Synthesis of Thermo-Responsive Monofunctionalized Diblock Copolymer Worms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3. Mass Spectroscopy (MS)
2.4. Gel Permeation Chromatography (GPC)
2.5. Transmission Electron Microscopy (TEM)
2.6. Dynamic Light Scattering (DLS)
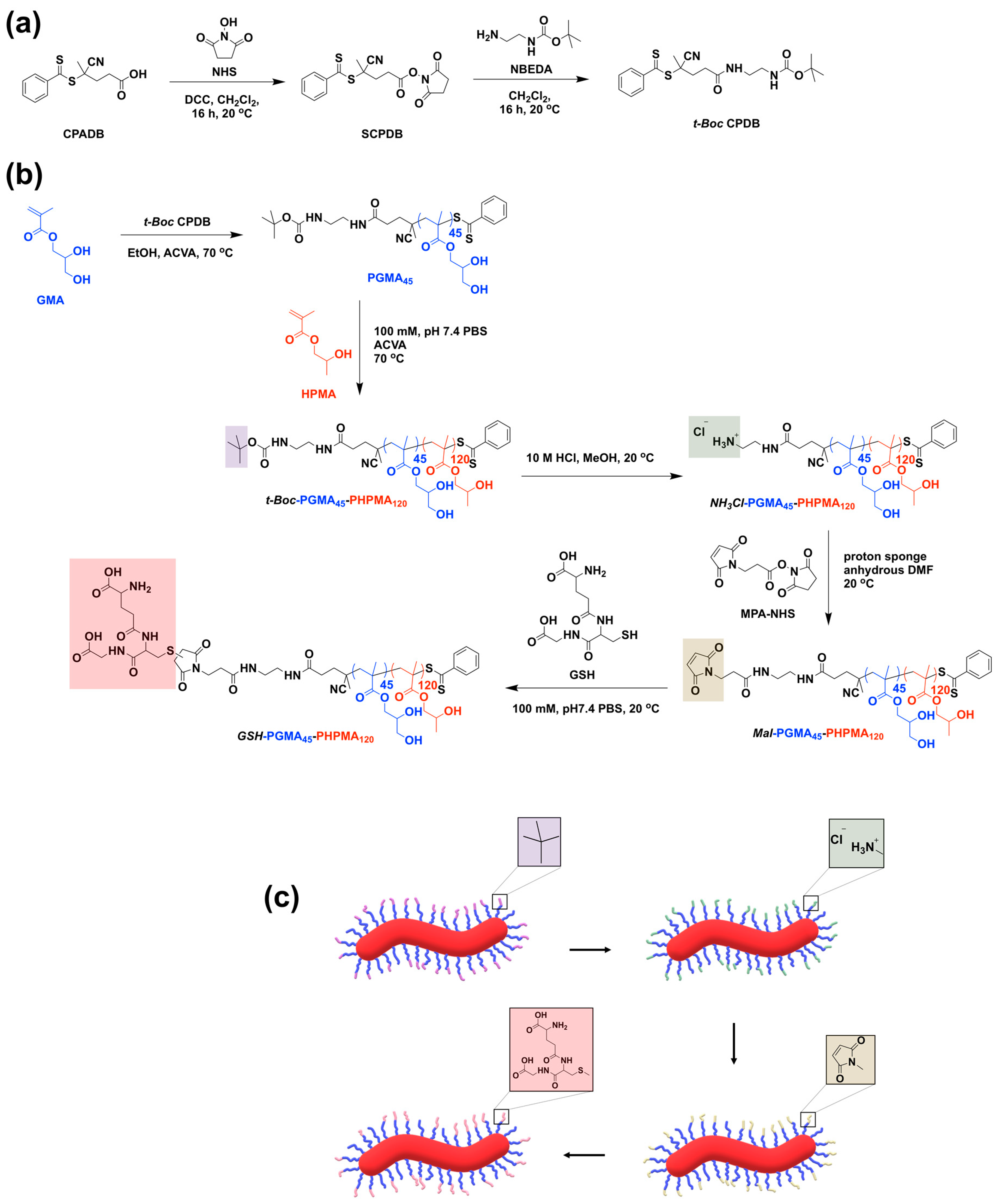
2.7. Synthesis of CTA 5-(2-(Tert-Butoxycarbonylamino)ethylamino)-2-cyano-5-oxopentan-2-yl benzo-dithioate, t-Boc CPDB
2.8. Synthesis of t-Boc ethylenediaminated Poly(glycerol methacrylate) (t-Boc-PGMA45) Macro-CTA and Purification
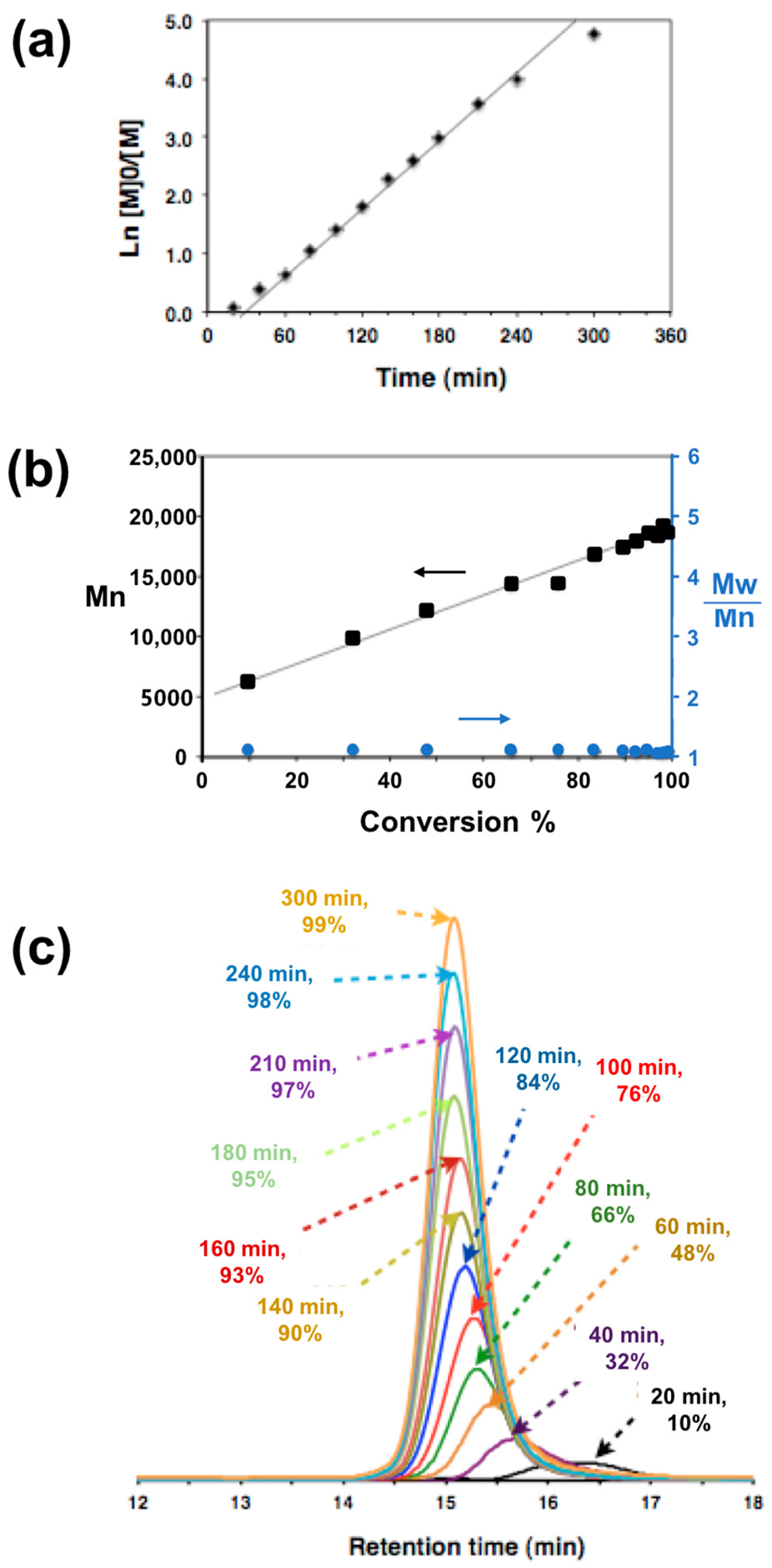
2.9. RAFT Dispersion Polymerization of 2-Hydroxypropyl Methacrylate (HPMA) Using t-Boc-PGMA45 Macro-CTA
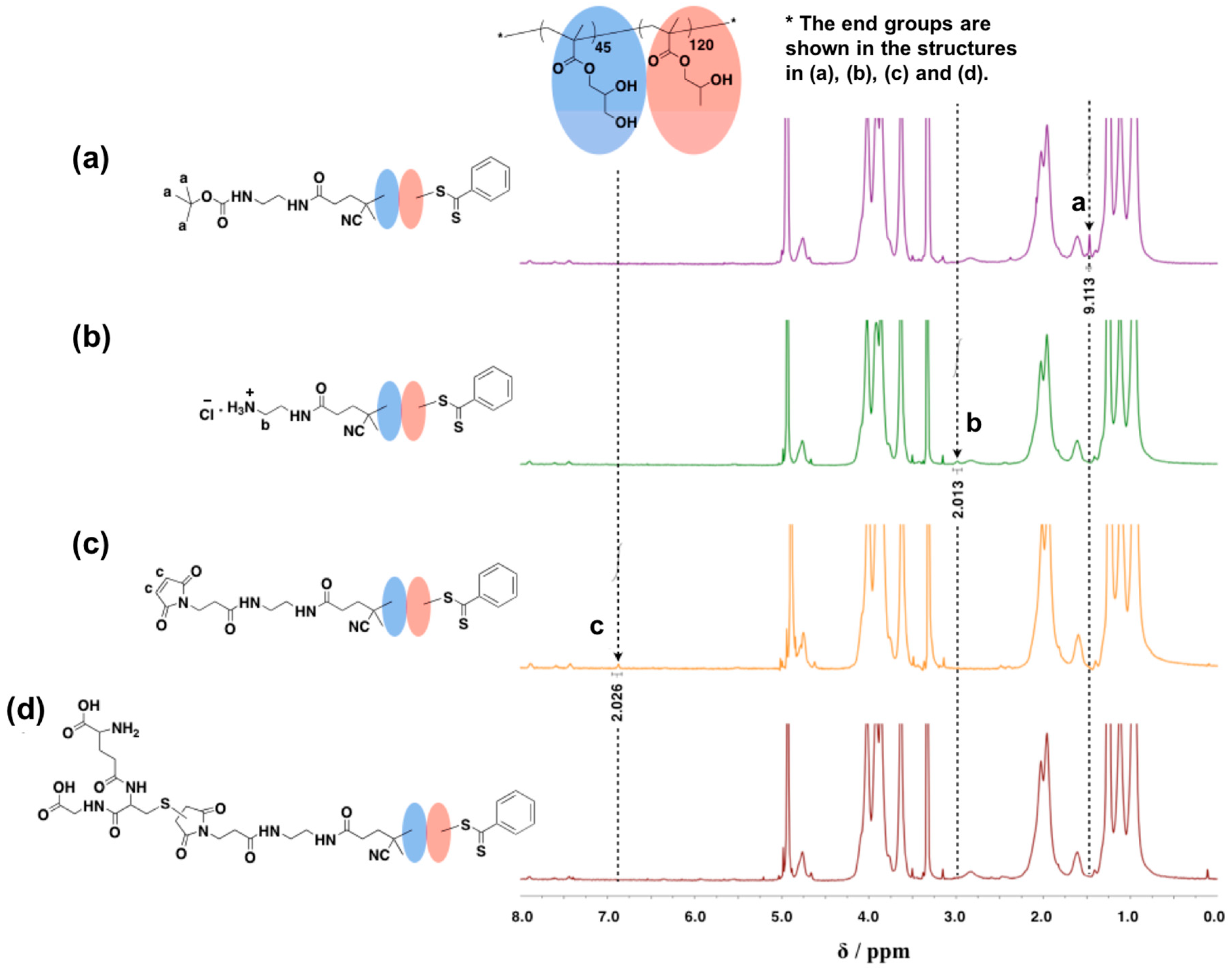
2.10. Synthesis of Amine Monofunctionalized PGMA45-PHPMA120 (NH3Cl-PGMA45-PHPMA120)
2.11. Synthesis of Maleimide Monofunctionalized PGMA45-PHPMA120 (Mal-PGMA45-PHPMA120)
2.12. Conjugation of Reduced L-Glutathione on PGMA45-PHPMA120 (GSH-PGMA45-PHPMA120)
3. Results and Discussion
3.1. t-Boc CTA Synthesis
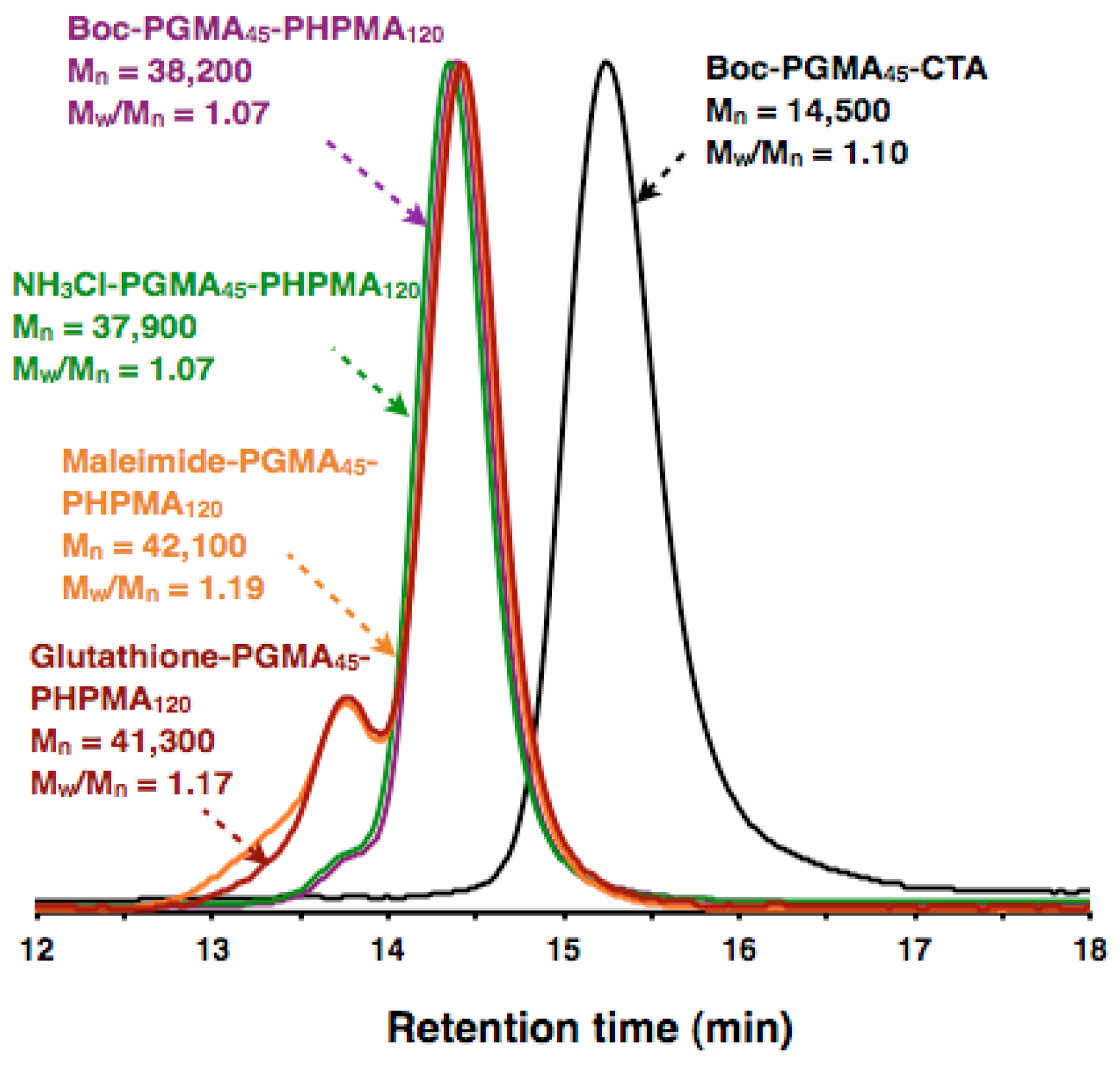
3.2. Macro-CTA Synthesis
3.3. Synthesis of NH3Cl-PGMA45-PHPMA120 with Worm-Like Morphology
3.4. Synthesis of Mal-PGMA45-PHPMA120 with Worm-Like Morphology
3.5. Synthesis of GSH-PGMA45-PHPMA120 with Worm-like Morphology
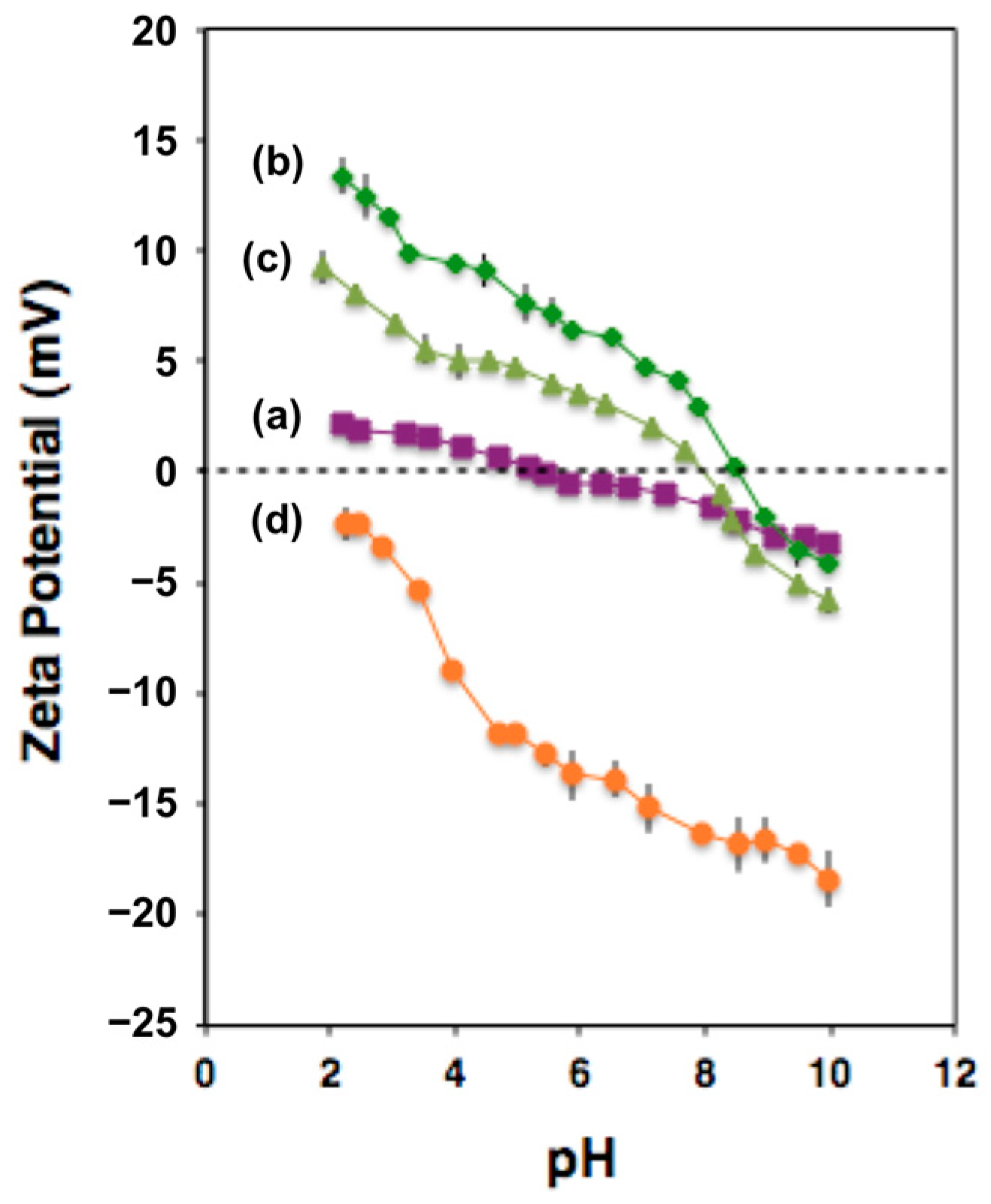
3.6. Zeta-Potential vs. pH Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Agent Design and Synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Decker, C.G.; Maynard, H.D. Degradable PEGylated protein conjugates utilizing RAFT polymerization. Eur. Polym. J. 2015, 65, 305–312. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Li, M.; Gondi, S.R.; Sumerlin, B.S. Temperature-Regulated Activity of Responsive Polymer−Protein Conjugates Prepared by Grafting-from via RAFT Polymerization. J. Am. Chem. Soc. 2008, 130, 11288–11289. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, K.; Zhao, J.; Gao, B.; Feng, Y. Polymeric nano-carriers for on-demand delivery of genes via specific responses to stimuli. J. Mater. Chem. B 2020, 8, 9621–9641. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Palumbo, R.N.; Nagarajan, L.; Krogstad, E.; Wang, C. Well-defined block copolymers for gene delivery to dendritic cells: Probing the effect of polycation chain-length. J. Control. Release 2010, 142, 229–237. [Google Scholar] [CrossRef][Green Version]
- Wojciechowski, K.; Gutarowicz, M.; Mierzejewska, J.; Parzuchowski, P. Antimicrobial films of poly(2-aminoethyl methacrylate) and its copolymers doped with TiO2 and CaCO3. Colloids Surf. B Biointerfaces 2020, 185, 110605. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; Figueiredo, A.G.P.R.; Silva, N.H.C.S.; Barros-Timmons, A.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Antimicrobial bacterial cellulose nanocomposites prepared by in situ polymerization of 2-aminoethyl methacrylate. Carbohydr. Polym. 2015, 123, 443–453. [Google Scholar] [CrossRef]
- Chen, C.; Ng, D.Y.W.; Weil, T. Polymer bioconjugates: Modern design concepts toward precision hybrid materials. Prog. Polym. Sci. 2020, 105, 101241. [Google Scholar] [CrossRef]
- Bulmus, V. RAFT polymerization mediated bioconjugation strategies. Polym. Chem. 2011, 2, 1463–1472. [Google Scholar] [CrossRef]
- He, L.; Read, E.S.; Armes, S.P.; Adams, D.J. Direct synthesis of controlled-structure primary amine-based methacrylic polymers by living radical polymerization. Macromolecules 2007, 40, 4429–4438. [Google Scholar] [CrossRef]
- Read, E.S.; Thompson, K.L.; Armes, S.P. Synthesis of well-defined primary amine-based homopolymers and block copolymers and their Michael addition reactions with acrylates and acrylamides. Polym. Chem. 2010, 1, 221–230. [Google Scholar] [CrossRef]
- Thompson, K.L.; Read, E.S.; Armes, S.P. Chemical degradation of poly(2-aminoethyl methacrylate). Polym. Degrad. Stab. 2008, 93, 1460–1466. [Google Scholar] [CrossRef]
- Kumar, S.; Acharya, R.; Chatterji, U.; De, P. Controlled synthesis of pH responsive cationic polymers containing side-chain peptide moieties via RAFT polymerization and their self-assembly. J. Mater. Chem. B 2013, 1, 946–957. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Salgado-Rodriguez, R.; Licea-Claverie, A. Preparation of alpha,omega-Telechelic Hexyl Acrylate Polymers with -OH, -COOH, and -NH2 Functional Groups by RAFT. J. Polym. Sci. Part A-Polym. Chem. 2010, 48, 3033–3051. [Google Scholar] [CrossRef]
- Chambon, P.; Blanazs, A.; Battaglia, G.; Armes, S.P. Facile Synthesis of Methacrylic ABC Triblock Copolymer Vesicles by RAFT Aqueous Dispersion Polymerization. Macromolecules 2012, 45, 5081–5090. [Google Scholar] [CrossRef]
- Fielding, L.A.; Derry, M.J.; Ladmiral, V.; Rosselgong, J.; Rodrigues, A.M.; Ratcliffe, L.P.D.; Sugihara, S.; Armes, S.P. RAFT dispersion polymerization in non-polar solvents: Facile production of block copolymer spheres, worms and vesicles in n-alkanes. Chem. Sci. 2013, 4, 2081–2087. [Google Scholar] [CrossRef]
- Warren, N.J.; Mykhaylyk, O.O.; Mahmood, D.; Ryan, A.J.; Armes, S.P. RAFT Aqueous Dispersion Polymerization Yields Poly(ethylene glycol)-Based Diblock Copolymer Nano-Objects with Predictable Single Phase Morphologies. J. Am. Chem. Soc. 2014, 136, 1023–1033. [Google Scholar] [CrossRef]
- Hayward, R.C.; Pochan, D.J. Tailored Assemblies of Block Copolymers in Solution: It Is All about the Process. Macromolecules 2010, 43, 3577–3584. [Google Scholar] [CrossRef]
- Blanazs, A.; Ryan, A.J.; Armes, S.P. Predictive Phase Diagrams for RAFT Aqueous Dispersion Polymerization: Effect of Block Copolymer Composition, Molecular Weight, and Copolymer Concentration. Macromolecules 2012, 45, 5099–5107. [Google Scholar] [CrossRef]
- Verber, R.; Blanazs, A.; Armes, S.P. Rheological studies of thermo-responsive diblock copolymer worm gels. Soft Matter 2012, 8, 9915–9922. [Google Scholar] [CrossRef]
- Blanazs, A.; Verber, R.; Mykhaylyk, O.O.; Ryan, A.J.; Heath, J.Z.; Douglas, C.W.I.; Armes, S.P. Sterilizable Gels from Thermoresponsive Block Copolymer Worms. J. Am. Chem. Soc. 2012, 134, 9741–9748. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Vijayan, K.; Cheng, D.; Lima, E.M.; Discher, D.E. Micelles of different morphologies—Advantages of worm-like filomicelles of PEO-PCL in paclitaxel delivery. Pharm. Res. 2007, 24, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Christian, D.A.; Cai, S.; Garbuzenko, O.B.; Harada, T.; Zajac, A.L.; Minko, T.; Discher, D.E. Flexible Filaments for in Vivo Imaging and Delivery: Persistent Circulation of Filomicelles Opens the Dosage Window for Sustained Tumor Shrinkage. Mol. Pharm. 2009, 6, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Moeinzadeh, S.; Barati, D.; He, X.; Jabbari, E. Gelation Characteristics and Osteogenic Differentiation of Stromal Cells in Inert Hydrolytically Degradable Micellar Polyethylene Glycol Hydrogels. Biomacromolecules 2012, 13, 2073–2086. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.P.; Pubill, S.; Ribeiro, A.; Leach, J.B. Hydrolytically Degradable Poly(ethylene glycol) Hydrogel Scaffolds as a Cell Delivery Vehicle: Characterization of PC12 Cell Response. Biotechnol. Prog. 2013, 29, 1255–1264. [Google Scholar] [CrossRef]
- DeVolder, R.; Kong, H.-J. Hydrogels for in vivo-like three-dimensional cellular studies. Wiley Interdiscip. Rev.-Syst. Biol. Med. 2012, 4, 351–365. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Goda, T.; Ishihara, K. Soft contact lens biomaterials from bioinspired phospholipid polymers. Expert Rev. Med. Devices 2006, 3, 167–174. [Google Scholar] [CrossRef]
- Shimizu, T.; Goda, T.; Minoura, N.; Takai, M.; Ishihara, K. Super-hydrophilic silicone hydrogels with interpenetrating poly(2-methacryloyloxyethyl phosphorylcholine) networks. Biomaterials 2010, 31, 3274–3280. [Google Scholar] [CrossRef]
- Deepa, G.; Thulasidasan, A.K.T.; Anto, R.J.; Pillai, J.J.; Kumar, G.S.V. Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy. Int. J. Nanomed. 2012, 7, 4077–4088. [Google Scholar]
- Lai, J.-Y. Biodegradable in situ gelling delivery systems containing pilocarpine as new antiglaucoma formulations: Effect of a mercaptoacetic acid/N-isopropylacrylamide molar ratio. Drug Des. Dev. Ther. 2013, 7, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Heaysman, C.L.; Willis, S.; Lewis, A.L. Physical hydrogels with self-assembled nanostructures as drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Canton, I.; Warren, N.J.; Chahal, A.; Amps, K.; Wood, A.; Weightman, R.; Wang, E.; Moore, H.; Armes, S.P. Mucin-Inspired Thermoresponsive Synthetic Hydrogels Induce Stasis in Human Pluripotent Stem Cells and Human Embryos. ACS Cent. Sci. 2016, 2, 65–74. [Google Scholar] [CrossRef]
- Mitchell, D.E.; Lovett, J.R.; Armes, S.P.; Gibson, M.I. Combining Biomimetic Block Copolymer Worms with an Ice-Inhibiting Polymer for the Solvent-Free Cryopreservation of Red Blood Cells. Angew. Chem. Int. Ed. 2016, 55, 2801–2804. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.A.; Warren, N.J.; Mosadegh, B.; Mohammady, M.R.; Whitesides, G.M.; Armes, S.P. Disulfide-Based Diblock Copolymer Worm Gels: A Wholly-Synthetic Thermoreversible 3D Matrix for Sheet-Based Cultures. Biomacromolecules 2015, 16, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Binch, A.L.A.; Ratcliffe, L.P.D.; Milani, A.H.; Saunders, B.R.; Armes, S.P.; Hoyland, J.A. Site-Directed Differentiation of Human Adipose-Derived Mesenchymal Stem Cells to Nucleus Pulposus Cells Using an Injectable Hydroxyl-Functional Diblock Copolymer Worm Gel. Biomacromolecules 2021, 22, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yu, K.; Kindrachuk, J.; Brooks, D.E.; Hancock, R.E.W.; Kizhakkedathu, J.N. Antibacterial Surfaces Based on Polymer Brushes: Investigation on the Influence of Brush Properties on Antimicrobial Peptide Immobilization and Antimicrobial Activity. Biomacromolecules 2011, 12, 3715–3727. [Google Scholar] [CrossRef]
- Kretschy, D.; Koellensperger, G.; Hann, S. Elemental labelling combined with liquid chromatography inductively coupled plasma mass spectrometry for quantification of biomolecules: A review. Anal. Chim. Acta 2012, 750, 98–110. [Google Scholar] [CrossRef]
- Kocik, M.K.; Mykhaylyk, O.O.; Armes, S.P. Aqueous worm gels can be reconstituted from freeze-dried diblock copolymer powder. Soft Matter 2014, 10, 3984–3992. [Google Scholar] [CrossRef]
- Zheng, Q.; Pan, C.Y. Synthesis and characterization of dendrimer-star polymer using dithiobenzoate-terminated poly(propylene imine) dendrimer via reversible addition-fragmentation transfer polymerization. Macromolecules 2005, 38, 6841–6848. [Google Scholar] [CrossRef]
- Convertine, A.J.; Lokitz, B.S.; Vasileva, Y.; Myrick, L.J.; Scales, C.W.; Lowe, A.B.; McCormick, C.L. Direct synthesis of thermally responsive DMA/NIPAM diblock and DMA/NIPAM/DMA triblock copolymers via aqueous, room temperature RAFT polymerization. Macromolecules 2006, 39, 1724–1730. [Google Scholar] [CrossRef]
- Favier, A.; Charreyre, M.T.; Chaumont, P.; Pichot, C. Study of the RAFT polymerization of a water-soluble bisubstituted acrylamide derivative. 1. Influence of the dithioester structure. Macromolecules 2002, 35, 8271–8280. [Google Scholar] [CrossRef]
- Blanazs, A.; Madsen, J.; Battaglia, G.; Ryan, A.J.; Armes, S.P. Mechanistic Insights for Block Copolymer Morphologies: How Do Worms Form Vesicles? J. Am. Chem. Soc. 2011, 133, 16581–16587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Armes, S.P. RAFT Synthesis of Sterically Stabilized Methacrylic Nanolatexes and Vesicles by Aqueous Dispersion Polymerization. Angew. Chem.-Int. Ed. 2010, 49, 4042–4046. [Google Scholar] [CrossRef] [PubMed]
- Bauri, K.; De, P.; Shah, P.N.; Li, R.; Faust, R. Polyisobutylene-Based Helical Block Copolymers with pH-Responsive Cationic Side-Chain Amino Acid Moieties by Tandem Living Polymerizations. Macromolecules 2013, 46, 5861–5870. [Google Scholar] [CrossRef]
- Themistou, E.; Battaglia, G.; Armes, S.P. Facile synthesis of thiol-functionalized amphiphilic polylactide-methacrylic diblock copolymers. Polym. Chem. 2014, 5, 1405–1417. [Google Scholar] [CrossRef]
- Oswald, M.; Geissler, S.; Goepferich, A. Determination of the activity of maleimide-functionalized phospholipids during preparation of liposomes. Int. J. Pharm. 2016, 514, 93–102. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Wang, F.; Shi, M.; Khan, F.I. Synthesis of Thermo-Responsive Monofunctionalized Diblock Copolymer Worms. Polymers 2023, 15, 4590. https://doi.org/10.3390/polym15234590
Xue X, Wang F, Shi M, Khan FI. Synthesis of Thermo-Responsive Monofunctionalized Diblock Copolymer Worms. Polymers. 2023; 15(23):4590. https://doi.org/10.3390/polym15234590
Chicago/Turabian StyleXue, Xuan, Feifei Wang, Minhao Shi, and Faez Iqbal Khan. 2023. "Synthesis of Thermo-Responsive Monofunctionalized Diblock Copolymer Worms" Polymers 15, no. 23: 4590. https://doi.org/10.3390/polym15234590
APA StyleXue, X., Wang, F., Shi, M., & Khan, F. I. (2023). Synthesis of Thermo-Responsive Monofunctionalized Diblock Copolymer Worms. Polymers, 15(23), 4590. https://doi.org/10.3390/polym15234590






