Effect of Glycerol Concentrations on the Characteristics of Cellulose Films from Cattail (Typha angustifolia L.) Flowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
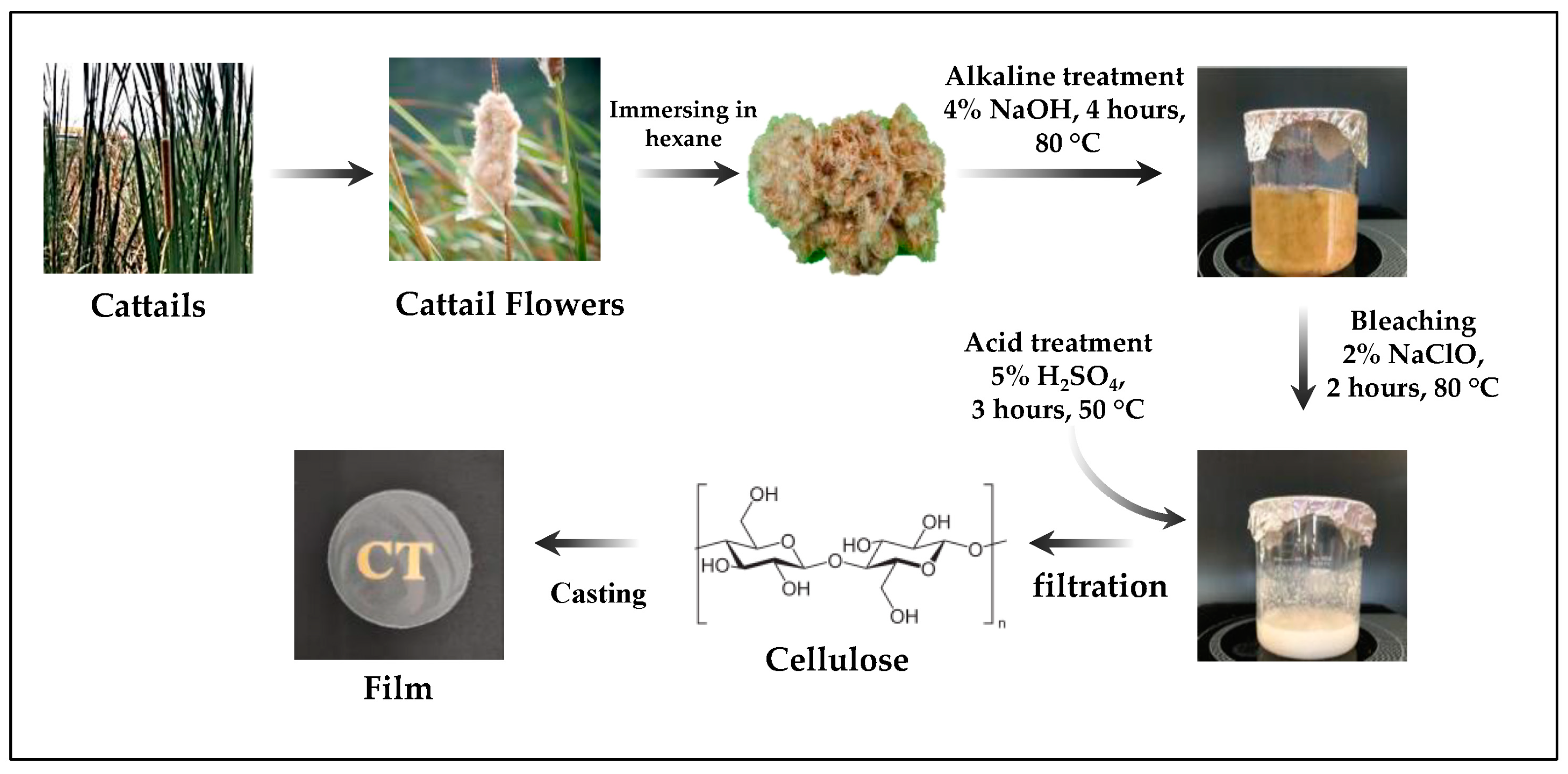
2.2. Cellulose Extraction from Cattail Flowers
2.3. Preparation of Cellulose Films from Cattail Flowers
2.4. Analysis of Film Characteristics
2.4.1. Transparency of Films
2.4.2. Morphology Observation
2.4.3. Functional Group Analysis
2.4.4. X-ray Diffraction Analysis
2.4.5. Thermal Behavior Determination
2.4.6. Hydrophilic Properties of CTC Films
3. Results and Discussion
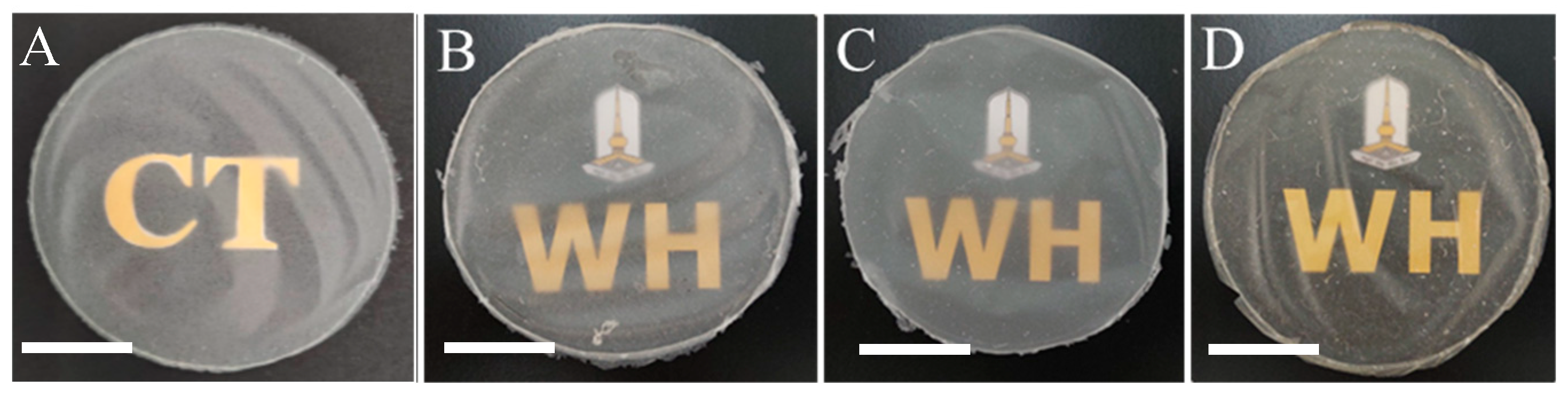
3.1. Appearance of CTC Films
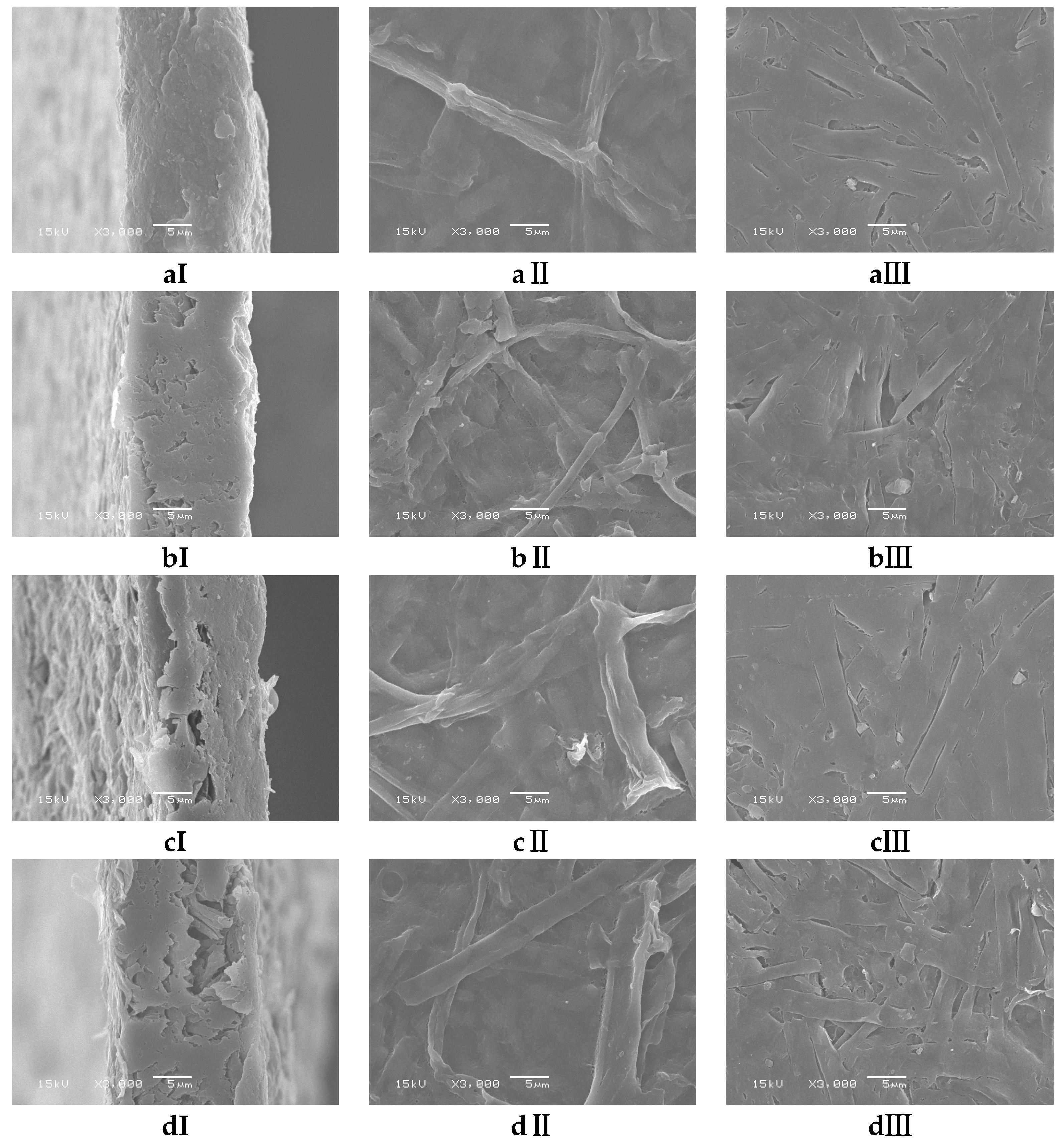
3.2. Morphological Determination
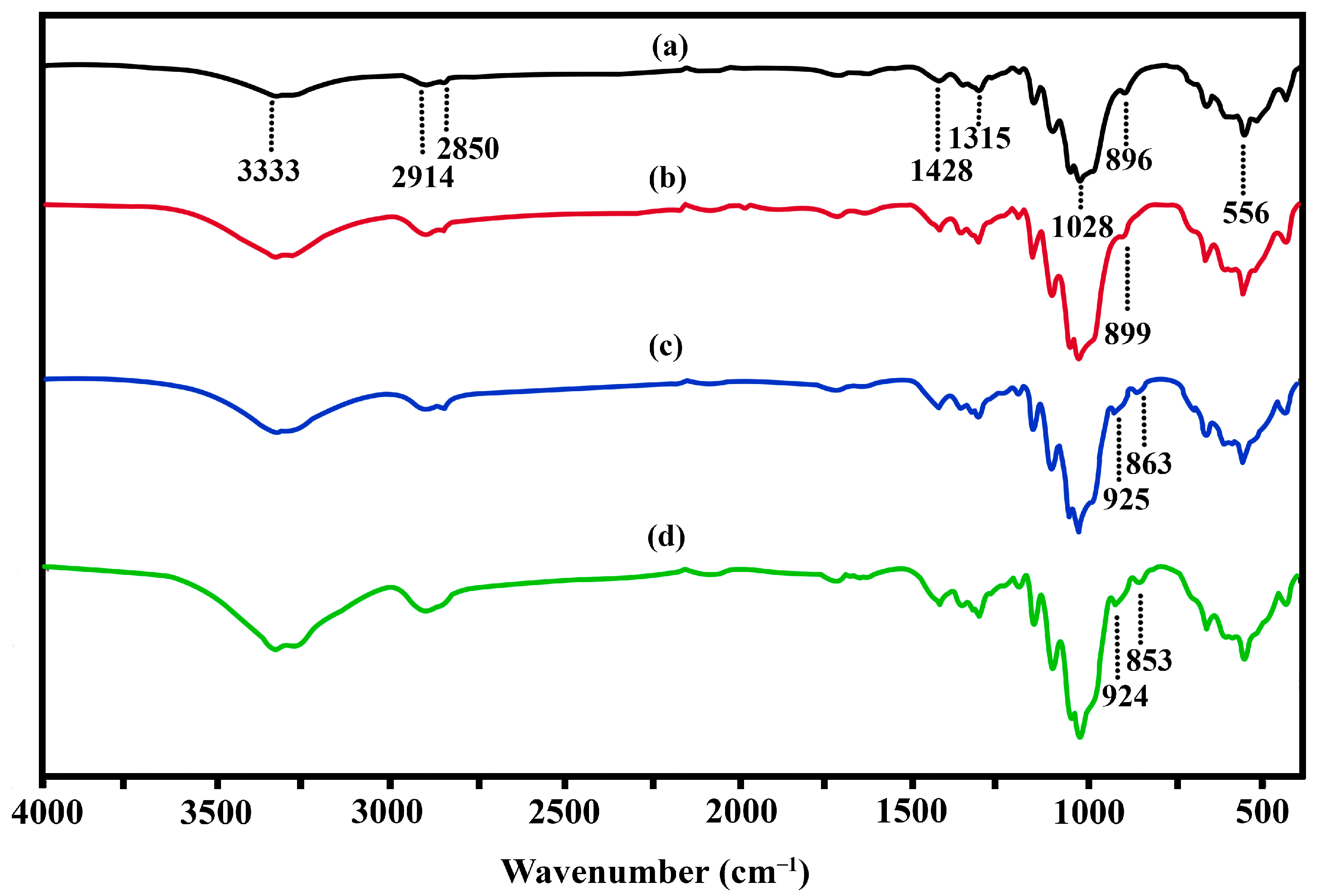
3.3. Functional Group Analysis
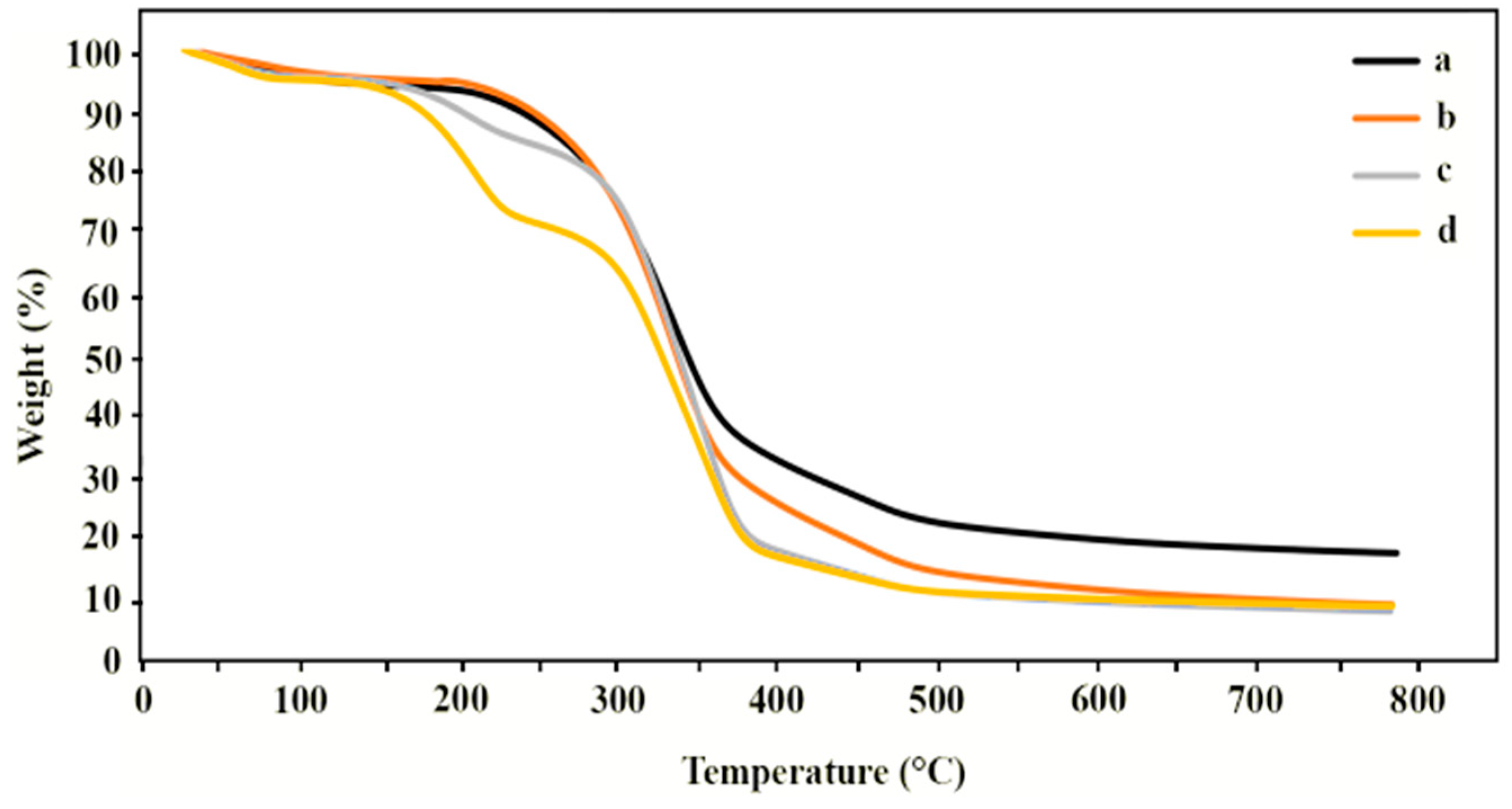
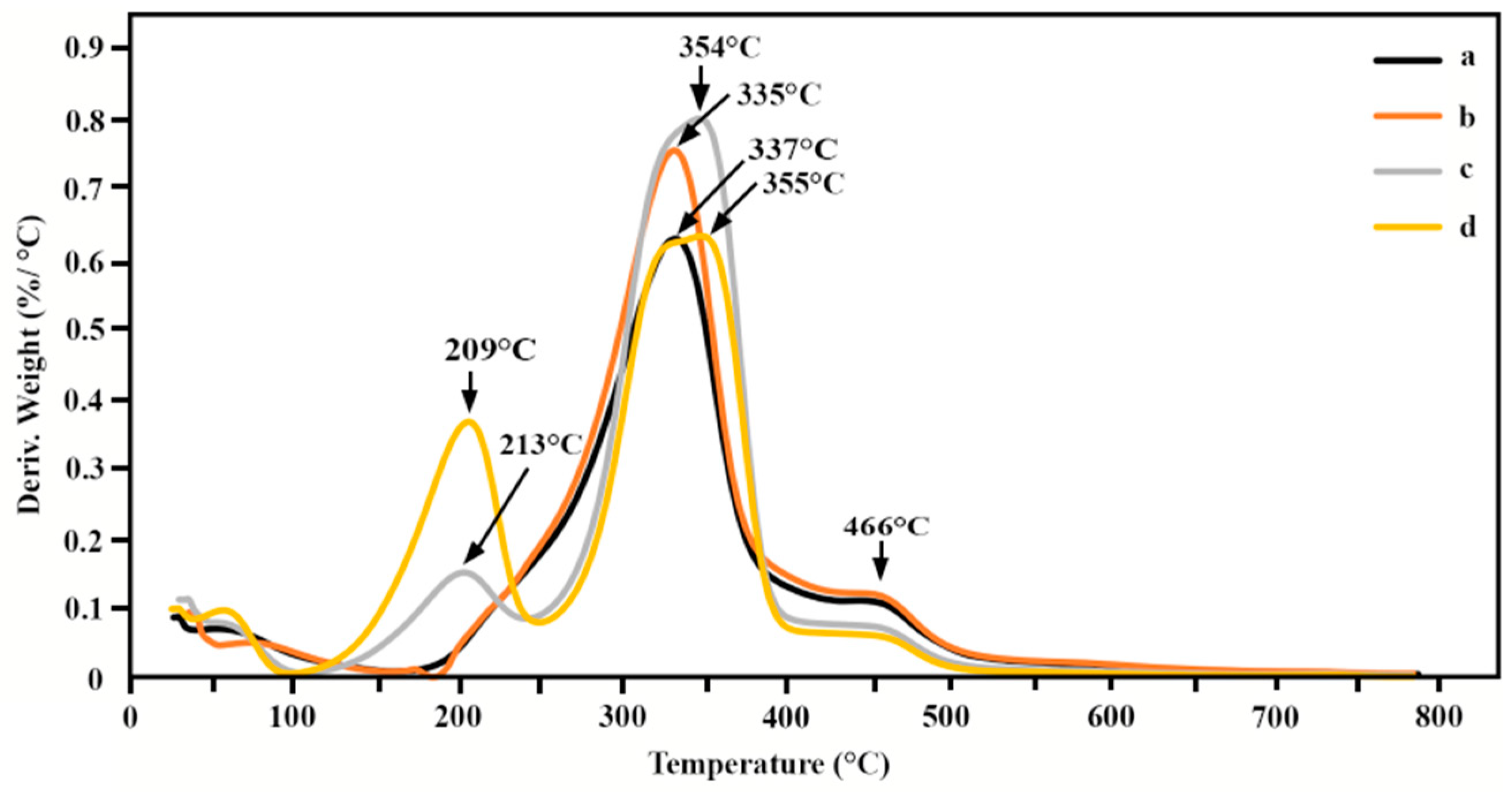
3.4. Determination of Thermal Properties
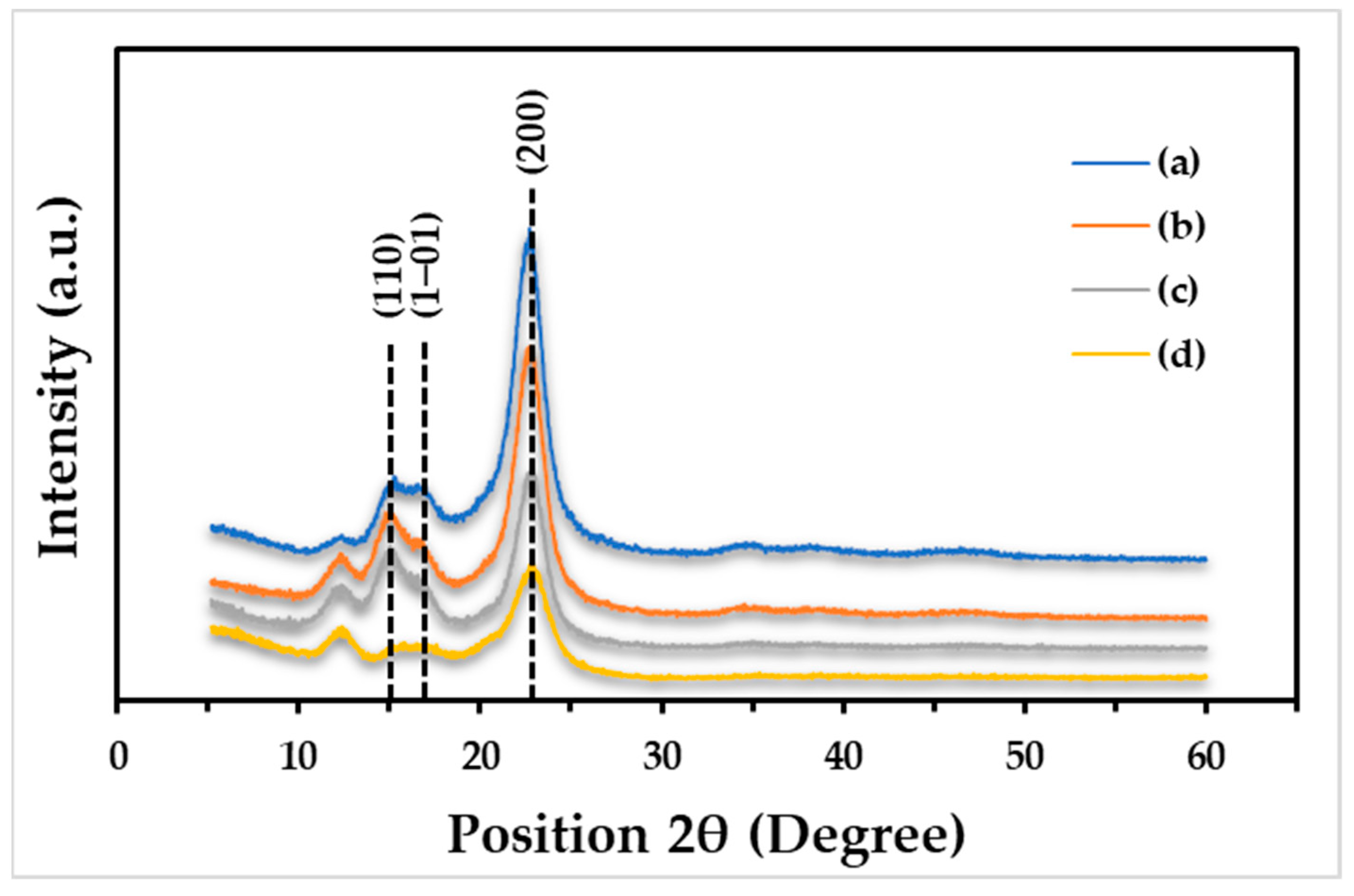
3.5. Crystallinity of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic Waste and Its Management Strategies for Environmental Sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Gourmelon, G. Global Plastic Production Rises, Recycling Lags. Vital Signs 2015, 22, 91–95. [Google Scholar]
- Haque, S.; Islam, S. Effectiveness of Waste Plastic Bottles as Construction Material in Rohingya Displacement Camps. Clean. Eng. Technol. 2021, 3, 100110. [Google Scholar] [CrossRef]
- Nayak, S.; Khuntia, S.K. Development and Study of Properties of Moringa oleifera Fruit Fibers/Polyethylene Terephthalate Composites for Packaging Applications. Compos. Commun. 2019, 15, 113–119. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; Matas, M.de; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-Based Biomaterials for Accelerated Diabetic Wound Healing: A Critical Review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic Pollution, a Threat to Marine Ecosystem and Human Health: A Short Review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.; Thornhill, S.G.; Shelton, S.; Kumar, M. Keratin-Based Biofilms, Hydrogels, and Biofibers. In Keratin as a Protein Biopolymer; Springer: Cham, Switzerland, 2019; pp. 187–200. [Google Scholar]
- Alashwal, B.Y.; Bala, M.S.; Gupta, A.; Sharma, S.; Mishra, P. Improved Properties of Keratin-Based Bioplastic Film Blended with Microcrystalline Cellulose: A Comparative Analysis. J. King Saud. Univ. Sci. 2020, 32, 853–857. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Nilsson, K.; Trifol, J.; Langton, M.; Gomez-Caturla, J.; Balart, R.; Garcia-Garcia, D.; Morina, R. Faba Bean Protein Films Reinforced with Cellulose Nanocrystals as Edible Food Packaging Material. Food Hydrocoll. 2021, 121, 107019. [Google Scholar] [CrossRef]
- Xie, X.; Liu, L.; Zhang, L.; Lu, A. Strong Cellulose Hydrogel as Underwater Superoleophobic Coating for Efficient Oil/Water Separation. Carbohydr. Polym. 2020, 229, 115467. [Google Scholar] [CrossRef]
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Comparison of Cellulose Nanowhiskers Extracted from Industrial Bio-residue and Commercial Microcrystalline Cellulose. Mater. Lett. 2012, 71, 28–31. [Google Scholar] [CrossRef]
- Khan, A.; Huq, T.; Khan, R.A.; Riedl, B.; Lacroix, M. Nanocellulose Based Composites and Bioactive Agents for Food Packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 163174. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Lee, K.; Jeon, Y.; Kim, D.; Kwon, G.; Kim, U.-J.; Hong, C.; Choung, J.W.; You, J. Double-Crosslinked Cellulose Nanofiber-Based Bioplastic Films for Practical Applications. Carbohydr. Polym. 2021, 260, 117817. [Google Scholar] [CrossRef] [PubMed]
- Filippo, M.F.D.; Dolci, L.S.; Liccardo, L.; Bigi, A.; Bonvicini, F.; Gentilomi, G.A.; Passerini, N.; Panzavolta, S.; Albertini, B. Cellulose Derivatives-Snail Slime Films: New Disposable Eco-Friendly Materials for Food Packaging. Food Hydrocoll. 2021, 111, 106247. [Google Scholar] [CrossRef]
- Cazón, P.; Velázquez, G.; Vázquez, M. Bacterial Cellulose Films: Evaluation of the Water Interaction. Food Packag. Shelf Life 2020, 25, 100526. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Ma, X.; Huang, L.; Ni, Y.; Chen, L.; Ouyang, X.; Li, J. Conductive Regenerated Cellulose Film and Its Electronic Devices- A Review. Carbohydr. Polym. 2020, 250, 116969. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y. Optimization of Bleaching Process for Cellulose Extraction from Apple and Kale Pomace and Evaluation of Their Potentials as Film Forming Materials. Carbohydr. Polym. 2021, 253, 117225. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Ide, Y.; Kim, J.H.; Hossain, M.S.A.; Bando, Y.; Yamauchi, Y.; Wu, K.C.-W. 3D Network of Cellulose-Based Energy Storage Devices and Related Emerging Applications. Mater. Horiz. 2017, 4, 522–545. [Google Scholar] [CrossRef]
- Kwon, G.; Lee, K.; Kim, D.; Jeon, Y.; Kim, U.-J.; You, J. Cellulose Nanocrystalcoated TEMPO-Oxidized Cellulose Nanofiber Films for High Performance All-Cellulose Nanocomposites. J. Hazard Mater. 2020, 398, 123100. [Google Scholar] [CrossRef]
- Rajeswari, A.; Jackcina Stobel Christy, E.; Swathi, E.; Pius, A. Fabrication of Improved Cellulose Acetate-Based Biodegradable Films for Food Packaging Applications. J. Environ. Chem. Ecotoxicol. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Baghaei, B.; Mikael Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.W.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Yu, Z.; Dhital, R.; Wang, W.; Sun, L.; Zeng, W.; Mustapha, A.; Lin, M. Development of Multifunctional Nanocomposites Containing Cellulose Nanofibrils and Soy Proteins as Food Packaging Materials. Food Packag. Shelf Life 2019, 21, 100366. [Google Scholar] [CrossRef]
- Jiang, Z.; Ho, S.-H.; Wang, X.; Li, Y.; Wang, C. Application of Biodegradable Cellulose-Based Biomass Materials in Wastewater Treatment. Environ. Pollut. 2021, 290, 118087. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Tang, Y.; Wang, X.; Zhu, P.; Chen, T.; Zhou, Y. Preparation of Polyaniline/Cellulose Nanocrystal Composite and Its Application in Surface Coating of Cellulosic Paper. Prog. Org. Coat. 2021, 159, 106452. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose Nanocomposites: Fabrication and Biomedical Applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Song, G.-G.; Yang, J.; Liu, K.-X.; Qin, Z.; Zheng, X.-C. Cattail Fiber-Derived Hierarchical Porous Carbon Materials for High-Performance Supercapacitors. Diam. Relat. Mater. 2021, 111, 108162. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, Z.; Xu, T.; Qu, R.; Yuan, J.; Cai, F.; Wang, Y.; Wang, X. An Environmentally Friendly Deproteinization and Decolorization Method for Polysaccharides of Typha angustifolia Based on a Metal Ion-Chelating Resin Adsorption. Ind. Crops Prod. 2019, 134, 160–167. [Google Scholar] [CrossRef]
- Cui, F.; Li, H.; Chen, C.; Wang, Z.; Liu, X.; Jiang, G.; Cheng, T.; Bai, R.; Song, L. Cattail Fibers as Source of Cellulose to Prepare a Novel Type of Composite Aerogel Adsorbent for the Removal of Enrofloxacin in Wastewater. Int. J. Biol. Macromol. 2021, 191, 171–181. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, J.; Jiang, L.; Zhu, Z.; He, S.; He, S.; Shao, W. Development of Intelligent/Active Food Packaging Film Based on TEMPO-Oxidized Bacterial Cellulose Containing Thymol and Anthocyanin-Rich Purple Potato Extract for Shelf Life Extension of Shrimp. Food Packag. Shelf Life 2021, 29, 100709. [Google Scholar] [CrossRef]
- Rebaque, D.; Martínez-Rubio, R.; Fornalé, S.; García-Angulo, P.; Alonso-Simón, A.; Álvarez, J.M.; Caparros-Ruiz, D.; Acebes, J.L.; Encina, A. Characterization of Structural Cell Wall Polysaccharides in Cattail (Typha latifolia): Evaluation as Potential Biofuel Feedstock. Carbohydr. Polym. 2017, 175, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in Bio-Based Plastics and Plasticizing Modifications. J. Mater. Chem. 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Behera, L.; Mohanta, M.; Thirugnanam, A. Intensification of Yam-Starch Based Biodegradable Bioplastic Film with Bentonite for Food Packaging Application. Environ. Technol. Innov. 2022, 25, 102180. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Water and Glycerol as Plasticizers Affect Mechanical and Water Vapor Barrier Properties of An Edible Wheat Gluten Film. J. Food. Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Pouplin, M.; Redl, A.; Gontard, N. Glass Transition of Wheat Gluten Plasticized with Water, Glycerol, or Sorbitol. J. Agric. Food Chem. 1999, 47, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Nephomnyshy, I.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The Development of a Direct Approach to Formulate High Oil Content Zein-Based Emulsion Gels Using Moderate Temperatures. Food Hydrocoll. 2020, 101, 105528. [Google Scholar] [CrossRef]
- Tyagi, V.; Bhattacharya, B. Role of Plasticizers in Bioplastics. MOJ Food Process. Technol. 2019, 7, 128–130. [Google Scholar] [CrossRef]
- Özeren, H.D.; Wei, X.-F.; Nilsson, F.; Olsson, R.T.; Hedenqvist, M.S. Role of Hydrogen Bonding in Wheat Gluten Protein Systems Plasticized with Glycerol and Water. Polymer 2021, 232, 124149. [Google Scholar] [CrossRef]
- Gao, C.; Stading, M.; Wellner, N.; Parker, M.L.; Noel, T.R.; Mills, E.N.C.; Belton, P.S. Plasticization of a Protein-Based Film by Glycerol: A Spectroscopic, Mechanical, and Thermal Study. J. Agric. Food Chem. 2006, 54, 4611–4616. [Google Scholar] [CrossRef]
- Xu, H.; Chai, Y.; Zhang, G. Synergistic Effect of Oleic Acid and Glycerol on Zein Film Plasticization. J. Agric. Food Chem. 2012, 60, 10075–10081. [Google Scholar] [CrossRef]
- Cunningham, P.; Ogale, A.A.; Dawson, P.L.; Acton, J.C. Tensile Properties of Soy Protein Isolate Films Produced by a Thermal Compaction Technique. J. Food Sci. 2000, 65, 668–671. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. The Role of Surfactants on Ethyl-Cellulose Oleogel Structure and Mechanical Properties. Carbohydr. Polym. 2015, 127, 355–362. [Google Scholar] [CrossRef]
- Keshanidokht, S.; Kumar, S.; Thulstrup, P.W.; Via, M.A.; Clausen, M.P.; Risbo, J. Thermo-Responsive Behavior of Glycerol-Plasticized Oleogels Stabilized by Zein. Food Hydrocoll. 2023, 139, 108582. [Google Scholar] [CrossRef]
- Rodríguez, M.; Os’es, J.; Ziani, K.; Mat’e, J.I. Combined Effect of Plasticizers and Surfactants on the Physical Properties of Starch Based Edible Films. Food Res. Inter. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Hidayati, S.; Maulidia, U.; Satyajaya, W.; Hadi, S. Effect of Glycerol Concentration and Carboxy Methyl Cellulose on Biodegradable Film Characteristics of Seaweed Waste. Heliyon 2021, 7, e07799. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of Glycerol and Sorbitol on Cellulose-Based Biodegradable Films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R.; Altstadt, V. Poly(Lactic Acid)/Coplasticized Thermoplastic Starch Blend: Effect of Plasticizer Migration on Rheological and Mechanical Properties. Polym. Adv. Technol. 2019, 30, 839–851. [Google Scholar] [CrossRef]
- Di Gioia, L.; Guilbert, S. Corn Protein-Based Thermoplastic Resins: Effect of some Polar and Amphiphilic Plasticizers. J. Agric. Food Chem. 1999, 47, 1254–1261. [Google Scholar] [CrossRef]
- Baimark, Y.; Niamsa, N.; Morakot, N.; Threeprom, J.; Srisuwan, Y. Preparation and Morphology Study of Biodegradable Chitosan/Methoxy Poly(Ethylene Glycol)-b-Poly(ε-Caprolactone) Nanocomposite Films. Int. J. Polym. Anal. Charact. 2007, 12, 457–467. [Google Scholar] [CrossRef]
- Adel, A.; El-Shafei, A.; Ibrahim, A.; Al-Shemy, M. Extraction of Oxidized Nanocellulose from Date Palm (Phoenix Dactylifera L.) Sheath Fibers: Influence of CI and CII Polymorphs on the Properties of Chitosan/Bionanocomposite Films. Ind. Crops Prod. 2018, 124, 155–165. [Google Scholar] [CrossRef]
- Mehanny, S.; Abu-El Magd, E.E.; Ibrahim, M.; Farag, M.; Gil-San-Millan, R.; Navarro, J.; El Habbak, A.E.H.; El-Kashif, E. Extraction and Characterization of Nanocellulose from Three Types of Palm Residues. J. Mater. Res. Technol. 2021, 10, 526–537. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F. Mechanical and Water Barrier Properties of Agar/κ-Carrageenan/Konjac Glucomannan Ternary Blend Biohydrogel Films. Carbohydr. Polym. 2013, 96, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Thongsomboon, W.; Baimark, Y.; Srihanam, P. Valorization of Cellulose-Based Materials from Agricultural Waste: Comparison between Sugarcane Bagasse and Rice Straw. Polymers 2023, 15, 3190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Yan, D.; Zhang, C.; Nie, S. Enzyme-Assisted Mechanical Production of Cellulose Nanofibrils: Thermal Stability. Cellulose 2018, 25, 5049–5061. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tapia-Blácido, D.R. Structural Modification of Fiber and Starch in Turmeric Residue by Chemical and Mechanical Treatment for Production of Biodegradable Films. Int. J. Biol. Macromol. 2019, 126, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.; Pires, A.S.; Mateus, N.; Freitas, V.; Cruz, L. Pyranoflavylium-Cellulose Acetate Films and the Glycerol Effect towards the Development of pH-Freshness Smart Label for Food Packaging. Food Hydrocoll. 2022, 127, 107501. [Google Scholar] [CrossRef]
- Lassoued, M.; Crispino, F.; Loranger, E. Design and Synthesis of Transparent and Flexible Nanofibrillated Cellulose Films to Replace Petroleum-Based Polymers. Carbohydr. Polym. 2021, 254, 117411. [Google Scholar] [CrossRef]
- Shabanpour, B.; Kazemi, M.; Ojagh, S.M.; Pourashouri, P. Bacterial Cellulose Nanofibers as Reinforce in Edible Fish Myofibrillar Protein Nanocomposite Films. Int. J. Biol. Macromol. 2018, 117, 742–751. [Google Scholar] [CrossRef]
- Sogut, E. Active Whey Protein Isolate Films Including Bergamot Oil Emulsion Stabilized by Nanocellulose. Food Packag. Shelf Life 2020, 23, 100430. [Google Scholar] [CrossRef]
- Karatzos, S.K.; Edye, L.A.; Orlando, W.; Doherty, S. Sugarcane Bagasse Pretreatment Using Three Imidazolium—Based Ionic Liquids: Mass Balances and Enzyme Kinetics. Biotechnol. Biofuels 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Qi, Y.; Lin, S.; Lan, J.; Zhan, Y.; Guo, J. Fabrication of Super-High Transparent Cellulose Films with Multifunctional Performances via Post-Modification Strategy. Carbohydr. Polym. 2021, 260, 117760. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rahaman, T.; Gupta, P.; Mitra, R.; Dutta, S.; Kharlyngdoh, E.; Guha, S.; Ganguly, J.; Pal, A.; Das, M. Cellulose and Lignin Profiling in Seven, Economically Important Bamboo Species of India by Anatomical, Biochemical, FTIR Spectroscopy and Thermogravimetric Analysis. Biomass Bioenerg. 2022, 158, 106362. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollaku, R. Effect of Cellulose Nanocrystals from Sugarcane Bagasse on Whey Protein Isolate-based Films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, Y.; Chen, M.; Li, J.; Li, J.; Luo, J.; Gao, Q. A Soy Protein-based Film by Mixed Covalent Cross-Linking and Flexibilizing Networks. Ind. Crops Prod. 2022, 183, 114952. [Google Scholar] [CrossRef]
- Osorio-Ruiz, A.; Avena-Bustillos, R.J.; Chiou, B.-S.; Rodríguez-González, F.; MartinezAyala, A.-L. Mechanical and Thermal Behavior of Canola Protein Isolate Films as Improved by Cellulose Nanocrystals. ACS Omega 2019, 4, 19172–19176. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.R.; Jian, R.; Zheng, P.; Yu, J.; Ma, X. Preparation and Properties of Glycerol Plasticized Starch (GPS)/Cellulose NanoParticle (CN) Composites. Carbohydr. Polym. 2010, 79, 301–305. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on Cellulose Nanocrystals Produced from Cellulose Sources with Various Polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Hemida, M.H.; Moustafa, H.; Mehanny, S.; Morsy, M.; Dufresne, A.; Abd EL Rahman, E.N.; Ibrahim, M.M. Cellulose NanoCrystals from Agricultural Residues (Eichhornia crassipes): Extraction and Characterization. Heliyon 2023, 6, e16436. [Google Scholar] [CrossRef]
| Films | T660 (%) | MC (%) | WS (%) | WCA (°) |
|---|---|---|---|---|
| Native CTC | 97.78 ± 0.23 | 3.13 ± 0.10 | 1.96 ± 1.75 | 90.53 ± 2.65 |
| CTC + 1.38% glycerol | 98.55 ± 0.35 | 3.96 ± 0.23 | 11.90 ± 2.05 | 75.20 ± 1.06 |
| CTC + 2.78% glycerol | 99.83 ± 0.55 | 4.13 ± 0.32 | 17.43 ± 1.84 | 59.33 ± 1.20 |
| CTC + 4.14% glycerol | 99.33 ± 0.92 | 4.54 ± 0.19 | 22.31 ± 2.23 | 51.73 ± 1.17 |
| Films | Onset of Decomposition (°C) | Td, max (°C) | Charred Residue Weight at 800 °C (%) |
|---|---|---|---|
| Native CTC | 175 | 337, 466 | 20 |
| CTC + 1.38% glycerol | 180 | 335, 466 | 12 |
| CTC + 2.78% glycerol | 120 | 209, 355, 466 | 10 |
| CTC + 4.14% glycerol | 120 | 213, 354, 466 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khotsaeng, N.; Simchuer, W.; Imsombut, T.; Srihanam, P. Effect of Glycerol Concentrations on the Characteristics of Cellulose Films from Cattail (Typha angustifolia L.) Flowers. Polymers 2023, 15, 4535. https://doi.org/10.3390/polym15234535
Khotsaeng N, Simchuer W, Imsombut T, Srihanam P. Effect of Glycerol Concentrations on the Characteristics of Cellulose Films from Cattail (Typha angustifolia L.) Flowers. Polymers. 2023; 15(23):4535. https://doi.org/10.3390/polym15234535
Chicago/Turabian StyleKhotsaeng, Nuanchai, Wilaiwan Simchuer, Thanonchat Imsombut, and Prasong Srihanam. 2023. "Effect of Glycerol Concentrations on the Characteristics of Cellulose Films from Cattail (Typha angustifolia L.) Flowers" Polymers 15, no. 23: 4535. https://doi.org/10.3390/polym15234535
APA StyleKhotsaeng, N., Simchuer, W., Imsombut, T., & Srihanam, P. (2023). Effect of Glycerol Concentrations on the Characteristics of Cellulose Films from Cattail (Typha angustifolia L.) Flowers. Polymers, 15(23), 4535. https://doi.org/10.3390/polym15234535







