Abstract
Increasing energy demands and growing environmental concerns regarding the consumption of fossil fuels are important motivations for the development of clean and sustainable energy sources. A triboelectric nanogenerator (TENG) is a promising energy technology that harnesses mechanical energy from the ambient environment by converting it into electrical energy. In this work, the enhancement of the energy conversion performance of a natural rubber (NR)-based TENG has been proposed by using modified activated carbon (AC). The effect of surface modification techniques, including acid treatments and plasma treatment for AC material on TENG performance, are investigated. The TENG fabricated from the NR incorporated with the modified AC using N2 plasma showed superior electrical output performance, which was attributed to the modification by N2 plasma introducing changes in the surface chemistry of AC, leading to the improved dielectric property of the NR-AC composite, which contributes to the enhanced triboelectric charge density. The highest power density of 2.65 mW/m2 was obtained from the NR-AC (N2 plasma-treated) TENG. This research provides a key insight into the modification of AC for the development of TENG with high energy conversion performance that could be useful for other future applications such as PM2.5 removal or CO2 capture.
1. Introduction
Due to a predominant reliance on limited and non-renewable energy sources, significant issues have arisen concerning energy shortages and environmental pollution. Consequently, clean and sustainable energy has received continuous attention and development. The triboelectric nanogenerator (TENG) is an innovative technology capable of harvesting mechanical energy and converting it into electrical energy, based on the principles of contact electrification and electrostatic induction [1]. TENG has been demonstrated to be highly efficient, environmentally friendly, low-cost, easy to manufacture, and suitable for large-scale applications. Its versatility extends to various applications, such as sensors [2], portable micro/nano power sources [3], raindrop energy harvesting [4], and air filtration systems [5].
Natural-based materials, such as leaves, wood, silk, and paper, have been developed for TENG applications. Most of them have shown promising energy production performance and potential applications. However, some of them experience difficulties in the modulation of their intrinsic property to enhance the power output of TENG due to processing constraints.
Natural rubber (NR) is a natural polymeric material with a chemical structure of cis-1,4-polyisoprene. NR latex is typically extracted from Hevea brasiliensis trees, which are grown in tropical areas such as Southeast Asia, India, and Africa. NR possesses many outstanding characteristics, including high elasticity, low thermal conductivity, good electrical insulation, and biodegradability [6]. In this regard, NR has been developed for diverse applications including examination gloves, car wheels, packaging, and shoes. In addition, NR film is made from NR latex, which is feasible for manufacturing processes and modifications such as the incorporation of filler materials to engineer the properties of the NR film.
NR-TENG has been developed and is gaining more attention. Various modification approaches have been proposed for improving the performance of NR-TENG by intensifying the triboelectric charge density on friction layers. These modifications include adding metal/metal oxide nanoparticles such as silver (Ag) [7,8], TiO2 nanoparticles [9], hybrid Ag–cellulose [10], and activated carbon (AC) [10,11]. These filler materials were found to enhance the charge capacitance of the NR triboelectric layer through dielectric constant modulation via the interfacial polarization of conductive nanoparticles in a dielectric NR polymer [12]. Other conductive nanostructured materials have been reported to effectively enhance the power output of polymer-based TENGs such as Au [13] and carbon nanotubes [14]; however, they are costly, which could be a major constraint for large-scale production and commercialization. Among these filler materials, AC is a carbon material with a highly porous structure, giving it incredibly high specific surface area, making it an excellent adsorbent for a wide range of substances [15], leading to a broad spectrum of applications, including in air and water filtration/purification [16], CO2 adsorption [17], environmental remediation [18], supercapacitors [19], and medical [20] and pharmaceutical [21] areas. In this regard, AC is a promising filler for NR since a large surface area is a key benefit required for enhancing the triboelectric charge density [22,23]. Moreover, AC is inexpensive and offers numerous ways to further modify surface structure and chemistry [24].
Many different methods have been proposed for modifying the surface of activated carbon, such as acid treatment, base treatment, impregnation treatment, ozone treatment, surfactant treatment, plasma treatment, and microwave treatment [24]. For acid treatments, HCl [25], H2SO4 [26], and HNO3 [27] have been reported. KOH [28], NaOH [29], and NH3 [30,31] are used as base treatments. In addition, other substances such as ZnCl2 [32], NaNH2 [33], and NH4Cl [34] are also used for the treatment. Furthermore, plasma treatments such as O2 [35], CO2 [36], and N2 [37,38] have also been reported.
Apart from surface morphology modifications, surface functional groups are important for increasing the surface charge density of TENG by increasing the ability to gain or lose electrons [39]. It has been confirmed by many studies that acid/base treatments are able to change surface chemistries when various chemical functional groups are introduced to AC materials [40,41]. For example, single-bond oxygen functional groups were obtained from HCl modification, carbonyl carboxyl and nitrate groups from HNO3, sulfur-containing groups from H2SO4 treatment, and a hydroxyl group from NaOH treatment [40]. In addition to the wet chemical process with acid/base treatments, the surface functional groups of AC materials can be modified by N2 plasma treatment, which introduces not only a micropore structure but also nitrogen functional groups to AC material [38].
In this work, the effects of a surface treatment method on the surface morphology, specific surface area, and surface chemistry of an AC material are investigated. According to the results from a previous study, it was shown that the acid treatments gave better improvement in terms of specific surface area compared to the basic treatment [42]. In this study, we use acid treatments with three different types of acid (HCl, H2SO4, and HNO3), and N2 plasma treatment to modify the surface of AC. These modified AC particles are subsequently used as fillers in NR material, aiming to enhance the energy production of TENG. The correlations of the specific surface area of AC, dielectric properties, and TENG performance of the NR-AC are studied. Furthermore, the applications of the fabricated NR-AC TENG as a power source for small electronic devices are demonstrated. This work has proposed an effective approach to enhance the energy conversion performance of the natural-based TENGs, addressing critical challenges in the development of large-scale renewable energy sources that are environmentally friendly and sustainable.
2. Materials and Methods
2.1. AC Modified with Acid Treatment
A commercial AC powder was purchased from SIGMA-ALDRICH, St. Louis, Missouri, USA. To achieve finer particles, the AC power underwent a 24-h ball milling process before either subsequent acid or plasma treatment. This initial AC material is referred to as AC (ball mill). A total of 37% hydrochloric acid (HCl, RCI Labscan, Bangkok, Thailand), 98% sulphuric acid (H2SO4, ANaPURE, Brightchem Sdn. Bhd., Selangor, Malaysia), and 65% nitric acid (HNO3, ANaPURE, Brightchem Sdn. Bhd., Selangor, Malaysia) were employed in the modification of AC powder. A total of 5 g of AC powder was mixed with a 50 ml acid solution and magnetically stirred for 6 h. Subsequently, the acid-treated products were washed with deionized (DI) water until they reached a pH of 5. The resulting materials were then dried at 150 °C overnight. The specimens were labelled as AC (HCl), AC (H2SO4), and AC (HNO3), corresponding to the types of acid used.
2.2. AC Modified with N2 Plasma Treatment
Nitrogen plasma with a pressure of 1.68 Torr was used to treat AC powders. AC powders were placed in a stainless steel lunchbox and positioned on the powered electrode. Plasmas were generated using an asymmetric bipolar pulse power supply (DC Pinnacle® Plus+, Advanced Energy, Shanghai, China) set at 500 W power, a frequency of 50 kHz, and a duty cycle of 10%. The treatment was sustained for 60 s.
2.3. Preparation of NR–AC Composite Film
The modified AC powder from each treatment, at a concentration of 0.4% (w/v), was added to 20 mL of NR latex obtained from the Thai Rubber Latex Group Public Co., Ltd. (Chonburi, Thailand), with a dry rubber content of 61%. The selection of 0.4% AC addition aligned with the optimum fraction determined in our previous work [11]. The mixtures underwent magnetic stirring for 20 min to obtain homogeneous suspensions. A 2.0 mL portion of the mixture was cast onto a 4 × 4 cm2 ITO substrate to produce a film thickness of approximately 0.7 mm. Three samples were prepared for each experimental condition. The specimens were then allowed to air-dry at room temperature for 1 day before being cured at 60 °C for 6 h. Subsequently, the samples were ready for the TENG performance test.
2.4. Material Characterizations
The morphologies and crystal structures of the modified AC powders and NR-AC composite films were examined using scanning electron microscopy (SEM) (Helios Nanolab, FEI, Waltham, MA, USA) and X-ray diffraction (XRD) (PANalytical EMPYREAN, Malvern, UK), respectively. The specific surface area was analyzed by the Brunauer–Emmett–Teller (BET) analysis, and the pore size distribution was analyzed using the N2-DFT method. Micropore and mesopore volumes were derived from the t-plot (TP) and Barrett–Joyner–Halenda (BJH) methods, respectively. The chemical functionalities of the specimens were characterized via Fourier-transform infrared spectroscopy (FTIR TENSOR27, Bangkok, Thailand).
2.5. TENG Output Measurement
The TENG performance of NR-AC composites was evaluated using a single electrode mode with a contact-separation configuration. A PTFE sheet with a lateral dimension of 4 × 4 cm2 and a thickness of 5 mm was used as a contact material. The separation distance between the triboelectric layer was 8 cm. The generated electrical outputs voltage and current were acquired using an oscilloscope (Tektronix DPO2002B, Tektronix China Ltd., Shanghai, China) with an input impedance of 10 MΩ and a digital ammeter (Keithley DMM6500, Tektronix China Ltd., Shanghai, China), respectively. Data were collected under an applied impact force of 3 N at a frequency of 5 Hz. The delivered power density of TENGs was determined by connecting a series of external load resistances ranging from 0.5 to 10 MΩ.
3. Results
A schematic diagram in Figure 1 shows a summary of the two different treatment methods employed to modify AC powders. SEM images in Figure 2 reveal the microstructures of the modified AC particles: AC (ball mill), AC (HCl), AC (H2SO4), AC (HNO3), and AC (N2 plasma). The particle sizes of the acid-treated and N2-plasma-treated AC specimens are relatively similar, with an average particle size comparable to that of the ball-milled AC (AC (ball mill)), except for HCl acid-treated AC, where the particles aggregated, resulting in a larger particle size.
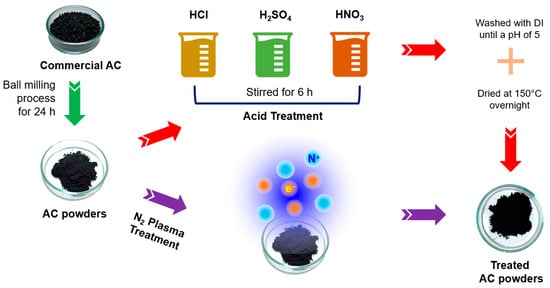
Figure 1.
Schematic diagram of the experiment using two different techniques: acid treatment and N2 plasma treatment to modify the AC powders.
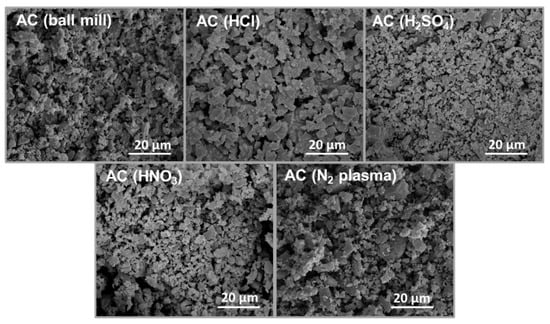
Figure 2.
SEM images of AC (ball mill), AC (HCl), AC (H2SO4), AC (HNO3), and AC (N2 plasma) powders.
The specific surface area, total pore volume, and average pore size were analyzed and displayed in Table 1. It was shown that the modification of AC by acid treatment and plasma treatment did not induce significant changes in surface area and pore structure, except for the AC(HNO3), which exhibited a slightly lower specific surface area and total pore volume. This discrepancy was attributed to the larger pore diameter compared to other samples. However, these alternations in specific surfaces were not found to be significant, consistent with findings from previous studies [40].

Table 1.
Specific surface area, total pore volume, and average pore size of the modified AC particles: AC (ball mill), AC (HCl), AC (H2SO4), AC (HNO3), and AC (N2 plasma).
The Raman spectra of all AC samples, including AC (ball mill) and the modified AC samples, are displayed in Figure 3. Two characteristic peaks of carbon are evident in all specimens at 1335 cm−1 and 1575 cm−1, assigned to the D and G peaks, respectively. These Raman spectra resemble those of activated carbon reported in other previous work [43].
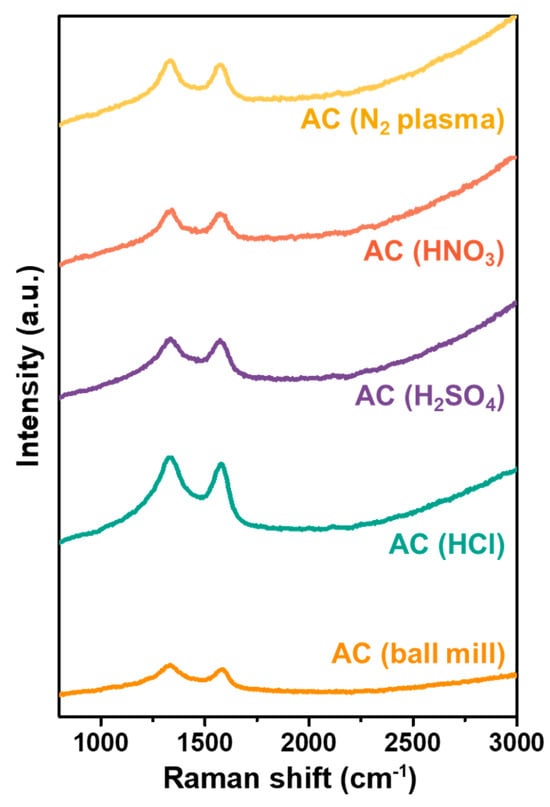
Figure 3.
Raman spectra of the unmodified AC powders (ball mill), and the modified AC including the acid-treated and N2 plasma-treated AC.
The NR and NR-AC composite films coated onto ITO-conductive glass substrates used for fabrication TENG are revealed in Figure 4. The NR composite, incorporating modified AC particles including AC (ball mill), AC (HCl), AC (H2SO4), AC (HNO3), and AC (N2 plasma), maintained a uniform black color characteristic of AC, whereas pristine NR film appeared transparent with light yellow color. All NR-AC composite films exhibit a homogeneous dispersion of AC particles, and the aggregation of AC particles into large clusters was not visible on the film surfaces.

Figure 4.
Digital photographs of pristine NR films (without AC) and NR–AC composite films on ITO substrates including NR-AC (ball mill), NR-AC (HCl), NR-AC (H2SO4), NR-AC (HNO3), and NR-AC (N2 plasma).
The surface morphologies of NR and all NR-AC composite films are presented in Figure 5. AC particles are clearly visible on the surfaces of all NR-AC composite films without significant differences, whereas the surface of NR appears flat with no observed particles. The sizes of AC particles in the composites are consistent with those observed in the SEM images of the AC particles in Figure 2.
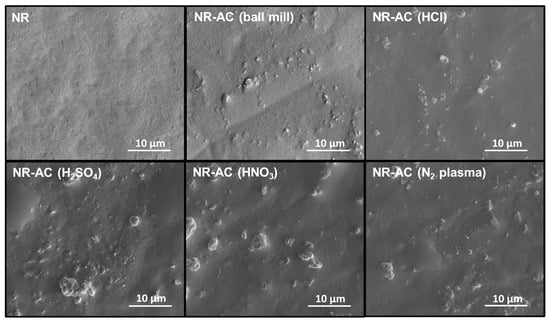
Figure 5.
Surface of pristine NR film, NR-AC (ball mill), NR-AC (HCl), NR-AC (H2SO4), NR-AC (HNO3), and NR-AC (N2 plasma) composite films.
The microstructures of the AC particles and NR-AC composite films were also probed using XRD analysis, as shown in Figure 6. The XRD patterns of all the modified AC particles in Figure 6a exhibited a consistent broad diffraction peak at 2θ~43°, indicating an amorphous carbon structure and the modification did not alter the crystal structure of AC. In the case of NR-AC composite films, broad peaks at 18° were observed in all XRD patterns (Figure 6b), corresponding to diffraction peaks from amorphous NR, consistent with findings from previous studies [44,45]. The diffraction peak from AC at ~43° was not observed in the XRD patterns of the composite films due to the small fraction of AC present in the composite (0.4%).
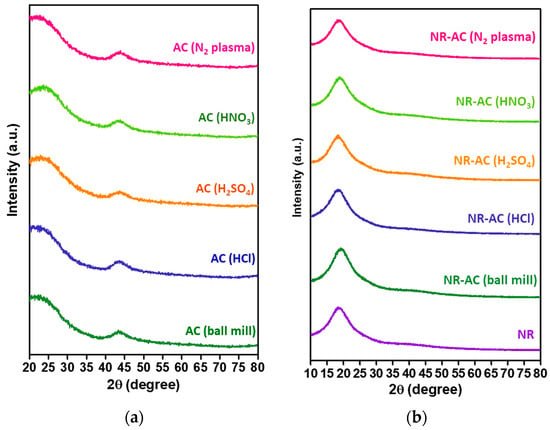
Figure 6.
(a) XRD patterns of the unmodified AC (AC (ball mill) and modified AC particles including AC (HCl), AC (H2SO4), AC (HNO3), and AC (N2 plasma). (b) XRD of all the NR-AC composite films.
The chemical structures of the fabricated composite films were inspected using FTIR analysis. FTIR spectra of pristine NR and NR–AC are presented in Figure 7. The FTIR spectra of pristine NR and NR-AC (ball mill) are similar but differ from those of NR-modified AC composites, which are almost identical. All NR composites show absorption peaks at 840 and 1645 cm−1, assigned to out-of-plane bending vibrations of C–H and C=C stretching of a cis-1,4-polyisoprene molecule of NR, respectively. The peaks at 1375 and 1444 cm−1 are associated with O-H bending vibration from water and C-H bending vibration from the methyl group, respectively. These peaks are more pronounced in the FTIR spectra of NR and NR-AC (ball-mill) composites than those in the NR-modified AC composites, so the multiple peaks at 2850–2920 and 2960 cm−1 correspond to the asymmetric–symmetric stretching vibration of CH2 and C–H in NR molecule, respectively. For all NR composites with modified AC, broad peaks at 3270 cm−1 and 1590 cm−1 are detected, associated with the stretching vibration of O–H from carboxylic acid and C=C from cyclic alkene in AC particles. These peaks are absent in the pristine NR and NR-AC (ball-mill) specimens, suggesting that the modification processes caused a change in the chemical structures of AC particles.
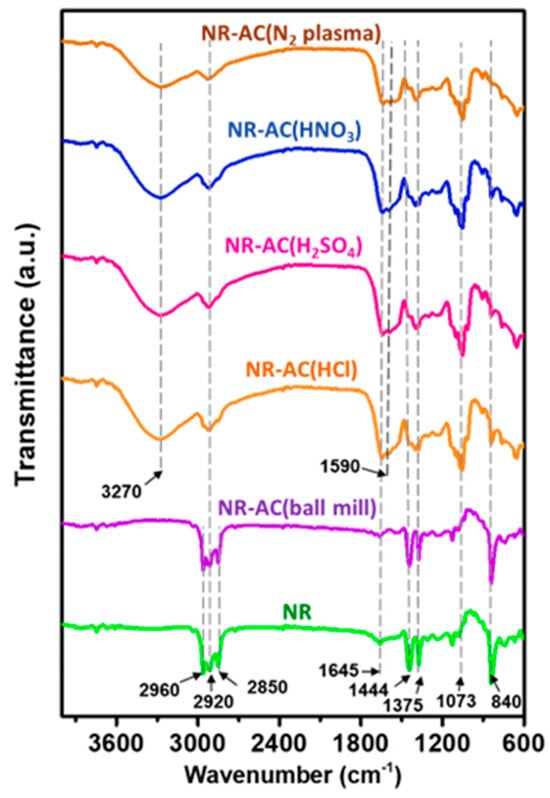
Figure 7.
FTIR spectra of pristine NR film and NR–AC composite films.
The electrical generation performance of the fabricated TENGs was examined using NR-AC films on ITO glasses (Figure 4) as the bottom positive tribo-electrode for TENG and a PTFE sheet as the contact tribo-negative materials. Performance testing was carried out under a consistent impact force of 3 N with a frequency of 5 N. The electricity generation during a contact-separation movement is illustrated in Figure 8. Upon initial contact between the surfaces of the NR-AC film and PTFE, electrons are transferred from the NR-AC composite to PFTE due to the electrification effect, resulting in the formation of positive surface charges on NR-AC and negative surface charges on PTFE surfaces. The subsequent separation of the two materials with opposite surface charges leads to the buildup of electrical potential, inducing free electrons to flow from the ground to the ITO glass, counterbalancing this potential and resulting in the generation of a positive current signal. When the two surfaces are brought back in contact, the potential decreases and disappears, causing electrons to return to the ground, thereby generating a negative current, as illustrated in an inset.
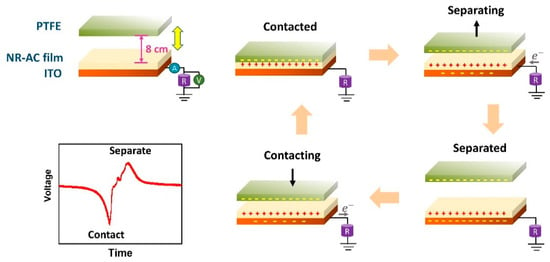
Figure 8.
Schematic diagram presenting the working mechanism of NR composite TENG in a single-electrode mode with the inset of the generated electrical signal.
The measured electrical outputs of the fabricated TENG are presented in Figure 9a,b, representing output voltage and current, respectively. It was seen that the incorporation of untreated (AC ball mill), acid-treated, as well as plasma-treated AC into NR substantially increased the electrical outputs of the TENGs. All acid-treated-AC TENGs exhibited a similar trend in electrical outputs, slightly lower than those of the N2 plasma-treated one. The NR–AC (N2 plasma) TENG showed the highest peak-to-peak voltage (Vpp) of 108 V and peak-to-peak current (Ipp) of 9.8 µA, whereas the NR-AC(H2SO4), NR-AC(HCl) and NR-AC(HNO3) TENGs had Vpp of 97, 95, and 94 V, and Ipp of 8.7, 8.6, and 8.5 µA, respectively. These TENGs exhibited superior energy conversion performance compared to the NR-AC (ball mill) and pristine NR TENGs, as summarized in Table 2 for Vpp and Ipp of all the fabricated TENGs.
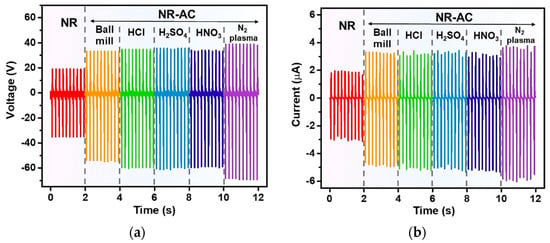
Figure 9.
(a) Output voltage and (b) current of the fabricated TENGs from NR and NR–AC composite films tested under the 3N impact force at a working frequency of 5 Hz.

Table 2.
Electrical output voltage (Vpp) and current (Ipp) of NR and NR–AC TENGs.
To elucidate the effect of AC surface modification on TENG performance, the dielectric constants of the specimens were examined. The dielectric constant indicates charge storing ability of the sample, which can be used to represent the changes in chemical structure resulting from surface modification processes. The plot of dielectric constant versus frequencies at room temperature is presented in Figure 10a. The results reveal that the NR-AC (N2 plasma) composite had the highest dielectric constant, followed by NR-AC(H2SO4), NR-AC(HNO3), NR-AC(HCl), NR-AC (ball mill), and pristine NR, respectively. The dielectric constants exhibited a relatively similar trend to that of the electrical outputs, as presented by the plot correlating TENG output performance, specific surface area, and dielectric constants of all specimens in Figure 10b. Despite the nearly unchanged specific surface areas, the modified AC, including NR-AC (N2 plasma) and all the NR-acid-treated AC TENGs, showed higher electrical outputs than the untreated AC. This suggests that surface modifications did not significantly alter the surface morphologies but led to changes in surface chemistries, which in turn increased the triboelectric charge density, as evidenced by the enhanced electrical output.
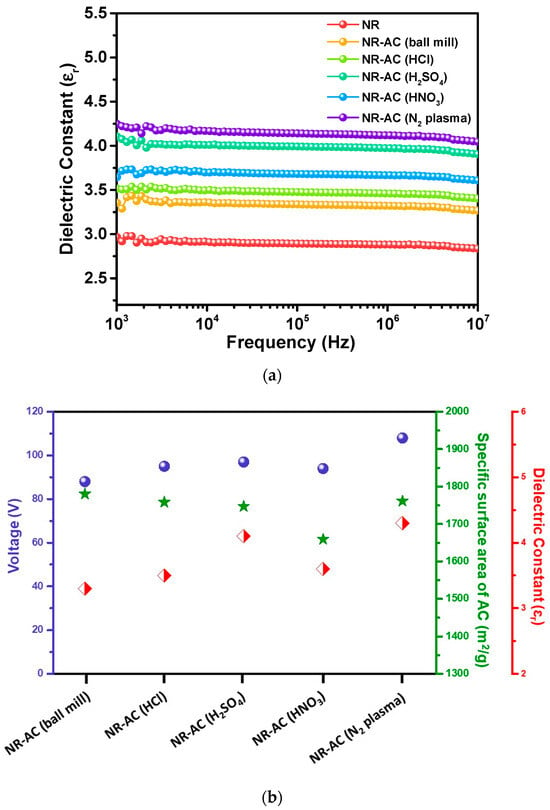
Figure 10.
(a) Dielectric constant of pristine NR and NR-AC composite films at various frequencies measured at room temperature. (b) Plot of TENG electrical voltage output, specific surface area, and dielectric constant of the NR-AC composites.
While FTIR and elemental analysis did not provide substantial information regarding changes in the chemical functional groups and elemental composition resulting from surface treatments, alteration in dielectric constant implies an enhanced capacity to retain triboelectric charges. This enhancement could be attributed to changes in the chemical and electronic properties of the surfaces. This aligns with the findings from previous studies on surface modification involving nitrogen and oxygen functional groups, known to promote the electron-donating ability of triboelectric materials [13,46].
The performance of electrical power generation of the NR-AC (N2 plasma) TENGs was further evaluated in terms of electrical power density. The electrical voltage and current outputs of the TENG were measured across a range of load resistances from 0.5 to 10 MΩ. The plots of the measured output voltage versus current at various load resistances are displayed in Figure 11a. Power density was subsequently calculated using the formula Pd = V × J, where J represents current density (J = I/area). The plot of power density at various load resistances of the NR-AC (N2 plasma) TENGs, compared to those of NR and NR-AC (ball mill) TENGs, is presented in Figure 11b, and their maximum power densities with the corresponding matched load resistances are presented in Table 3. The NR–AC (N2 plasma) TENG exhibited the highest power density of 2.65 W/m2, surpassing those of NR-AC (ball mill) and NR TENGs, which were 1.39 and 0.56 W/m2, respectively. The performance of the NR-AC TENG in this work showed superior output performance compared to many reported natural-based TENGs, as shown in Table 4 [47,48,49,50,51,52,53,54,55,56,57,58].
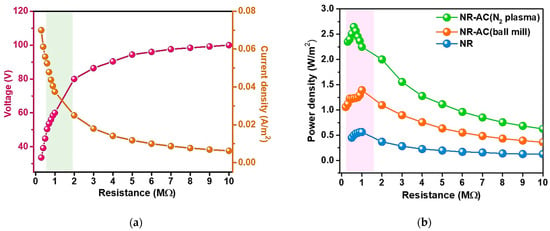
Figure 11.
(a) Dependence of output voltage and current on the connected load resistances of the NR–AC (N2 plasma) TENG and (b) power densities of NR–AC (N2 plasma) TENG compared to those of NR and NR–AC (ball mill) TENGs.

Table 3.
Maximum power density of NR, NR–AC (ball mill), and NR–AC (N2 plasma) TENGs with their corresponding matched loads.
It was also observed that the matched load of the NR-AC (N2 plasma) TENG was lower than those of NR and NR-AC (ball mill) TENGs, suggesting that the NR–AC (N2 plasma) composite had lower internal resistance. This was attributed to the fact that N2 plasma generates electric charges, including free radicals and electrons during the treatment. Typically, three possible events occur on the material surface when treated with N2 plasma; (i) a change in surface roughness with a hierarchical nanostructure, (ii) improved hydrophilic properties, and (iii) the introduction of nitrogen-containing functional groups [59]. In this work, the surface roughness (surface area) did not undergo dramatic changes (Table 1), and nitrogen-containing functional groups were not detected via EDS and FTIR. However, it was possible that the AC powders treated with a high power of bipolar pulse plasma resulted in bond breaking, producing free radicals and electrons [60]. In this regard, N2 plasma is considered a powerful technique for modifying the surface chemistry of AC, leading to the superior electrical output of TENG.

Table 4.
Summary of power output performances of TENGs fabricated from natural-based materials compared to the NR-AC TENG from this study [61].
Table 4.
Summary of power output performances of TENGs fabricated from natural-based materials compared to the NR-AC TENG from this study [61].
| Natural-Material-Based TENGs | Power Density | Applications | References |
|---|---|---|---|
| Natural rubber-activated carbon/PTFE | 2.65 W/m2 | Energy harvesting | This work |
| silk fibroin/rice paper | 21.6 mW/m2 | Power implantation device | [47] |
| Chitosan/Kapton | 2.1 µW/m2 | Energy harvesting | [48] |
| Silk/Si-rubber | 16.6 μW/cm2 | Energy Harvesting | [49] |
| fish gelatin/PTFE-coated PDMS | 45.8 μW/cm2 | Energy Harvesting | [50] |
| cyclo-phenylalanine peptide/PTFE | 73.7 mW/m2 | Energy harvesting | [51] |
| Cellulose/FEP | 14 μW/cm2 | Power electronics device | [52] |
| Ag-doped Cellulose/FEP | 7.68 µW/cm2 | Air filter and Antibacterial patch | [53] |
| BaTiO3-doped bacteria cellulose/PDMS | 4.8 W/m2 | Human motion energy harvesting | [54] |
| Cellulose/Nitrocellulose | 16.1 W/m2 | Paper piano | [55] |
| Cellulose/PTFE | 18.4 W/m2 | Velocity and Force Sensor | [56] |
| Leaf/PVDF | 1.1 mW/cm2 | Energy harvesting | [57] |
| Rice paper/PVC | 37.64 μW/cm2 | Power electronics device | [58] |
The working stability of the NR-AC (N2 plasma TENG) TENG was assessed by measuring the output retention under the impact force of 3 N at 5 Hz frequency, as presented in Figure 12. The NR-AC composite TENG demonstrated consistent output retention over 10,000 cycles or approximately 30 min. This indicates that AC powder possesses a notable ability to retain charges and maintain film deformation, contributing to the overall stability of the NR composite TENG.
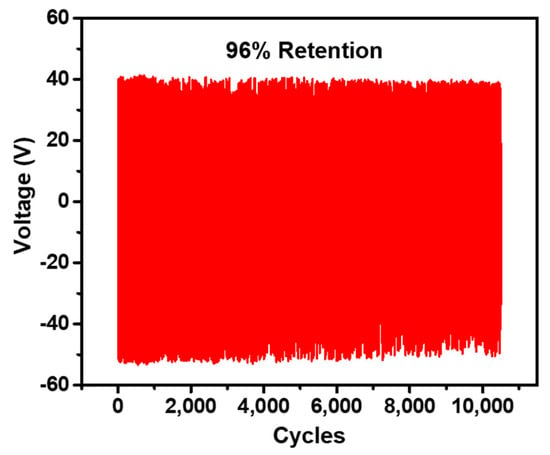
Figure 12.
Electrical output voltage of the NR-AC (N2 plasma) TENG measured over 10,000 cycles.
The applications of the fabricated TENG were demonstrated as a power source, capable of directly powering small electronics such as lighting up LED or charging commercial capacitors, as presented in Figure 13a,b, respectively. A total of 83 commercial green LEDs were successfully illuminated by the instantaneously generated electrical power from the TENG (Figure 13a). The TENG was able to charge commercial capacitors with capacitances of 22, 47, and 100 µF through a bride rectifier, taking 55, 143, and 240 s to reach 1.5 V, respectively (Figure 13b). Furthermore, with the high surface area and good adsorption properties of AC materials, the modified AC embedded in NR film could be further developed as a triboelectric filter membrane for the removal of PM2.5 [62], CO2, and NO2 gases [17]. This presents a significant challenge for future research on TENG.
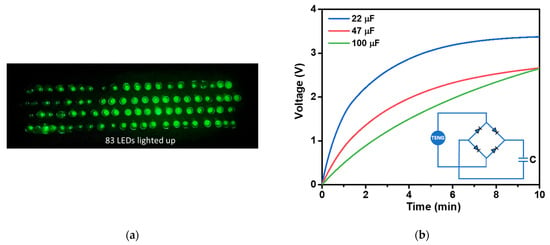
Figure 13.
(a) Demonstration of the NR-AC TENG a power source for illuminating 83 green LEDs. (b) Voltage profiles of 22, 47, and 100 µF capacitors charged by the fabricated NR–AC TENG with a bridge rectifier.
4. Conclusions
The AC modified via acid treatment and N2 plasma treatment were found to enhance the performance of the NR TENG. Despite causing no significant change in specific surface area, all modifications resulted in an increase in dielectric constant, which correlated with TENG performance. The NR composite with AC particles modified by N2 plasma exhibited the highest electrical output. This achievement was attributed to the modified chemical structure caused by the high power of plasma radiation, introducing free radicals and electrons creating dipole formation in the NR composite film. This modification also reduced the internal resistance of the NR-AC composite film compared to both pristine NR and the NR-AC (unmodified). The highest power density of 2.65 W/m2 was achieved from the NR-AC (N2 plasma) TENG, surpassing that of the pristine NR TENG by 4.5 times.
Author Contributions
Conceptualization, V.H.; methodology, P.M., S.K. (Sirima Kongpet), V.H. and A.C.; performing experiments, P.M., S.K. (Sirima Kongpet), W.K. and P.L.; validation, V.H. and P.T.; investigation, P.M., S.K. (Sirima Kongpet). A.C., K.C., S.K. (Suninad Kaewnisai) and V.H.; writing—original draft preparation, V.H.; writing—review and editing, V.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Council of Thailand (NRCT) (Grant No. N41A640143); the Research and Graduate Studies, Khon Kaen University; the Fundamental Fund of Khon Kaen University, National Science, Research and Innovation Fund (NSRF); the Basic Research Fund, Khon Kaen University; the NSRF via the Program Management Unit for Human Resources and Institutional Development, Research and Innovation (grant number B40G660038).
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research was supported by the Fundamental Fund of Khon Kaen University, National Science, Research and Innovation Fund (NSRF); the National Research Council of Thailand (NRCT) (Grant No. N41A640143); the Research and Graduate Studies, Khon Kaen University; the NSRF via the Program Management Unit for Human Resources and Institutional Development, Research and Innovation (grant number B40G660038).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, G.; Peng, B.; Chen, J.; Jing, Q.; Wang, Z.L. Triboelectric nanogenerators as a new energy technology: From fundamentals, devices, to applications. Nano Energy 2015, 14, 126–138. [Google Scholar] [CrossRef]
- Wang, S.; Lin, L.; Wang, Z.L. Triboelectric nanogenerators as self-powered active sensors. Nano Energy 2015, 11, 436–462. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.L. Recent progress of triboelectric nanogenerators: From fundamental theory to practical applications. EcoMat 2020, 2, e12059. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, N.; Liu, J.; Wen, Z.; Sun, X.; Lee, S.-T.; Sun, B. Integrating a Silicon Solar Cell with a Triboelectric Nanogenerator via a Mutual Electrode for Harvesting Energy from Sunlight and Raindrops. ACS Nano 2018, 12, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Han, C.B.; He, C.; Gu, G.Q.; Nie, J.H.; Shao, J.J.; Xiao, T.X.; Deng, C.R.; Wang, Z.L. Washable Multilayer Triboelectric Air Filter for Efficient Particulate Matter PM2.5 Removal. Adv. Funct. Mater. 2018, 28, 1706680. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tarachiwin, L. Recent Advances in Structural Characterization of Natural Rubber. Rubber Chem. Technol. 2009, 82, 283–314. [Google Scholar] [CrossRef]
- Appamato, I.; Bunriw, W.; Harnchana, V.; Siriwong, C.; Mongkolthanaruk, W.; Thongbai, P.; Chanthad, C.; Chompoosor, A.; Ruangchai, S.; Prada, T.; et al. Engineering Triboelectric Charge in Natural Rubber–Ag Nanocomposite for Enhancing Electrical Output of a Triboelectric Nanogenerator. ACS Appl. Mater. Interfaces 2023, 15, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Suphasorn, P.; Appamato, I.; Harnchana, V.; Thongbai, P.; Chanthad, C.; Siriwong, C.; Amornkitbamrung, V. Ag Nanoparticle-Incorporated Natural Rubber for Mechanical Energy Harvesting Application. Molecules 2021, 26, 2720. [Google Scholar] [CrossRef]
- Bunriw, W.; Harnchana, V.; Chanthad, C.; Huynh, V.N. Natural Rubber-TiO2 Nanocomposite Film for Triboelectric Nanogenerator Application. Polymers 2021, 13, 2213. [Google Scholar] [CrossRef]
- Chomjun, T.; Appamato, I.; Harnchana, V.; Amornkitbamrung, V. Eco-Friendly Triboelectric Material Based on Natural Rubber and Activated Carbon from Human Hair. Polymers 2022, 14, 1110. [Google Scholar] [CrossRef]
- Mekbuntoon, P.; Kaeochana, W.; Prada, T.; Appamato, I.; Harnchana, V. Power Output Enhancement of Natural Rubber Based Triboelectric Nanogenerator with Cellulose Nanofibers and Activated Carbon. Polymers 2022, 14, 4495. [Google Scholar] [CrossRef]
- Du, G.; Wang, J.; Liu, Y.; Yuan, J.; Liu, T.; Cai, C.; Luo, B.; Zhu, S.; Wei, Z.; Wang, S.; et al. Fabrication of Advanced Cellulosic Triboelectric Materials via Dielectric Modulation. Adv. Sci. 2023, 10, 2206243. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, W.; Xu, J.; Chen, J. Engineering Materials at the Nanoscale for Triboelectric Nanogenerators. Cell Rep. Phys. Sci. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Jang, S.-H.; Shchegolkov, A.V.; Rodionov, Y.V.; Glivenkova, O.A. Multistage Mechanical Activation of Multilayer Carbon Nanotubes in Creation of Electric Heaters with Self-Regulating Temperature. Materials 2021, 14, 4654. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High Hydrogen Storage Capacity of Porous Carbons Prepared by Using Activated Carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.S. Characterization and performance evaluation of a full-scale activated carbon-based dynamic botanical air filtration system for improving indoor air quality. Build. Environ. 2011, 46, 758–768. [Google Scholar] [CrossRef]
- Acevedo, S.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of CO2 on Activated Carbons Prepared by Chemical Activation with Cupric Nitrate. ACS Omega 2020, 5, 10423–10432. [Google Scholar] [CrossRef]
- Pereira, M.C.; Coelho, F.S.; Nascentes, C.C.; Fabris, J.D.; Araújo, M.H.; Sapag, K.; Oliveira, L.C.A.; Lago, R.M. Use of activated carbon as a reactive support to produce highly active-regenerable Fe-based reduction system for environmental remediation. Chemosphere 2010, 81, 7–12. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.L.; Simon, P.; Fauvarque, J.F.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Ho, S.; Muhammad, A.; Abid, R.; Umme, L.; Rida, Z. Uses of activated carbon in medicine area: Short review. EPRA Int. J. Res. Dev. (IJRD) 2022, 7, 34–39. [Google Scholar]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Mishra, S.; Supraja, P.; Haranath, D.; Kumar, R.R.; Pola, S. Effect of surface and contact points modification on the output performance of triboelectric nanogenerator. Nano Energy 2022, 104, 107964. [Google Scholar] [CrossRef]
- Niu, S.; Wang, S.; Lin, L.; Liu, Y.; Zhou, Y.S.; Hu, Y.; Wang, Z.L. Theoretical study of contact-mode triboelectric nanogenerators as an effective power source. Energy Environ. Sci. 2013, 6, 3576–3583. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Effects of acidic treatment of activated carbons on dye adsorption. Dye. Pigment. 2007, 75, 306–314. [Google Scholar] [CrossRef]
- Rahman, A.; Hango, H.J.; Daniel, L.S.; Uahengo, V.; Jaime, S.J.; Bhaskaruni, S.V.H.S.; Jonnalagadda, S.B. Chemical preparation of activated carbon from Acacia erioloba seed pods using H2SO4 as impregnating agent for water treatment: An environmentally benevolent approach. J. Clean. Prod. 2019, 237, 117689. [Google Scholar] [CrossRef]
- Ternero-Hidalgo, J.J.; Rosas, J.M.; Palomo, J.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Functionalization of activated carbons by HNO3 treatment: Influence of phosphorus surface groups. Carbon 2016, 101, 409–419. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hwang, S.Y.; Park, J.E.; Lee, G.B.; Kim, H.; Kim, S.; Hong, B.U. Impact of the oxygen functional group of nitric acid-treated activated carbon on KOH activation reaction. Carbon Lett. 2019, 29, 281–287. [Google Scholar] [CrossRef]
- Shim, J.-W.; Park, S.-J.; Ryu, S.-K. Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers. Carbon 2001, 39, 1635–1642. [Google Scholar] [CrossRef]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface modification of activated carbons for CO2 capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Hamdi, N.; Kriaa, A.; Srasra, E. Adsorption of methyl orange using activated carbon prepared from lignin by ZnCl2 treatment. Russ. J. Phys. Chem. A 2012, 86, 1294–1300. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, G.; Xu, Y.; Zhao, P. One step N-doping and activation of biomass carbon at low temperature through NaNH2: An effective approach to CO2 adsorbents. J. CO2 Util. 2019, 33, 320–329. [Google Scholar] [CrossRef]
- Moussavi, G.; Alahabadi, A.; Yaghmaeian, K.; Eskandari, M. Preparation, characterization and adsorption potential of the NH4Cl-induced activated carbon for the removal of amoxicillin antibiotic from water. Chem. Eng. J. 2013, 217, 119–128. [Google Scholar] [CrossRef]
- García, A.B.; Martínez-Alonso, A.; Leon y Leon, C.A.; Tascón, J.M.D. Modification of the surface properties of an activated carbon by oxygen plasma treatment. Fuel 1998, 77, 613–624. [Google Scholar] [CrossRef]
- Şahin, Ö.; Yardim, Y.; Baytar, O.; Saka, C. Enhanced electrochemical double-layer capacitive performance with CO2 plasma treatment on activated carbon prepared from pyrolysis of pistachio shells. Int. J. Hydrogen Energy 2020, 45, 8843–8852. [Google Scholar] [CrossRef]
- Bai, B.C.; Lee, H.-U.; Lee, C.W.; Lee, Y.-S.; Im, J.S. N2 plasma treatment on activated carbon fibers for toxic gas removal: Mechanism study by electrochemical investigation. Chem. Eng. J. 2016, 306, 260–268. [Google Scholar] [CrossRef]
- Lim, C.; Kwak, C.H.; Jeong, S.G.; Kim, D.; Lee, Y.-S. Enhanced CO2 adsorption of activated carbon with simultaneous surface etching and functionalization by nitrogen plasma treatment. Carbon Lett. 2023, 33, 139–145. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Q.; Mo, J.; Lu, Y.; Cai, C.; Luo, B.; Nie, S. Chemically tailored molecular surface modification of cellulose nanofibrils for manipulating the charge density of triboelectric nanogenerators. Nano Energy 2021, 89, 106369. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S. Acid/Base-Treated Activated Carbons: Characterization of Functional Groups and Metal Adsorptive Properties. Langmuir 2004, 20, 2233–2242. [Google Scholar] [CrossRef]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Silva, A.M.T.; Faria, J.L. Activated carbons treated with sulphuric acid: Catalysts for catalytic wet peroxide oxidation. Catal. Today 2010, 151, 153–158. [Google Scholar] [CrossRef]
- Saleh, T.A.; Danmaliki, G.I. Influence of acidic and basic treatments of activated carbon derived from waste rubber tires on adsorptive desulfurization of thiophenes. J. Taiwan Inst. Chem. Eng. 2016, 60, 460–468. [Google Scholar] [CrossRef]
- Lay, M.; Rusli, A.; Abdullah, M.K.; Abdul Hamid, Z.A.; Shuib, R.K. Converting dead leaf biomass into activated carbon as a potential replacement for carbon black filler in rubber composites. Compos. Part B Eng. 2020, 201, 108366. [Google Scholar] [CrossRef]
- Johns, J.; Rao, V. Characterization of Natural Rubber Latex/Chitosan Blends. Int. J. Polym. Anal. Charact. 2008, 13, 280–291. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Phudkrachang, P.; Mulsow, L.-o.; Kunchornsup, W.; Sirivat, A. Removal of water extractable proteins from concentrated natural rubber latex by eggshells. J. Elastomers Plast. 2012, 45, 253–269. [Google Scholar] [CrossRef]
- Pace, G.; Serri, M.; Castillo, A.E.d.R.; Ansaldo, A.; Lauciello, S.; Prato, M.; Pasquale, L.; Luxa, J.; Mazánek, V.; Sofer, Z.; et al. Nitrogen-doped graphene based triboelectric nanogenerators. Nano Energy 2021, 87, 106173. [Google Scholar] [CrossRef]
- Jiang, W.; Li, H.; Liu, Z.; Li, Z.; Tian, J.; Shi, B.; Zou, Y.; Ouyang, H.; Zhao, C.; Zhao, L.; et al. Fully Bioabsorbable Natural-Materials-Based Triboelectric Nanogenerators. Adv. Mater. 2018, 30, 1801895. [Google Scholar] [CrossRef]
- Wang, R.; Gao, S.; Yang, Z.; Li, Y.; Chen, W.; Wu, B.; Wu, W. Engineered and Laser-Processed Chitosan Biopolymers for Sustainable and Biodegradable Triboelectric Power Generation. Adv. Mater. 2018, 30, 1706267. [Google Scholar] [CrossRef]
- Choi, A.Y.; Lee, C.J.; Park, J.; Kim, D.; Kim, Y.T. Corrugated Textile based Triboelectric Generator for Wearable Energy Harvesting. Sci. Rep. 2017, 7, 45583. [Google Scholar] [CrossRef]
- Han, Y.; Han, Y.; Zhang, X.; Li, L.; Zhang, C.; Liu, J.; Lu, G.; Yu, H.-D.; Huang, W. Fish Gelatin Based Triboelectric Nanogenerator for Harvesting Biomechanical Energy and Self-Powered Sensing of Human Physiological Signals. ACS Appl. Mater. Interfaces 2020, 12, 16442–16450. [Google Scholar] [CrossRef]
- Park, I.W.; Choi, J.; Kim, K.Y.; Jeong, J.; Gwak, D.; Lee, Y.; Ahn, Y.H.; Choi, Y.J.; Hong, Y.J.; Chung, W.-J.; et al. Vertically aligned cyclo-phenylalanine peptide nanowire-based high-performance triboelectric energy generator. Nano Energy 2019, 57, 737–745. [Google Scholar] [CrossRef]
- Yao, C.; Hernandez, A.; Yu, Y.; Cai, Z.; Wang, X. Triboelectric nanogenerators and power-boards from cellulose nanofibrils and recycled materials. Nano Energy 2016, 30, 103–108. [Google Scholar] [CrossRef]
- He, X.; Zou, H.; Geng, Z.; Wang, X.; Ding, W.; Hu, F.; Zi, Y.; Xu, C.; Zhang, S.L.; Yu, H.; et al. A Hierarchically Nanostructured Cellulose Fiber-Based Triboelectric Nanogenerator for Self-Powered Healthcare Products. Adv. Funct. Mater. 2018, 28, 1805540. [Google Scholar] [CrossRef]
- Shao, Y.; Feng, C.-p.; Deng, B.-w.; Yin, B.; Yang, M.-b. Facile method to enhance output performance of bacterial cellulose nanofiber based triboelectric nanogenerator by controlling micro-nano structure and dielectric constant. Nano Energy 2019, 62, 620–627. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, J.; Xu, F.; Gong, S. Crepe cellulose paper and nitrocellulose membrane-based triboelectric nanogenerators for energy harvesting and self-powered human-machine interaction. Nano Energy 2019, 61, 69–77. [Google Scholar] [CrossRef]
- Xia, K.; Du, C.; Zhu, Z.; Wang, R.; Zhang, H.; Xu, Z. Sliding-mode triboelectric nanogenerator based on paper and as a self-powered velocity and force sensor. Appl. Mater. Today 2018, 13, 190–197. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Zheng, Y.; Wang, D.; Zhou, F.; Liu, W. Leaves based triboelectric nanogenerator (TENG) and TENG tree for wind energy harvesting. Nano Energy 2019, 55, 260–268. [Google Scholar] [CrossRef]
- Chi, Y.; Xia, K.; Zhu, Z.; Fu, J.; Zhang, H.; Du, C.; Xu, Z. Rice paper-based biodegradable triboelectric nanogenerator. Microelectron. Eng. 2019, 216, 111059. [Google Scholar] [CrossRef]
- Akdoğan, E.; Şirin, H.T. Plasma surface modification strategies for the preparation of antibacterial biomaterials: A review of the recent literature. Mater. Sci. Eng. C 2021, 131, 112474. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Cheng, J.-H.; Sun, D.-W. Effects of plasma chemistry on the interfacial performance of protein and polysaccharide in emulsion. Trends Food Sci. Technol. 2020, 98, 129–139. [Google Scholar] [CrossRef]
- Chao, S.; Ouyang, H.; Jiang, D.; Fan, Y.; Li, Z. Triboelectric nanogenerator based on degradable materials. EcoMat 2021, 3, e12072. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, Z.; Wei, F.; Yang, X. Carbon nanotubes/activated carbon fiber based air filter media for simultaneous removal of particulate matter and ozone. Build. Environ. 2017, 125, 60–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).