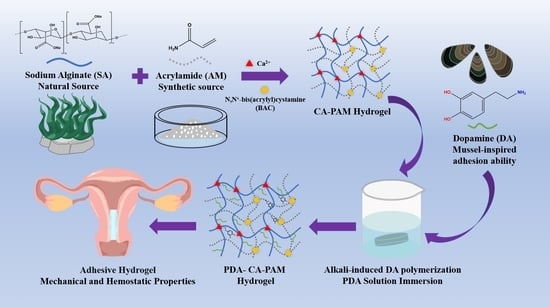
Mussel-Inspired Calcium Alginate/Polyacrylamide Dual Network Hydrogel: A Physical Barrier to Prevent Postoperative Re-Adhesion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
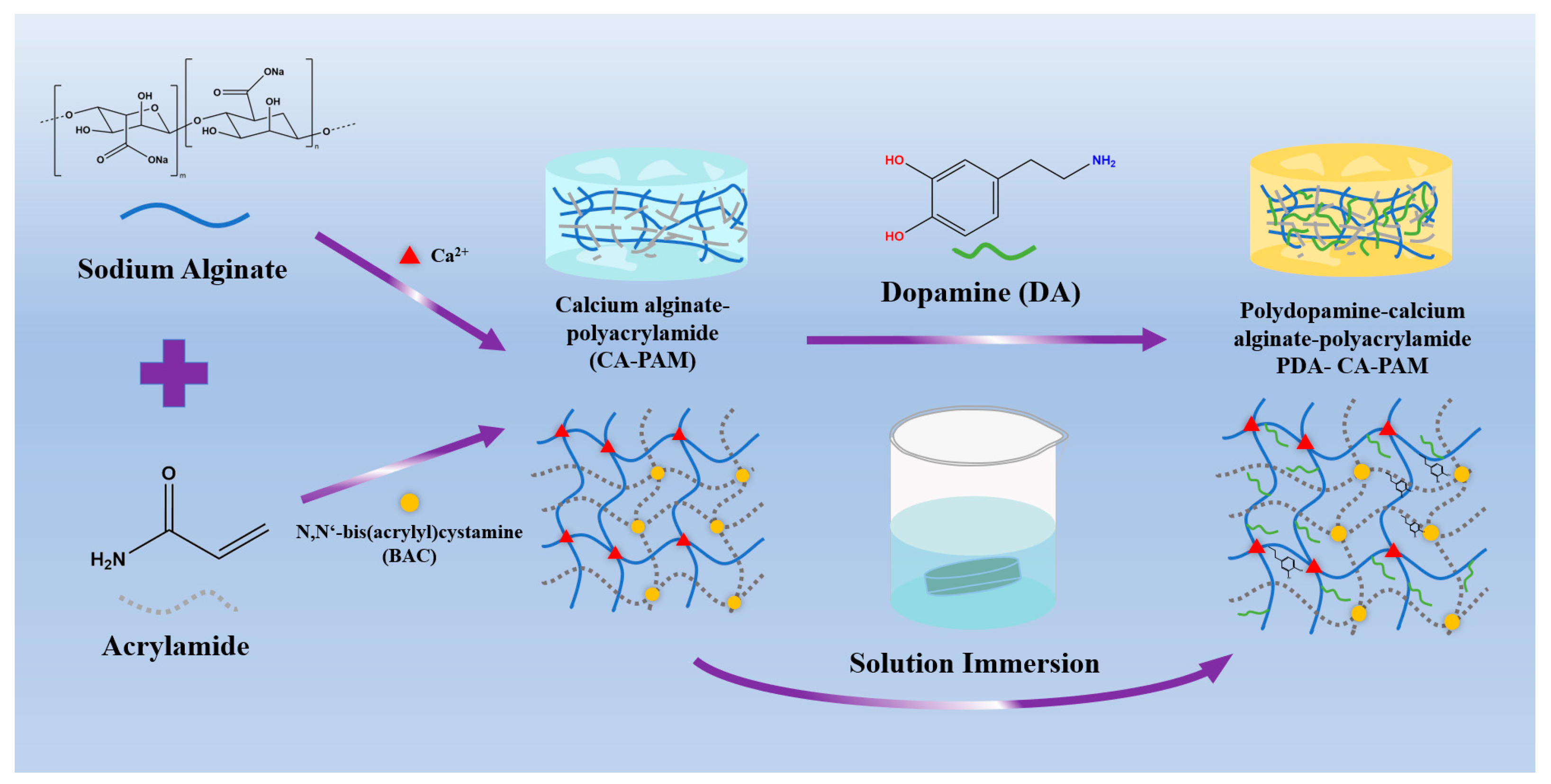
2.2. Preparation of PDA-CA-PAM Hydrogels
2.3. Characterization
2.4. Adhesion Measurements
2.5. Swelling Behavior
2.6. Biodegradation Behavior
2.7. Hydrophilicity Assay
2.8. Hemolysis Assay
2.9. Hemostasis Assay
2.10. Cytocompatibility
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of PDA-CA-PAM Hydrogel
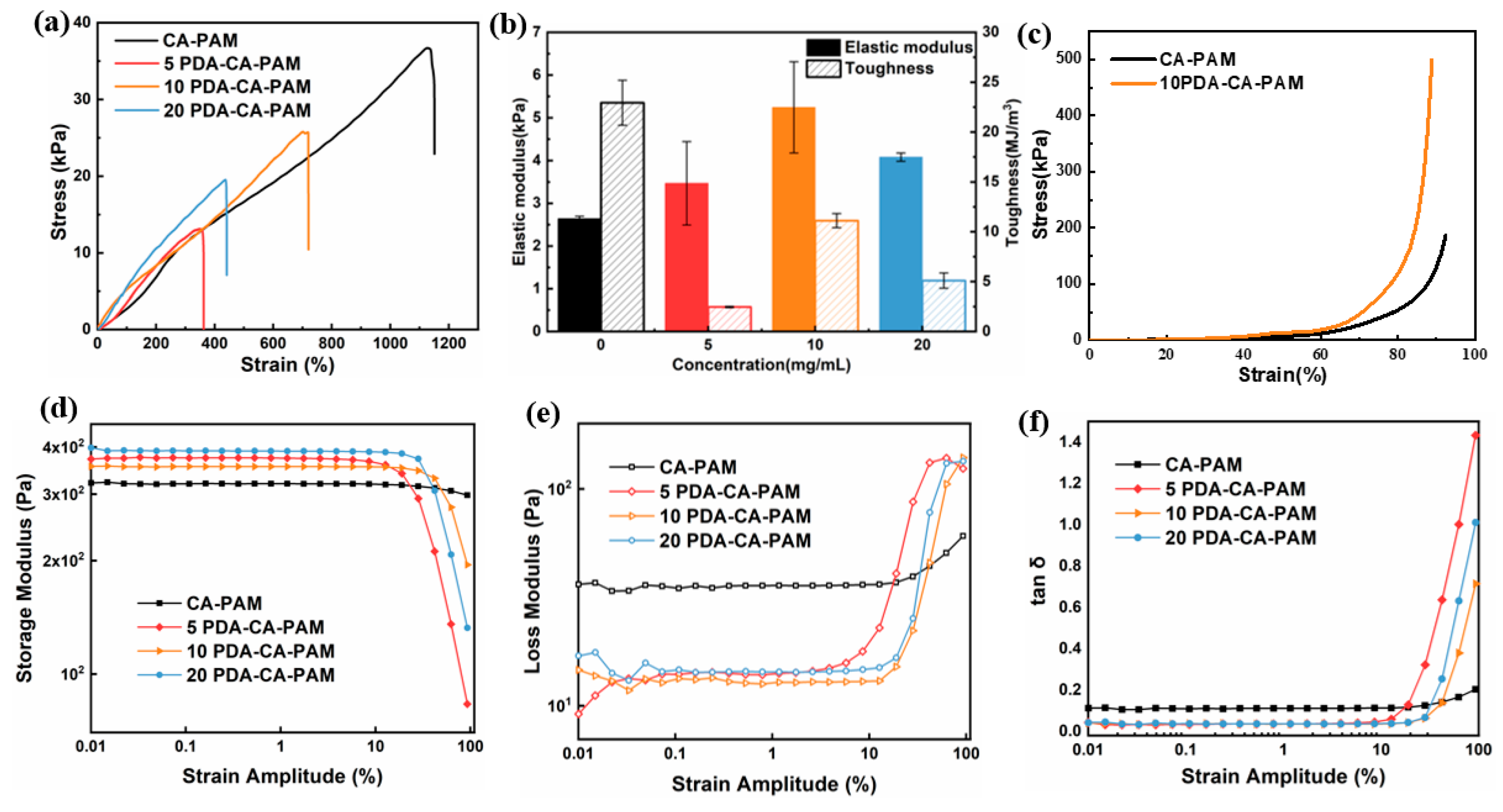
3.2. Mechanical Properties and Rheological Analysis
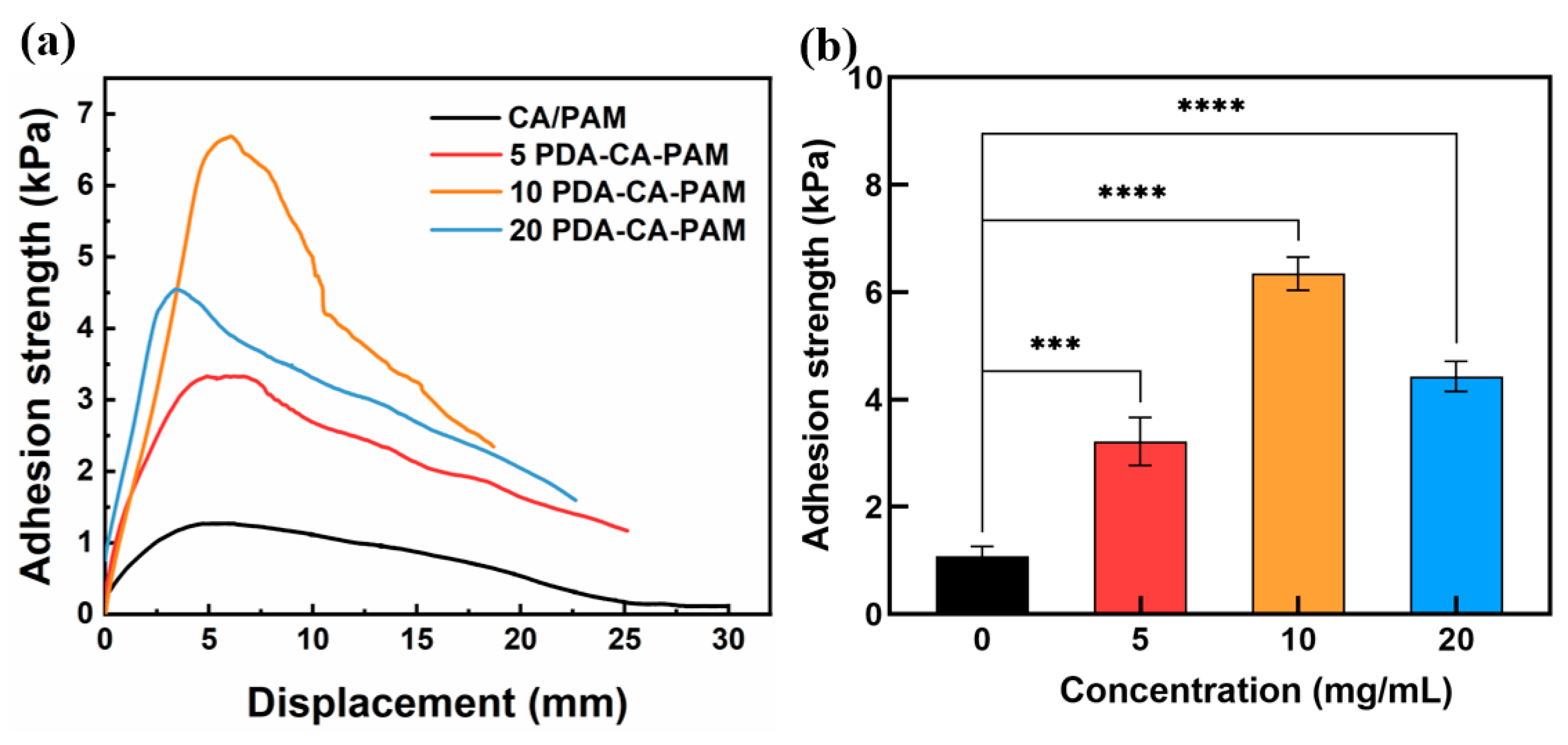
3.3. Adhesive Performance
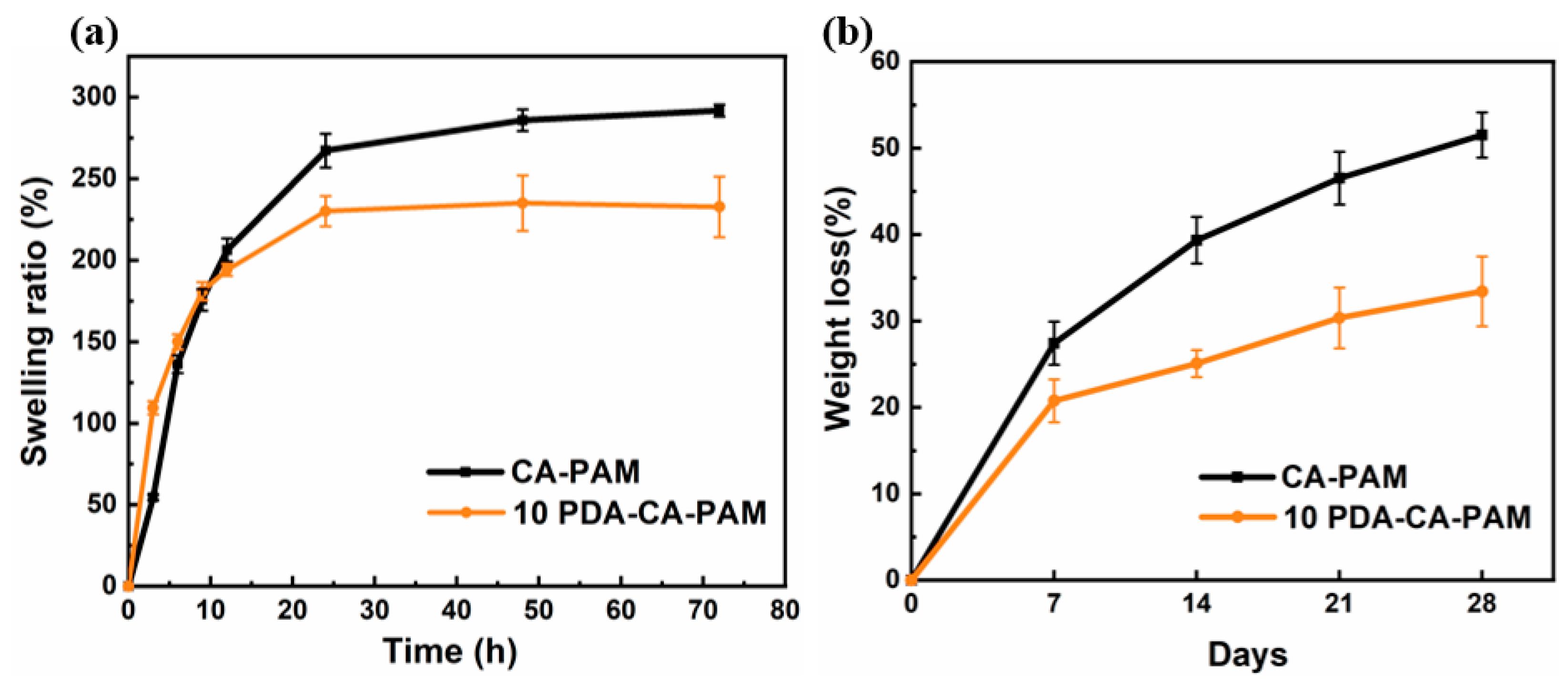
3.4. Swelling and Biodegradation
3.5. Hydrophilicity and Hemostasis Performance
3.6. Cell Proliferation, Viability, and Attachment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, D.; Wong, Y.M.; Cheong, Y.; Xia, E.L.; Li, T.C. Asherman syndrome-one century later. Fertil. Steril. 2008, 89, 759–779. [Google Scholar] [CrossRef] [PubMed]
- Sardo, A.D.; Calagna, G.; Scognamiglio, M.; O’Donovan, P.; Campo, R.; De Wilde, R.L. Prevention of intrauterine post-surgical adhesions in hysteroscopy. A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Pan, Y.B.; Zhang, Y.L.; Dai, Y.D.; Jiang, L.L.; Shi, L.B.; Yang, W.J.; Xu, S.Q.; Zhang, Y.Y.; Xu, W.Z.; et al. Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells. Cell Death Dis. 2020, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Deans, R.; Abbott, J. Review of Intrauterine Adhesions. J. Minim. Invasive Gynecol. 2010, 17, 555–569. [Google Scholar] [CrossRef]
- Salma, U.; Xue, M.; Sayed, A.S.M.; Xu, D.B. Efficacy of Intrauterine Device in the Treatment of Intrauterine Adhesions. Biomed. Res. Int. 2014, 2014, 589296. [Google Scholar] [CrossRef]
- Lin, X.N.; Zhou, F.; Wei, M.L.; Yang, Y.; Li, Y.; Li, T.C.; Zhang, S.Y. Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. Fertil. Steril. 2015, 104, 235–240. [Google Scholar] [CrossRef]
- Zhang, E.S.; Yang, J.H.; Wang, K.; Song, B.Y.; Zhu, H.; Han, X.F.; Shi, Y.J.; Yang, C.B.; Zeng, Z.P.; Cao, Z.Q. Biodegradable Zwitterionic Cream Gel for Effective Prevention of Postoperative Adhesion. Adv. Funct. Mater. 2021, 31, 2009431. [Google Scholar] [CrossRef]
- Tonguc, E.A.; Var, T.; Yilmaz, N.; Batioglu, S. Intrauterine device or estrogen treatment after hysteroscopic uterine septum resection. Int. J. Gynecol. Obstet. 2010, 109, 226–229. [Google Scholar] [CrossRef]
- Erdi, M.; Saruwatari, M.S.; Rozyyev, S.; Acha, C.; Ayyub, O.B.; Sandler, A.D.; Kofinas, P. Controlled Release of a Therapeutic Peptide in Sprayable Surgical Sealant for Prevention of Postoperative Abdominal Adhesions. ACS Appl. Mater. Interfaces 2023, 15, 14089–14098. [Google Scholar] [CrossRef]
- Zou, Y.K.; Yue, P.P.; Cao, H.K.; Wu, L.Q.; Xu, L.; Liu, Z.Z.; Wu, S.Q.; Ye, Q.F. Biocompatible and biodegradable chitin-based hydrogels crosslinked by BDDE with excellent mechanical properties for effective prevention of postoperative peritoneal adhesion. Carbohyd. Polym. 2023, 305, 120543. [Google Scholar] [CrossRef]
- Orhue, A.A.E.; Aziken, M.E.; Igbefoh, J.O. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int. J. Gynecol. Obs. 2003, 82, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.M.R.; Black, C.R.M.; Dawson, J.I.; Oreffo, R.O.C. A review of hydrogel use in fracture healing and bone regeneration. J. Tissue Eng. Regen. Med. 2016, 10, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.J.; Yuan, Y.F.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Murphy, W.L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Fei, Z.; Xin, X.; Fei, H.; Cui, Y.C. Meta-analysis of the use of hyaluronic acid gel to prevent intrauterine adhesions after miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 1–4. [Google Scholar] [CrossRef]
- Kim, T.; Ahn, K.H.; Choi, D.S.; Hwang, K.J.; Lee, B.I.; Jung, M.H.; Kim, J.W.; Kim, J.H.; Cha, S.H.; Lee, K.H.; et al. A Randomized, Multi-Center, Clinical Trial to Assess the Efficacy and Safety of Alginate Carboxymethylcellulose Hyaluronic Acid Compared to Carboxymethylcellulose Hyaluronic Acid to Prevent Postoperative Intrauterine Adhesion. J. Minim. Invasive Gynecol. 2012, 19, 731–736. [Google Scholar] [CrossRef]
- Park, D.Y.; Chung, H.J.; Sim, N.S.; Jo, K.H.; Kim, D.H.; Kim, C.H.; Yoon, J.H. Comparison of calcium alginate and carboxymethyl cellulose for nasal packing after endoscopic sinus surgery: A prospective, randomised, controlled single-blinded trial. Clin. Otolaryngol. 2016, 41, 234–240. [Google Scholar] [CrossRef]
- Carruthers, A.; Carruthers, J. Non-animal-based hyaluronic acid fillers: Scientific and technical considerations. Plast. Reconstr. Surg. 2007, 120, 33s–40s. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.H.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z.G. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.P.; Zhou, G.S.; Chen, Y.Y.; Mao, C.B.; Yang, M.Y. Polydopamine-Coated Antheraea pernyi (A. pernyi) Silk Fibroin Films Promote Cell Adhesion and Wound Healing in Skin Tissue Repair. ACS Appl. Mater. Interfaces 2019, 11, 34736–34743. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Lin, Y.J.; Enriquez, E.; Peng, X.F.; Turng, L.S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Y.; Wang, J.; Yao, X.; Peng, Y.; Tu, X.X.; Du, P.F.; Zheng, Z.; Wang, X.L. Facile Preparation of Mussel-Inspired Polyurethane Hydrogel and Its Rapid Curing Behavior. ACS Appl. Mater. Interfaces 2014, 6, 12495–12504. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Suneetha, M.; Rao, K.M.; Han, S.S. Mussel-Inspired Cell/Tissue-Adhesive, Hemostatic Hydrogels for Tissue Engineering Applications. Acs Omega 2019, 4, 12647–12656. [Google Scholar] [CrossRef]
- Xie, Z.H.; Li, H.; Mi, H.Y.; Feng, P.Y.; Liu, Y.J.; Jing, X. Freezing-tolerant, widely detectable and ultra-sensitive composite organohydrogel for multiple sensing applications. J. Mater. Chem. C 2021, 9, 10127–10137. [Google Scholar] [CrossRef]
- Fan, L.; Xie, L.; Zheng, Y.P.; Wei, D.X.; Yao, D.D.; Zhang, J.; Zhang, T.D. Antibacterial, Self-Adhesive, Recyclable, and Tough Conductive Composite Hydrogels for Ultrasensitive Strain Sensing. ACS Appl. Mater. Interfaces 2020, 12, 22225–22236. [Google Scholar] [CrossRef]
- Yan, M.; Shi, J.F.; Tang, S.; Zhou, G.H.; Zeng, J.X.; Zhang, Y.X.; Zhang, H.; Yu, Y.; Guo, J. Preparation of high-strength and high-toughness biomass medical films based on a polydopamine dynamically united calcium alginate/carboxymethyl chitosan dual network. New J. Chem. 2021, 45, 14469–14482. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, W.J.; Lei, D.; Huang, J.F.; Yin, Y.P.; Zhu, Y.Q.; You, Z.W.; Wang, F.; Sun, S.H. PGS Scaffolds Promote the In Vivo Survival and Directional Differentiation of Bone Marrow Mesenchymal Stem Cells Restoring the Morphology and Function of Wounded Rat Uterus. Adv. Healthc. Mater. 2019, 8, 1801455. [Google Scholar] [CrossRef] [PubMed]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef]
- Liang, M.; He, C.P.; Dai, J.D.; Ren, P.F.; Fu, Y.F.; Wang, F.M.; Ge, X.; Zhang, T.Z.; Lu, Z.H. A high-strength double network polydopamine nanocomposite hydrogel for adhesion under seawater. J. Mater. Chem. B 2020, 8, 8232–8241. [Google Scholar] [CrossRef]
- Bu, Y.Z.; Pandit, A. Cohesion mechanisms for bioadhesives. Bioact. Mater. 2022, 13, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Pang, Y.; Zhang, S.Y.; Cleveland, C.; Yin, X.L.; Booth, L.; Lin, J.Q.; Lee, Y.A.L.; Mazdiyasni, H.; Saxton, S.; et al. Triggerable tough hydrogels for gastric resident dosage forms. Nat. Commun. 2017, 8, 124. [Google Scholar] [CrossRef]
- Li, J.Y.; Suo, Z.G.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.P.; Zhang, T.L.; Ma, P.X.; Guo, B.L. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Correia, A.; Hasany, M.; Figueiredo, P.; Dobakhti, F.; Eskandari, M.R.; Hosseini, S.H.; Abiri, R.; Khorshid, S.; Hirvonen, J.; et al. A Hydrogen-Bonded Extracellular Matrix-Mimicking Bactericidal Hydrogel with Radical Scavenging and Hemostatic Function for pH-Responsive Wound Healing Acceleration. Adv. Healthc. Mater. 2021, 10, 2001122. [Google Scholar] [CrossRef]
- Liu, X.M.; Wang, H.H.; She, J.J.; Zhang, Q.; Hu, Q.Y.; Li, D.D.; Wu, H.L.; Ye, X.F.; Diao, R.Y.; Shi, X.T.; et al. An anti-fibroblast adhesion and anti-inflammatory hydrogel film combined with VEGF for intrauterine adhesion prevention. Chem. Eng. J. 2023, 466, 143144. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Xue, B.; Xu, C.; Dong, X. Mussel-Inspired Calcium Alginate/Polyacrylamide Dual Network Hydrogel: A Physical Barrier to Prevent Postoperative Re-Adhesion. Polymers 2023, 15, 4498. https://doi.org/10.3390/polym15234498
Su Z, Xue B, Xu C, Dong X. Mussel-Inspired Calcium Alginate/Polyacrylamide Dual Network Hydrogel: A Physical Barrier to Prevent Postoperative Re-Adhesion. Polymers. 2023; 15(23):4498. https://doi.org/10.3390/polym15234498
Chicago/Turabian StyleSu, Zekun, Beibei Xue, Chang Xu, and Xufeng Dong. 2023. "Mussel-Inspired Calcium Alginate/Polyacrylamide Dual Network Hydrogel: A Physical Barrier to Prevent Postoperative Re-Adhesion" Polymers 15, no. 23: 4498. https://doi.org/10.3390/polym15234498
APA StyleSu, Z., Xue, B., Xu, C., & Dong, X. (2023). Mussel-Inspired Calcium Alginate/Polyacrylamide Dual Network Hydrogel: A Physical Barrier to Prevent Postoperative Re-Adhesion. Polymers, 15(23), 4498. https://doi.org/10.3390/polym15234498