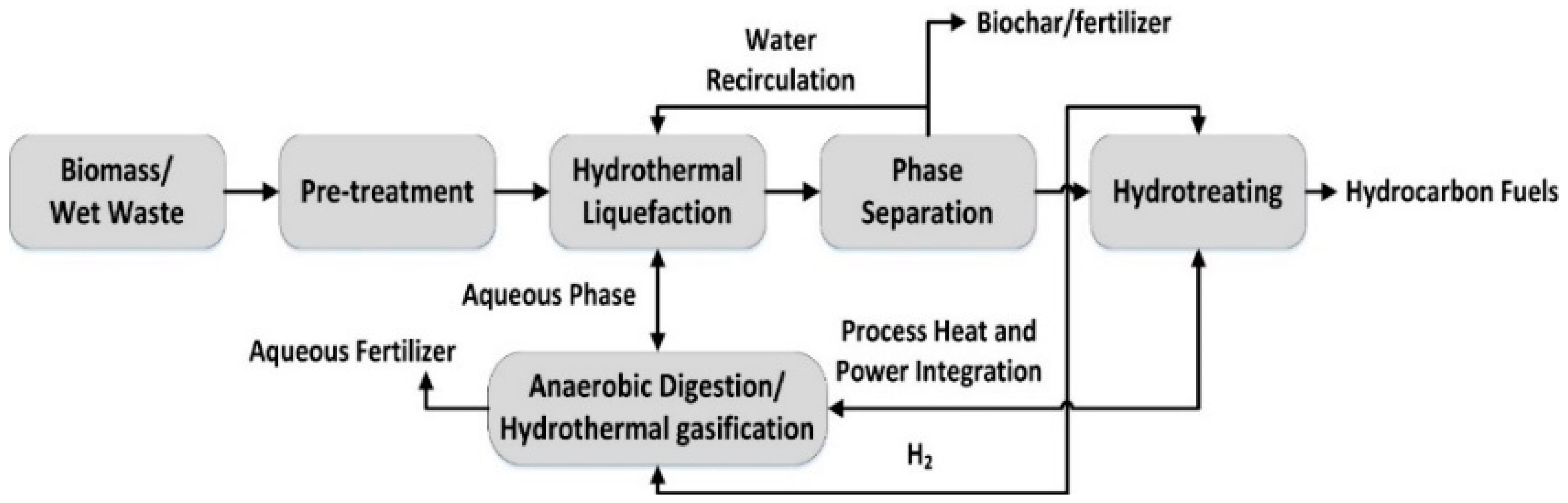
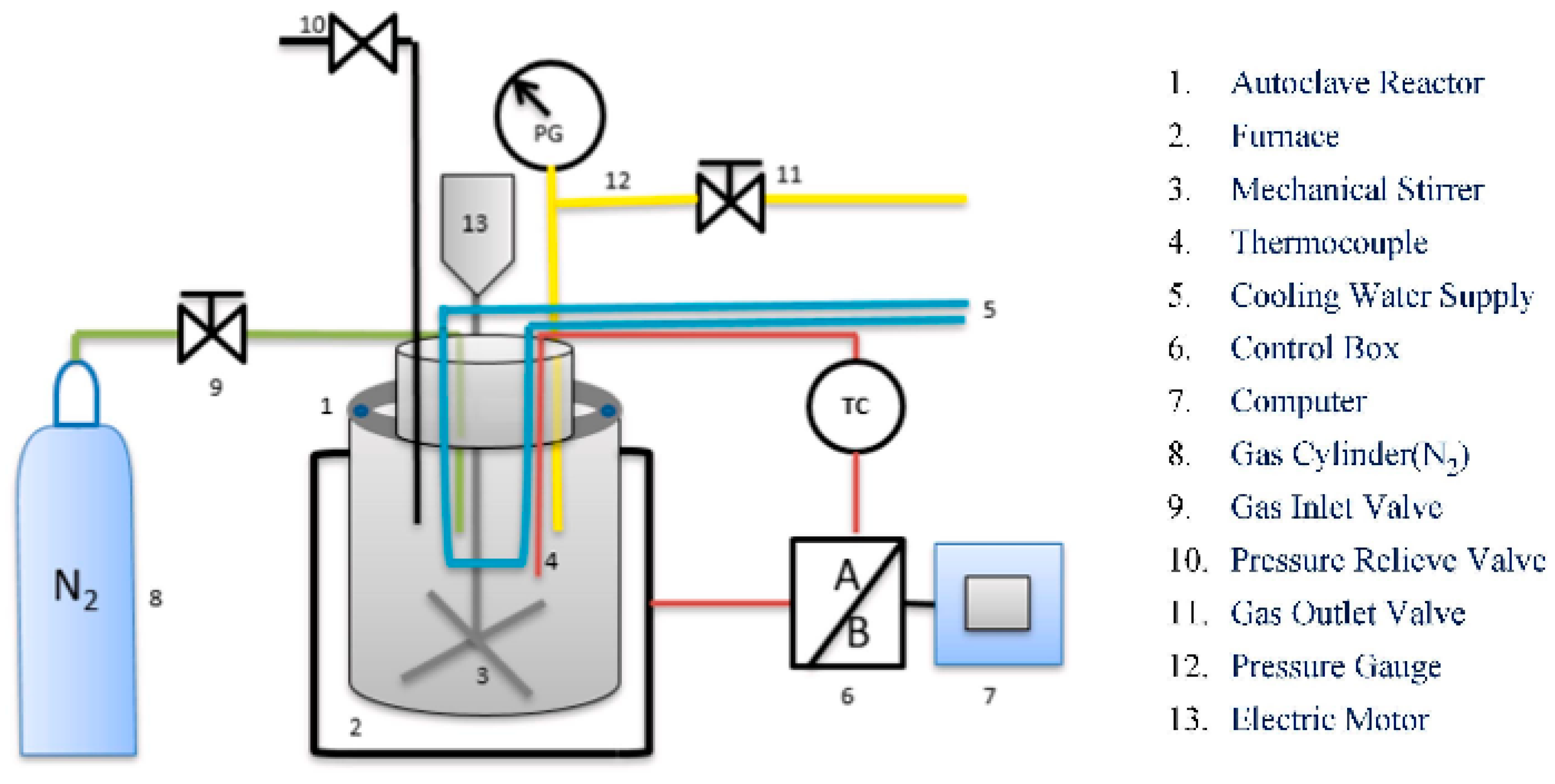

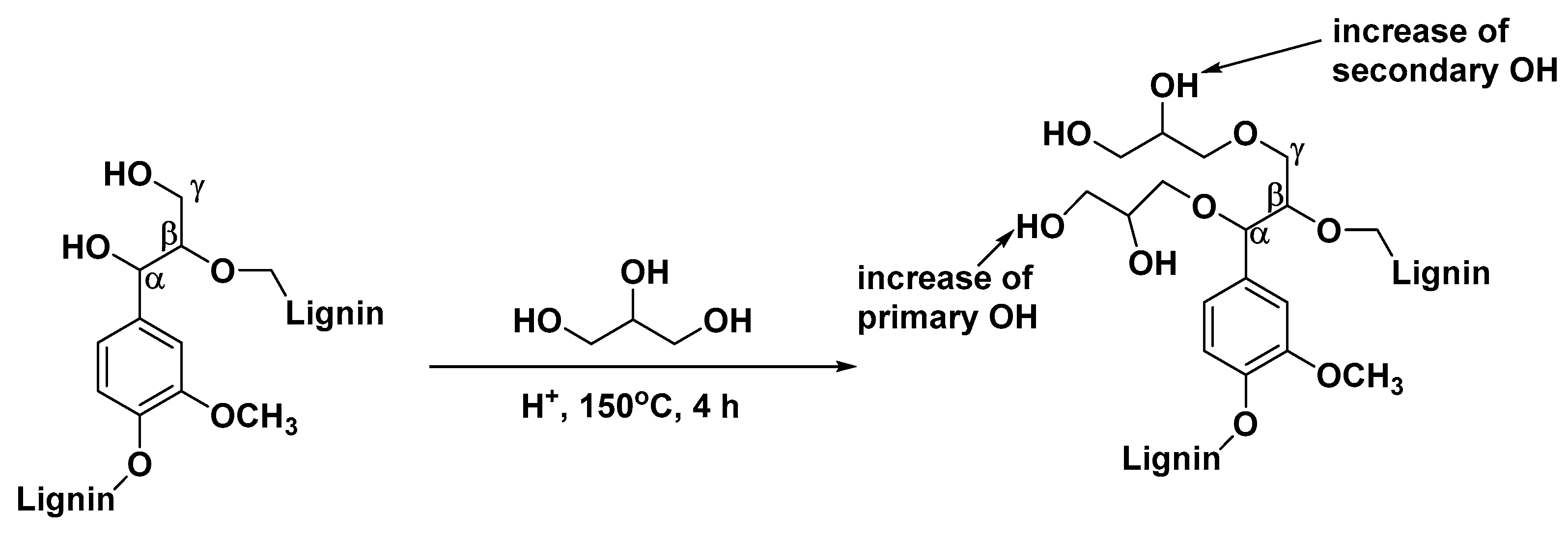
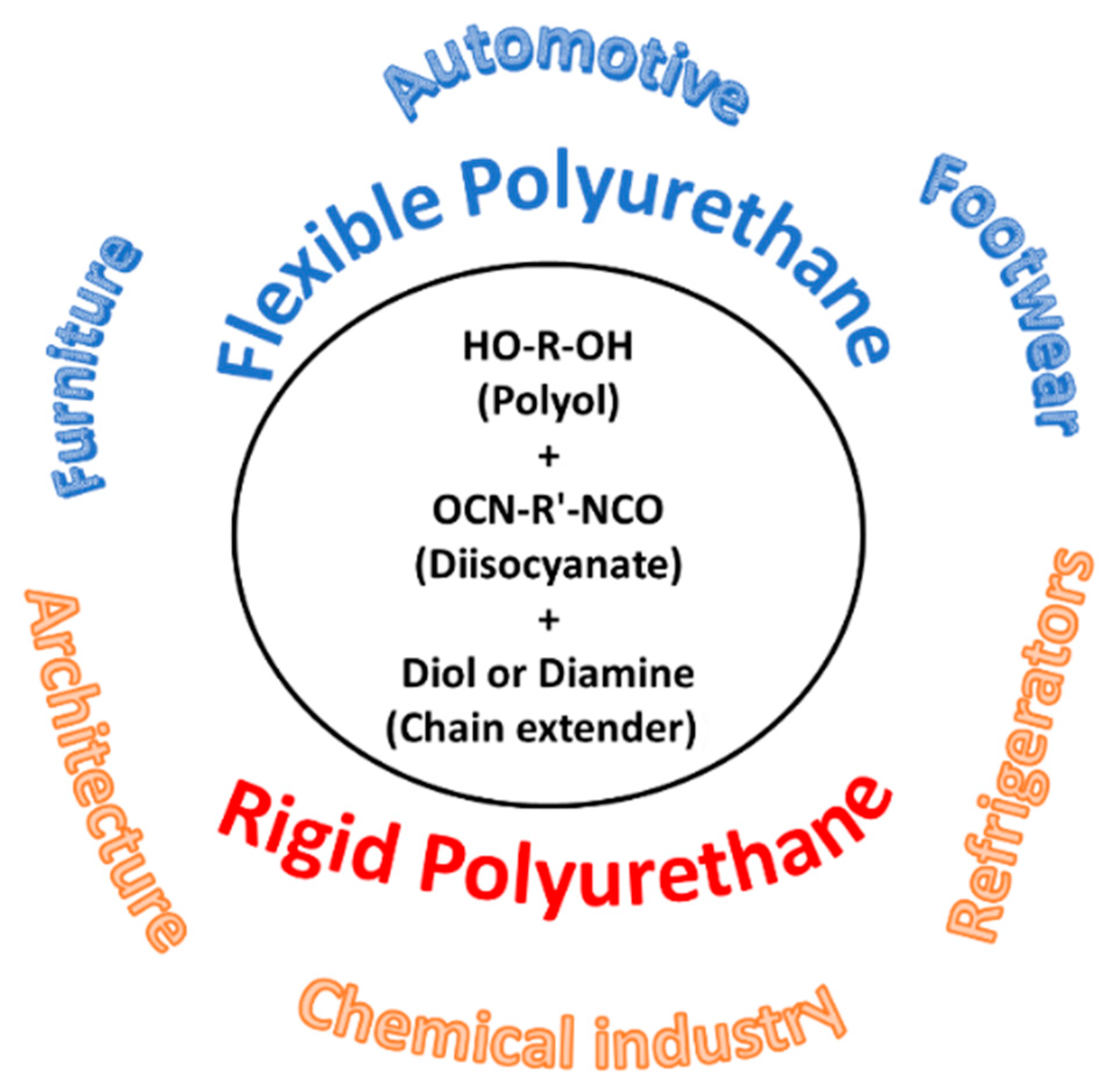
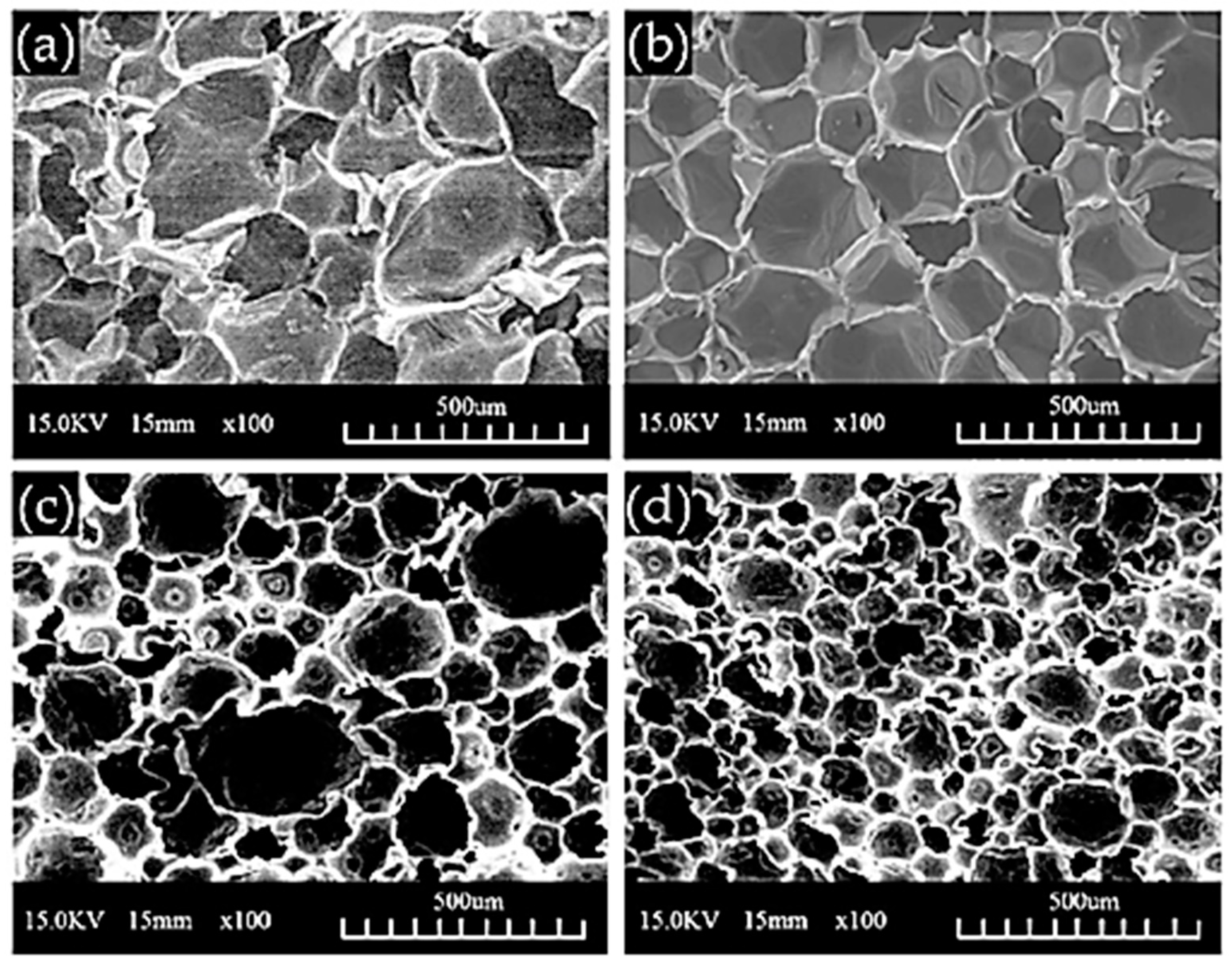
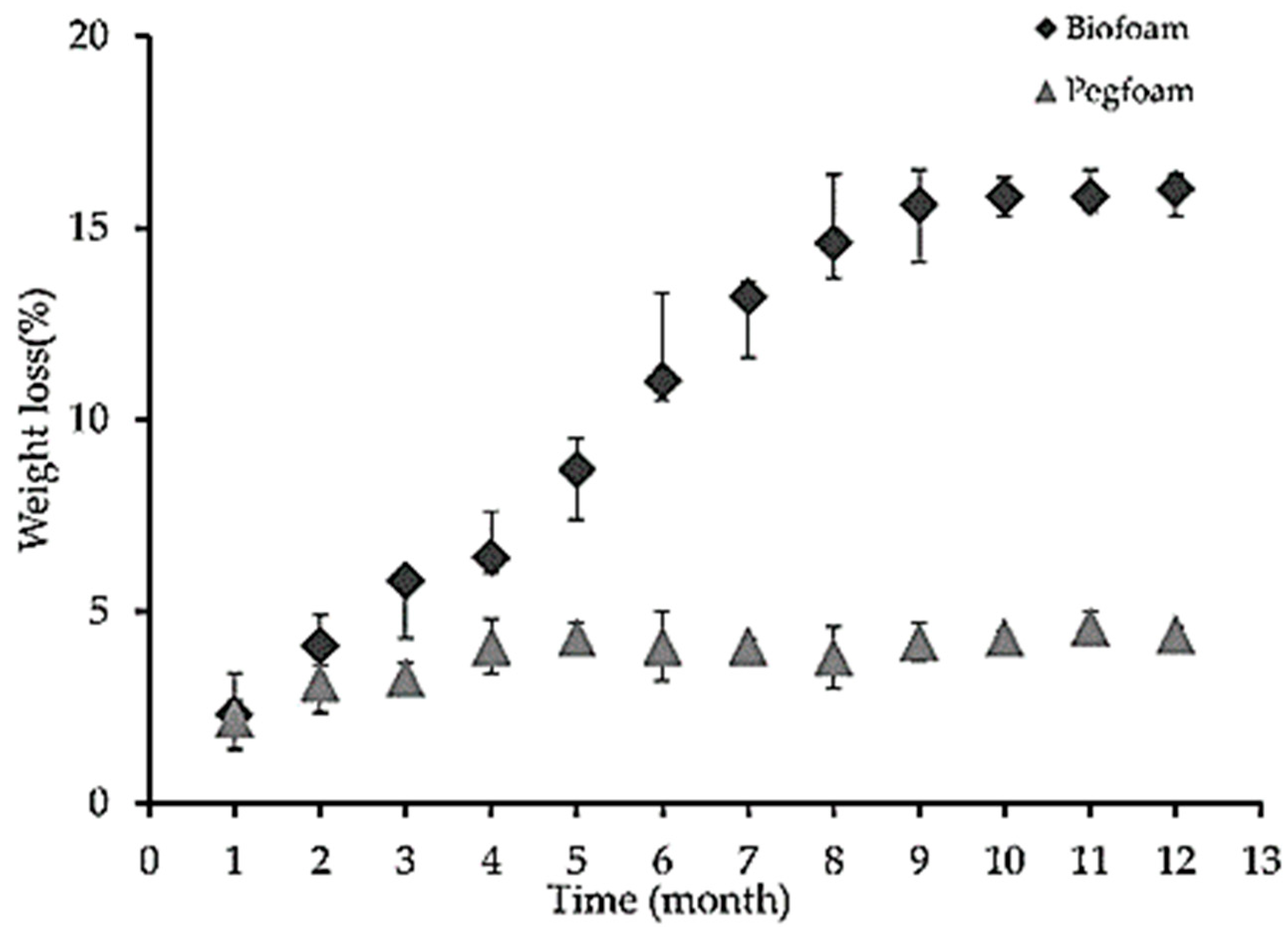
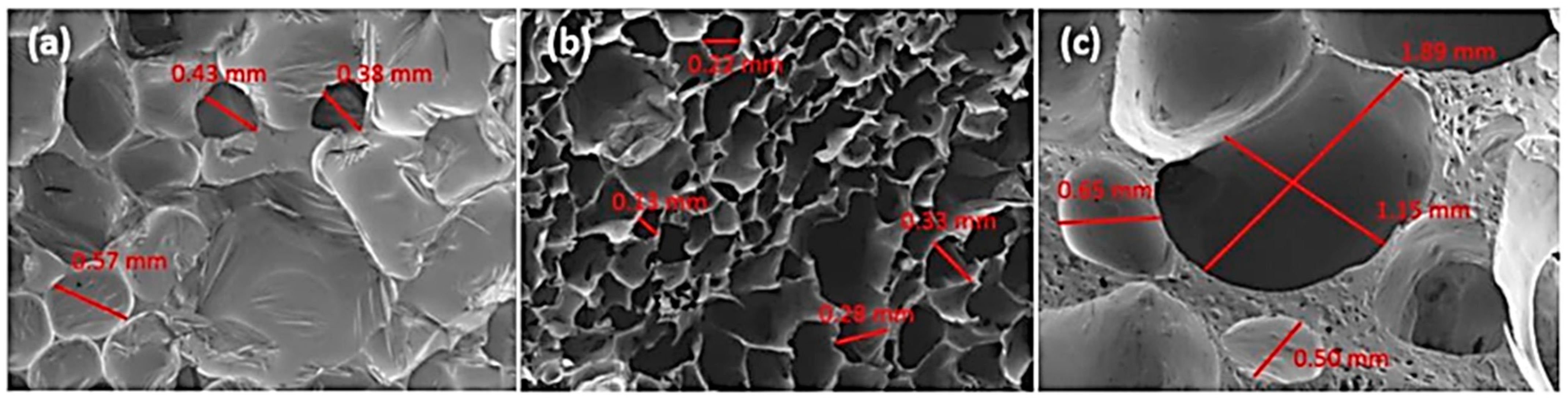
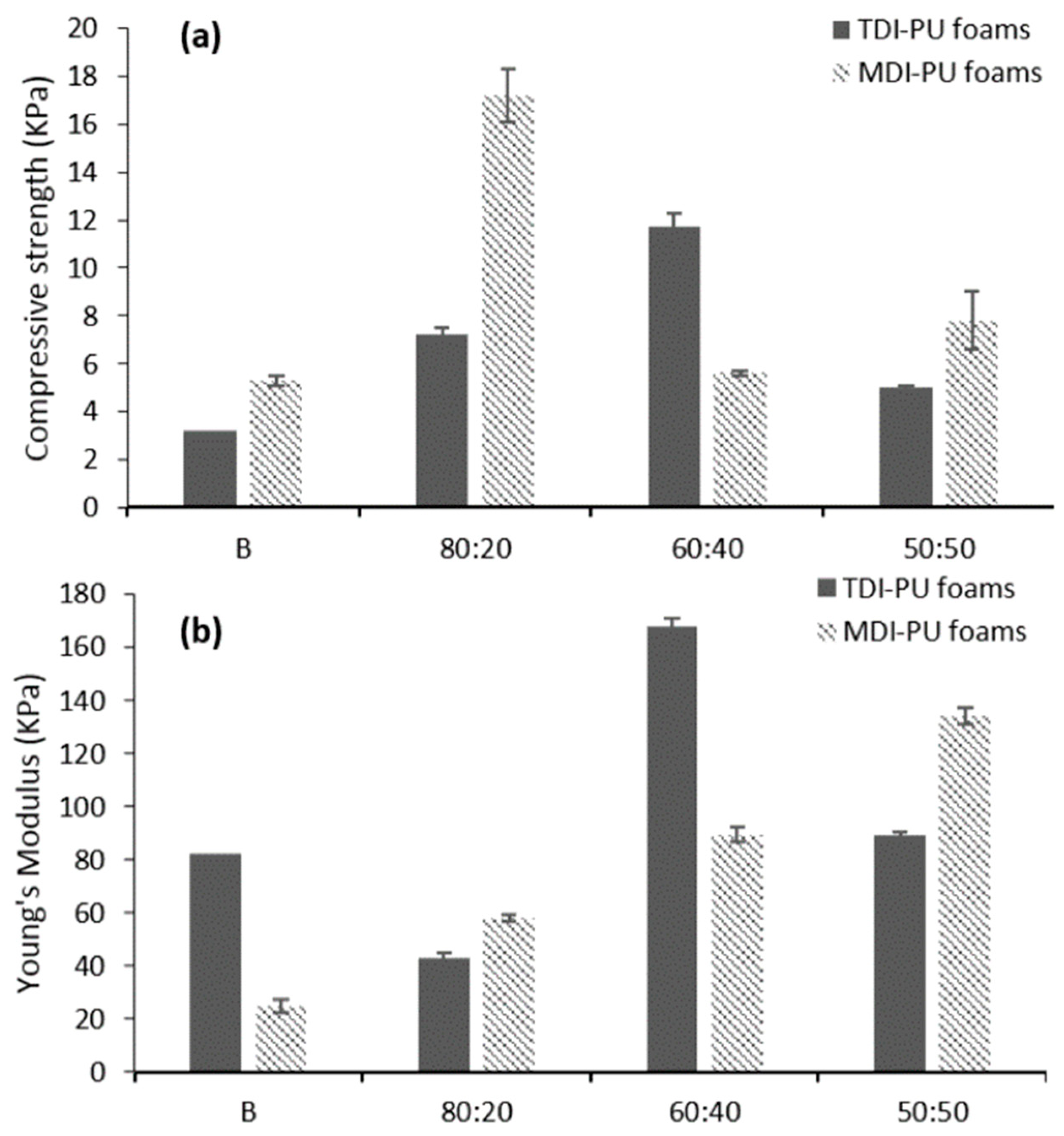
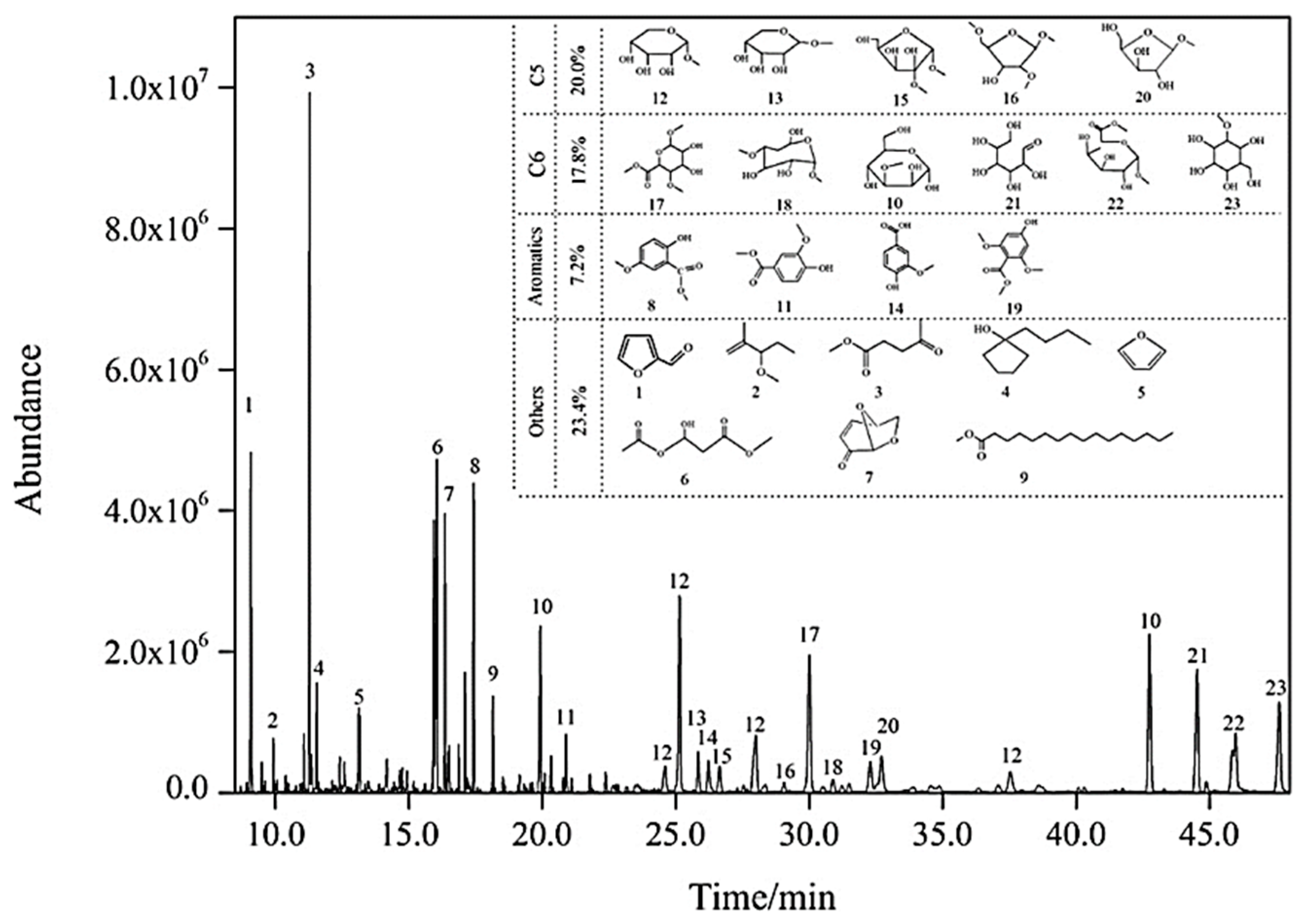

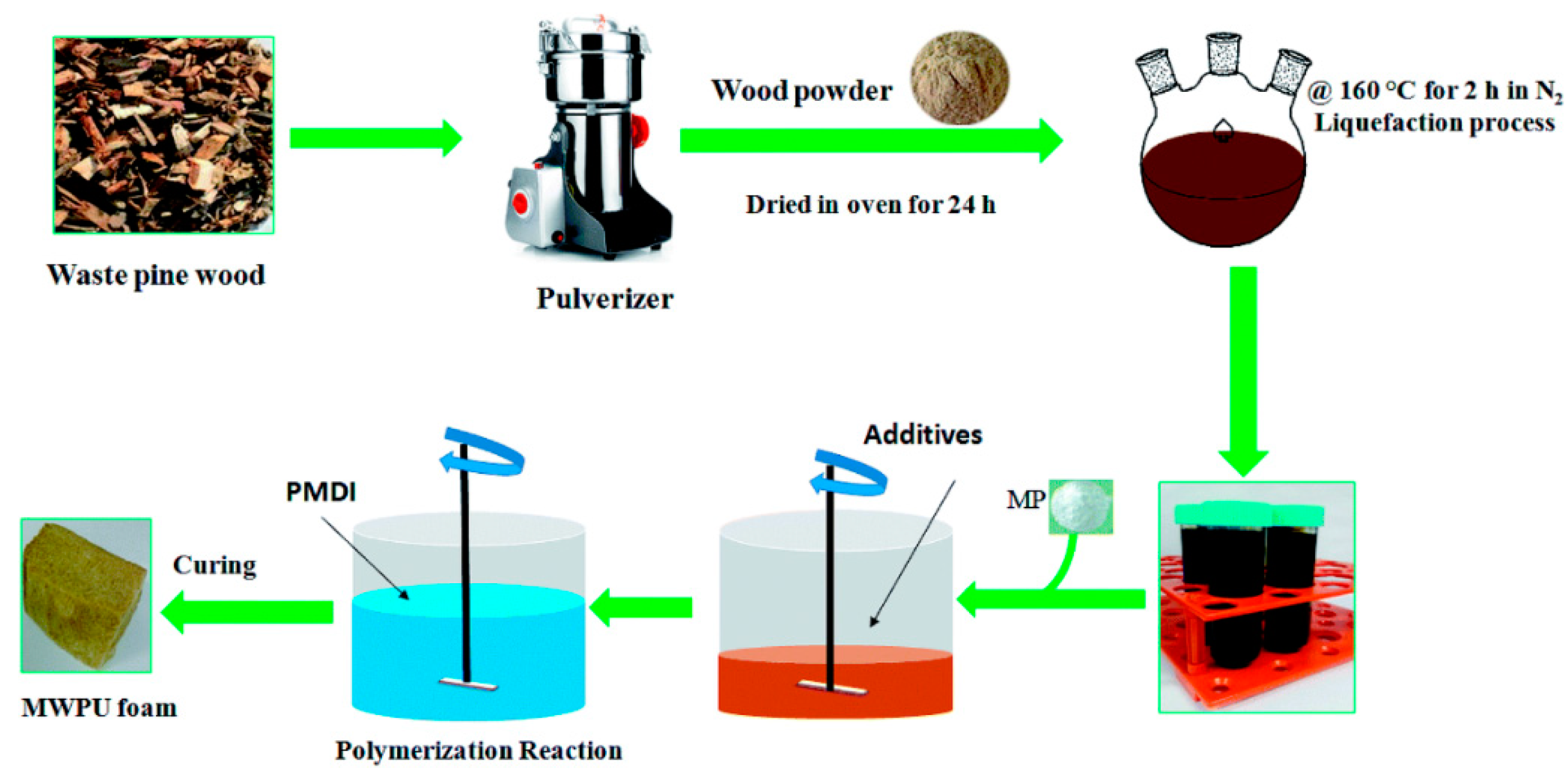
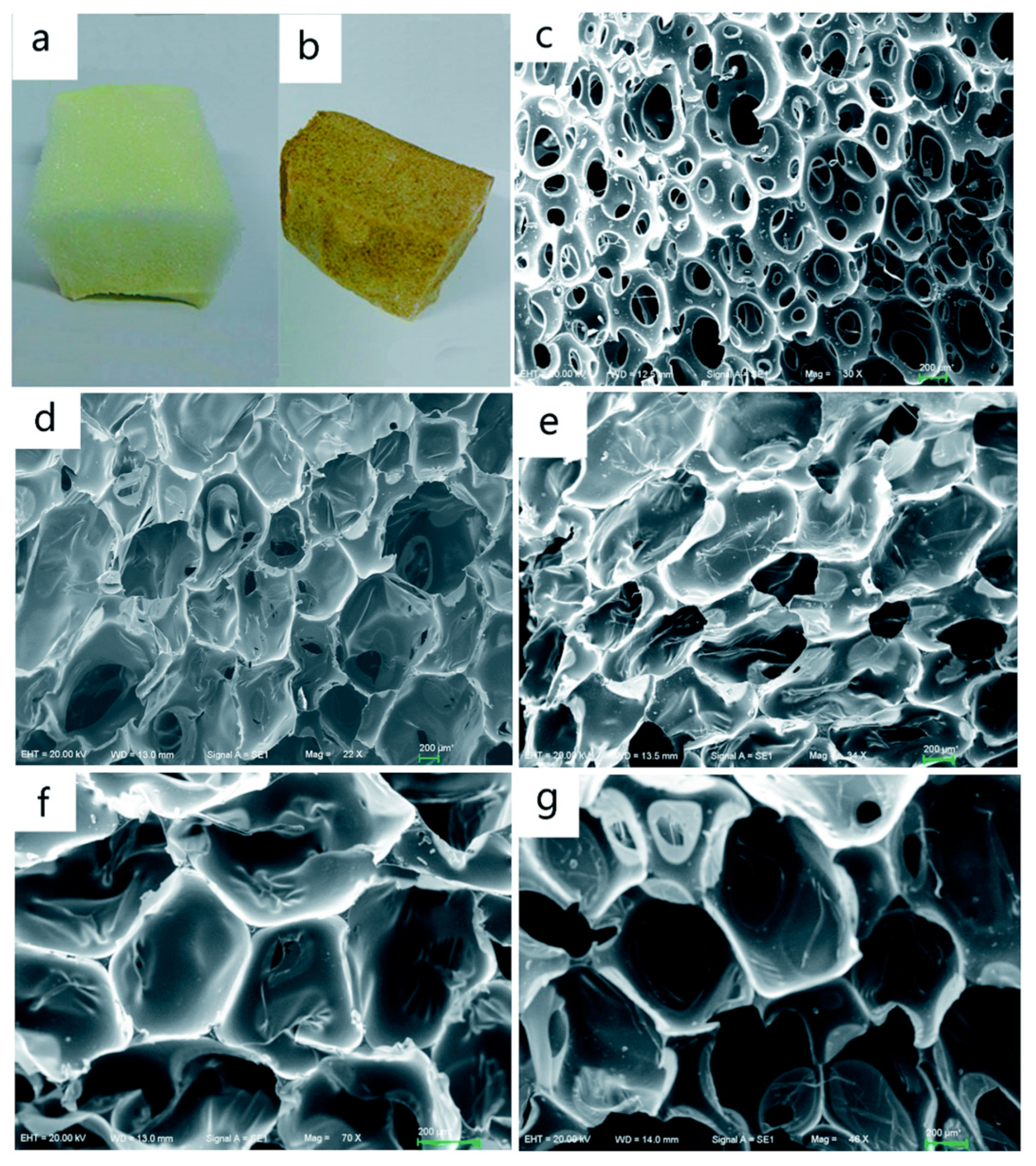
| T (°C)│RT (min) | Pinitial/Pfinal (psi) | HC 1 wt.% | Conversion 2 wt.% | Oil Phase | Gas 5 wt.% | WSH 6 wt.% | |
|---|---|---|---|---|---|---|---|
| AO 3 wt.% | HO 4 wt.% | ||||||
| 250│15 | 300/1100 | 23.496 | 76.504 | 13.50 | 19.35 | 12.291 | 31.363 |
| 300│15 | 300/1650 | 23.396 | 76.604 | 10.20 | 21.75 | 14.436 | 30.218 |
| 350│15 | 300/2600 | 30.206 | 69.794 | 9.00 | 17.70 | 10.742 | 32.352 |
| 375│15 | 300/2850 | 25.520 | 74.480 | 8.55 | 14.25 | 13.504 | 38.176 |
| 250│15 | 600/1600 | 22.286 | 77.71 | 15.60 | 22.20 | 12.895 | 27.019 |
| 300│15 | 600/2200 | 21.406 | 78.594 | 13.00 | 27.15 | 9.578 | 28.866 |
| 350│15 | 600/3150 | 26.840 | 73.160 | 9.00 | 17.70 | 11.201 | 35.259 |
| 375│15 | 600/3500 | 22.440 | 77.560 | 6.66 | 14.25 | 18.073 | 38.577 |
| 300│0 | 600/2200 | 17.380 | 82.620 | 13.36 | 29.25 | 10.674 | 29.336 |
| 300│30 | 600/2200 | 20.020 | 79.980 | 10.50 | 25.20 | 17.369 | 26.911 |
| 300│60 | 600/2200 | 19.360 | 80.640 | 8.10 | 23.55 | 17.541 | 31.449 |
| Ultrasounds Amplitude % | Rate Constant (k) (×10−2) | Correlation Coefficient (R2) |
| 0 (Conventional) | 1.20 | 0.83 |
| 60 | 1.59 | 0.95 |
| 80 | 2.47 | 0.87 |
| 100 | 5.03 | 0.98 |
| Sample | Tg (°C) | E’ (MPa) at 25 °C |
|---|---|---|
| Ag_PU | −34.9 | 839.9 |
| AG_1.2PU | −42.3 | 651.6 |
| OG_PU | −52.3 | 505.1 |
| OG_1.2PU | −36.1 | 671.1 |
| AGP_PU | −43.5 | 609.8 |
| AGP_1.2PU | −47.1 | 339.9 |
| OGP_PU | −48.5 | 665.3 |
| OGP_1.2PU | −49.7 | 351.8 |
| AP_PU | −51.8 | 114.9 |
| AP_1.2PU | −47.9 | 62.4 |
| OP_PU | −45.2 | 358.4 |
| OP_1.2PU | −54.6 | 232.6 |
| Sample | Number-Average Molecular Weight (Mn) | Weight-Average Molecular Weight (Mw) | Peak Molecular Weight (Mp) | Polydispersity |
|---|---|---|---|---|
| 20 min | 144 | 149 | 145 | 1.04 |
| 50 min | 232 | 241 | 233 | 1.04 |
| 80 min | 209 | 218 | 210 | 1.04 |
| 110 min | 237 | 246 | 238 | 1.04 |
| 140 min | 219 | 228 | 220 | 1.04 |
| Ingredients | Parts by Weight | |
|---|---|---|
| A liquid | Biomass-based polyol (12.5% biomass con.) | 100 |
| Catalyst (DBTL) | 1 to 1.5 | |
| Surfactant (SH-193) | 2 to 2.5 | |
| Blowing agent (water, including water from Neutralization with NaOH solution) | 2 to 4.25 | |
| Additives (PEG 400) | 15 | |
| B liquid | MDI | 100 to 240 |
| (Isocyanate index) | 80 to 120 | |
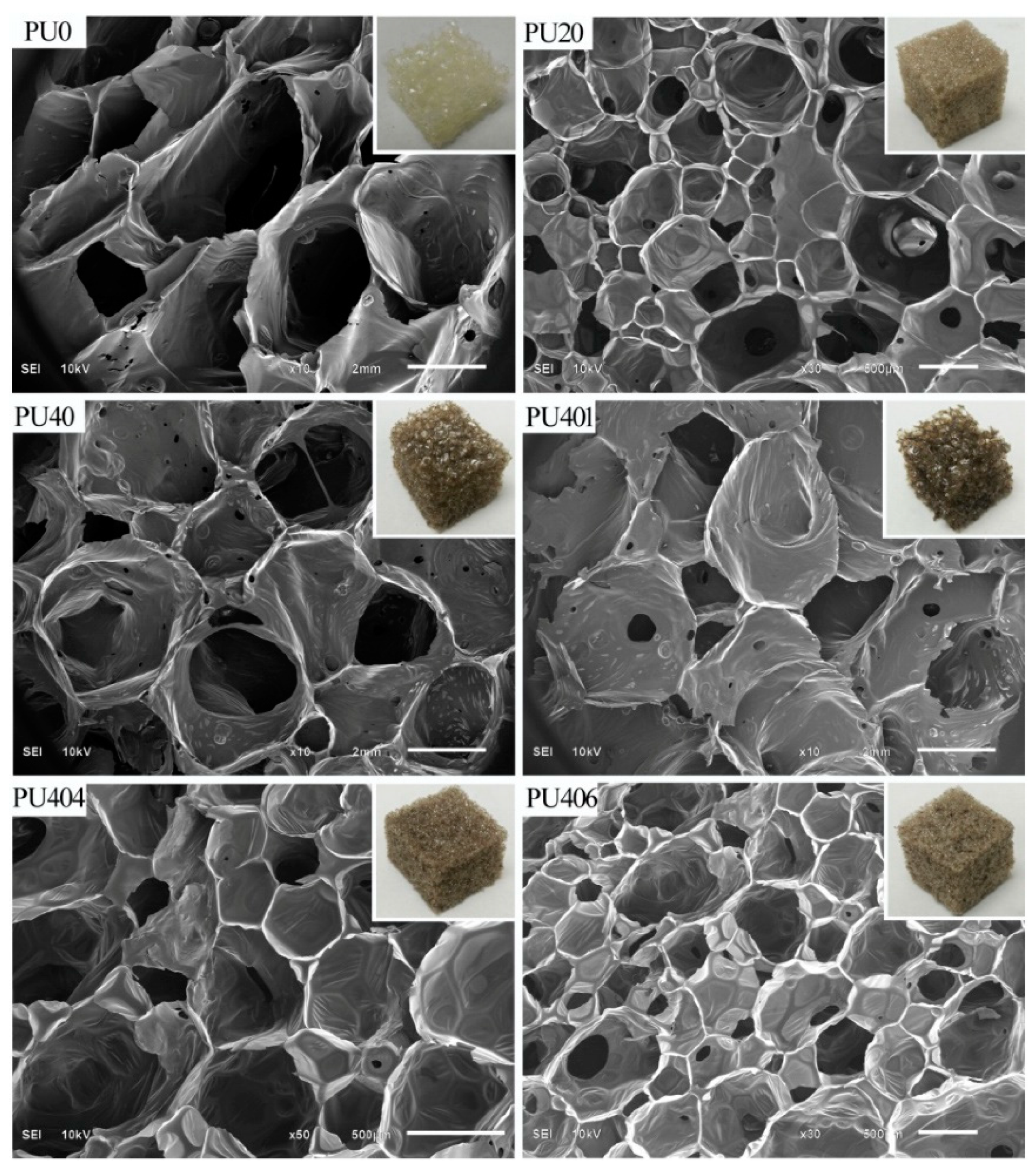
| Sample ID | Cell Diameter (mm) | Density (kg∙m−3) | Thermal Conductivity (mW∙m−1∙K−1) | Young’s Modulus (kPa) | Compressive Stress (δ 10% kPa) |
|---|---|---|---|---|---|
| PU0 | 4.1±1.1 | 26.8 ± 1.6 | 40.4 ± 1.7 | 272.1 ± 67.6 | 25.3 ± 2.5 |
| PU5 | 3.9 ± 0.8 | 26.1 ± 1.0 | 39.9 ± 1.8 | 258.3 ± 65.1 | 24.8 ± 3.5 |
| PU10 | 3.3 ± 0.7 | 20.7 ± 1.3 | 38.1 ± 1.9 | 151.4 ± 15.6 | 12.1 ± 1.4 |
| PU15 | 0.5 ± 0.1 | 21.0 ± 1.5 | 38.0 ± 1.2 | 500.3 ± 81.7 | 35.7 ± 4.8 |
| PU20 | 0.6 ± 0.3 | 22.1 ± 2.1 | 37.2 ± 1.7 | 592.1 ± 81.1 | 37.3 ± 4.8 |
| PU25 | 1.9 ± 0.4 | 23.3 ± 2.3 | 39.7 ± 1.9 | 197.8 ± 36.8 | 14.3 ± 2.1 |
| PU30 | 2.4 ± 0.5 | 23.1 ± 3.0 | 39.6 ± 0.8 | 171.7 ± 43.9 | 12.0 ± 1.7 |
| PU35 | 2.8 ± 0.8 | 24.2 ± 2.6 | 39.8 ± 1.9 | 172.7 ± 46.4 | 11.6 ± 1.8 |
| PU401 | 3.1 ± 0.7 | 26.6 ± 2.0 | 41.1 ± 1.4 | 327.2 ± 46.4 | 17.8 ± 1.7 |
| PU402 | 0.9 ± 0.3 | 24.7 ± 2.6 | 36.1 ± 1.9 | 459.2 ± 28.2 | 22.0 ± 1.3 |
| PU403 | 0.3 ± 0.1 | 24.8 ± 1.1 | 35.8 ± 1.9 | 681.4 ± 132.9 | 22.3 ± 2.9 |
| PU404 | 0.2 ± 0.1 | 24.2 ± 0.7 | 34.4 ± 0.8 | 1186.6 ± 163.3 | 28.7 ± 4.5 |
| PU405 | 0.2 ± 0.1 | 23.3 ± 2.3 | 34.4 ± 1.3 | 976.6 ± 137.4 | 26.6 ± 3.2 |
| PU406 | 0.2 ± 0.1 | 20.6 ± 0.8 | 34.0 ± 1.1 | 857.8 ± 114.5 | 25.6 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontaş, M.G.; Diacon, A.; Călinescu, I.; Rusen, E. Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams. Polymers 2023, 15, 563. https://doi.org/10.3390/polym15030563
Bontaş MG, Diacon A, Călinescu I, Rusen E. Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams. Polymers. 2023; 15(3):563. https://doi.org/10.3390/polym15030563
Chicago/Turabian StyleBontaş, Marius Gabriel, Aurel Diacon, Ioan Călinescu, and Edina Rusen. 2023. "Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams" Polymers 15, no. 3: 563. https://doi.org/10.3390/polym15030563
APA StyleBontaş, M. G., Diacon, A., Călinescu, I., & Rusen, E. (2023). Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams. Polymers, 15(3), 563. https://doi.org/10.3390/polym15030563









