Angle-Independent Color Change in Thermoresponsive Gel-Immobilized Colloidal Amorphous Film Attached to PET Substrate
Abstract
1. Introduction
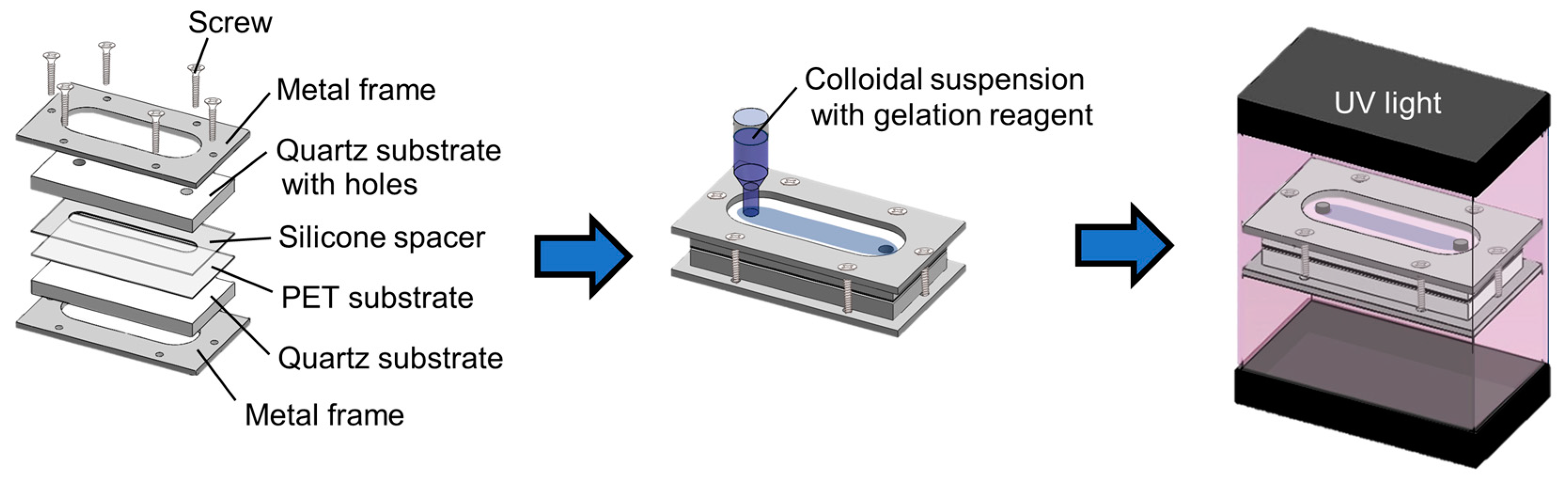
2. Materials and Methods
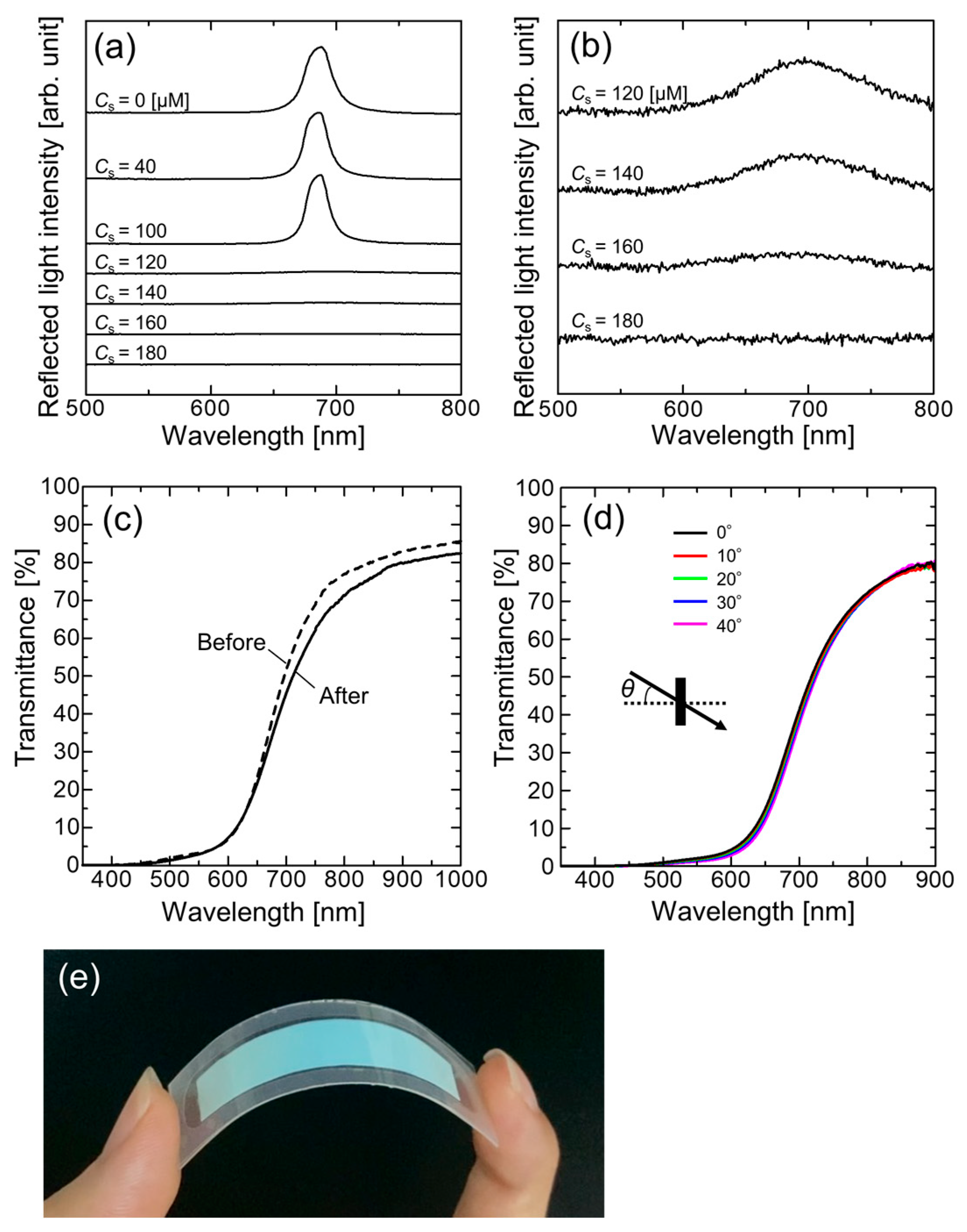
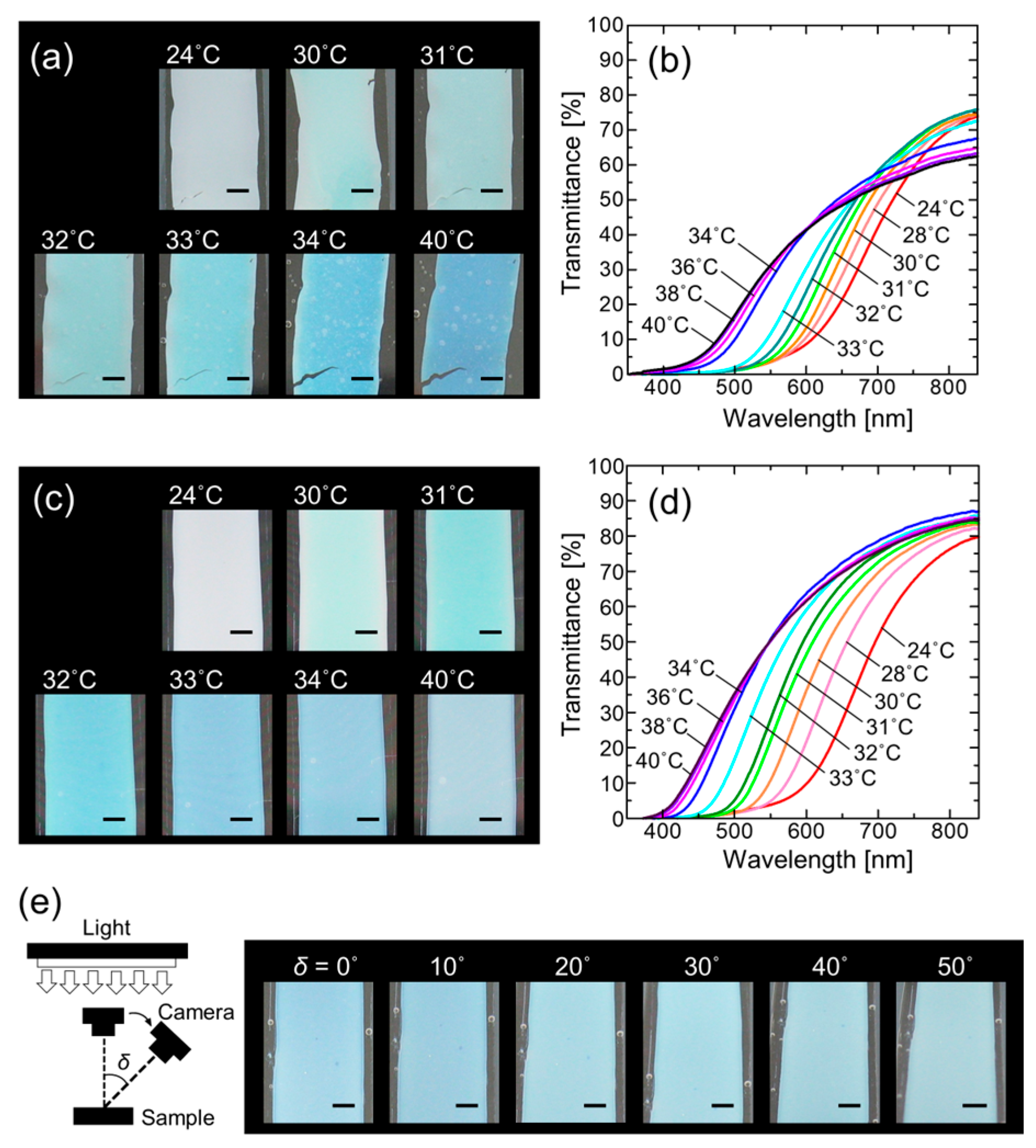
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezaei, S.D.; Dong, Z.; Chan, J.Y.E.; Trisno, J.; Ng, R.J.H.; Ruan, Q.; Qiu, C.-W.; Mortensen, N.A.; Yang, J.K.W. Nanophotonic Structural Colors. ACS Photonics 2021, 8, 18–33. [Google Scholar] [CrossRef]
- Xuan, Z.; Li, J.; Liu, Q.; Yi, F.; Wang, S.; Lu, W. Artificial Structural Colors and Applications. Innovation 2021, 2, 100081. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hong, W. Mechanochromism of Structural-Colored Materials. Adv. Opt. Mater. 2020, 8, 2000984. [Google Scholar] [CrossRef]
- Quan, Y.-J.; Kim, Y.-G.; Kim, M.-S.; Min, S.-H.; Ahn, S.-H. Stretchable Biaxial and Shear Strain Sensors Using Diffractive Structural Colors. ACS Nano 2020, 14, 5392–5399. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Sun, M.; Hua, M.; He, X. Bioinspired structural color sensors based on responsive soft materials. Curr. Opin. Solid State Mat. Sci. 2019, 23, 13–27. [Google Scholar] [CrossRef]
- Li, S.; Kou, D.; Zhang, S.; Ma, W. Large-Area Fabrication of Structurally Colored and Humidity Sensitive Composite Nanofilm via Ultrasonic Spray-Coating. Polymers 2021, 13, 3768. [Google Scholar] [CrossRef] [PubMed]
- Pariente, J.Á.; Blanco, Á.; López, C. Colloidal photonic crystals formation studied by real-time light diffraction. Nanophotonics 2022, 11, 3257–3267. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Chu, Z.; Tang, W.; Qiu, Y.; Zhao, X.; Jiang, W.; Ye, J.; Chen, C. Rapid Coating of Ultraviolet Shielding Colloidal Crystals. Crystals 2020, 10, 502. [Google Scholar] [CrossRef]
- Lai, C.-F.; Li, J.-S. Self-assembly of colloidal Poly(St-MMA-AA) core/shell photonic crystals with tunable structural colors of the full visible spectrum. Opt. Mater. 2019, 88, 128–133. [Google Scholar] [CrossRef]
- Gao, W.; Rigout, M.; Owens, H. Self-assembly of silica colloidal crystal thin films with tuneable structural colours over a wide visible spectrum. Appl. Surf. Sci. 2016, 380, 12–15. [Google Scholar] [CrossRef]
- Takeoka, Y. Structural Colored Gel. J. Photopolym. Sci. Technol. 2009, 22, 123–132. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Nero, M.; Jansson, K.; Willhammar, T.; Sipponen, M.H. Photonic crystals with rainbow colors by centrifugation-assisted assembly of colloidal lignin nanoparticles. Nat. Commun. 2023, 14, 3099. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Hirano, Y.; Tanaka, M.; Kanai, T. Practical Preparation of Elastomer-Immobilized Nonclose-Packed Colloidal Photonic Crystal Films with Various Uniform Colors. Polymers 2023, 15, 2294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Zhao, O.; Du, X. Chameleon-Inspired Structural-Color Actuators. Matter 2019, 1, 626–638. [Google Scholar] [CrossRef]
- Liao, J.; Zhu, C.; Gao, B.; Zhao, Z.; Liu, X.; Tian, L.; Zeng, Y.; Zhou, X.; Xie, Z.; Gu, Z. Multiresponsive Elastic Colloidal Crystals for Reversible Structural Color Patterns. Adv. Funct. Mater. 2019, 29, 1902954. [Google Scholar] [CrossRef]
- Qi, F.; Meng, Z.; Xue, M.; Qiu, L. Recent advances in self-assemblies and sensing applications of colloidal photonic crystals. Anal. Chim. Acta 2020, 1123, 91–112. [Google Scholar] [CrossRef]
- Yu, S.; Dong, S.; Jiao, X.; Li, C.; Chen, D. Ultrathin Photonic Polymer Gel Films Templated by Non-Close-Packed Monolayer Colloidal Crystals to Enhance Colorimetric Sensing. Polymers 2019, 11, 534. [Google Scholar] [CrossRef]
- Kredel, J.; Gallei, M. Compression-Responsive Photonic Crystals Based on Fluorine-Containing Polymers. Polymers 2019, 11, 2114. [Google Scholar] [CrossRef]
- Ruan, J.-L.; Chen, C.; Shen, J.-H.; Zhao, X.-L.; Qian, S.-H.; Zhu, Z.-G. A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef]
- Yin, H.; Dong, B.; Liu, X.; Zhan, T.; Shi, L.; Zi, J.; Yablonovitch, E. Amorphous diamond-structured photonic crystal in the feather barbs of the scarlet macaw. Proc. Natl. Acad. Sci. USA 2012, 109, 10798–10801. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Y.; Dong, B.; Zhan, T.; Liu, X.; Zi, J. Amorphous Photonic Crystals with Only Short-Range Order. Adv. Mater. 2013, 25, 5314–5320. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Y.; Yang, D.; Ma, D.; Huang, S. Self-assembly of colloidal particles into amorphous photonic crystals. Mater. Adv. 2021, 2, 6499–6518. [Google Scholar]
- Takeoka, Y.; Yoshioka, S.; Takano, A.; Arai, S.; Khanin, N.; Nishihara, H.; Teshima, M.; Ohtsuka, Y.; Seki, T. Production of Colored Pigments with Amorphous Arrays of Black and White Colloidal Particles. Angew. Chem. Int. Ed. 2013, 52, 7261–7265. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Shi, L.; Qiu, H.; Zhang, S.; Qi, N.; Hu, J.; Yuan, W.; Zhang, X.; Zhang, K.-Q. Additive Mixing and Conformal Coating of Noniridescent Structural Colors with Robust Mechanical Properties Fabricated by Atomization Deposition. ACS Nano 2018, 12, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Lu, X.; Wei, C.; Li, J.; Li, Y.; Yang, S. Bright, Angle-Independent, Solvent-Responsive, and Structurally Colored Coatings and Rewritable Photonic Paper Based on High-Refractive-Index Colloidal Quasi-Amorphous Arrays. ACS Appl. Nano Mater. 2021, 4, 9855–9865. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Imran, A.B.; Seki, T.; Ishii, M.; Nakamura, H.; Takeoka, Y. Angle-Independent Structural Color in Colloidal Amorphous Arrays. ChemPhysChem 2010, 11, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.D.; Noh, H.; Liew, S.F.; Saranathan, V.; Schreck, C.F.; Yang, L.; Park, J.-G.; Prum, R.O.; Mochrie, S.G.J.; O’Hern, C.S.; et al. Biomimetic Isotropic Nanostructures for Structural Coloration. Adv. Mater. 2010, 22, 2939–2944. [Google Scholar] [CrossRef]
- Chen, G.; Yi, B.; Huang, Y.; Liang, Q.; Shen, H. Development of bright and low angle dependence structural colors from order-disorder hierarchical photonic structure. Dyes Pigment. 2019, 161, 464–469. [Google Scholar] [CrossRef]
- Kanai, T.; Nakagawa, S.; Takeshima, H.; Hirano, Y. Amorphous photonic structure in a charged colloidal system showing angle-independent uniform color. J. Colloid Interface Sci. 2023, 629, 225–232. [Google Scholar] [CrossRef]
- Sugiyama, K.; Kato, K.; Kido, M.; Shiraishi, K.; Ohga, K.; Okada, K.; Matsuo, O. Grafting of vinyl monomers on the surface of a poly(ethylene terephthalate) film using Ar plasma-post polymerization technique to increase biocompatibility. Macromol. Chem. Phys. 1998, 199, 1201–1208. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: Theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Kanai, T.; Yano, H.; Kobayashi, N.; Sawada, T. Enhancement of Thermosensitivity of Gel-Immobilized Tunable Colloidal Photonic Crystals with Anisotropic Contraction. ACS Macro Lett. 2017, 6, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Kobayashi, N.; Tajima, H. Enhanced linear thermosensitivity of gel-immobilized colloidal photonic crystal film bound on glass substrate. Mater. Adv. 2021, 2, 2600–2603. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, S.; Hirano, Y.; Tanaka, M.; Kanai, T. Angle-Independent Color Change in Thermoresponsive Gel-Immobilized Colloidal Amorphous Film Attached to PET Substrate. Polymers 2023, 15, 4661. https://doi.org/10.3390/polym15244661
Nakagawa S, Hirano Y, Tanaka M, Kanai T. Angle-Independent Color Change in Thermoresponsive Gel-Immobilized Colloidal Amorphous Film Attached to PET Substrate. Polymers. 2023; 15(24):4661. https://doi.org/10.3390/polym15244661
Chicago/Turabian StyleNakagawa, Sato, Yuna Hirano, Mikako Tanaka, and Toshimitsu Kanai. 2023. "Angle-Independent Color Change in Thermoresponsive Gel-Immobilized Colloidal Amorphous Film Attached to PET Substrate" Polymers 15, no. 24: 4661. https://doi.org/10.3390/polym15244661
APA StyleNakagawa, S., Hirano, Y., Tanaka, M., & Kanai, T. (2023). Angle-Independent Color Change in Thermoresponsive Gel-Immobilized Colloidal Amorphous Film Attached to PET Substrate. Polymers, 15(24), 4661. https://doi.org/10.3390/polym15244661




